Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Population
2.2. Associations between Plasma Calprotectin Levels and Suspected MAFLD
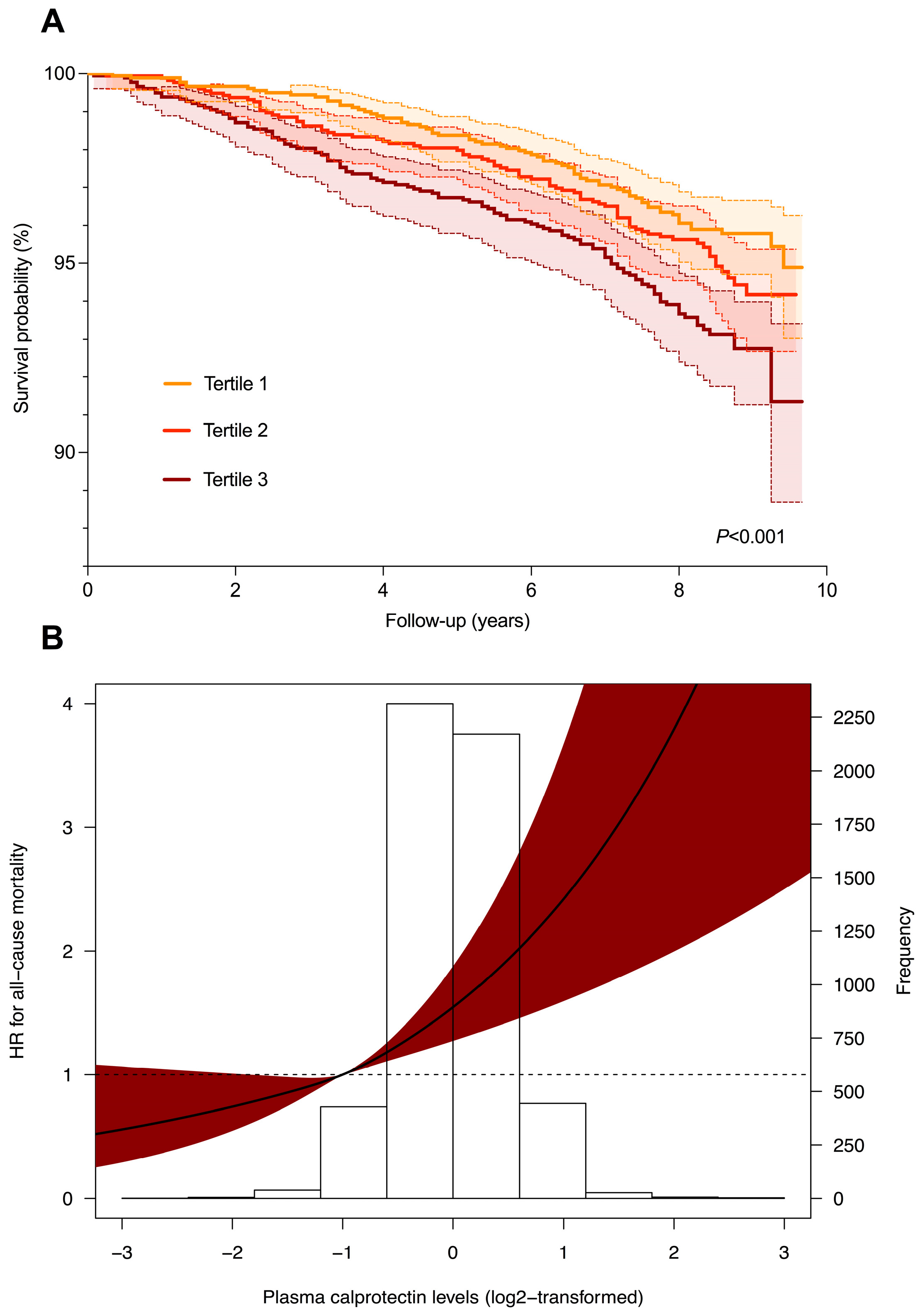
2.3. Plasma Calprotectin Levels and Risk of All-Cause Mortality in Subjects with and without Suspected MAFLD
3. Discussion
4. Materials and Methods
4.1. Study Population and Study Design
4.2. Data Collection
4.3. Study Outcomes and Definitions
4.4. Laboratory Measurements
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- van den Berg, E.H.; Amini, M.; Schreuder, T.C.M.A.; Dullaart, R.P.F.; Faber, K.N.; Alizadeh, B.Z.; Blokzijl, H. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS ONE 2017, 12, e0171502. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J. Endocrinol. 2013, 218, R25–R36. [Google Scholar] [CrossRef] [PubMed]
- Gambino, R.; Musso, G.; Cassader, M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: Mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1325–1365. [Google Scholar] [CrossRef]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; van den Berg, E.H.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef]
- Liu, K.; Wang, F.S.; Xu, R. Neutrophils in liver diseases: Pathogenesis and therapeutic targets. Cell. Mol. Immunol. 2021, 18, 38–44. [Google Scholar] [CrossRef]
- Khoury, T.; Mari, A.; Nseir, W.; Kadah, A.; Sbeit, W.; Mahamid, M. Neutrophil-to-lymphocyte ratio is independently associated with inflammatory activity and fibrosis grade in nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1110–1115. [Google Scholar] [CrossRef]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Mirea, A.M.; Toonen, E.J.M.; van den Munckhof, I.; Munsterman, I.D.; Tjwa, E.T.T.L.; Jaeger, M.; Oosting, M.; Schraa, K.; Rutten, J.H.W.; van der Graaf, M.; et al. Increased proteinase 3 and neutrophil elastase plasma concentrations are associated with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes. Mol. Med. 2019, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Wittkowski, H.; Roth, J. Monitoring disease activity by stool analyses: From occult blood to molecular markers of intestinal inflammation and damage. Gut 2009, 58, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Teng, T.S.; Ji, A.L.; Ji, X.Y.; Li, Y.Z. Neutrophils and Immunity: From Bactericidal Action to Being Conquered. J. Immunol. Res. 2017, 2017, 9671604. [Google Scholar] [CrossRef]
- de Seny, D.; Fillet, M.; Ribbens, C.; Marée, R.; Meuwis, M.A.; Lutteri, L.; Chapelle, J.-P.; Wehenkel, L.; Louis, E.; Merville, M.P.; et al. Monomeric calgranulins measured by SELDI-TOF mass spectrometry and calprotectin measured by ELISA as biomarkers in arthritis. Clin. Chem. 2008, 54, 1066–1075. [Google Scholar] [CrossRef]
