A Tea Plant (Camellia sinensis) FLOWERING LOCUS C-like Gene, CsFLC1, Is Correlated to Bud Dormancy and Triggers Early Flowering in Arabidopsis
Abstract
1. Introduction
2. Results
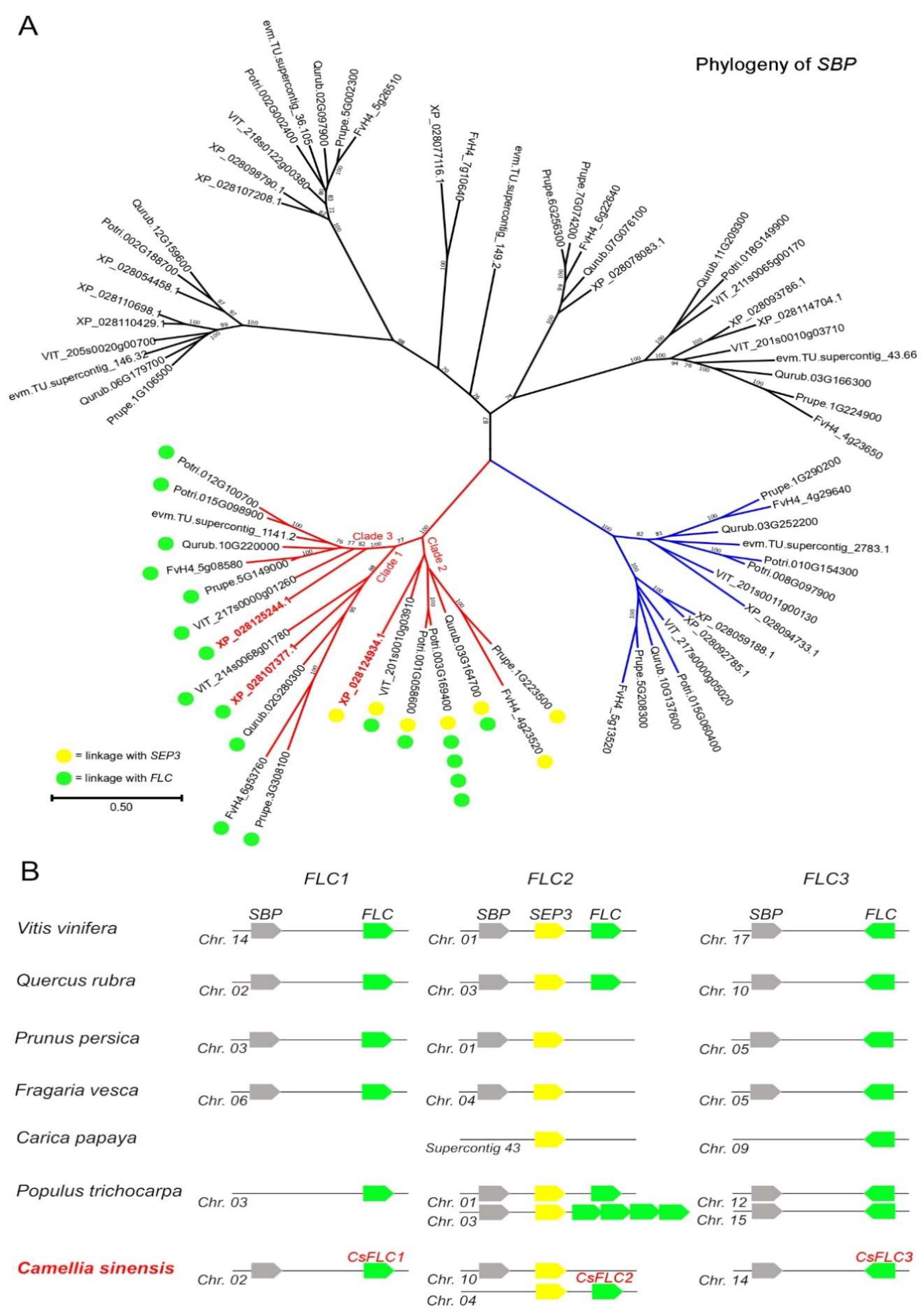
2.1. Identification of CsFLC-Like Genes in Tea Genome
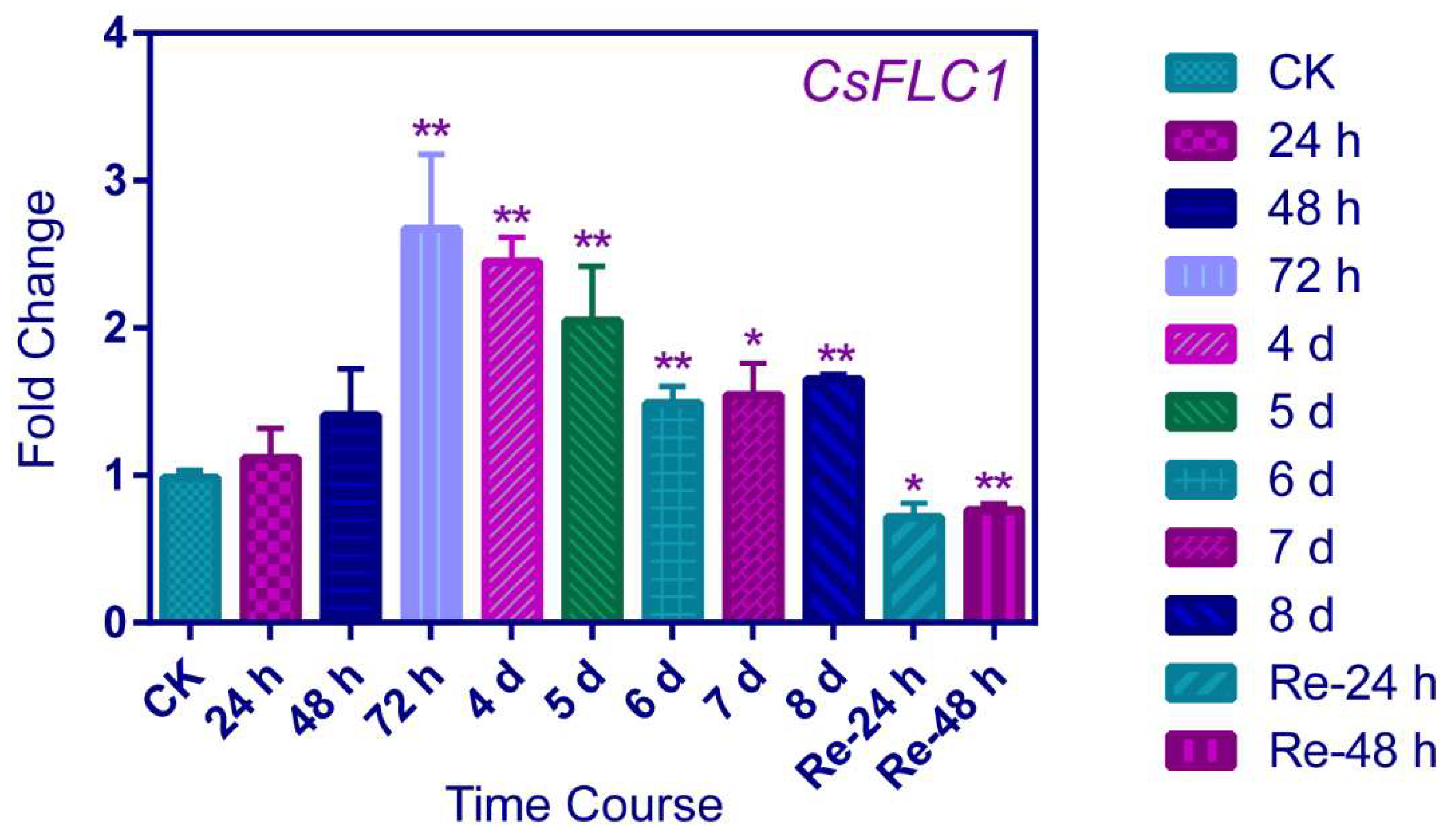
2.2. CsFLC1 Gene Expression Patterns
2.3. The CsFLC1 Promoter Is Responsive to Low Temperature and Photoperiod
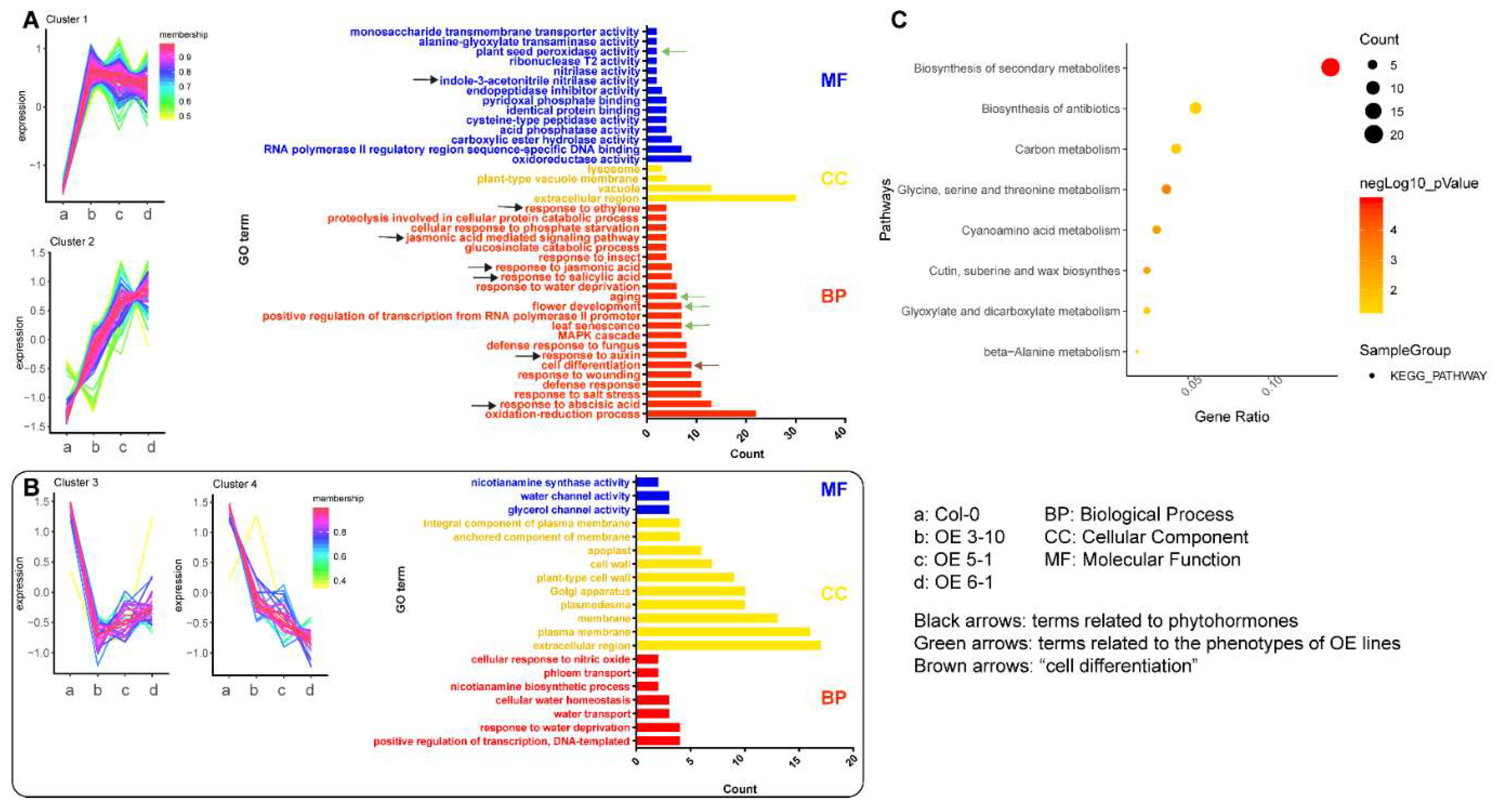
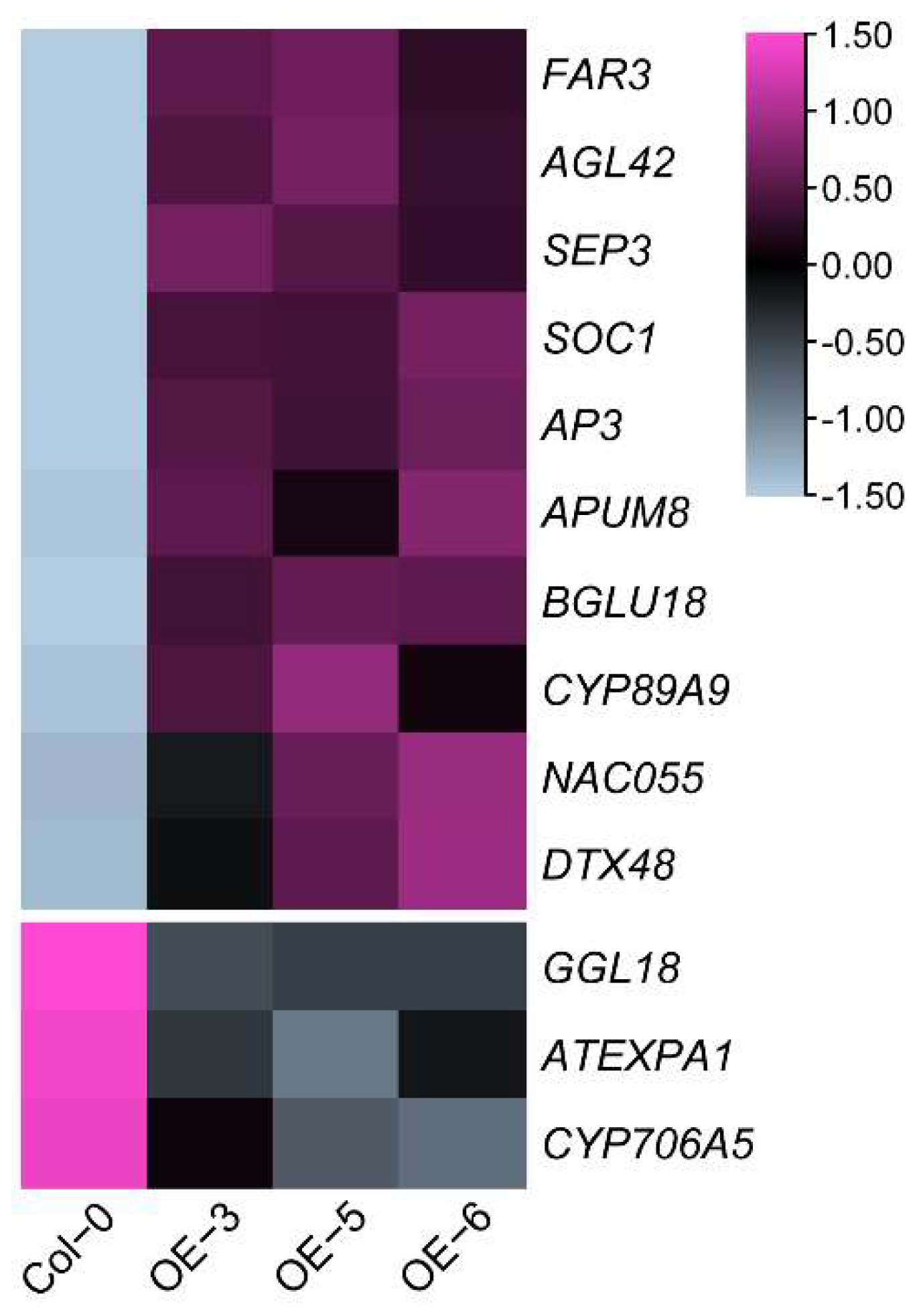
2.4. CsFLC1 Affects Flowering Time and Seed Germination in Transgenic Arabidopsis thaliana
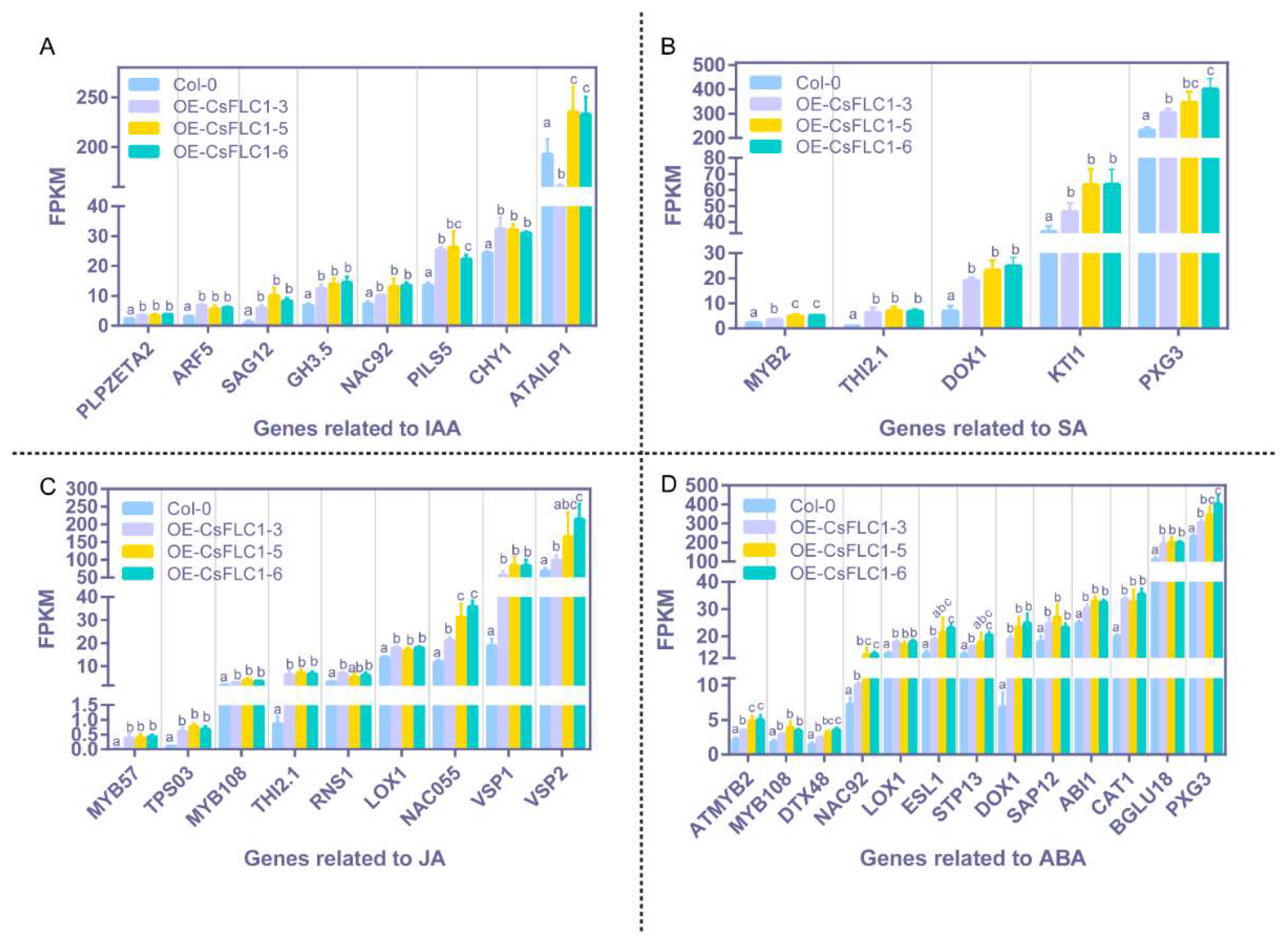
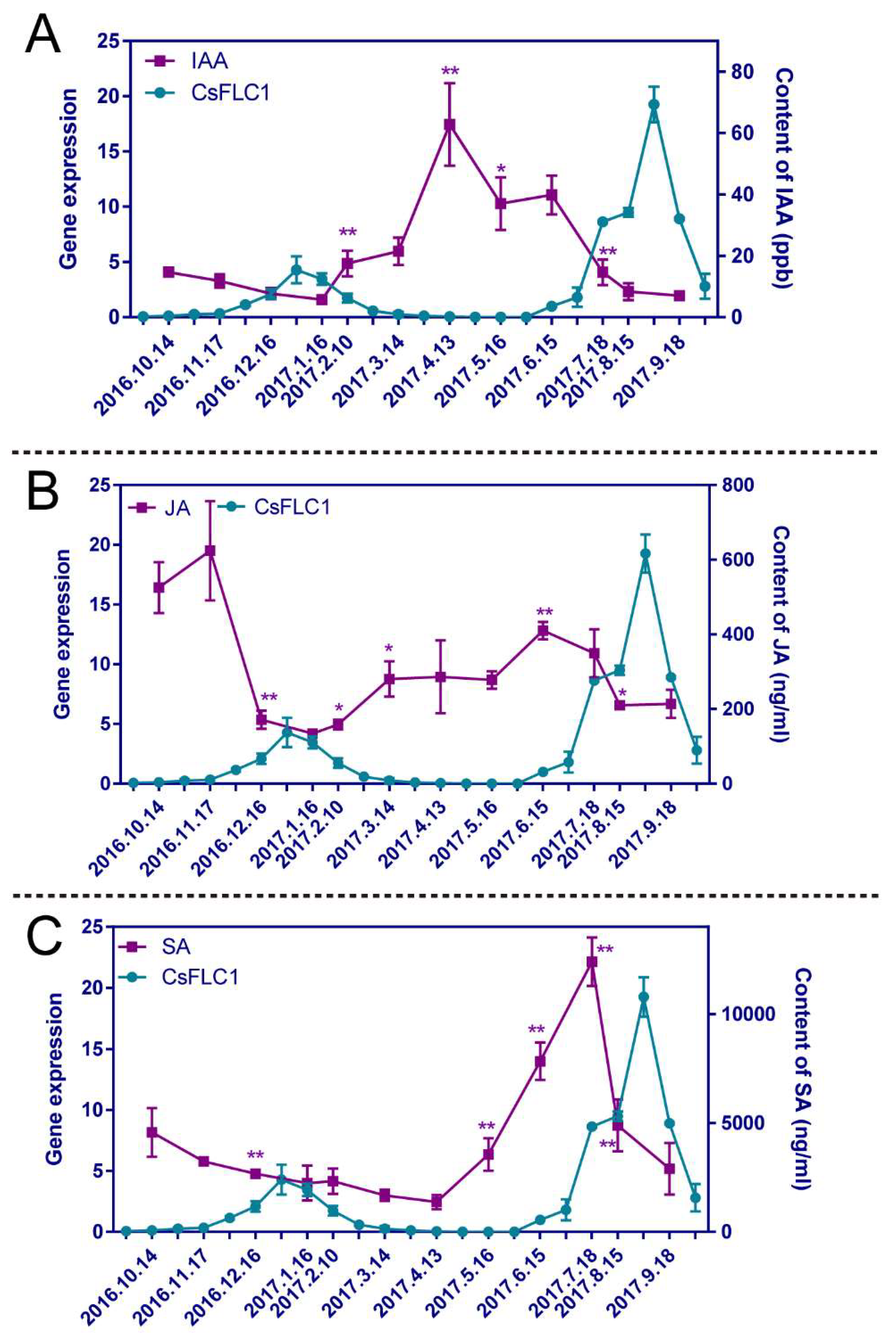
2.5. Phytohormone Contents of Tea Plants in the Whole Year
3. Discussion
3.1. Function of CsFLC in Reproduction Processes
3.2. CsFLC1 Putative Function in Bud Dormancy
3.3. Relationship between CsFLC1 Expression and Phytohormones’ Concentration and Response
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Phylogenetic Analysis
4.3. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.4. Cloning, Plasmid Construction and Transformation
4.5. Subcellular Localization
4.6. GUS Staining
4.7. RNA Sequencing and Transcriptome Data Analysis of Arabidopsis Transgenic Lines
4.8. Measurements of Phytohormone Contents in Tea Plant Buds
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| AP3 | APETALA3 |
| ARF5/MP | AUXIN RESPOSE FACTOR 5/MONOPTEROS |
| CAT1 | CATALASE 1 |
| CAL | CAULIFLOWER |
| CDS | Coding sequences |
| DEGs | Differentially expressed genes |
| DOX1 | DIOXYGENASE 1 |
| FT | FLOWERING LOCUS T |
| FLC | FLOWERING LOCUS C |
| GO | Gene ontology |
| GH3.5 | Gretchen Hagen 3.5 |
| IAA | Indoleacetic acid |
| JA | Jasmonic acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LD | Long day |
| MD | Middle day |
| MF | Molecular function |
| OE | Overexpression |
| SA | Salicylic acid |
| SAG12 | SENESCENCE-ASSOCIATED GENE 12 |
| SHP | SHATTERPROOF |
| SAMs | Shoot apical meristems |
| SD | Short day |
| SOC1 | SUPPRESSOR OF OVEREXPRESSION OF CO 1 |
| TPS03 | TERPENE SYNTHASE 03 |
| THI2.1 | THIONIN 2.1 |
| TF | Transcription factor |
| VSP1 | VEGETATIVE STORAGE PROTEIN 1 |
References
