Metabolomics and Proteomics Characterizing Hepatic Reactions to Dietary Linseed Oil in Duck
Abstract
1. Introduction
2. Results
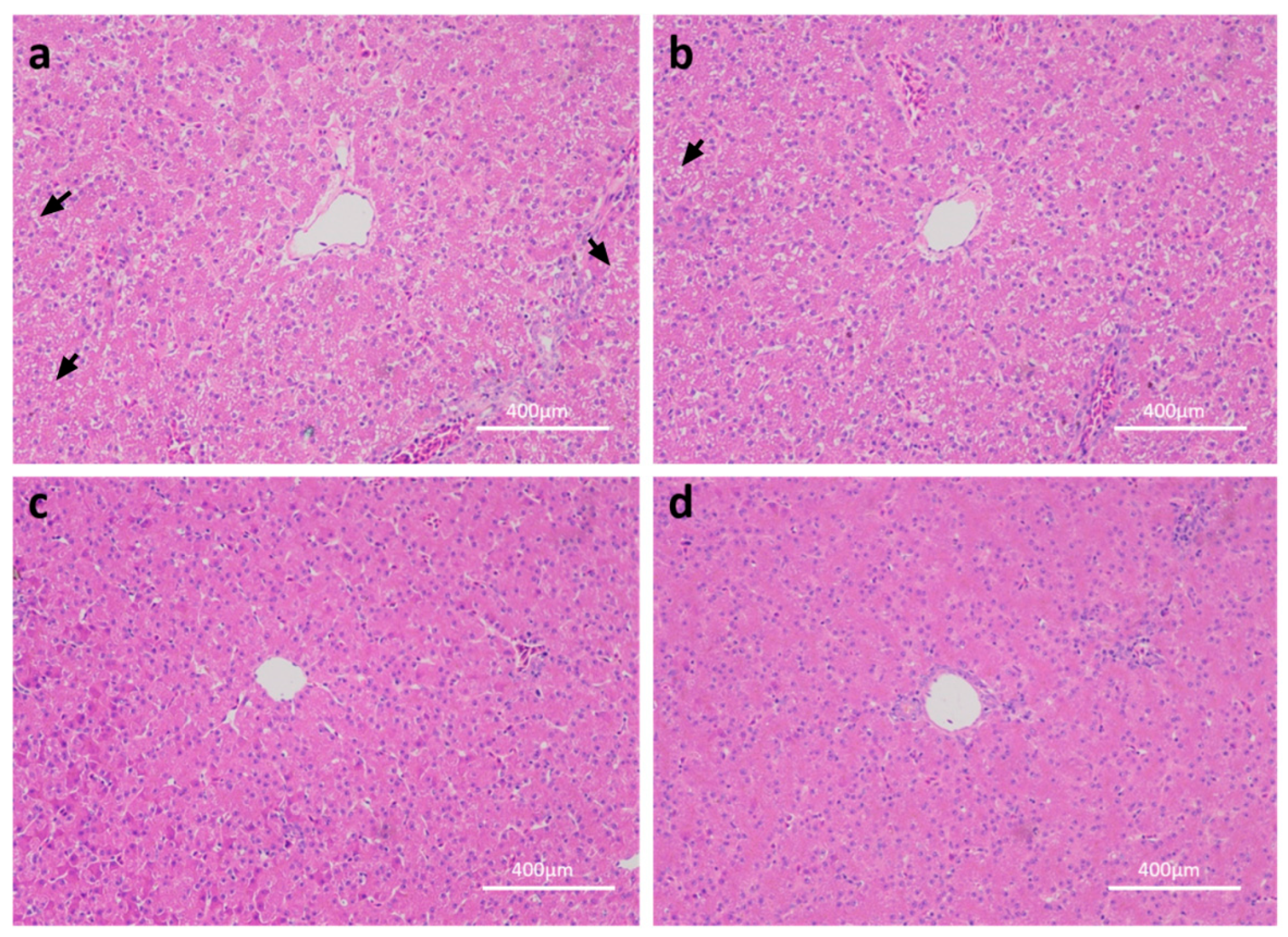
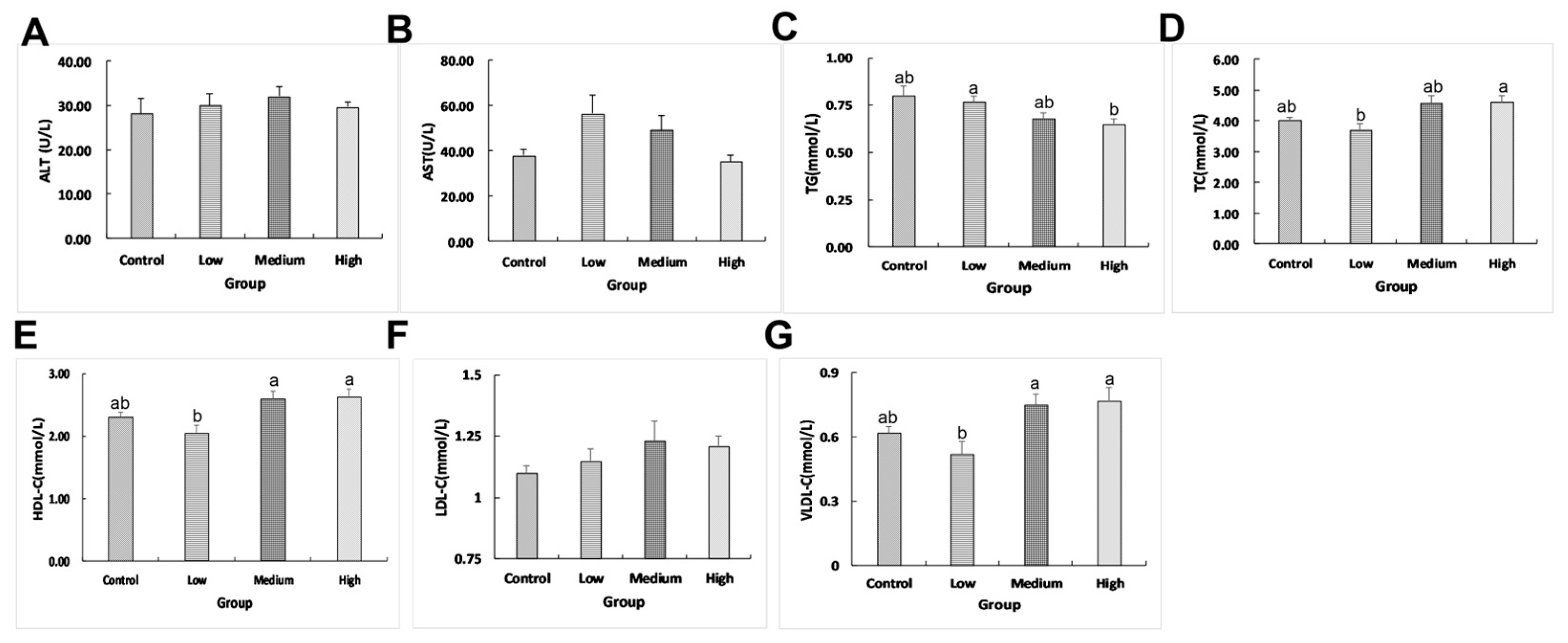
2.1. Effects of Linseed Oil on Liver Morphology and Blood Parameters
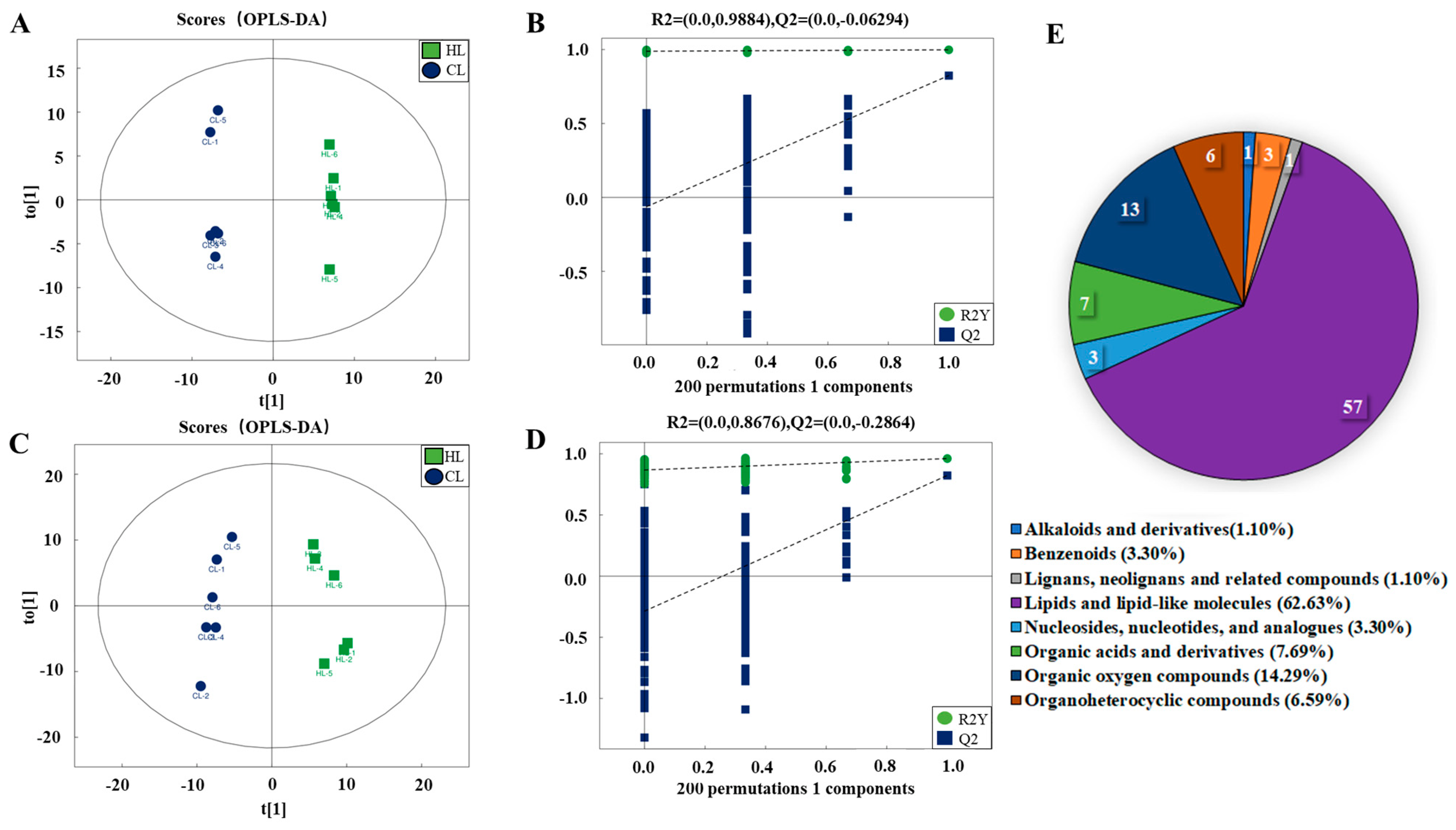
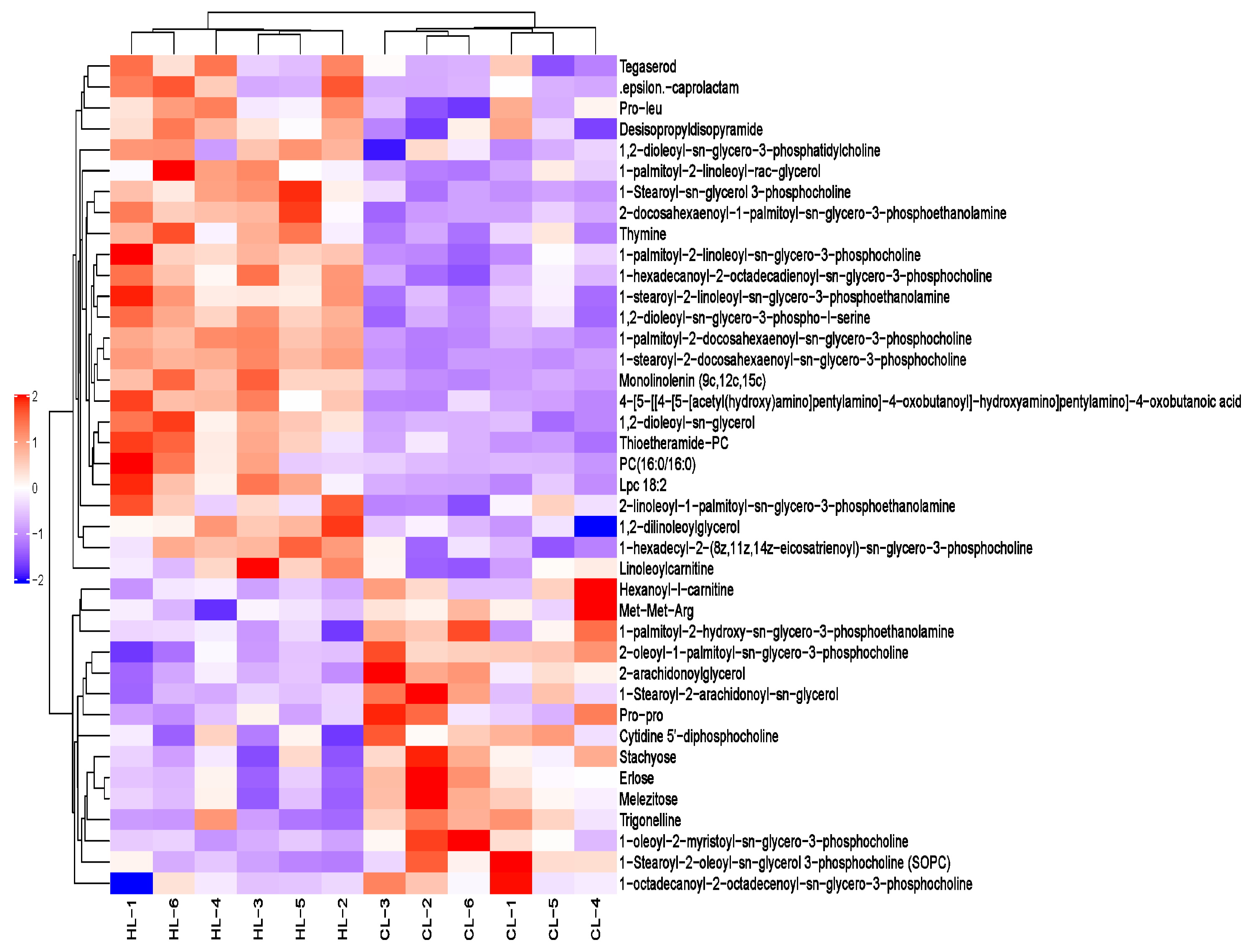
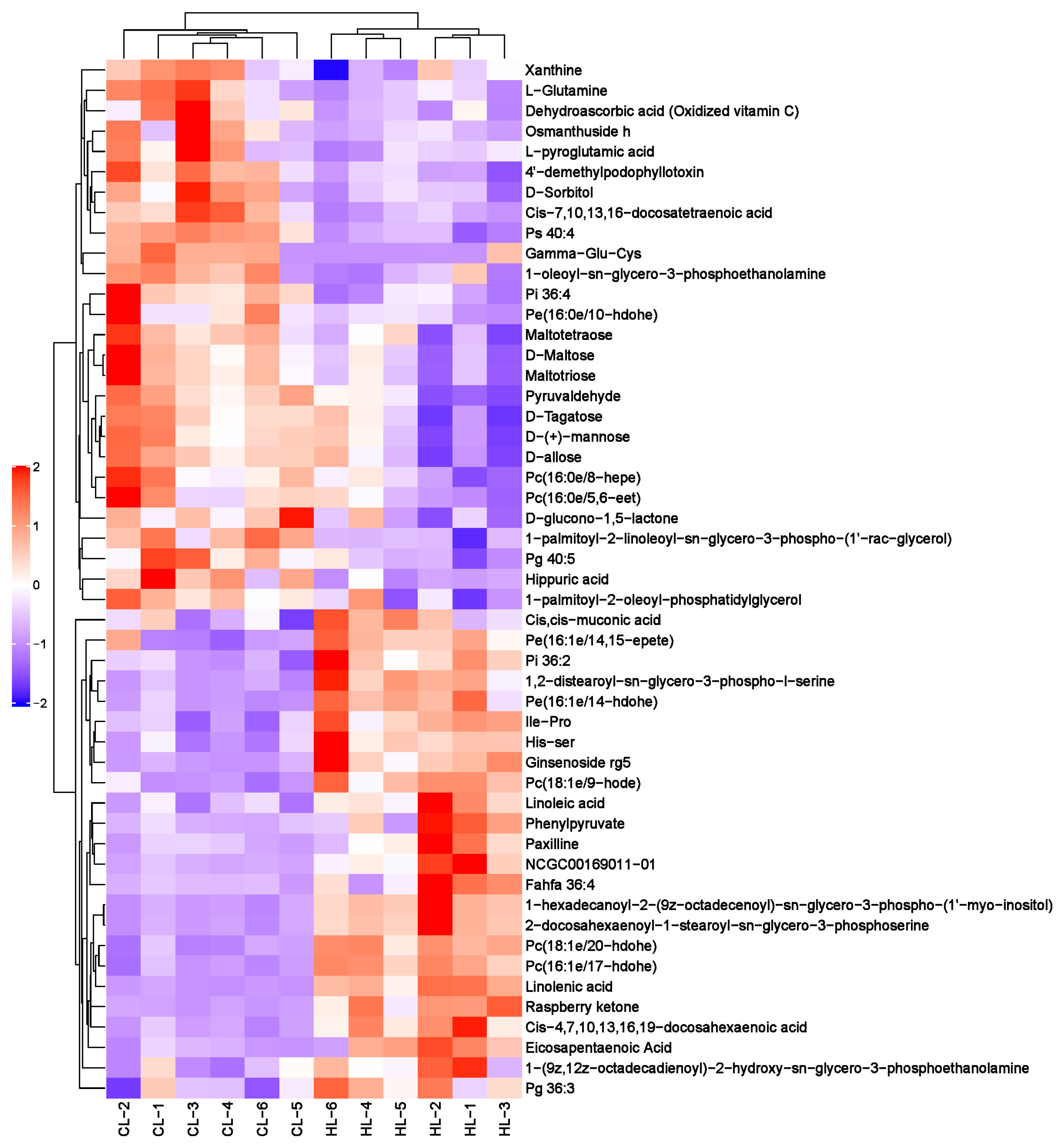
2.2. Metabolomic Changes in Linseed-Oil-Supplemented Duck Livers
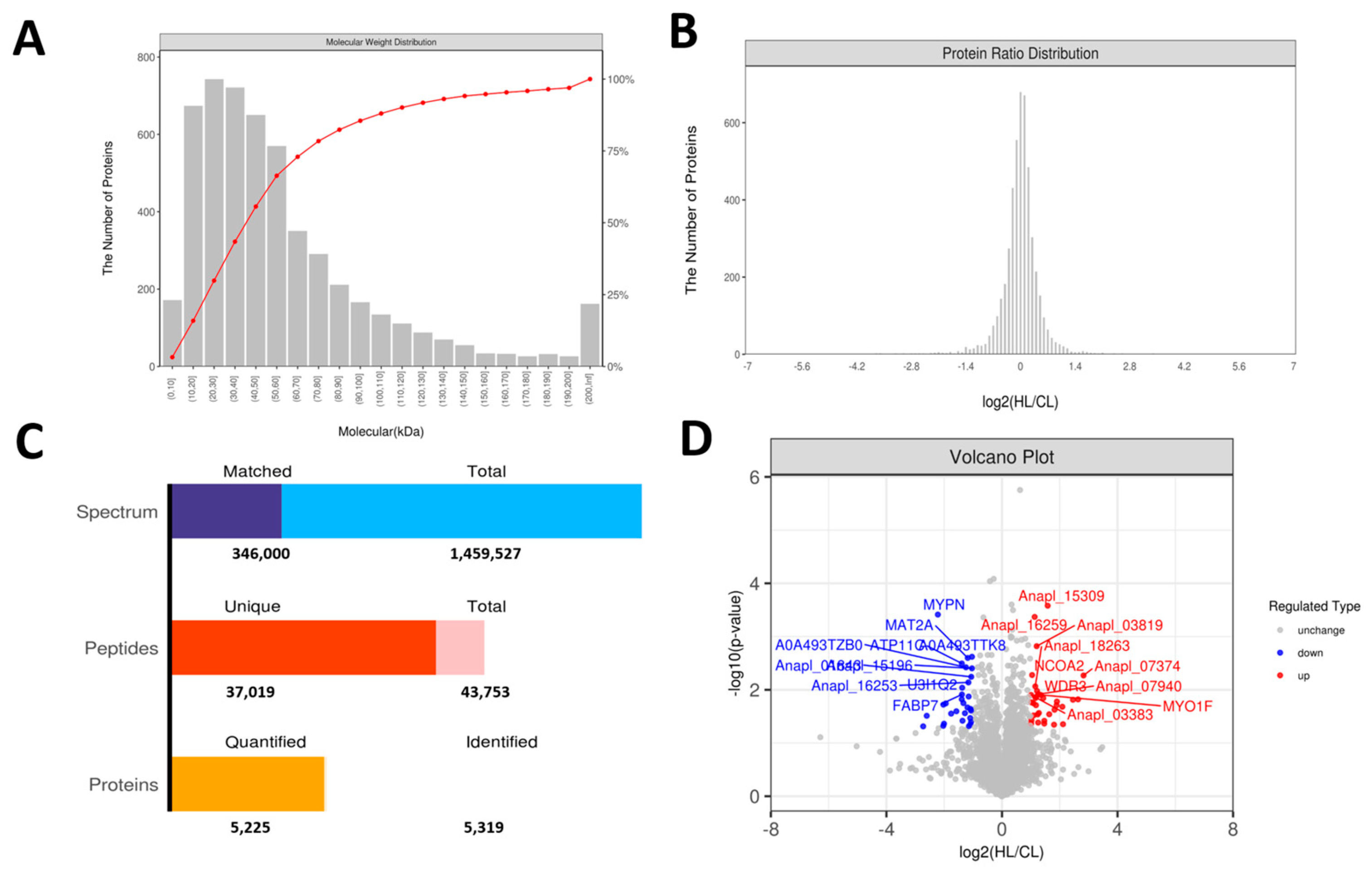
2.3. Proteomics Changes in Linseed-Oil-Supplemented Duck Livers
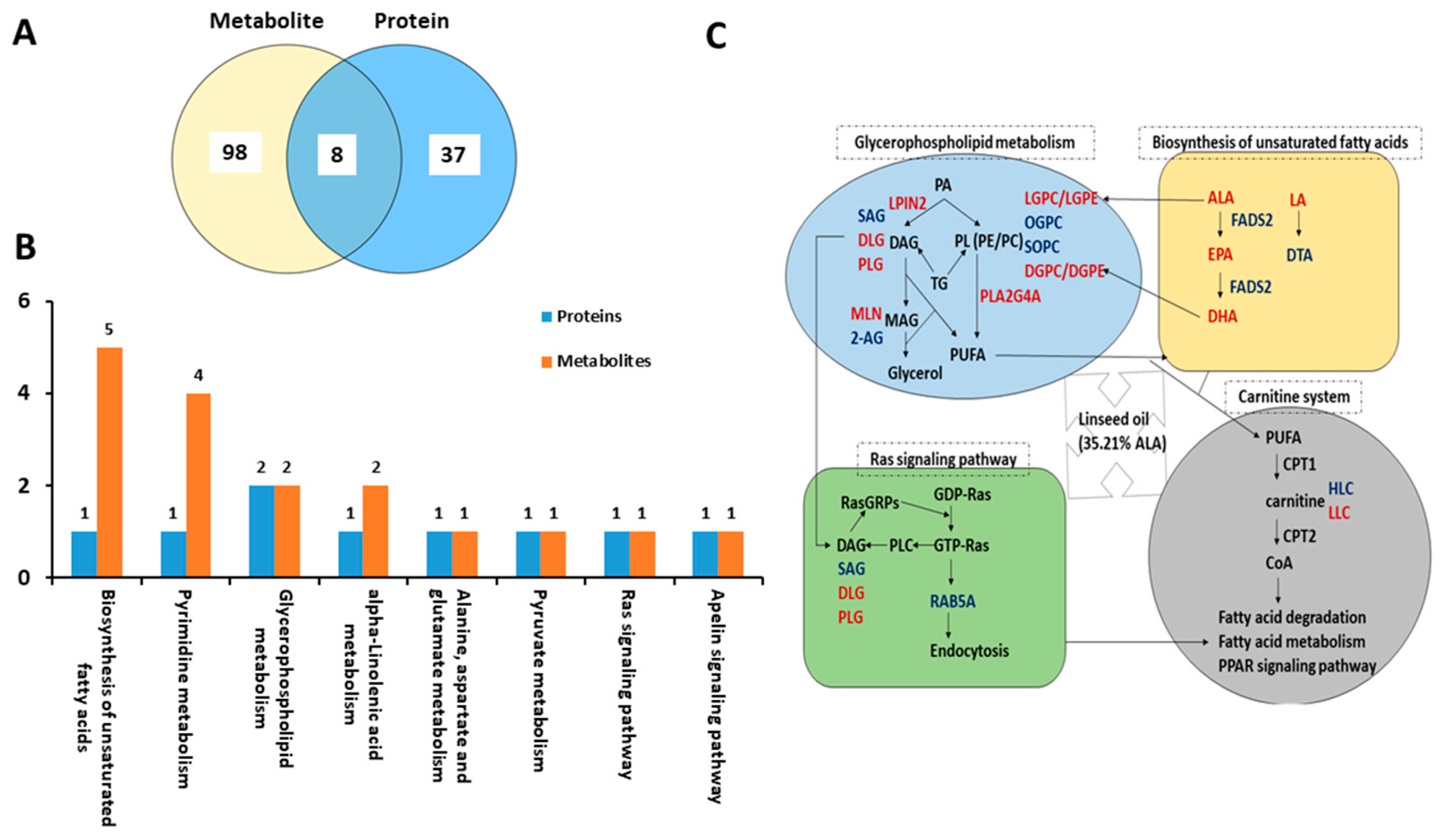
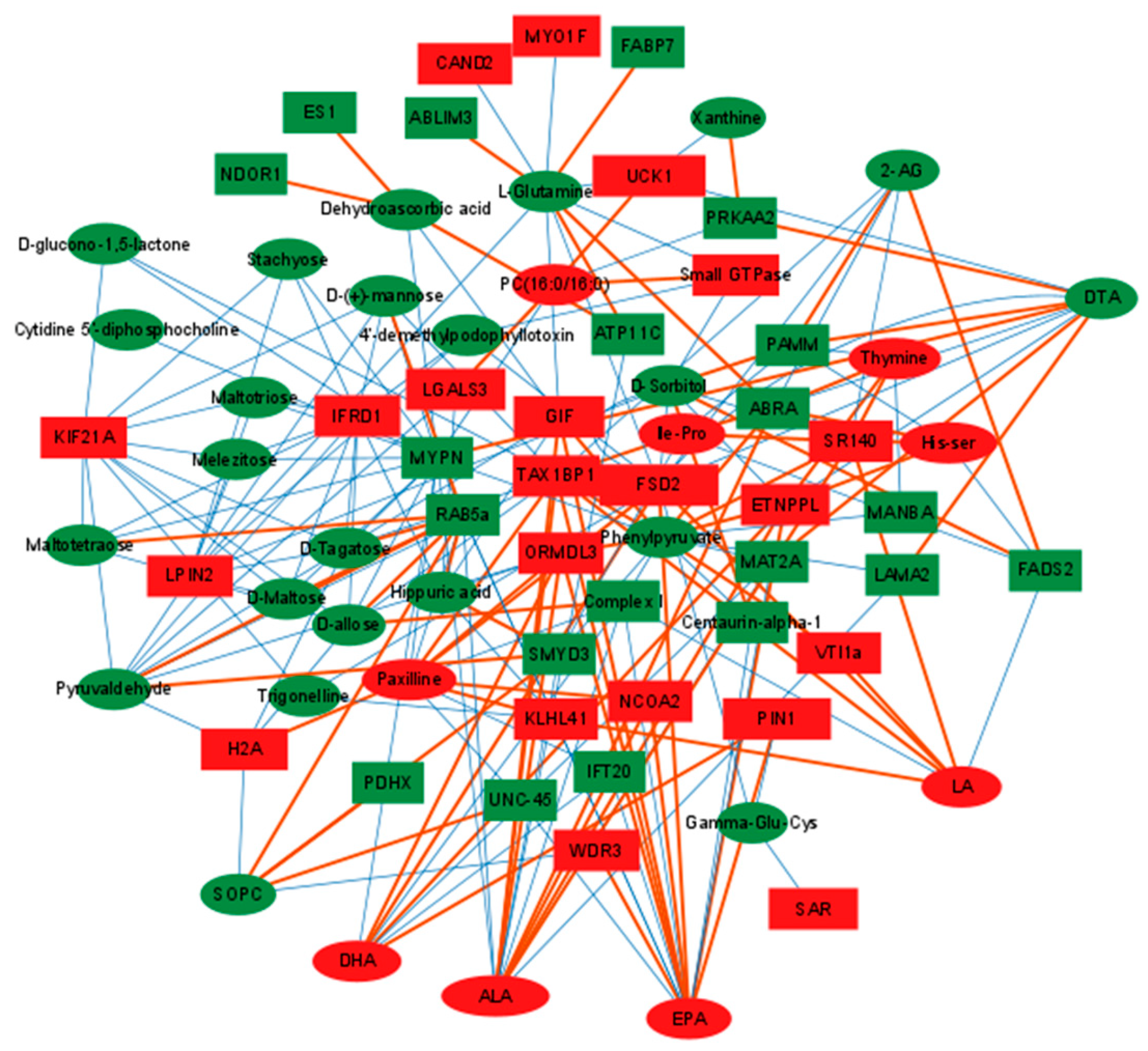
2.4. Conjoint Analysis of Differential Metabolites and Proteins
3. Discussion
3.1. Linseed Oil Improved Blood Parameters
3.2. Dietary Linseed Oil Altered the Lipid Composition of the Liver
3.3. Dietary Linseed Oil Altered the Level of Hepatic Lipid-like Molecules
3.4. Dietary Linseed Oil Altered the Metabolism of PUFA in Liver
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Blood and Sample Collection
4.3. Plasma Biochemical Parameters
4.4. Liver Morphological Observation
4.5. Untargeted Metabolomic Analysis of Liver
4.6. The 4D Label-Free Proteome Analysis of Liver
4.7. Association Analyses of Differentially Expressed Metabolites and Proteins
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can we improve the nutritional quality of meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef]
- Longo, V.D.; Anderson, R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell 2022, 185, 1455–1470. [Google Scholar] [CrossRef]