- Ho, G.T.; Lee, H.M.; Brydon, G.; Ting, T.; Hare, N.; Drummond, H.; Shand, A.G.; Bartolo, D.C.; Wilson, R.G.; Dunlop, M.G.; et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 673–678. [Google Scholar]
- Kunutsor, S.K.; Flores-Guerrero, J.L.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Sundrehagen, E.; Seidu, S.; Dullaart, R.P.; Bakker, S.J. Plasma calprotectin and risk of cardiovascular disease: Findings from the PREVEND prospective cohort study. Atherosclerosis 2018, 275, 205–213. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; von Martels, J.Z.H.; de Vos, P.; Faber, K.N.; Dijkstra, G. Increased fecal calprotectin levels in Crohn’s disease correlate with elevated serum Th1- and Th17-associated cytokines. PLoS ONE 2018, 13, e0193202. [Google Scholar] [CrossRef]
- Bıçakçı, E.; Demirtaş, C.O.; Çelikel, Ç.; Haklar, G.; Duman, D.G. Myeloperoxidase and calprotectin; Any role as non-invasive markers for the prediction of inflammation and fibrosis in non-alcoholic steatohepatitis. Turk. J. Gastroenterol. 2020, 31, 681–687. [Google Scholar] [CrossRef]
- Cai, Q.; Zhu, J.; Cui, X.; Xia, Y.; Gao, H.; Wang, X.; Cheng, M. S100A9 promotes inflammatory response in diabetic nonalcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2022, 618, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Ming, Y.; Song, Y.; Zhang, J.; Chen, X.; Zeng, M.; Mao, Y. S100A9: A Potential Biomarker for the Progression of Non-Alcoholic Fatty Liver Disease and the Diagnosis of Non-Alcoholic Steatohepatitis. PLoS ONE 2015, 10, e0127352. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Ahmad, M.F.; Nagy, L.E.; Tsukamoto, H. Inflammatory pathways in alcoholic steatohepatitis. J. Hepatol. 2019, 70, 249–259. [Google Scholar] [CrossRef]
- Hessian, P.A.; Edgeworth, J.; Hogg, N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J. Leukoc. Biol. 1993, 53, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Yun, H.; Moon, S.; Cho, Y.E.; Gao, B. Role of Neutrophils in the Pathogenesis of Nonalcoholic Steatohepatitis. Front. Endocrinol. 2021, 12, 751802. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Alkhouri, N.; Morris-Stiff, G.; Campbell, C.; Lopez, R.; Tamimi, T.A.-R.; Yerian, L.; Zein, N.N.; Feldstein, A.E. Neutrophil to lymphocyte ratio: A new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012, 32, 297–302. [Google Scholar] [CrossRef]
- Yilmaz, H.; Yalcin, K.S.; Namuslu, M.; Celik, H.T.; Sozen, M.; Inan, O.; Nadir, I.; Turkay, C.; Akcay, A.; Kosar, A. Neutrophil-Lymphocyte Ratio (NLR) Could Be Better Predictor than C-reactive Protein (CRP) for Liver Fibrosis in Non-alcoholic Steatohepatitis (NASH). Ann. Clin. Lab. Sci. 2015, 45, 278–286. [Google Scholar]
- Gao, B.; Tsukamoto, H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef]
- Nemeth, T.; Mocsai, A. Feedback Amplification of Neutrophil Function. Trends Immunol. 2016, 37, 412–424. [Google Scholar] [CrossRef]