- Balanzà:, V.; Martinez-Fernandez, I.; Sato, S.; Yanofsky, M.F.; Kaufmann, K.; Angenent, G.C.; Bemer, M.; Ferrandiz, C. Genetic control of meristem arrest and life span in Arabidopsis by a FRUITFULL-APETALA2 pathway. Nat. Commun. 2018, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, Y.; Guan, Y.; Wang, S.; Luo, H.; Li, X.; Li, H.; Zhang, Z. Auxin-induced AUXIN RESPONSE FACTOR4 activates APETALA1 and FRUITFULL to promote flowering in woodland strawberry. Hort. Res. 2021, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, C.C.; Rouse, D.T.; Finnegan, E.J.; Peacock, W.J.; Dennis, E.S. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC). Proc. Natl. Acad. Sci. USA. 2000, 97, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Michaels, S.D.; Amasino, R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef]
- Ratcliffe, O.J. Regulation of flowering in Arabidopsis by an FLC homologue. Plant Physiol. 2001, 126, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, B.S.; Kwon, S.J.; Kim, J.; Lim, M.H.; Park, Y.D.; Kim, D.Y.; Suh, S.C.; Jin, Y.M.; Ji, H.A. Delayed flowering time in Arabidopsis and Brassica rapa by the overexpression of FLOWERING LOCUS C (FLC) homologs isolated from Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Cell Rep. 2007, 26, 327–336. [Google Scholar] [CrossRef]
- Nakano, Y.; Kawashima, H.; Kinoshita, T.; Yoshikawa, H.; Hisamatsu, T. Characterization of FLC, SOC1 and FT homologs in Eustoma grandiflorum: Effects of vernalization and post-vernalization conditions on flowering and gene expression. Physiol. Plant. 2011, 141, 383–393. [Google Scholar] [CrossRef]
- Searle, I. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 2006, 20, 898–912. [Google Scholar] [CrossRef]
- Seo, E.; Lee, H.; Jin, J.; Park, H.; Kim, J.; Noh, Y.S.; Lee, I. Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. Plant Cell 2009, 21, 3185–3197. [Google Scholar] [CrossRef]
- Sheldon, C.C.; Burn, J.E.; Perez, P.P.; Metzger, J.; Dennis, E.S. The FLF MADS Box gene: A repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 1999, 11, 445–458. [Google Scholar] [CrossRef]
- Questa, J.I.; Song, J.; Geraldo, N.; An, H.; Dean, C. Arabidopsis transcriptional repressor VAL1 triggers Polycomb silencing at FLC during vernalization. Science 2016, 353, 485–488. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Amasino, R.M. Role of chromatin modification in flowering-time control. Trends Plant Sci. 2005, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Greb, T.; Mylne, J.S.; Crevillen, P.; Geraldo, N.; An, H.; Gendall, A.R.; Dean, C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr. Biol. 2007, 17, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, C.C.; Hills, M.J.; Lister, C.; Dean, C.; Dennis, E.S.; Peacock, W.J. Resetting of FLOWERING LOCUS C expression after epigenetic repression by vernalization. Proc. Natl. Acad. Sci. USA. 2008, 105, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, H.; Xing, L.; Xu, S.; Liu, H.; Chong, K.; Xu, Y. Requirement of histone acetyltransferases HAM1 and HAM2 for epigenetic modification of FLC in regulating flowering in Arabidopsis. Plant Physiol. 2013, 170, 444–451. [Google Scholar] [CrossRef]