- Hermier, D. Lipoprotein metabolism and fattening in poultry. J. Nutr. 1997, 127, 805s–808s. [Google Scholar] [CrossRef]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian liver: The forgotten organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef]
- Wei, R.; Han, C.; Deng, D.; Ye, F.; Gan, X.; Liu, H.; Li, L.; Xu, H.; Wei, S. Research progress into the physiological changes in metabolic pathways in waterfowl with hepatic steatosis. Br. Poult. Sci. 2021, 62, 118–124. [Google Scholar] [CrossRef]
- Prasad, K. Flaxseed and cardiovascular health. J. Cardiovasc. Pharmacol. 2009, 54, 369–377. [Google Scholar] [CrossRef]
- Shahid, M.S.; Wu, Y.; Xiao, Z.; Raza, T.; Dong, X.; Yuan, J. Duration of the flaxseed diet promotes deposition of n-3 fatty acids in the meat and skin of Peking ducks. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, Y.Y.; Wang, J.P.; Yan, B.X.; Huang, Y.Q.; Wang, Z.X. Effect of flaxseed on the fatty acid profile of egg yolk and antioxidant status of their neonatal offspring in Huoyan geese. Animal 2015, 9, 1749–1755. [Google Scholar] [CrossRef]
- Kalakuntla, S.; Nagireddy, N.K.; Panda, A.K.; Jatoth, N.; Thirunahari, R.; Vangoor, R.R. Effect of dietary incorporation of n-3 polyunsaturated fatty acids rich oil sources on fatty acid profile, keeping quality and sensory attributes of broiler chicken meat. Anim. Nutr. 2017, 3, 386–391. [Google Scholar] [CrossRef]
- Wang, L.; Dong, B.; Yang, T.; Zhang, A.; Hu, X.; Wang, Z.; Chang, G.; Chen, G. Dietary linseed oil affects the polyunsaturated fatty acid and transcriptome profiles in the livers and breast muscles of ducks. Front. Nutr. 2022, 9, 1030712. [Google Scholar] [CrossRef]
