3. Methods and Materials
General
The NMR spectra,
1H, and
13C were measured in solutions of CDCl
3 or DMSO-
d6 on a Bruker AVANCE-III HD instrument (at 400.40 or 100.61 MHz, respectively). The residual solvent signals were used as internal standards in DMSO-
d6 (2.50 ppm for
1H, and 40.45 ppm for
13C nuclei) or in CDCl
3 (7.26 ppm for
1H, and 77.16 ppm for
13C nuclei). The high-resolution mass spectra were registered with a Bruker Maxis spectrometer (electrospray ionization, in MeCN solution, using HCO
2Na–HCO
2H for calibration). See
Supplementary Materials for NMR (
Figures S1–S32) and HRMS (
Figures S33–S48) spectral charts. The IR spectra were measured on FT-IR spectrometer Shimadzu IR Affinity-1S equipped with an ATR sampling module. The melting points were measured with a Stuart SMP30 apparatus. The reaction progress and purity of isolated compounds were controlled by TLC on ALUGRAM Xtra SIL G UV 254 plates. The column chromatography was performed with Macherey Nagel Silica gel 60 (particle size: 0.063–0.2 mm). The pyrimidines were synthesized by published methods [
22,
23] and the synthesis of 5-ethynylpyrimidines is described in our recent report [
24]. All other reagents and solvents were purchased from commercial vendors and used as received.
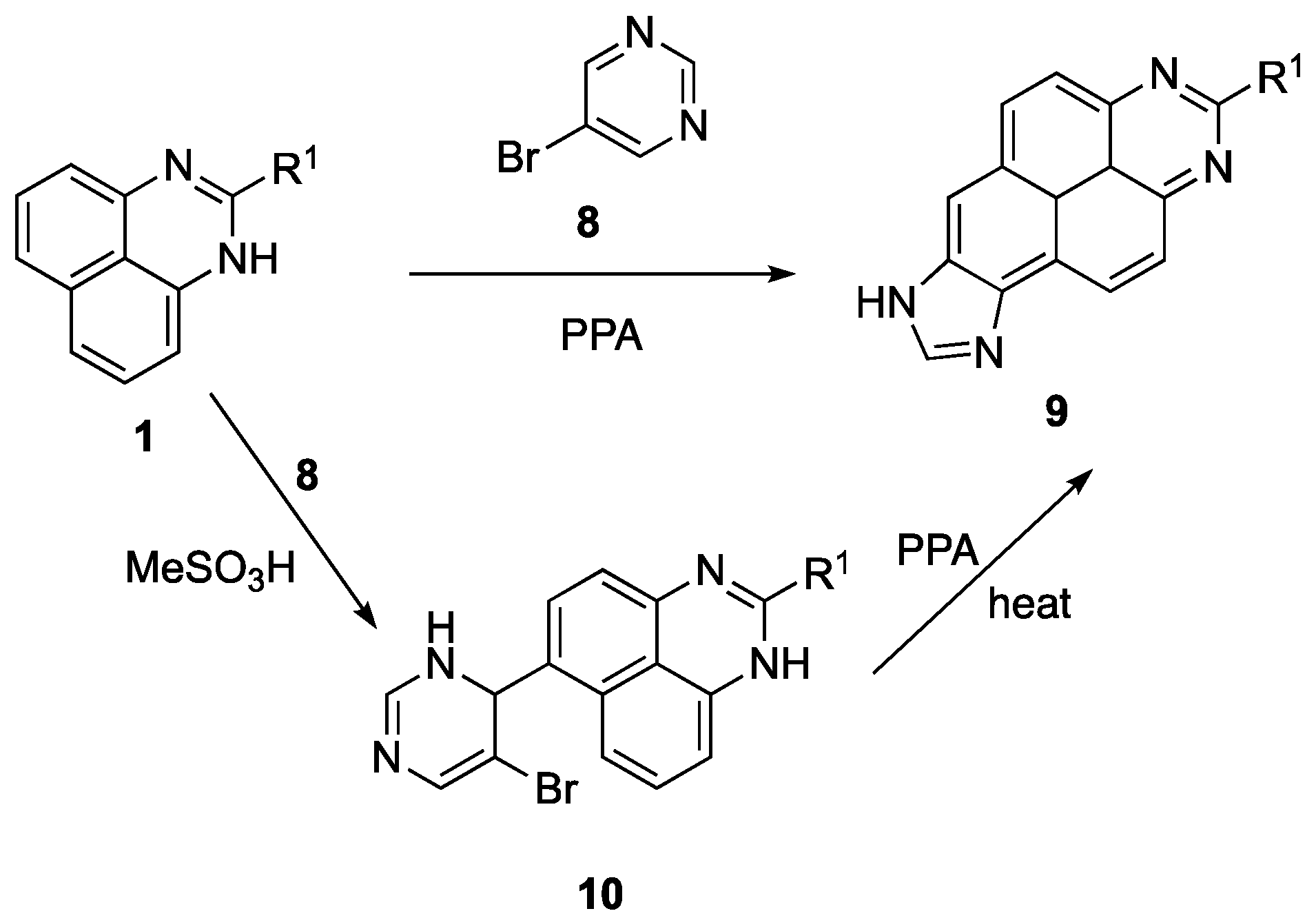
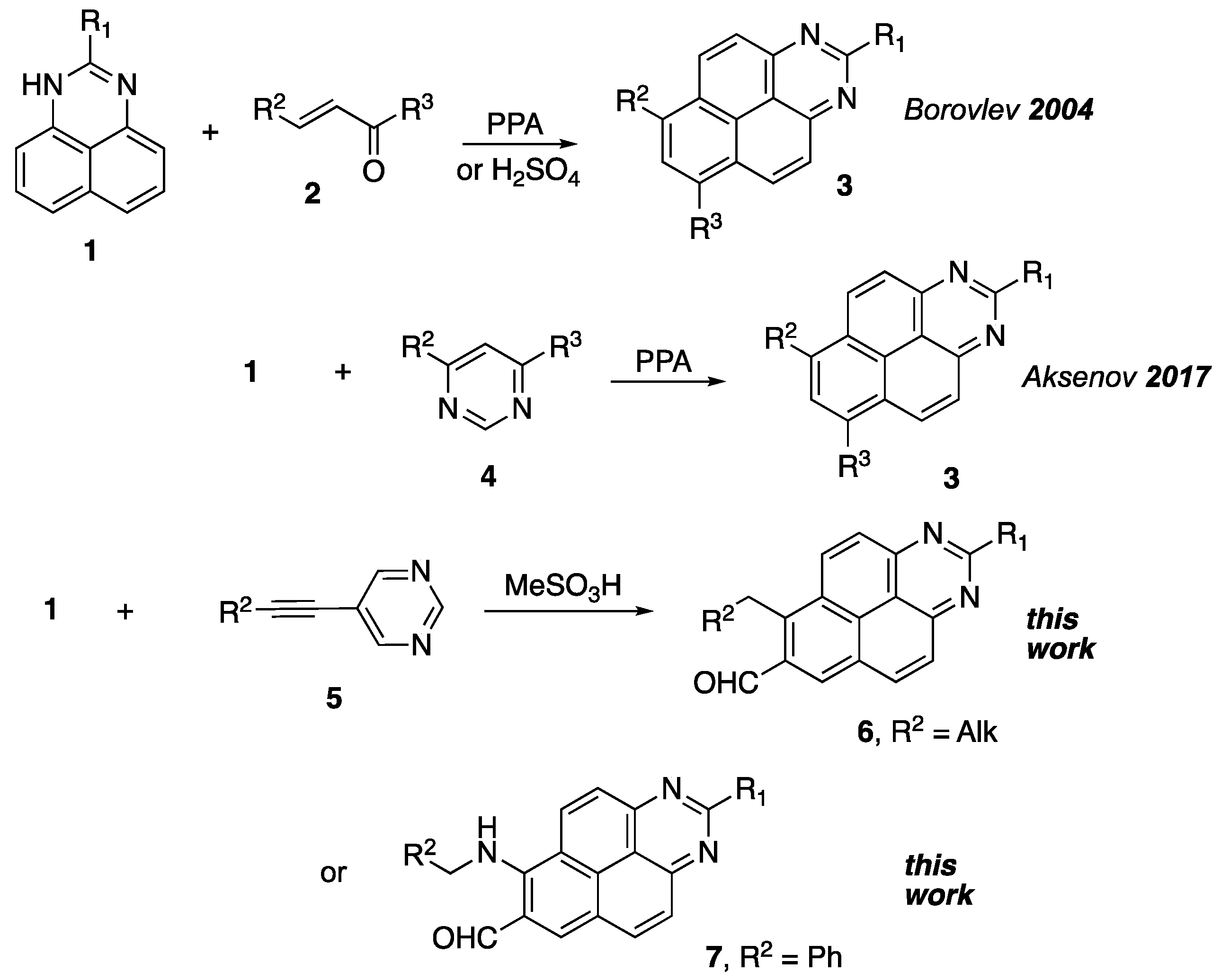
Method for preparation of benzo[gh]perimidine-7-carbaldehyde:
A round bottom flask (10 mL) was charged with the appropriate pyrimidine 5 (1.00 mmol) and the corresponding 1H-perimidine 1 (1.00 mmol) was added, followed by 5 mL of methanesulfonic