- Kwak, J.S.; Son, G.H.; Song, J.T.; Seo, H.S. Post-translational modifications of FLOWERING LOCUS C modulate its activity. J. Exp. Bot. 2016, 68, 383–389. [Google Scholar] [CrossRef]
- Kwak, J.S.; Son, G.H.; Sung-Il, K.; Song, J.T.; Seo, H.S. Arabidopsis HIGH PLOIDY2 sumoylates and stabilizes FLOWERING LOCUS C through its E3 ligase activity. Front. Plant Sci. 2016, 7, 530. [Google Scholar] [CrossRef]
- Nishio, H.; Buzas, D.M.; Nagano, A.J.; Suzuki, Y.; Sugano, S.; Ito, M.; Morinaga, S.I.; Kudoh, H. From the laboratory to the field: Assaying histone methylation at FLOWERING LOCUS C in naturally growing Arabidopsis halleri. Genes Genet. Syst. 2016, 91, 15–26. [Google Scholar] [CrossRef]
- Soichiro, N.; Cecile, M.M.; Hisayo, Y.; Chikako, H.; Kazuma, O.; Yosuke, T.; Shigeki, M.; Tao, R. Functional and expressional analyses of apple FLC-like in relation to dormancy progress and flower bud development. Tree Physiol. 2019, 41, 562–570. [Google Scholar] [CrossRef]
- Voogd, C.; Brian, L.A.; Wu, R.; Wang, T.; Allan, A.C.; Varkonyi-Gasic, E. A MADS-box gene with similarity to FLC is induced by cold and correlated with epigenetic changes to control budbreak in kiwifruit. N. Phytol. 2022, 233, 2111–2126. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Chang, Y.; Ma, C.; Wang, L.; Hao, X.; Li, A.; Cheng, H.; Wang, L.; Cui, P.; et al. Population sequencing enhances understanding of tea plant evolution. Nat. Commun. 2020, 11, 4447. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Prasanth, M.I.; Brimson, J.M.; Sharika, R.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. Neuroprotective properties of green tea (Camellia sinensis) in Parkinson’s Disease: A Review. Molecules 2020, 25, 3926. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Zhao, R.; Wan, Q.; Du, L.; Zhou, Y. Tea consumption and health outcomes: Umbrella review of meta-analyses of observational studies in humans. Mol. Nutr. Food Res. 2019, 63, e1900389. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Zhao, C.N.; Cao, S.Y.; Tang, G.Y.; Gan, R.Y.; Li, H.B. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1693–1705. [Google Scholar] [CrossRef]