- Alvarenga, R.R.; Zangeronimo, M.G.; Pereira, L.J.; Rodrigues, P.B.; Gomide, E.M. Lipoprotein metabolism in poultry. World’s Poult. Sci. J. 2011, 67, 431–440. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A unique multifunctional receptor for cholesterol Influx and efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef]
- Gracia-Rubio, I.M.C.; Civeira, F.; Cenarro, A. SR-B1, a Key Receptor Involved in the Progression of Cardiovascular Disease: A Perspective from Mice and Human Genetic Studies. Biomedicines 2021, 9, 612. [Google Scholar] [CrossRef]
- Shi, C.M.; Zhao, H.; Zhai, X.L.; Chen, Y.J.; Lin, S.M. Linseed oil can decrease liver fat deposition and improve antioxidant ability of juvenile largemouth bass, Micropterus salmoides. Fish Physiol. Biochem. 2019, 45, 1513–1521. [Google Scholar] [CrossRef]
- Bovet, P.; Faeh, D.; Madeleine, G.; Viswanathan, B.; Paccaud, F. Decrease in blood triglycerides associated with the consumption of eggs of hens fed with food supplemented with fish oil. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 280–287. [Google Scholar] [CrossRef]
- Ayerza, R., Jr.; Coates, W. Effect of dietary alpha-linolenic fatty acid derived from chia when fed as ground seed, whole seed and oil on lipid content and fatty acid composition of rat plasma. Ann. Nutr. Metab. 2007, 51, 27–34. [Google Scholar] [CrossRef]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; West, S.G.; Gillies, P.J.; Kris-Etherton, P.M. