New Gd3+ and Mn2+-Co-Doped Scheelite-Type Ceramics—Their Structural, Optical and Magnetic Properties
Abstract
1. Introduction
2. Results and Discussion
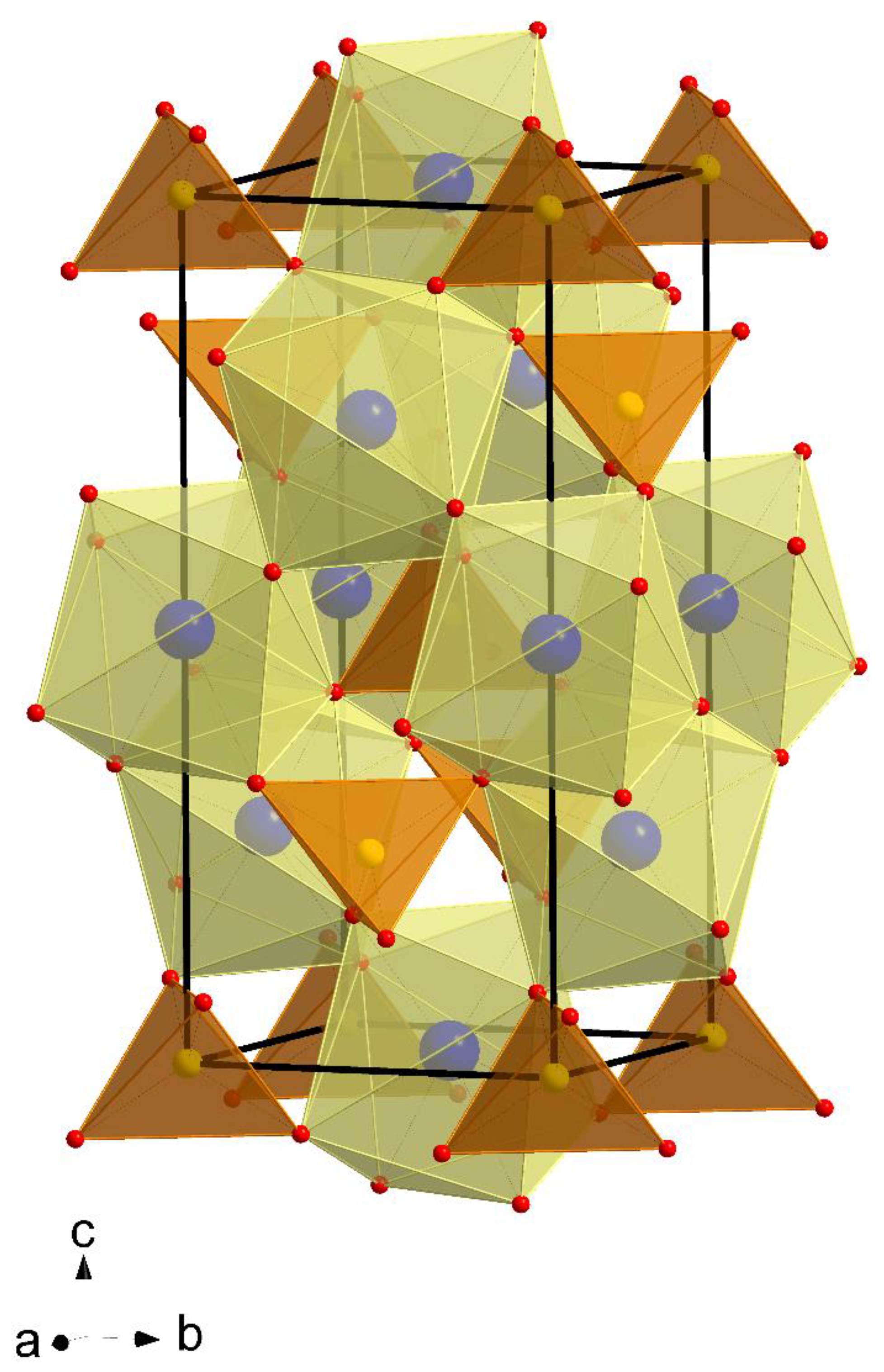