- Serhal, R.; Hilal, G.; Boutros, G.; Sidaoui, J.; Wardi, L.; Ezzeddine, S.; Alaaeddine, N. Nonalcoholic Steatohepatitis: Involvement of the Telomerase and Proinflammatory Mediators. BioMed Res. Int. 2015, 2015, 850246. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; He, Y.; Hwang, S.; Bertola, A.; Mackowiak, B.; Ahmed, Y.A.; Seo, W.; Ma, J.; Wang, X.; Park, S.H.; et al. E-Selectin-Dependent Inflammation and Lipolysis in Adipose Tissue Exacerbate Steatosis-to-NASH Progression via S100A8/9. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, A.; Cotoi, O.S. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediat. Inflamm. 2013, 2013, 828354. [Google Scholar] [CrossRef] [PubMed]
- Nacken, W.; Roth, J.; Sorg, C.; Kerkhoff, C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech. 2003, 60, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arter. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Ludwig, S.; Goebeler, M.; Strey, A.; Thorey, I.S.; Reichelt, R.; Foell, D.; Gerke, V.; Manitz, M.P.; Nacken, W.; et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 2004, 104, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Viemann, D.; Barczyk, K.; Vogl, T.; Fischer, U.; Sunderkötter, C.; Schulze-Osthoff, K.; Roth, J. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood 2007, 109, 2453–2460. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, G.C.; Tan, H.Y.; Hao, F.B.; Hu, J.J. Nonalcoholic fatty liver disease and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis. Sci. Rep. 2019, 9, 11124. [Google Scholar] [CrossRef]
- Stefan, N. Nonalcoholic Fatty Liver Disease and Mortality. Clin. Gastroenterol. Hepatol. 2018, 16, 1043–1045. [Google Scholar] [CrossRef]
- Post, A.; Garcia, E.; van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; Groothof, D.; Westenbrink, B.D.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Nonalcoholic fatty liver disease, circulating ketone bodies and all-cause mortality in a general population-based cohort. Eur. J. Clin. Investig. 2021, 51, e13627. [Google Scholar] [CrossRef] [PubMed]
- Hillege, H.L.; Janssen, W.M.; Bak, A.A.; Diercks, G.F.; Grobbee, D.E.; Crijns, H.J.G.M.; van Gilst, W.; de Zeeuw, D.; de Jong, P.E. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, J.L.; Wolffenbuttel, B.H.; Dullaart, R.P.; on behalf of the PREVEND study group. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J. Intern. Med. 2011, 269, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Borggreve, S.E.; Hillege, H.L.; Wolffenbuttel, B.H.R.; de Jong, P.E.; Bakker, S.J.L.; van der Steege, G.; Van Tol, A.; Dullaart, R.P.F. The effect of cholesteryl ester transfer protein −629C→A promoter polymorphism on high-density lipoprotein cholesterol is dependent on serum triglycerides. J. Clin. Endocrinol. Metab. 2005, 90, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016, 59, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef]