acid. The reaction mixture was stirred at room temperature and the reaction progress was monitored by thin-layer chromatography (EtOAc/Hexane, 2:1, v/v). After one hour, when TLC confirmed that all starting substances were consumed, the reaction mixture was poured isolated into cold water (50 mL) and neutralized with an aqueous ammonia solution (20%, 15 mL). The crystalline precipitate was filtered off and washed with a small amount of water to remove the excess ammonia. The resulting product was separated and purified by column chromatography (EtOAc/Hexane, 1:3, v/v).
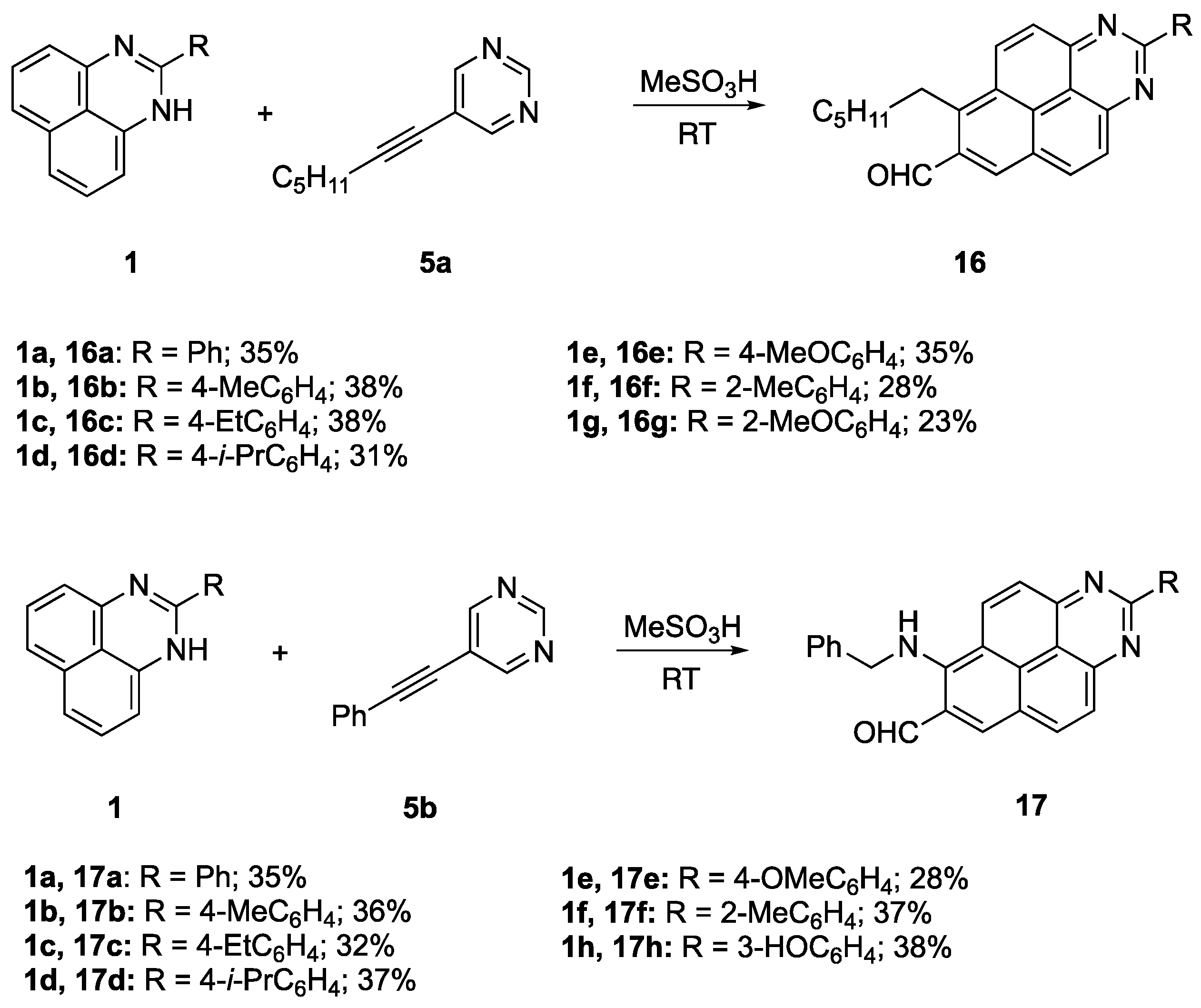
6-Hexyl-2-phenylbenzo[gh]perimidine-7-carbaldehyde (16a): This compound was prepared by employing 2-phenyl-1H-perimidine 1a and 5-(hept-1-yn-1-yl)pyrimidine 5a in a yield of 