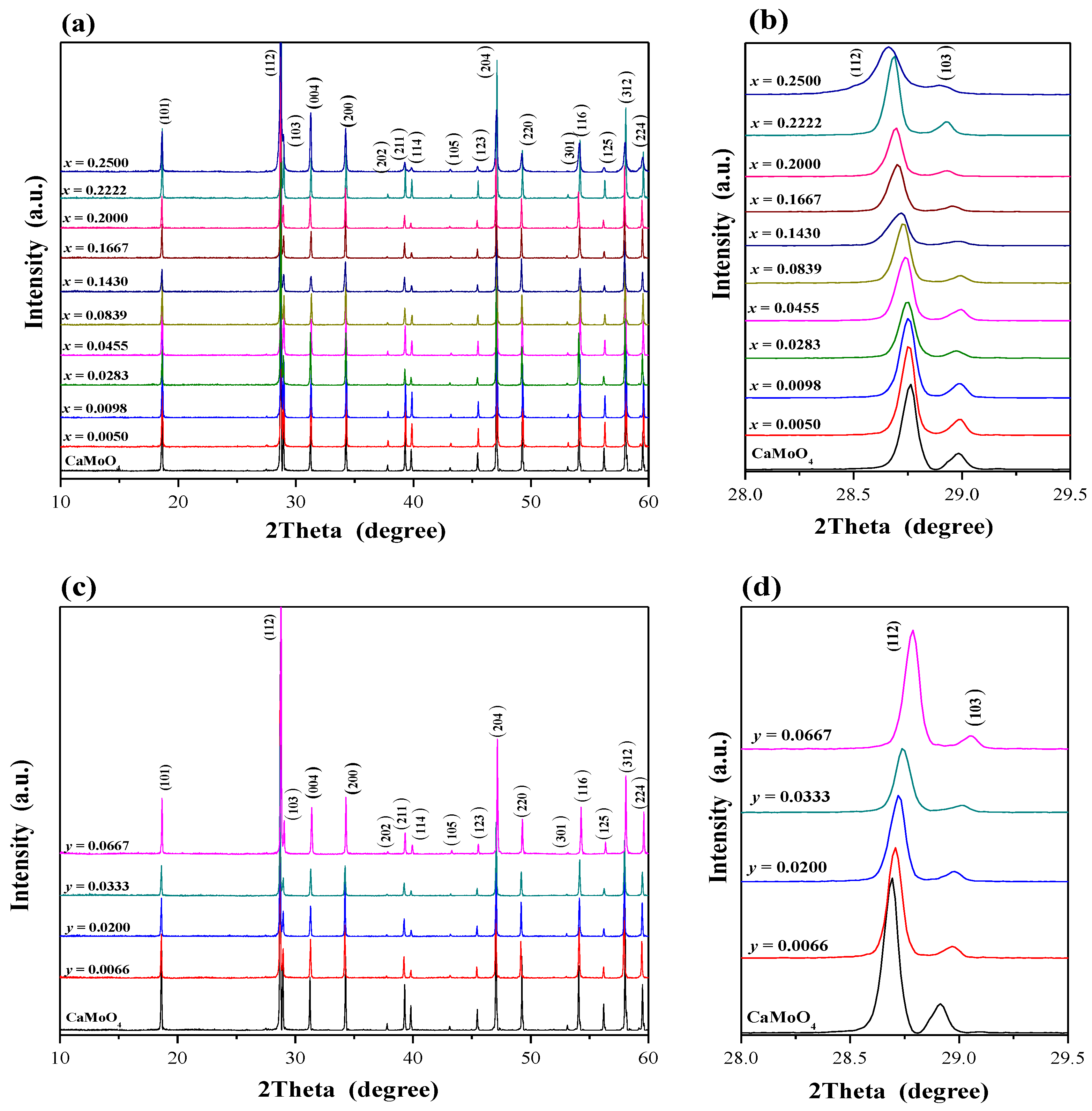
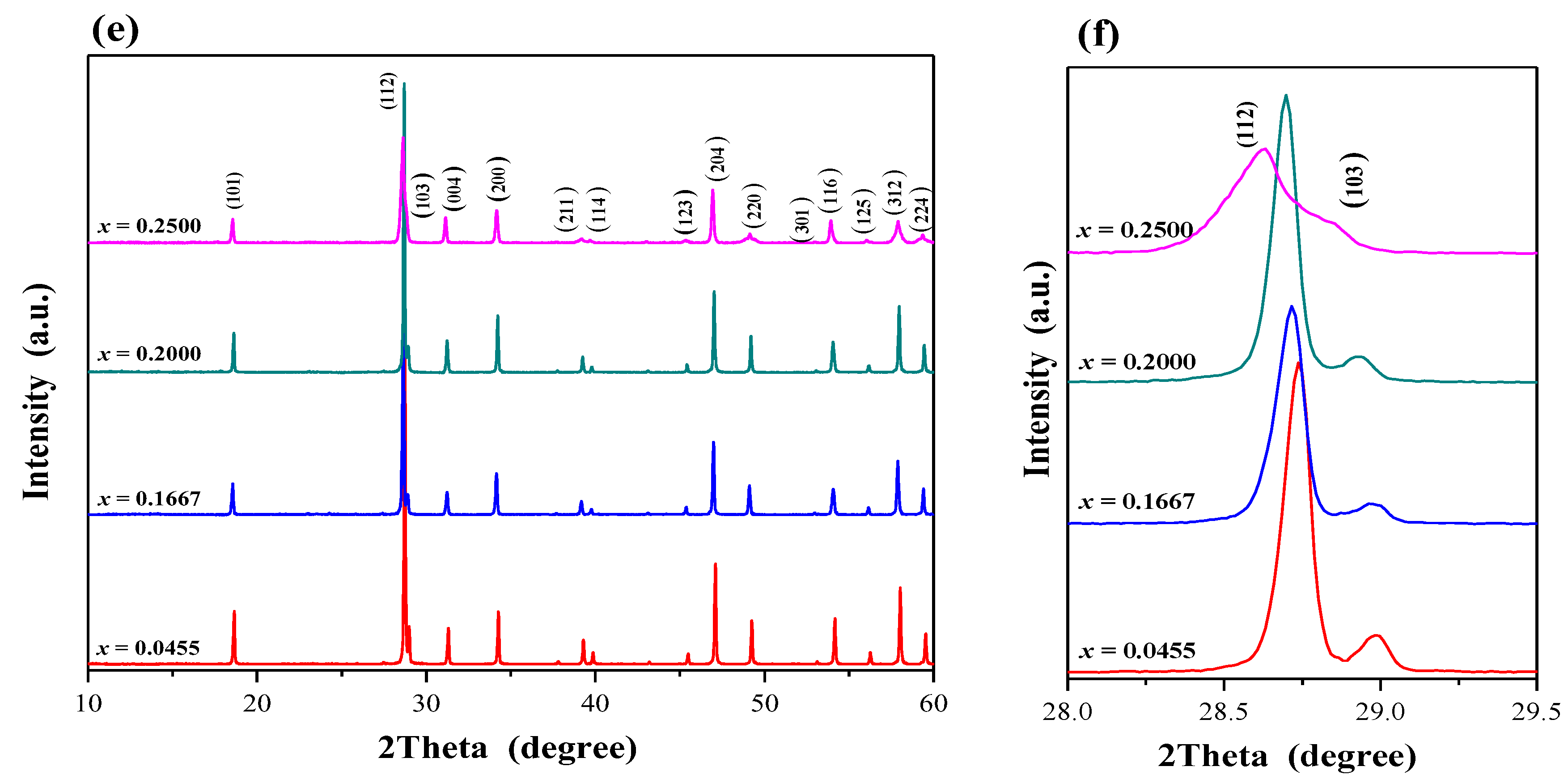
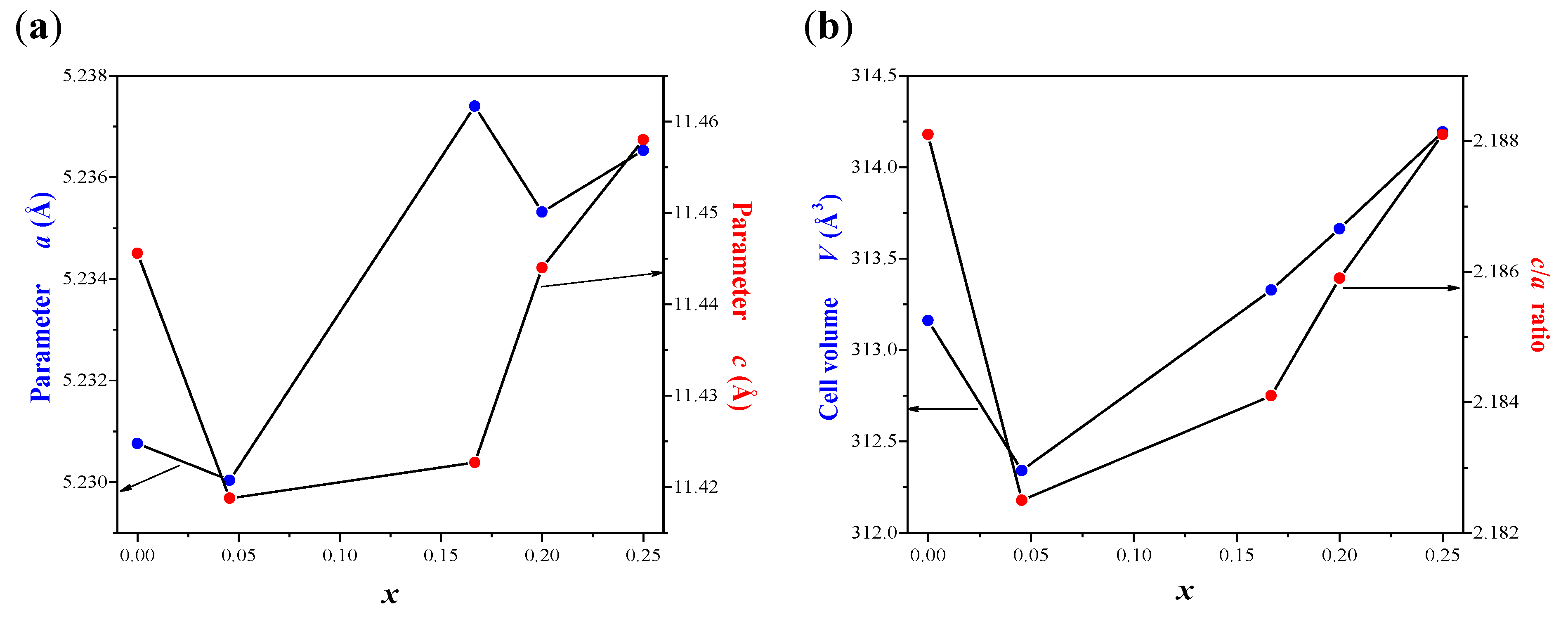
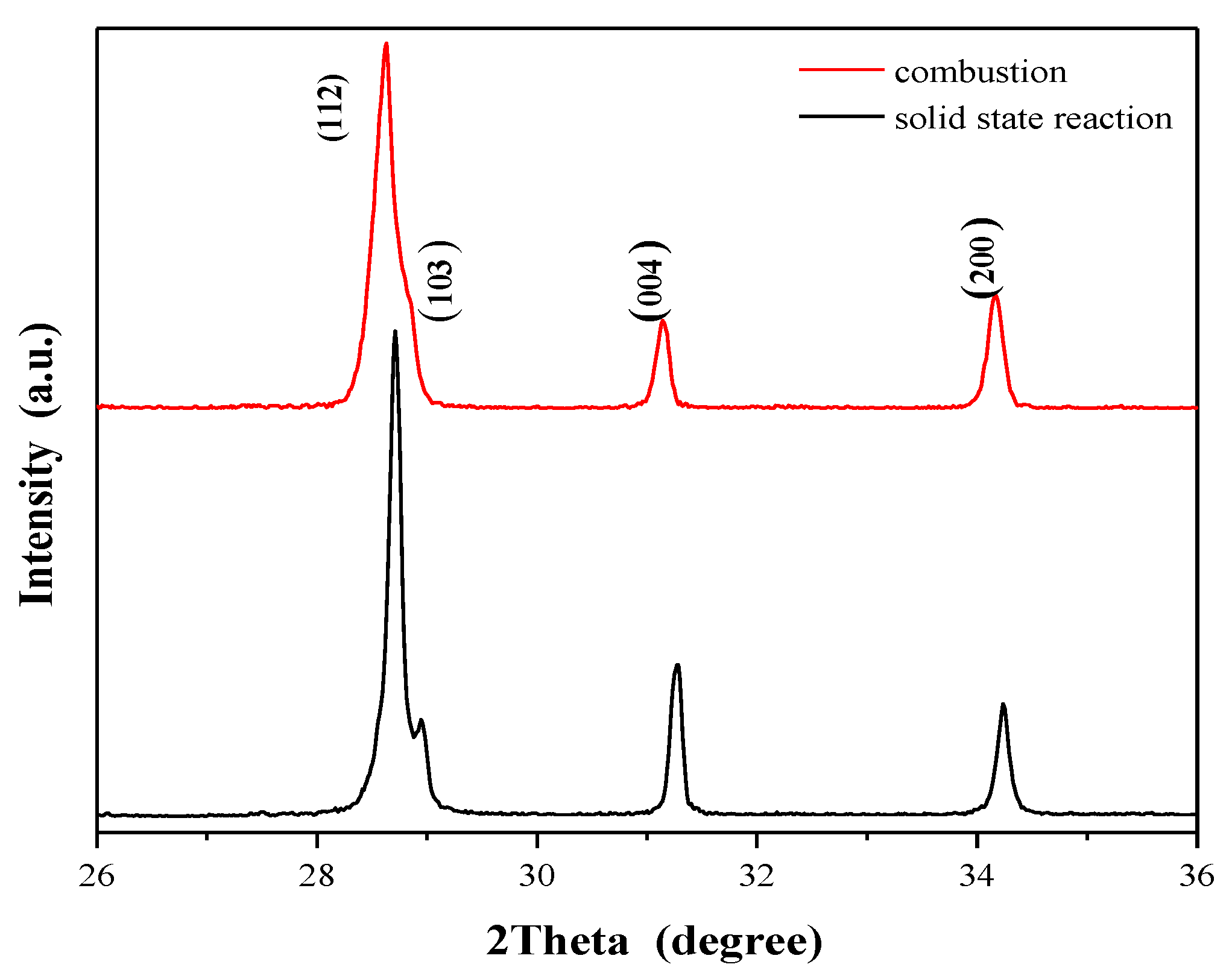
2.1. X-ray Diffraction Studies of CaMnGdMoWO Materials