- Williams, J.L.; Everett, J.M.; D’Cunha, N.M.; Sergi, D.; Georgousopoulou, E.N.; Keegan, R.J.; McKune, A.J.; Mellor, D.D.; Anstice, N.; Naumovski, N. The effects of green tea amino acid L-theanine consumption on the ability to manage stress and anxiety levels: A systematic review. Plant Foods Hum. Nutr. 2019, 75, 12–23. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, X.; Lu, Q.; Zhang, W.; Zhang, H.; Wang, L.; Yang, Y.; Xiao, B.; Wang, X. Genome-wide identification and expression analysis of flowering-related genes reveal putative floral induction and differentiation mechanisms in tea plant (Camellia sinensis). Genomics 2020, 112, 2318–2326. [Google Scholar] [CrossRef]
- Wang, X.C.; Hao, X.Y.; Ma, C.L.; Cao, H.L.; Yue, C.; Wang, L.; Zeng, J.M.; Yang, Y.J. Identification of differential gene expression profiles between winter dormant and sprouting axillary buds in tea plant (Camellia sinensis) by suppression subtractive hybridization. Tree Genet. Genomes 2014, 10, 1149–1159. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Ren, H.; Zhou, J.; Wang, L.; Yang, Y.; Hao, X.; Wang, X. Genome-wide identification and expression profiling reveal the diverse role of Methyl-CpG-binding domain proteins in tea plant (Camellia sinensis). Beverage Plant Res. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Hao, X.; Yang, Y.; Yue, C.; Wang, L.; Horvath, D.P.; Wang, X. Comprehensive transcriptome analyses reveal differential gene expression profiles of Camellia sinensis axillary buds at para-, endo-, ecodormancy, and bud flush stages. Front. Plant Sci. 2017, 8, 553. [Google Scholar] [CrossRef]
- Ruelens, P.; Maagd, R.A.D.; Proost, S.; Theien, G.; Geuten, K.; Kaufmann, K. FLOWERING LOCUS C in monocots and the tandem origin of angiosperm-specific MADS-box genes. Nat. Commun. 2013, 4, 2280. [Google Scholar] [CrossRef]
- Zhao, T.; Holmer, R.; Bruijn, S.D.; Angenent, G.C.; Van, D.; Schranz, M.E. Phylogenomic synteny network analysis of MADS-Box transcription factor genes reveals lineage-specific transpositions, ancient tandem duplications, and deep positional conservation. Plant Cell 2017, 29, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Zheng, C.; Ma, J.Q.; Jiang, C.K.; Ercisli, S.; Yao, M.J.; Chen, L. The chromosome-scale genome reveals the evolution and diversification after the recent tetraploidization event in tea plant. Hortic. Res. 2020, 7, 63. [Google Scholar] [CrossRef]