- WHO. International Statistical Classification of Diseases and Related Health Problems, 10th revision; World Health Organization: Geneva, Switzerland, 2011; p. 2. [Google Scholar]
- Grubb, A.; Blirup-Jensen, S.; Lindström, V.; Schmidt, C.; Althaus, H.; Zegers, I.; on behalf of the IFCC Working Group on Standardisation of Cystatin C (WG-SCC). First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin. Chem. Lab. Med. 2010, 48, 1619–1621. [Google Scholar] [CrossRef]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin. Chem. Lab. Med. 2002, 40, 725–733. [Google Scholar]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin. Chem. Lab. Med. 2002, 40, 718–724. [Google Scholar]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 6. Reference procedure for the measurement of catalytic concentration of gamma-glutamyltransferase. Clin. Chem. Lab. Med. 2002, 40, 734–738. [Google Scholar] [PubMed]
- Schumann, G.; Bonora, R.; Ceriotti, F.; Férard, G.; Ferrero, C.A.; Franck, P.F.; Gella, F.J.; Hoelzel, W.; Jørgensen, P.J.; Kanno, T.; et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. Part 1. The concept of reference procedures for the measurement of catalytic activity concentrations of enzymes. Clin. Chem. Lab. Med. 2002, 40, 631–634. [Google Scholar]

| FLI < 60 | FLI ≥ 60 | p-Value | |
|---|---|---|---|
| n = 3854 | n = 1592 | ||
| Demographics | |||
| Age (years) | 49.8 [42.1–59.4] | 56.0 [47.9–65.8] | <0.001 |
| Sex | <0.001 | ||
| Male, n (%) | 1570 (40.7) | 1066 (67.0) | |
| Female, n (%) | 2284 (59.3) | 526 (33.0) | |
| Ethnicity | 0.233 | ||
| White, n (%) | 3676 (96.1) | 1520 (96.1) | |
| Black, n (%) | 28 (0.7) | 19 (1.2) | |
| Asian, n (%) | 82 (2.1) | 26 (1.6) | |
| Other, n (%) | 41 (1.1) | 16 (1.0) | |
| Anthropometrics | |||
| BMI, kg/m2 | 24.6 [22.8–26.7] | 30.1 [28.0–32.9] | <0.001 |
| Waist circumference (cm) | 86 [79–93] | 104 [99–110] | <0.001 |
| Waist/hip ratio | 0.87 [0.82–0.92] | 0.97 [0.91–1.01] | <0.001 |
| Cardiovascular risk factors | |||
| Current smokers, n (%) | 1076 (28.2) | 423 (26.9) | 0.303 |
| Alcohol consumption, n (%) | 2930 (76.7) | 1158 (73.2) | 0.006 |
| None, n (%) | 891 (67.7) | 425 (32.3) | |
| 1–4 drinks per month, n (%) | 657 (72.2) | 253 (27.8) | |
| 2–7 drinks per week, n (%) | 1289 (74.0) | 453 (26.0) | |
| 1–3 drinks per day, n (%) | 856 (71.2) | 347 (28.8) | |
| 4 or more drinks per day, n (%) | 128 (54.9) | 105 (45.1) | |
| SBP (mmHg) | 118 [109–131] | 133 [123–144] | <0.001 |
| DBP (mmHg) | 71 [65–77] | 77 [71–83] | <0.001 |
| Heart rate (bpm) | 67 [61–74] | 69 [63–76] | <0.001 |
| Comorbidities | |||
| History of cardiovascular disease, n (%) | 96 (2.5) | 84 (5.3) | <0.001 |