2.2. Thermal Stability and Morphology of CaMnGdMoWO Solid Solution
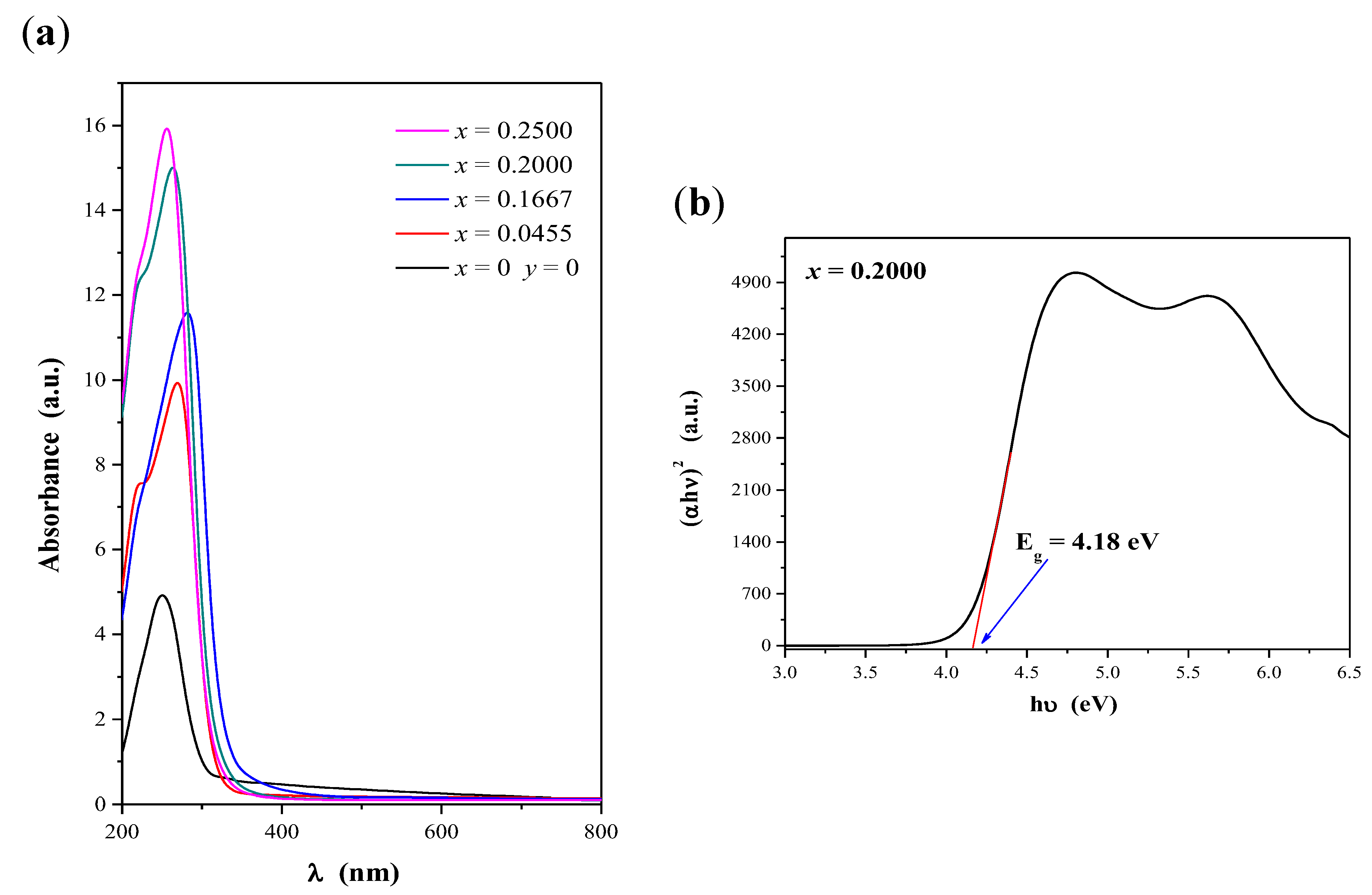
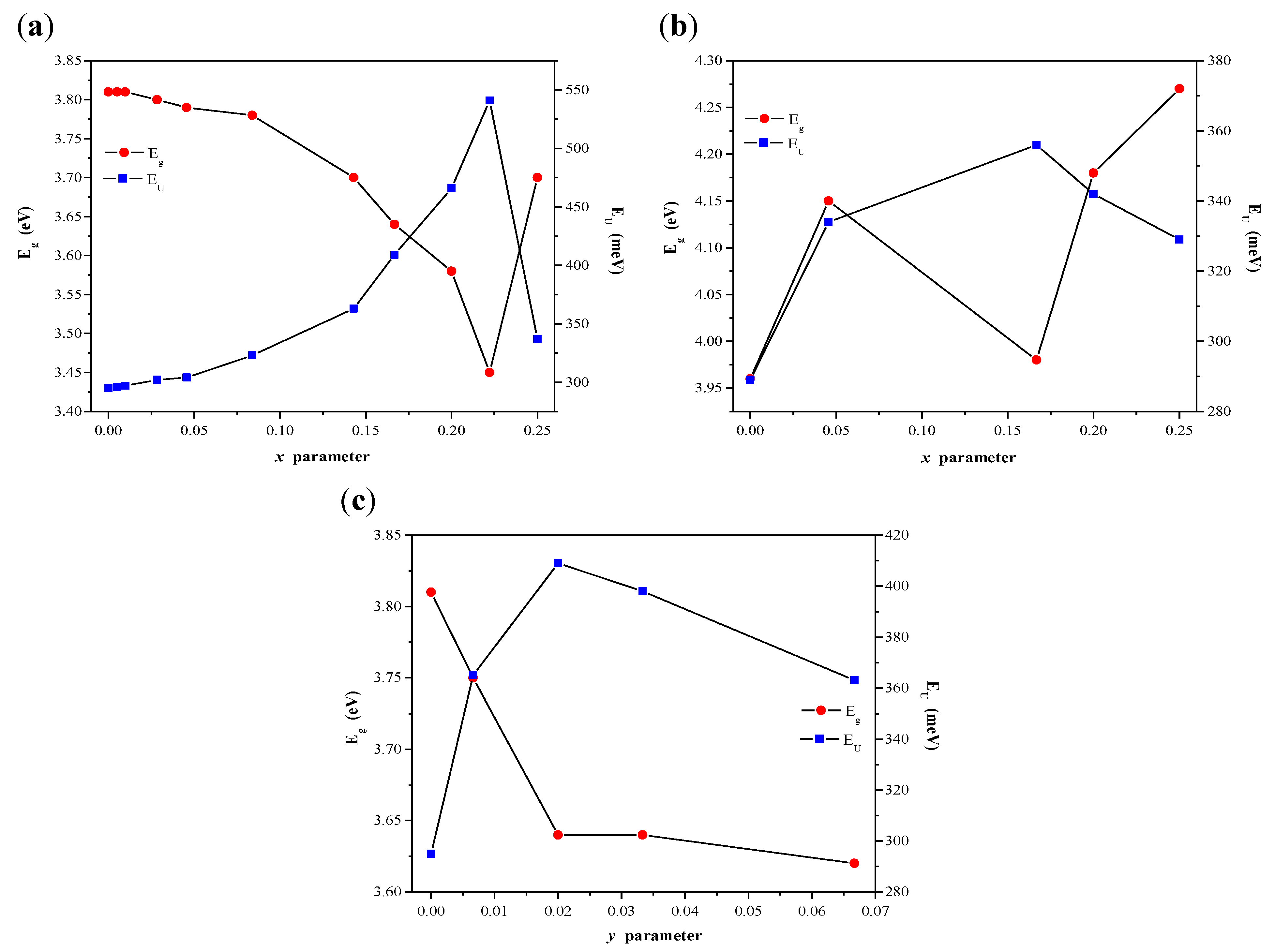