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J. Nutr. 2004, 134, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhai, S.; Xie, Q.; Tian, L.; Li, X.; Zhang, J.; Ye, H.; Zhu, Y.; Yang, L.; Wang, W. Effects of Dietary n-6:n-3 PUFA Ratios on Lipid Levels and Fatty Acid Profile of Cherry Valley Ducks at 15–42 Days of Age. J. Agric. Food Chem. 2017, 65, 9995–10002. [Google Scholar] [CrossRef] [PubMed]
- Chorner, Z.; Barbeau, P.A.; Castellani, L.; Wright, D.C.; Chabowski, A.; Holloway, G.P. Dietary α-linolenic acid supplementation alters skeletal muscle plasma membrane lipid composition, sarcolemmal FAT/CD36 abundance, and palmitate transport rates. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R1234–R1242. [Google Scholar] [CrossRef] [PubMed]
- Crespo, N.; Esteve-Garcia, E. Dietary linseed oil produces lower abdominal fat deposition but higher de novo fatty acid synthesis in broiler chickens. Poult. Sci. 2002, 81, 1555–1562. [Google Scholar] [CrossRef]
- Qi, X.; Shang, M.; Chen, C.; Chen, Y.; Hua, J.; Sheng, X.; Wang, X.; Xing, K.; Ni, H.; Guo, Y. Dietary supplementation with linseed oil improves semen quality, reproductive hormone, gene and protein expression related to testosterone synthesis in aging layer breeder roosters. Theriogenology 2019, 131, 9–15. [Google Scholar] [CrossRef]
- Qi, K.K.; Chen, J.L.; Zhao, G.P.; Zheng, M.Q.; Wen, J. Effect of dietary omega6/omega3 on growth performance, carcass traits, meat quality and fatty acid profiles of Beijing-you chicken. J. Anim. Physiol. Anim. Nutr. 2010, 94, 474–485. [Google Scholar] [CrossRef]
- Mortimer, B.C.; Kenrick, M.A.; Holthouse, D.J.; Stick, R.V.; Redgrave, T.G. Plasma clearance of model lipoproteins containing saturated and polyunsaturated monoacylglycerols injected intravenously in the rat. Biochim. Biophys. Acta 1992, 1127, 67–73. [Google Scholar] [CrossRef]