| History of diabetes, n (%) | 47 (1.2) | 82 (5.2) | <0.001 |
| Metabolic syndrome, n (%) | 1195 (31.0) | 933 (58.6) | <0.001 |
| Medication usage | |||
| Antihypertensive medication, n (%) | 491 (12.7) | 496 (31.2) | <0.001 |
| Lipid-lowering drugs, n (%) | 193 (5.0) | 191 (12.0) | <0.001 |
| Oral glucose-lowering drugs, n (%) | 24 (0.7) | 32 (4.5) | <0.001 |
| Laboratory parameters | |||
| Glucose (mmol/L) | 4.7 [4.4–5.1] | 5.1 [4.6–5.6] | <0.001 |
| Insulin (mU/L) | 6.8 [5.1–9.2] | 12.6 [9.4–18.2] | <0.001 |
| HOMA-IR (mU × mmol/L2/22.5) | 1.43 [1.04–2.00] | 2.87 [2.04–4.26] | <0.001 |
| UAE (mg/24 h) | 7.8 [5.7–12.5] | 10.8 [7.1–23.1] | <0.001 |
| hs-CRP (mg/L) | 1.02 [0.49–2.27] | 2.33 [1.17–4.19] | <0.001 |
| eGFR (mL/min/1.73 m2) | 96.3 [84.9–106.1] | 89.3 [77.4–100.6] | <0.001 |
| Cystatin C (mg/dL) | 0.85 [0.77–0.95] | 0.93 [0.84–1.04] | <0.001 |
| AST (U/L) | 21 [19–25] | 25 [21–30] | <0.001 |
| ALT (U/L) | 15 [12–20] | 23 [17–32] | <0.001 |
| ALP (U/L) | 63 [52–75] | 72 [61–85] | <0.001 |
| GGT (U/L) | 19 [14–27] | 41 [29–62] | <0.001 |
| Total cholesterol (mmol/L) | 5.25 [4.63–5.98] | 5.76 [5.08–6.45] | <0.001 |
| LDL-cholesterol (mmol/L) | 3.44 [2.87–4.08] | 3.78 [3.19–4.42] | <0.001 |
| HDL-cholesterol (mmol/L) | 1.30 [1.12–1.52] | 1.06 [0.92–1.23] | <0.001 |
| Triglycerides (mmol/L) | 0.94 [0.71–1.25] | 1.73 [1.29–2.34] | <0.001 |
| Plasma calprotectin (mg/L) | 0.46 [0.34–0.65] | 0.57 [0.42–0.79] | <0.001 |
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Plasma calprotectin (2-log) | 1.68 (1.56–1.82) | <0.001 | 1.58 (1.45–1.71) | <0.001 | 1.51 (1.38–1.64) | <0.001 | 1.19 (1.06–1.33) | 0.003 |
| Age | 1.03 (1.03–1.04) | <0.001 | 1.01 (1.00–1.02) | <0.001 | 0.99 (0.98–1.00) | 0.005 | ||
| Sex (ref = male) | 0.36 (0.32–0.41) | <0.001 | 0.34 (0.30–0.39) | <0.001 | 0.28 (0.23–0.33) | <0.001 | ||
| Diabetes (ref = no) | 3.01 (2.02–4.47) | <0.001 | 0.78 (0.44–1.40) | 0.410 | ||||
| History of CVD (ref = no) | 0.96 (0.69–1.35) | 0.822 | 0.98 (0.65–1.49) | 0.928 | ||||
| Hypertension (ref = no) | 2.68 (2.31–3.11) | <0.001 | 1.95 (1.63–2.34) | <0.001 | ||||
| Current smoking (ref = no) | 0.94 (0.81–1.09) | 0.404 | 1.01 (0.85–1.21) | 0.892 | ||||
| Alcohol use (ref = no) | 0.78 (0.67–0.90) | 0.001 | 0.94 (0.78–1.14) | 0.544 | ||||
| Total cholesterol | 1.53 (1.41–1.65) | <0.001 | ||||||
| eGFR | 0.99 (0.98–0.99) | <0.001 | ||||||
| HOMA-IR | 2.22 (2.07–2.38) | <0.001 | ||||||
| hs-CRP | 1.03 (1.02–1.05) | <0.001 | ||||||
| HR per Doubling | T1 | T2 | T3 | |
|---|---|---|---|---|
| <0.41 mg/L | 0.41–0.61 mg/L | >0.61 mg/L | ||
| A. Total cohort (n = 5446) | ||||
| Model 1 | 1.48 (1.28–1.71), p < 0.001 | 1.00 (reference) | 1.25 (0.91–1.70), p = 0.172 | 1.73 (1.29–2.32), p < 0.001 |