137 mg (0.35 mmol, 35%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-yellow powder, m.p. 180.7–181.9 °C, Rf 0.58 (EtOAc/Hexane, 1:3). 1H NMR (400 MHz, CDCl3) δ 10.76 (s, 1H), 8.88–8.79 (m, 4H), 8.53 (d, J = 9.1 Hz, 1H), 8.35 (d, J = 9.8 Hz, 1H), 8.25 (d, J = 9.1 Hz, 1H), 7.65–7.55 (m, 3H), 3.89–3.70 (m, 2H), 1.90–1.79 (m, 2H), 1.68–1.53 (m, 2H), 1.46–1.32 (m, 4H), 0.92 (t, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.2, 161.7, 154.9, 154.1, 144.8, 138.1, 137.5, 136.5, 133.4, 131.1, 131.0, 128.8(2C), 128.6(2C), 127.9, 127.7, 127.4, 127.2, 124.6, 114.2, 33.4, 31.1, 28.9, 26.6, 22.1, 14.0; FTIR, vmax: 3068, 2954, 1951, 1689, 1628, 1511, 1398, 1306, 909, 845, 794, 714, 695, 679, 668 cm−1; HRMS (ESI TOF) m/z: calc’d for C27H25N2O [M + H]+: 393.1961, found 393.1959 (−0.5 ppm).