- Weintraub, M.S.; Zechner, R.; Brown, A.; Eisenberg, S.; Breslow, J.L. Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J. Clin. Investig. 1988, 82, 1884–1893. [Google Scholar] [CrossRef]
- Liisberg, U.; Fauske, K.R.; Kuda, O.; Fjære, E.; Myrmel, L.S.; Norberg, N.; Frøyland, L.; Graff, I.E.; Liaset, B.; Kristiansen, K.; et al. Intake of a Western diet containing cod instead of pork alters fatty acid composition in tissue phospholipids and attenuates obesity and hepatic lipid accumulation in mice. J. Nutr. Biochem. 2016, 33, 119–127. [Google Scholar] [CrossRef]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Fatty Acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol. 2013, 2013, 361895. [Google Scholar] [CrossRef]
- Gundermann, K.J.; Kuenker, A.; Kuntz, E.; Droździk, M. Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacol. Rep. 2011, 63, 643–659. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Turner, N.; Storlien, L.H.; Else, P.L. Dietary fats and membrane function: Implications for metabolism and disease. Biol. Rev. Camb. Philos. Soc. 2005, 80, 155–169. [Google Scholar] [CrossRef]
- Leaf, A.; Xiao, Y.F.; Kang, J.X.; Billman, G.E. Membrane effects of the n-3 fish oil fatty acids, which prevent fatal ventricular arrhythmias. J. Membr. Biol. 2005, 206, 129–139. [Google Scholar] [CrossRef]