- Deng, W.; Ying, H.; Helliwell, C.A.; Taylor, J.M.; Peacock, W.J.; Dennis, E.S. FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011, 108, 6680–6685. [Google Scholar] [CrossRef]
- Amasino, R.M.; Michaels, S.D. The timing of flowering. Plant Physiol. 2010, 154, 516–520. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, T.; Zeng, X. Genetic and epigenetic understanding of the seasonal timing of flowering. Plant Commun. 2020, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.H.; Dean, C. Fine-tuning timing: Natural variation informs the mechanistic basis of the switch to flowering in Arabidopsis thaliana. J. Exp. Bot. 2017, 68, 5439–5452. [Google Scholar] [CrossRef]
- Michaels, S.D. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 2001, 13, 935–941. [Google Scholar] [CrossRef]
- Salathia, N.; Davis, S.J.; Lynn, J.R.; Michaels, S.D.; Millar, A.J. FLOWERING LOCUS C-dependent and -independent regulation of the circadian clock by the autonomous and vernalization pathways. BMC Plant Biol. 2006, 6, 10. [Google Scholar] [CrossRef][Green Version]
- Dorca-Fornell, C.; Gregis, V.; Grandi, V.; Coupland, G.; Colombo, L.; Kater, M.M. The Arabidopsis SOC1-like genes AGL42, AGL71 and AGL72 promote flowering in the shoot apical and axillary meristems. Plant J. 2011, 67, 1006–1017. [Google Scholar] [CrossRef]
- Véronique, H.; Silva, C.S.; Agnès, J.; Arnaud, S.; Quentin, C.; Vanessa, C.; Conn, S.J.; Carles, C.C.; François, P.; Chloe, Z. Tetramerization of MADS family transcription factors SEPALLATA3 and AGAMOUS is required for floral meristem determinacy in Arabidopsis. Nucleic Acids Res. 2018, 46, 4966–4977. [Google Scholar] [CrossRef]
- Wuest, S.E.; O"Maoileidigh, D.S.; Rae, L.; Kwasniewska, K.; Raganelli, A.; Hanczaryk, K.; Lohan, A.J.; Loftus, B.; Graciet, E.; Wellmer, F. Molecular basis for the specification of floral organs by APETALA3 and PISTILLATA. Proc. Natl. Acad. Sci. USA. 2012, 109, 13452–13457. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Tuan, P.A.; Saito, T.; Bai, S.; Kita, M.; Moriguchi, T. Changes in phytohormone content and associated gene expression throughout the stages of pear (Pyrus pyrifolia Nakai) dormancy. Tree Physiol. 2021, 41, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Tang, H.; Wang, B.; Wang, L.; Cao, H.; Wang, Y.; Zeng, J.; Fang, S.; Chu, J.; Yang, Y.; et al. Gene characterization and expression analysis reveal the importance of auxin signaling in bud dormancy regulation in tea plant. J. Plant Growth Regul. 2019, 38, 225–240. [Google Scholar] [CrossRef]