6-Hexyl-2-(p-tolyl)benzo[gh]perimidine-7-carbaldehyde (16b): This compound was prepared by employing 2-(p-tolyl)-1H-perimidine 1b and 5-(hept-1-yn-1-yl)pyrimidine 5a in a yield of 154 mg (0.38 mmol, 38%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-brown powder, m.p. 192.1–193.9 °C, Rf 0.62 (EtOAc/Hexane, 1:3). 1H NMR (400 MHz, CDCl3) δ 10.77 (s, 1H), 8.92–8.79 (m, 2H), 8.73 (d, J = 7.9 Hz, 2H), 8.54 (d, J = 9.2 Hz, 1H), 8.36 (d, J = 9.5 Hz, 1H), 8.26 (d, J = 9.2 Hz, 1H), 7.41 (d, J = 7.9 Hz, 2H), 3.89–3.72 (m, 2H), 2.49 (s, 3H), 1.91–1.78 (m, 2H), 1.66–1.54 (m, 2H), 1.46–1.29 (m, 4H), 0.92 (t, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 190.8, 161.3, 157.6, 155.3, 149.3, 145.1, 141.9, 137.4, 134.7, 133.6, 130.8, 130.3(2С), 128.4, 127.4(2С), 125.8, 124.7, 123.9, 113.8, 34.4, 31.6, 29.8, 29.7, 28.0, 22.6, 21.9, 14.1; FTIR, vmax: 2960, 2926, 2858, 1954, 1761, 1699, 1687, 1628, 1512, 1408, 1399, 1308, 1179, 957, 854, 830, 725, 669 cm−1; HRMS (ESI TOF) m/z: calc’d for C28H27N2O [M + H]+: 407.2118, found 407.2114 (−1.0 ppm).
2-(4-Ethylphenyl)-6-hexylbenzo[gh]perimidin-7-carbaldehyde (16c): This compound was prepared by employing 2-(4-ethylphenyl)-1H-perimidine 1c and 5-(hept-1-yn-1-yl)pyrimidine 5a in a yield of 154 mg (0.38 mmol, 38%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-yellow powder, m.p. 176–177.2 °C, Rf 0.64 (EtOAc/Hexane, 1:3). 1H NMR (400 MHz, CDCl3) δ 10.70 (s, 1H), 8.77–8.68 (m, 4H), 8.43 (d, J = 9.2 Hz, 1H), 8.24 (d, J = 9.5 Hz, 1H), 8.14 (d, J = 9.1 Hz, 1H), 7.42 (d, J = 8.0 Hz, 2H), 3.77–3.67 (m, 2H), 2.79 (q, J = 7.6 Hz, 2H), 1.81 (p, J = 7.7 Hz, 2H), 1.58 (p, J = 7.1 Hz, 2H), 1.43–1.30 (m, 7H), 0.92 (t, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.0, 162.8, 155.3, 154.4, 147.7, 144.7, 136.7, 135.8, 132.7, 132.0, 131.0, 129.3(2C), 128.5(2C), 128.2, 128.1, 127.7, 127.6, 125.6, 114.6, 33.8, 31.8, 29.9, 29.1, 27.6, 22.8, 15.6, 14.2; FTIR, vmax: 2963, 2928, 2858, 1918, 1772, 1684, 1626, 1509, 1407, 1396, 1307, 1177, 1017, 957, 844, 687, 668 cm−1; HRMS (ESI TOF) m/z: calc’d for C29H29N2O [M + H]+: 421.2274, found 421.2271 (−0.7 ppm).
6-Hexyl-2-(4-isopropylphenyl)benzo[gh]perimidin-7-carbaldehyde (16d): This compound was prepared by employing 2-(4-isopropylphenyl)-1H-perimidine 1d and 5-(hept-1-yn-1-yl)pyrimidine 5a in a yield of 134 mg (0.31 mmol, 31%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-brown powder, m.p. 186.2–187.9 °C, Rf 0.63 (EtOAc/Pe, 1:3). 1H NMR (400 MHz, CDCl3) δ 10.68 (s, 1H), 8.69 (d, J = 8.2 Hz, 4H), 8.39 (d, J = 9.0 Hz, 1H), 8.19 (d, J = 9.4 Hz, 1H), 8.09 (d, J = 9.0 Hz, 1H), 7.45 (d, J = 8.3 Hz, 2H), 3.69 (t, J = 8.2 Hz, 2H), 3.05 (hept, J = 6.8 Hz, 1H), 1.79 (p, J = 8.0 Hz, 2H), 1.57 (p, J = 7.1 Hz, 2H), 1.43–1.30 (m, 10H), 0.92 (t, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.0, 162.8, 155.2, 154.3, 152.3, 144.6, 136.6, 136.1, 132.2, 132.0, 131.0, 129.3(2C), 128.2, 128.1, 127.7, 127.6, 127.0(2C), 125.5, 114.6, 34.3, 33.8, 31.8, 29.9, 27.6, 24.04, 22.8, 14.2(2C); FTIR, vmax: 2957, 2859, 1942, 1774, 1685, 1627, 1508, 1396, 1306, 1017, 912, 849, 690 cm−1; HRMS (ESI TOF) m/z: calc’d for C30H31N2O [M + H]+: 435.2431, found 435.2429 (−0.4 ppm).