- Croset, M.; Brossard, N.; Polette, A.; Lagarde, M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000, 345 Pt 1, 61–67. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; Moaddel, R.; Sun, K.; Fabbri, E.; Zhang, P.; Khadeer, M.; Salem, N., Jr.; Ferrucci, L.; Semba, R.D. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: The baltimore longitudinal study of aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 62–67. [Google Scholar] [CrossRef]
- Huwiler, A.; Pfeilschifter, J. Lipids as targets for novel anti-inflammatory therapies. Pharmacol. Ther. 2009, 124, 96–112. [Google Scholar] [CrossRef]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of inflammation in brain: A matter of fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef]
- Balogun, K.A.; Albert, C.J.; Ford, D.A.; Brown, R.J.; Cheema, S.K. Dietary omega-3 polyunsaturated fatty acids alter the fatty acid composition of hepatic and plasma bioactive lipids in C57BL/6 mice: A lipidomic approach. PLoS ONE 2013, 8, e82399. [Google Scholar] [CrossRef]
- Singer, P.; Berger, I.; Moritz, V.; Förster, D.; Taube, C. N-6 and N-3 PUFA in liver lipids, thromboxane formation and blood pressure from SHR during diets supplemented with evening primrose, sunflowerseed or fish oil. Prostaglandins Leukot. Essent. Fat. Acids 1990, 39, 207–211. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, T. Functional Lipids in Metabolic Syndrome. J. Nutr. Sci. Vitaminol. 2015, 61, S159–S161. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Levy, B.D. Resolvins and protectins: Mediating solutions to inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, X.; Liu, B. Molecular characterization, chromosomal localization and association analysis with back-fat thickness of porcine LPIN2 and LPIN3. Mol. Biol. Rep. 