- Leopold, A.; Thimann, K. The effect of auxin on flower initiation. Am. J. Bot. 1949, 36, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 2003, 15, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, M.; Cavalleri, A.; Guazzotti, A.; Astori, C.; Manrique, S.; Bombarely, A.; Oliveto, S.; Biffo, S.; Weijers, D.; Kater, M.M. Alternative splicing generates a MONOPTEROS isoform required for ovule development. Curr. Biol. 2021, 31, 892–899. [Google Scholar] [CrossRef]
- Noh, Y.S.; Amasino, R.M. Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol. Biol. 1999, 41, 181–194. [Google Scholar] [CrossRef]
- Grbić, V. SAG2 and SAG12 protein expression in senescing Arabidopsis plants. Physiol. Plant. 2003, 119, 263–269. [Google Scholar] [CrossRef]
- James, M.; Poret, M.; Masclaux-Daubresse, C.; Marmagne, A.; Coquet, L.; Jouenne, T.; Chan, P.; Trouverie, J.; Etienne, P. SAG12, a major cysteine protease involved in nitrogen allocation during senescence for seed production in Arabidopsis Thaliana. Plant Cell Physiol. 2018, 59, 2052–2063. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I.; Rowe, M.L. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 2002, 14, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Q.; Li, Z.; Staswick, P.E.; Wang, M.; Zhu, Y.; He, Z. Dual regulation role of GH3. 5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiol. 2007, 145, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, M.; Li, Z.; Li, Q.; He, Z. Arabidopsis GH3. 5 regulates salicylic acid-dependent and both NPR1-dependent and independent defense responses. Plant Signal. Behav. 2008, 3, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, H.; Li, X.; Xiao, J.; Wang, S. Rice GH3 gene family: Regulators of growth and development. Plant Signal. Behav. 2011, 6, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Forner, S.; Strnad, M.; Hause, B. Jasmonates in flower and seed development. Biochimie 2013, 95, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhang, D. Roles of jasmonate signalling in plant inflorescence and flower development. Curr. Opin. Plant Biol. 2015, 27, 44–51. [Google Scholar] [CrossRef]
- Cheng, H.; Song, S.; Xiao, L.; Soo, H.M.; Cheng, Z.; Xie, D.; Peng, J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 2009, 5, e1000440. [Google Scholar] [CrossRef]
- Hickman, R.; Van Verk, M.C.; Van Dijken, A.; Mendes, M.P.; Vroegop-Vos, I.A.; Caarls, L.; Steenbergen, M.; Van der Nagel, I.; Wesselink, G.J.; Jironkin, A.; et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 2017, 29, 2086–2105. [Google Scholar] [CrossRef]