6-Hexyl-2-(4-methoxyphenyl)benzo[gh]perimidine-7-carbaldehyde (16e): This compound was prepared by employing 2-(4-methoxyphenyl)-1H-perimidine 1e and 5-(hept-1-yn-1-yl)pyrimidine 5a in a yield of 148 mg (0.35 mmol, 35%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-yellow powder, m.p. 178.4–179.7 °C, Rf 0.41 (EtOAc/Hexane, 1:3). 1H NMR (400 MHz, CDCl3) δ 10.77 (s, 1H), 9.10 (d, J = 9.5 Hz, 1H), 9.05 (s, 1H), 8.85 (d, J = 9.3 Hz, 1H), 8.74 (d, J = 9.0 Hz, 2H), 8.33 (d, J = 9.5 Hz, 1H) 8.24 (d, J = 9.3 Hz, 1H), 7.18 (d, J = 9.0 Hz, 2H), 3.90 (s, 3H), 3.86 (t, J = 8.1 Hz, 2H), 1.82–1.70 (m, 2H), 1.63–1.49 (m, 2H), 1.43–1.26 (m, 4H), 0.88 (t, J = 7.0 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.2, 161.8, 161.7, 154.9, 154.1, 144.7, 137.4, 137.1, 133.2, 132.1, 131.1, 130.6, 130.3(2С), 127.7, 127.2, 127.1, 124.8, 114.2(2С), 113.9, 55.4, 33.4, 31.1, 28.9, 26.6, 22.1, 14.0; FTIR, vmax: 2958, 2923, 2724, 2048, 1946, 1802, 1696, 1628, 1603, 1510, 1399, 1303, 1249, 1168, 1038, 957, 907, 849, 729 cm−1; HRMS (ESI TOF) m/z: calc’d for C28H27N2O2 [M + H]+: 423.2068, found 423.2067 (0.1 ppm).
6-Hexyl-2-(o-tolyl)benzo[gh]perimidin-7-carbaldehyde (16f): This compound was prepared by employing 2-(o-tolyl)-1H-perimidine 1f and 5-(hept-1-yn-1-yl)pyrimidine 5a in a yield of 114 mg (0.28 mmol, 28%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-yellow powder, m.p. 120.5–121.4 °C, Rf 0.55 (EtOAc/Hexane, 1:3). 1H NMR (400 MHz, CDCl3) δ 10.81 (s, 1H), 8.97–8.86 (m, 2H), 8.59 (d, J = 8.6 Hz, 1H), 8.42 (d, J = 9.5 Hz, 1H), 8.31 (d, J = 9.1 Hz, 1H), 8.07–7.97 (m, 1H), 7.51–7.33 (m, 3H), 3.94–3.75 (m, 2H), 2.66 (s, 3H), 1.84 (m, 2H), 1.60 (p, J = 7.3 Hz, 2H), 1.47–1.29 (m, 4H), 0.92 (t, J = 6.6 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 192.0, 165.6, 155.0, 154.1, 145.2(2C), 137.6, 137.4, 133.0, 132.4, 131.5, 131.4, 131.3, 129.8, 128.6(2C), 127.9, 127.5, 126.4, 125.5, 114.3, 34.0, 31.8, 29.8, 27.7, 22.8, 21.1, 14.2; FTIR, vmax: 3353, 3062, 2958, 2855, 1946, 1683, 1628, 1509, 1396, 1308, 1198, 1077, 956, 908, 853, 821, 791, 741, 724, 673 cm−1; HRMS (ESI TOF) m/z: calc’d for C28H27N2O [M + H]+: 407.2118, found 407.2112 (1.4 ppm).