2009, 36, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.J.C.; Corrigan, E.; Melnyk, N.; Hilker, R.; Van Der Kraak, G. Nuclear progesterone receptor regulates ptger4b and PLA2G4A expression in zebrafish (Danio rerio) ovulation. Gen. Comp. Endocrinol. 2021, 311, 113842. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeon, J.H.; Lee, M.J. Docosahexaenoic Acid, a Potential Treatment for Sarcopenia, Modulates the Ubiquitin-Proteasome and the Autophagy-Lysosome Systems. Nutrients 2020, 12, 2597. [Google Scholar] [CrossRef]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.; Hamilton, D.L. Omega-3 fatty acids and skeletal muscle health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef]
- Filomena, M.C.; Yamamoto, D.L.; Caremani, M.; Kadarla, V.K.; Mastrototaro, G.; Serio, S.; Vydyanath, A.; Mutarelli, M.; Garofalo, A.; Pertici, I.; et al. Myopalladin promotes muscle growth through modulation of the serum response factor pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 169–194. [Google Scholar] [CrossRef]
- Ramirez-Martinez, A.; Cenik, B.K.; Bezprozvannaya, S.; Chen, B.; Bassel-Duby, R.; Liu, N.; Olson, E.N. KLHL41 stabilizes skeletal muscle sarcomeres by nonproteolytic ubiquitination. eLife 2017, 6, e26439. [Google Scholar] [CrossRef]
- Sun, W.; Ma, X.; Wang, H.; Du, Y.; Chen, J.; Hu, H.; Gao, R.; He, R.; Peng, Q.; Cui, Z.; et al. MYO1F regulates antifungal immunity by regulating acetylation of microtubules. Proc. Natl. Acad. Sci. USA 2021, 118, e2100230118. [Google Scholar] [CrossRef]