2.3. Optical Properties of CaMnGdMoWO Ceramic Materials
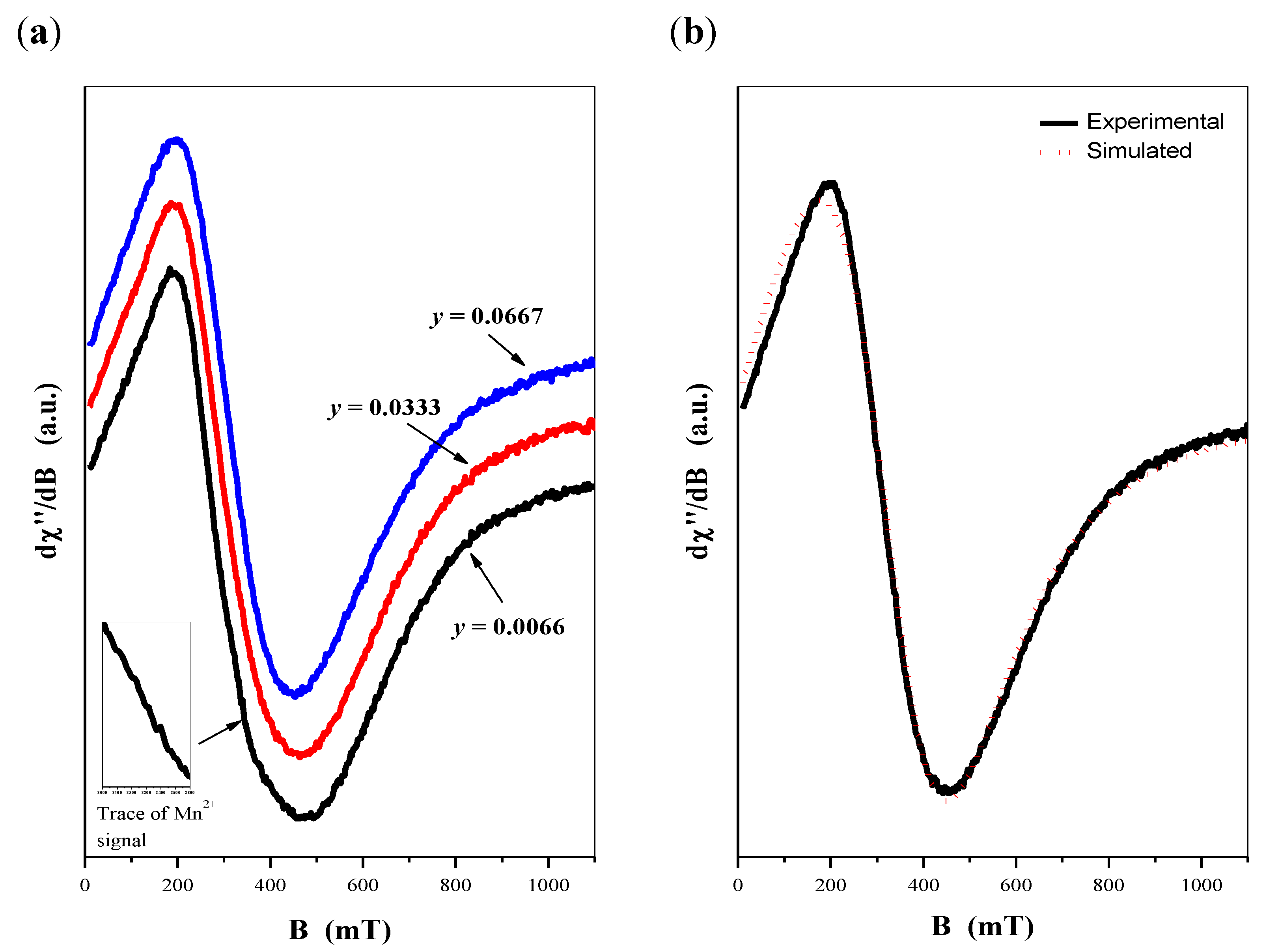
2.4. EPR Spectra of CaMnGdMoWO Materials
- -
- obtained using solid-state reaction method (fixed Mn2+ contribution, i.e., y = 0.0200),
- -
- obtained using solid sta-te reaction method (fixed Gd3+ contribution, i.e., x = 0.1667) and,
- -
- obtained using combustion synthesis (fixed Mn2+ contribution, i.e., y = 0.0200).