| Model 2 | 1.28 (1.10–1.50), p = 0.002 | 1.00 (reference) | 1.08 (0.79–1.48), p = 0.632 | 1.24 (0.92–1.66), p = 0.155 |
| Model 3 | 1.17 (1.00–1.38), p = 0.049 | 1.00 (reference) | 1.00 (0.73–1.38), p = 0.982 | 1.07 (0.80–1.45), p = 0.643 |
| Model 4 | 1.10 (0.91–1.33), p = 0.348 | 1.00 (reference) | 0.93 (0.66–1.31), p = 0.685 | 0.89 (0.64–1.24), p = 0.498 |
| B. FLI < 60 (n = 3854) | ||||
| Model 1 | 1.66 (1.37–2.00), p < 0.001 | 1.00 (reference) | 1.42 (0.94–2.15), p = 0.095 | 2.10 (1.42–3.10), p < 0.001 |
| Model 2 | 1.44 (1.18–1.77), p < 0.001 | 1.00 (reference) | 1.16 (0.77–1.75), p = 0.492 | 1.41 (0.95–2.09), p = 0.088 |
| Model 3 | 1.33 (1.07–1.65), p = 0.010 | 1.00 (reference) | 1.03 (0.68–1.56), p = 0.895 | 1.22 (0.81–1.81), p = 0.341 |
| Model 4 | 1.34 (1.05–1.72), p = 0.019 | 1.00 (reference) | 1.04 (0.67–1.63), p = 0.863 | 1.18 (0.76–1.83), p = 0.463 |
| C. FLI ≥ 60 (n = 1592) | ||||
| Model 1 | 1.07 (0.84–1.36), p = 0.604 | 1.00 (reference) | 0.83 (0.51–1.34), p = 0.435 | 0.97 (0.62–1.50), p = 0.875 |
| Model 2 | 1.07 (0.84–1.35), p = 0.598 | 1.00 (reference) | 0.99 (0.61–1.61), p = 0.967 | 1.02 (0.65–1.58), p = 0.947 |
| Model 3 | 1.01 (0.80–1.28), p = 0.942 | 1.00 (reference) | 1.03 (0.63–1.69), p = 0.892 | 0.95 (0.61–1.49), p = 0.815 |
| Model 4 | 0.88 (0.64–1.20), p = 0.406 | 1.00 (reference) | 0.90 (0.53–1.53), p = 0.686 | 0.70 (0.42–1.17), p = 0.170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgonje, A.R.; van den Berg, E.H.; Kieneker, L.M.; Nilsen, T.; Hidden, C.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; van Goor, H.; Abdulle, A.E. Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population. Int. J. Mol. Sci. 2022, 23, 15708. https://doi.org/10.3390/ijms232415708
Bourgonje AR, van den Berg EH, Kieneker LM, Nilsen T, Hidden C, Bakker SJL, Blokzijl H, Dullaart RPF, van Goor H, Abdulle AE. Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population. International Journal of Molecular Sciences. 2022; 23(24):15708. https://doi.org/10.3390/ijms232415708
Chicago/Turabian StyleBourgonje, Arno R., Eline H. van den Berg, Lyanne M. Kieneker, Tom Nilsen, Clara Hidden, Stephan J. L. Bakker, Hans Blokzijl, Robin P. F. Dullaart, Harry van Goor, and Amaal E. Abdulle. 2022. "Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population" International Journal of Molecular Sciences 23, no. 24: 15708. https://doi.org/10.3390/ijms232415708
APA StyleBourgonje, A. R., van den Berg, E. H., Kieneker, L. M., Nilsen, T., Hidden, C., Bakker, S. J. L., Blokzijl, H., Dullaart, R. P. F., van Goor, H., & Abdulle, A. E. (2022). Plasma Calprotectin Levels Associate with Suspected Metabolic-Associated Fatty Liver Disease and All-Cause Mortality in the General Population. International Journal of Molecular Sciences, 23(24), 15708. https://doi.org/10.3390/ijms232415708