- Proserpio, V.; Fittipaldi, R.; Ryall, J.G.; Sartorelli, V.; Caretti, G. The methyltransferase SMYD3 mediates the recruitment of transcriptional cofactors at the myostatin and c-Met genes and regulates skeletal muscle atrophy. Genes Dev. 2013, 27, 1299–1312. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Z.; Zhang, R.; Yan, Y.; Peng, Y.; Ye, H.; Lin, L.; Xu, J.; Li, W.; Huang, P. SMYD3 promotes hepatocellular carcinoma progression by methylating S1PR1 promoters. Cell Death Dis. 2021, 12, 731. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, A.; Wang, L.; Yang, T.; Dong, B.; Wang, Z.; Bi, Y.; Chen, G.; Chang, G. Metabolomics and Proteomics Characterizing Hepatic Reactions to Dietary Linseed Oil in Duck. Int. J. Mol. Sci. 2022, 23, 15690. https://doi.org/10.3390/ijms232415690
Zhang Y, Zhang A, Wang L, Yang T, Dong B, Wang Z, Bi Y, Chen G, Chang G. Metabolomics and Proteomics Characterizing Hepatic Reactions to Dietary Linseed Oil in Duck. International Journal of Molecular Sciences. 2022; 23(24):15690. https://doi.org/10.3390/ijms232415690
Chicago/Turabian StyleZhang, Yang, Ao Zhang, Laidi Wang, Ting Yang, Bingqiang Dong, Zhixiu Wang, Yulin Bi, Guohong Chen, and Guobin Chang. 2022. "Metabolomics and Proteomics Characterizing Hepatic Reactions to Dietary Linseed Oil in Duck" International Journal of Molecular Sciences 23, no. 24: 15690. https://doi.org/10.3390/ijms232415690
APA StyleZhang, Y., Zhang, A., Wang, L., Yang, T., Dong, B., Wang, Z., Bi, Y., Chen, G., & Chang, G. (2022). Metabolomics and Proteomics Characterizing Hepatic Reactions to Dietary Linseed Oil in Duck. International Journal of Molecular Sciences, 23(24), 15690. https://doi.org/10.3390/ijms232415690