3. Materials and Methods
3.1. Synthesis of CaMnGdMoWO Solid Solution
Ca1−3x−yMny▯xGd2x(MoO4)1−3x(WO4)3x
3.2. Characterization of Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gd2(WO4)3 Content [mol%] | MnMoO4 Content [mol%] | Formula of CaMnGdMoWO Solid Solution, Values of x and y Parameters | Lattice Parameters | Density [g·cm−3] | Eg [eV] | EU [meV] | |||
|---|---|---|---|---|---|---|---|---|---|
| a [Å] | c [Å] | c/a | |||||||
| CaMnGdMoWO when y = 0.0200 (solid state reaction method) | |||||||||
| 0 | 0 | x = 0; y = 0 | CaMoO4 | 5.22961(12) | 11.4410(4) | 2.1877 | 4.24(2) | 3.81 | 295 |
| 0.50 | 3.00 | x = 0.0050 | Ca0.9650Mn0.0200▯0.0050Gd0.0100(MoO4)0.9850(WO4)0.0150 | 5.22301(9) | 11.4310(8) | 2.1886 | 4.32(1) | 3.81 | 296 |
| 1.00 | 3.00 | x = 0.0098 | Ca0.9506Mn0.0200▯0.0098Gd0.0196(MoO4)0.9706(WO4)0.0294 | 5.22829(4) | 11.4300(12) | 2.1862 | 4.33(2) | 3.81 | 297 |
| 2.50 | 3.00 | x = 0.0283 | Ca0.8951Mn0.0200▯0.0283Gd0.0566(MoO4)0.9151(WO4)0.0849 | 5.22997(5) | 11.4299(6) | 2.1855 | 4.48(1) | 3.80 | 302 |
| 5.00 | 3.00 | x = 0.0455 | Ca0.8435Mn0.0200▯0.0455Gd0.0910(MoO4)0.8635(WO4)0.1365 | 5.23236(4) | 11.4234(7) | 2.1832 | 4.72(1) | 3.79 | 304 |
| 10.00 | 3.00 | x = 0.0839 | Ca0.7283Mn0.0200▯0.0839Gd0.1678(MoO4)0.7483(WO4)0.2517 | 5.23612(6) | 11.4173(7) | 2.1805 | 4.99(2) | 3.78 | 323 |
| 20.00 | 3.00 | x = 0.1430 | Ca0.5510Mn0.0200▯0.1430Gd0.2860(MoO4)0.5710(WO4)0.4290 | 5.23958(6) | 11.4246(8) | 2.1804 | 5.51(2) | 3.70 | 363 |
| 25.00 | 3.00 | x = 0.1667 | Ca0.4799Mn0.0200▯0.1667Gd0.3334(MoO4)0.4999(WO4)0.5001 | 5.23861(5) | 11.4279(5) | 2.1815 | 5.63(1) | 3.64 | 409 |
| 33.33 | 3.00 | x = 0.2000 | Ca0.3800Mn0.0200▯0.2000Gd0.4000(MoO4)0.4000(WO4)0.6000 | 5.23821(8) | 11.4573(9) | 2.1873 | 5.94(1) | 3.58 | 466 |
| 40.00 | 3.00 | x = 0.2222 | Ca0.3134Mn0.0200▯0.2222Gd0.4444(MoO4)0.3334(WO4)0.6666 | 5.23517(7) | 11.4513(5) | 2.1874 | 6.38(2) | 3.45 | 541 |
| 50.00 | 3.00 | x = 0.2500 | Ca0.2300Mn0.0200▯0.2500Gd0.5000(MoO4)0.2500(WO4)0.7500 | 5.23567(12) | 11.4391(6) | 2.1848 | 6.67(1) | 3.70 | 337 |
| CaMnGdMoWO when y = 0.0200 (combustion method) | |||||||||
| x = 0; y = 0 | CaMoO4 | 5.23076(9) | 11.4456(7) | 2.1881 | 4.23(2) | 3.96 | 289 | ||
| x = 0.0455 | Ca0.8435Mn0.0200▯0.0455Gd0.0910(MoO4)0.8635(WO4)0.1365 | 5.23004(11) | 11.4188(9) | 2.1825 | 4.05(3) | 4.15 | 334 | ||
| x = 0.1667 | Ca0.4799Mn0.0200▯0.1667Gd0.3334(MoO4)0.4999(WO4)0.5001 | 5.23740(8) | 11.4227(7) | 2.1841 | 5.79(3) | 3.98 | 356 | ||
| x = 0.2000 | Ca0.3800Mn0.0200▯0.2000Gd0.4000(MoO4)0.4000(WO4)0.6000 | 5.23532(9) | 11.4440(8) | 2.1859 | 6.05(2) | 4.18 | 342 | ||
| x = 0.2500 | Ca0.2300Mn0.0200▯0.2500Gd0.5000(MoO4)0.2500(WO4)0.7500 | 5.23653(6) | 11.4580(9) | 2.1881 | 6.49(3) | 4.27 | 329 | ||
| CaMnGdMoWO when x = 0.1667 (solid state reaction method) | |||||||||
| 25.00 | 1.00 | y = 0.0066 | Ca0.4933Mn0.0066▯0.1667Gd0.3334(MoO4)0.4999(WO4)0.5001 | 5.24167(6) | 11.4309(6) | 2.1808 | 5.86(2) | 3.75 | 365 |
| 25.00 | 5.00 | y = 0.0333 | Ca0.4668Mn0.0333▯0.1667Gd0.3334(MoO4)0.4999(WO4)0.5001 | 5.23624(7) | 11.4233(7) | 2.1816 | 5.87(3) | 3.64 | 398 |
| 25.00 | 10.00 | y = 0.0667 | Ca0.4332Mn0.0667▯0.1667Gd0.3334(MoO4)0.4999(WO4)0.5001 | 5.22944(6) | 11.4047(8) | 2.1809 | 5.92(1) | 3.62 | 363 |
References
- Danevich, F.A.; Georgadze, A.S.; Kobychev, V.V.; Kropivyansky, B.N.; Nagorny, S.S.; Nikolaiko, A.S.; Poda, D.V.; Tretyak, V.I.; Vyshnevskyi, I.M.; Yurchenko, S.S.; et al. Application of PbWO4 crystal scintillators in experiment to search for 2β decay of 116Cd. Nucl. Instr. Meth. Phys. Res. A 2006, 556, 259–265. [Google Scholar] [CrossRef]
- Belogurov, S.; Kornoukhov, V.; Annenkov, A.; Borisevich, A.; Fedorov, A.; Korzhik, M.; Ligoun, V.; Missevitch, O.; Kim, S.K.; Kim, S.C.; et al. CaMoO4 scintillation crystal for the search of 100Mo double beta decay. IEEE Trans. Nucl. Sci. 2005, 52, 1131. [Google Scholar] [CrossRef]
- Xi, H.; Zhou, D.; He, B.; Xie, H. Microwave dielectric properties of scheelite structured PbMoO4 ceramic with ultralow sintering temperature. J. Am. Ceram. Soc. 2014, 97, 1375–1378. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, S.H.; Lee, B.I. Low-temperature sintering and microwave dielectric properties of CaWO4 ceramics for LTCC applications. J. Eur. Ceram. Soc. 2006, 26, 2101–2104. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, J.; Mikhalik, V.B.; Kraus, H. Studies of scintillation properties of CaMoO4 at milikelvin temperatures. Appl. Phys. Lett. 2015, 106, 241904. [Google Scholar] [CrossRef]
- Lei, F.; Yan, B. Hydrothermal synthesis and luminescence of CaMoO4:RE3+ (M = W, Mo; RE = Eu, Tb) submicro-phosphors. J. Solid State Chem. 2008, 181, 855–862. [Google Scholar] [CrossRef]
- Sleight, A.W. Accurate cell dimensions for ABO4 molybdates and tungstates. Acta Crystallogr. B 1972, 28, 2899–2902. [Google Scholar] [CrossRef]