- Cleland, C.F.; Ajami, A. Identification of the flower-inducing factor isolated from aphid honeydew as being salicylic acid. Plant Physiol. 1974, 54, 904–906. [Google Scholar] [CrossRef][Green Version]
- Martínez, C.; Pons, E.; Prats, G.; León, J. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 2004, 37, 209–217. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Spivey, N.W.; Zeng, W.; Liu, P.P.; Fu, Z.Q.; Klessig, D.F.; He, S.Y.; Dong, X. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 2012, 11, 587–596. [Google Scholar] [CrossRef] [PubMed]
- De León, I.P.; Sanz, A.; Hamberg, M.; Castresana, C. Involvement of the Arabidopsis α-DOX1 fatty acid dioxygenase in protection against oxidative stress and cell death. Plant J. 2002, 29, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Giraudat, J. Abscisic acid signaling. Curr. Opin. Cell Biol. 1995, 7, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, K.E.; Nishimura, N.; Hitomi, K.; Getzoff, E.D.; Schroeder, J.I. Early abscisic acid signal transduction mechanisms: Newly discovered components and newly emerging questions. Genes Dev. 2010, 24, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.H.; Shrestha, N.; Bhandari, J.; Crowell, D.N. Roles for farnesol and ABA in Arabidopsis flower development. Plant Signal. Behav. 2011, 6, 1189–1191. [Google Scholar] [CrossRef][Green Version]
- Conti, L.; Galbiati, M.; Tonelli, C. ABA and the floral transition. In Abscisic acid: Metabolism, transport and signaling; Zhang, D.P., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 365–384. [Google Scholar]
- Guan, L.M.; Zhao, J.; Scandalios, J.G. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant, J. 2000, 22, 87–95. [Google Scholar] [CrossRef]
- Jun, J.H.; Liu, C.; Xiao, X.; Dixon, R.A. The transcriptional repressor MYB2 regulates both spatial and temporal patterns of proanthocyandin and anthocyanin pigmentation in Medicago truncatula. Plant Cell 2015, 27, 2860–2879. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA. 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Qiu, H.; Guo, Y.; Wan, H.; Zhang, X.; Scossa, F.; Alseekh, S.; Zhang, Q.; Wang, P.; et al. Genome assembly of wild tea tree DASZ reveals pedigree and selection history of tea varieties. Nat. Commun. 2020, 11, 3719. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hao, X.; Horvath, D.P.; Chao, W.S.; Yang, Y.; Wang, X.; Xiao, B. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Bent, A. Arabidopsis thaliana floral dip transformation method. Methods Mol. Biol. 2006, 343, 87. [Google Scholar] [CrossRef]