- Botelho, G.; Nogueira, I.C.; Moraes, E.; Longo, E. Study of structural and optical properties of CaMoO4 nanoparticles synthesized by the microwave-assisted solvothermal method. Mat. Chem. Phys. 2016, 183, 110–120. [Google Scholar] [CrossRef]
- Parchur, A.K.; Ningthoujam, R.S.; Rai, S.B.; Okram, G.S.; Singh, R.A.; Tyagi, M.; Gadkari, S.C.; Tewari, R.; Vatsab, R.K. Luminescence properties of Eu3+ doped CaMoO4 nanoparticles. Dalton Trans. 2011, 40, 7595–7601. [Google Scholar] [CrossRef]
- Xiao, B.; Schmidt, M. Incorporation of europium(III) into scheelite-related host matrixes ABO4 (A = Ca2+, Sr2+, Ba2+;B = W6+, Mo6+): Role of A and B sites on the dopant site distribution and photoluminescence. Inorg. Chem. 2017, 56, 14948–14959. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Rout, S.K.; Tiwari, A.; Yadav, P.; Sczancoski, J.C.; Filho, M.G.R.; Cavalcante, L.S. Structural refinement, Raman spectroscopy, optical and electrical properties of (Ba1−xSrx)MoO4 ceramics. J. Mater. Sci. 2015, 26, 8319–8335. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Jantunen, H. Low loss dielectric materials for LTCC applications: A review. Int. Mater. Rev. 2008, 53, 57–90. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Xu, W.; Kim, S.J.; Baeck, J.S.; Chang, Y.; Bae, J.E.; Chae, K.S.; Parl, J.A.; Kim, T.J.; Lee, G.H. Potential dual imaging nanoparticle: Gd2O3 nanoparticle. Sci. Rep. 2015, 5, 8549. [Google Scholar] [CrossRef] [PubMed]
- Lauffer, R.B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: Theory and design. Chem. Rev. 1987, 87, 901–927. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Kawano, T.; Ishijima, H.; Nakajima, T.; Aoki, J.; Endo, K. Gd-DTPA: A possible alternative contrast agent for use in CT during intraarterial administration. J. Comput. Assist. Tomogr. 1999, 23, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Zhu, L.; Kurokawa, A.; Takeuchi, H.; Yano, S.; Yanoh, T.; Wada, N.; Taira, S.; Hosokai, Y.; Usui, A. Synthesis of Gd2O3 nanoparticles for MRI contrast agents. J. Phys. Conf. Ser. 2012, 352, 012008. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Huang, D.T.; Bao, J.F.; Chen, Q.; Liu, G.; Chen, Z.; Chen, X.; Gao, J. A synergistically enhanced T1–T2 dual-modal contrast agent. Adv. Mater. 2012, 24, 6223–6228. [Google Scholar] [CrossRef]
- Tomaszewicz, E.; Filipek, E.; Fuks, H.; Typek, J. Thermal and magnetic properties of new scheelite type Cd1-3x▯xGd2xMoO4 ceramic materials. J. Eur. Ceram. Soc. 2014, 34, 1511–1522. [Google Scholar] [CrossRef]
- Godlewska, P.; Tomaszewicz, E.; Macalik, L.; Hanuza, J.; Ptak, M.; Tomaszewski, P.E.; Ropuszyńska-Robak, P. Structure and vibrational properties of scheelite type Cd0.25RE0.5▯0.25MoO4 solid solutions where ▯ is the cationic vacancy and RE = Pr, Nd, Sm–Dy. J. Mol. Struct. 2013, 1037, 332–337. [Google Scholar] [CrossRef]
- Macalik, L.; Tomaszewicz, E.; Ptak, M.; Hanuza, J.; Berkowski, M.; Mączka, M.; Ropuszyńska-Robak, P. Polarized Raman and IR spectra of oriented Cd0.9577Gd0.0282▯0.0141MoO4 and Cd0.9346Dy0.0436▯0.0218MoO4 single crystals where ▯ denotes the cationic vacancies. Spectrochim. Acta A 2015, 148, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Guzik, M.; Tomaszewicz, E.; Guyot, Y.; Legendziewicz, J.; Boulon, G. Structural and spectroscopic characterizations of new Cd1-3xNd2x▯xMoO4 scheelite-type molybdates with vacancies as potential optical materials. J. Mater. Chem. C 2015, 3, 4057–4069. [Google Scholar] [CrossRef]
- Guzik, M.; Tomaszewicz, E.; Guyot, Y.; Legendziewicz, J.; Boulon, G. Eu3+ luminescence from different sites in scheelite-type cadmium molybdate red phosphor with vacancies. J. Mater. Chem. C 2015, 3, 8582–8594. [Google Scholar] [CrossRef]
- Tomaszewicz, E.; Piątkowska, M.; Pawlikowska, M.; Groń, T.; Oboz, M.; Sawicki, B.; Urbanowicz, P. New vacancied and Dy3+-doped molybdates—Their structure, thermal stability, electrical and magnetic properties. Ceram. Int. 2016, 42, 18357–18367. [Google Scholar] [CrossRef]
- Sawicki, B.; Groń, T.; Tomaszewicz, E.; Duda, H.; Górny, K. Some optical and transport properties of a new subclass of ceramic tungstates and molybdates. Ceram. Int. 2015, 41, 13080–13089. [Google Scholar] [CrossRef]
- Kukuła, Z.; Maciejkowicz, M.; Tomaszewicz, E.; Pawlus, S.; Oboz, M.; Groń, T.; Guzik, M. Electric relaxation of superparamagnetic Gd-doped lead molybdato-tungstates. Ceram. Int. 2019, 45, 4437–4447. [Google Scholar] [CrossRef]
- Groń, T.; Maciejkowicz, M.; Tomaszewicz, E.; Guzik, M.; Oboz, M.; Sawicki, B.; Pawlus, S.; Nowok, A.; Kukuła, Z. Combustion synthesis, structural, magnetic and dielectric properties of Gd3+-doped lead molybdato-tungstates. J. Adv. Ceram. 2020, 9, 255–268. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Karolewicz, M.; Fuks, H.; Tomaszewicz, E. Synthesis, thermal, optical and magnetic properties of new Mn2+ doped and Eu3+ co-doped scheelites. J. Therm. Anal. Cal. 2019, 138, 2219–2231. [Google Scholar] [CrossRef]