- Kapila, J.; De Rycke, R.; Van Montagu, M.; Angenon, G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997, 122, 101–108. [Google Scholar] [CrossRef]
- Wang, L.; Yao, L.; Hao, X.; Li, N.; Qian, W.; Yue, C.; Ding, C.; Zeng, J.; Yang, Y.; Wang, X. Tea plant SWEET transporters: Expression profiling, sugar transport, and the involvement of CsSWEET16 in modifying cold tolerance in Arabidopsis. Plant Mol. Biol. 2018, 96, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, X.J. GOEAST: A web-based software toolkit for Gene Ontology enrichment analysis. Nucleic Acids Res. 2008, 36, W358–W363. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, Y.; Xiong, F.; Hao, X.; Zhang, X.; Li, N.; Wang, L.; Zeng, J.; Yang, Y.; Wang, X. Integrated transcriptomic and metabolomic analyses reveal the effects of callose deposition and multihormone signal transduction pathways on the tea plant-Colletotrichum camelliae interaction. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Dreni, L.; Zhang, H.; Zhang, X.; Li, N.; Zhang, K.; Di, T.; Wang, L.; Yang, Y.; Hao, X.; et al. A Tea Plant (Camellia sinensis) FLOWERING LOCUS C-like Gene, CsFLC1, Is Correlated to Bud Dormancy and Triggers Early Flowering in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 15711. https://doi.org/10.3390/ijms232415711
Liu Y, Dreni L, Zhang H, Zhang X, Li N, Zhang K, Di T, Wang L, Yang Y, Hao X, et al. A Tea Plant (Camellia sinensis) FLOWERING LOCUS C-like Gene, CsFLC1, Is Correlated to Bud Dormancy and Triggers Early Flowering in Arabidopsis. International Journal of Molecular Sciences. 2022; 23(24):15711. https://doi.org/10.3390/ijms232415711
Chicago/Turabian StyleLiu, Ying, Ludovico Dreni, Haojie Zhang, Xinzhong Zhang, Nana Li, Kexin Zhang, Taimei Di, Lu Wang, Yajun Yang, Xinyuan Hao, and et al. 2022. "A Tea Plant (Camellia sinensis) FLOWERING LOCUS C-like Gene, CsFLC1, Is Correlated to Bud Dormancy and Triggers Early Flowering in Arabidopsis" International Journal of Molecular Sciences 23, no. 24: 15711. https://doi.org/10.3390/ijms232415711
APA StyleLiu, Y., Dreni, L., Zhang, H., Zhang, X., Li, N., Zhang, K., Di, T., Wang, L., Yang, Y., Hao, X., & Wang, X. (2022). A Tea Plant (Camellia sinensis) FLOWERING LOCUS C-like Gene, CsFLC1, Is Correlated to Bud Dormancy and Triggers Early Flowering in Arabidopsis. International Journal of Molecular Sciences, 23(24), 15711. https://doi.org/10.3390/ijms232415711