- Pawlikowska, M.; Fuks, H.; Tomaszewicz, E. Solid state and combustion synthesis of Mn2+-doped scheelites–Their optical and magnetic properties. Ceram. Int. 2017, 43, 14135–14145. [Google Scholar] [CrossRef]
- Zhang, Y.; Holwarth, N.A.W.; Wiliams, R.T. Electronic band structure of the scheelite materials CaMoO4, CaWO4, PbMoO4 and PbWO4. Phys. Rev. B 1998, 20, 12738–12750. [Google Scholar] [CrossRef]
- Maurera, M.A.M.A.; Souza, A.G.; Soledade, L.E.B.; Pontes, F.M.; Longo, E.; Leite, E.R.; Varela, J.A. Microstructural and optical characterization of CaWO4 and SrWO4 thin films prepared by a chemical solution method. Mater. Lett. 2004, 58, 727–732. [Google Scholar] [CrossRef]
- Pontes, F.M.; Maurera, M.A.M.A.; Souza, A.G.; Longo, E.; Leite, E.R.; Magnani, R.; Machado, M.A.C.; Pizani, P.S.; Varela, J.A. Preparation, structural and optical characterization of BaWO4 and PbWO4 thin films prepared by a chemical route. J. Eur. Ceram. Soc. 2003, 23, 3001–3007. [Google Scholar] [CrossRef]
- Lacomba-Perales, R.; Ruiz-Fuertes, J.; Errandonea, D.; Martinez-Garcia, D.; Segura, A. Optical absorption of divalent metal tungstates: Correlation between the band-gap energy and the cation ionic radius. EPL 2008, 83, 37002. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag zur Optic der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Urbanowicz, P.; Piątkowska, M.; Sawicki, S.; Groń, T.; Kukuła, Z.; Tomaszewicz, E. Dielectric properties of RE2W2O9 (RE = Pr, Sm-Gd) ceramics. J. Eur. Ceram. Soc. 2015, 35, 4189–4193. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structures of amorphous germanium. Phys. Status Solidi. 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non-Cryst. Solids 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Solids; Abels, F., Ed.; Elsevier: Amsterdam, The Netherlands, 1969. [Google Scholar]
- Duman, S.; Gurbulak, B.; Dogan, S.; Ozcelik, F.S. The effect of Sn doping Urbach Tail and optical absorbtion measurements of InSe crystal. J. Phys. Conf. Series 2016, 707, 012027. [Google Scholar] [CrossRef]
- Studenyak, I.; Kranjčec, M.; Kurik, M. Urbach rule in solid state physics. Int. J. Opt. Appl. 2014, 4, 76–83. [Google Scholar]
- Singh, S.; Li, C.; Panzer, F.; Narasimhan, K.L.; Graeser, A.P.; Gujar, T.P.; Koöhler, A.; Thelakkat, M.; Huettner, S.; Kabr, D. Effect of thermal and structural disorder on the electronic structure of hybrid perovskite semiconductor CH3NH3PbI3. J. Phys. Chem. Lett. 2016, 7, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Saad, I.B.; Hannachi, N.; Roisnely, T.; Hlel, F. Optical, UV-Vis spectroscopy studies, electrical and dielectric properties of transition metal-based of the novel organic–inorganic hybrid (C6H10N2)(Hg2Cl5) 2.3H2O. J. Adv. Dielectr. 2019, 9, 1950040. [Google Scholar] [CrossRef]
- Sayyeda, M.I.; Rammah, Y.S.; Laariedha, F.; Abouhaswa, A.S.; Badeche, T.B. Lead borate glasses doped by lanthanum: Synthesis, physical, optical, and gamma photon shielding properties. J. Non-Crystal. Solids 2020, 527, 119731. [Google Scholar] [CrossRef]
- Turgut, G.; Sonmez, E.; Aydm, S.; Dilber, R.; Turgut, U. The effect of Mo and F double doping on structural, morphological, electrical and optical properties of spray deposited SnO2 thin films. Ceram. Int. 2014, 40, 12891–12898. [Google Scholar] [CrossRef]
- Rao, G.V.; Shashikala, H.D. Structural, optical and mechanical properties of ternary CaO–CaF2–P2O5 glasses. J. Adv. Ceram. 2014, 3, 109–116. [Google Scholar]
- Piątkowska, M.; Fuks, H.; Tomaszewicz, E.; Kochmańska, A.E. New vacancied and Gd3+-doped lead molybdato-tungstates and tungstates prepared via solid state and citrate-nitrate combustion method. Ceram. Int. 2017, 43, 7839–7850. [Google Scholar] [CrossRef]
- Taupin, D. Une methode generale pour l’indexation des diagrammes de poudres. J. Appl. Crystallogr. 1968, 1, 87. [Google Scholar] [CrossRef]
- Taupin, D. A powder—Diagram authomatic—Indexing routine. J. Appl. Crystallogr. 1973, 6, 380–385. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuks, H.; Kochmański, P.; Tomaszewicz, E. New Gd3+ and Mn2+-Co-Doped Scheelite-Type Ceramics—Their Structural, Optical and Magnetic Properties. Int. J. Mol. Sci. 2022, 23, 15740. https://doi.org/10.3390/ijms232415740
Fuks H, Kochmański P, Tomaszewicz E. New Gd3+ and Mn2+-Co-Doped Scheelite-Type Ceramics—Their Structural, Optical and Magnetic Properties. International Journal of Molecular Sciences. 2022; 23(24):15740. https://doi.org/10.3390/ijms232415740
Chicago/Turabian StyleFuks, Hubert, Paweł Kochmański, and Elżbieta Tomaszewicz. 2022. "New Gd3+ and Mn2+-Co-Doped Scheelite-Type Ceramics—Their Structural, Optical and Magnetic Properties" International Journal of Molecular Sciences 23, no. 24: 15740. https://doi.org/10.3390/ijms232415740
APA StyleFuks, H., Kochmański, P., & Tomaszewicz, E. (2022). New Gd3+ and Mn2+-Co-Doped Scheelite-Type Ceramics—Their Structural, Optical and Magnetic Properties. International Journal of Molecular Sciences, 23(24), 15740. https://doi.org/10.3390/ijms232415740