6-Hexyl-2-(2-methoxyphenyl)benzo[gh]perimidine-7-carbaldehyde (16g): This compound was prepared by employing 2-(2-methoxyphenyl)-1H-perimidine1g and5-(hept-1-yn-1-yl)pyrimidine 5a in a yield of 97 mg (0.23 mmol, 23%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-red powder, m.p. 138.5–140.6 °C, Rf 0.15 (EtOAc/Hexane, 1:3). 1H NMR (400 MHz, CDCl3) δ 10.76 (s, 1H), 9.12–9.03 (m, 2H), 8.85 (d, J = 9.2 Hz, 1H), 8.31 (d, J = 9.5 Hz, 1H), 8.21 (d, J = 9.2 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 7.54 (t, J = 7.6 Hz, 1H), 7.24 (d, J = 8.4 Hz, 1H), 7.14 (t, J = 7.4 Hz, 1H), 3.87–3.79 (m, 5H), 1.73 (brs, 2H), 1.53 (brs, 2H), 1.31 (brs, 4H), 0.87 (t, J = 6.9 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 193.3, 163.9, 157.4, 154.5, 153.7, 144.6, 137.3, 133.2, 132.3, 131.6, 131.0, 130.7, 129.8, 127.9, 127.6, 127.4, 127.0, 124.5, 120.3, 113.6, 112.3, 55.8, 33.5, 31.2, 29.0, 26.7, 22.2, 14.0; FTIR, vmax: 2925, 2843, 1766, 1690, 1628, 1512, 1248, 1023, 954, 856, 746, 665 cm−1; HRMS (ESI TOF) m/z: calc’d for C28H27N2O2 [M + H]+: 423.2067, found 423.2067 (0.1 ppm).
6-(Benzylamino)-2-phenylbenzo[gh]perimidin-7-carbaldehyde (17a): This compound was prepared by employing 2-phenyl-1H-perimidine 1a and 5-(phenylethynyl)pyrimidine 5b in a yield of 144 mg (0.35 mmol, 35%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as gray powder, m.p. 241–242.2 °C, Rf 0.16 (EtOAc/Hexane, 1:1). 1H NMR (400 MHz, CDCl3) δ 8.79 (d, J = 6.7 Hz, 2H), 8.63 (d, J = 9.4 Hz, 1H), 8.49 (d, J = 9.4 Hz, 1H), 8.37 (s, 1H), 8.23 (d, J = 9.4 Hz, 1H), 8.09 (d, J = 9.4 Hz, 1H), 7.92 (s, 1H), 7.67–7.49 (m, 8H), 6.36 (m, 1H), 5.22 (d, J = 5.7 Hz, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 160.9, 154.2, 154.0, 142.3, 139.3, 139.0, 135.0, 134.3, 131.3, 130.8(3C), 129.6, 129.1(2C), 129.0(2C), 128.8(2C), 128.4, 128.0, 127.5, 126.9, 126.6, 123.4, 121.6, 115.0, 39.9; FTIR, vmax: 3304, 3065, 2889, 2714, 1937, 1798, 1732, 1632, 1504, 1412, 1352, 1265, 1207, 899, 851, 835, 775 cm−1; HRMS (ESI TOF) m/z: calc’d for C28H20N3O [M + H]+: 414.1601, found 414.1599 (0.4 ppm).
6-(Benzylamino)-2-(p-tolyl)benzo[gh]perimidine-7-carbaldehyde (17b): This compound was prepared by employing 2-(p-tolyl)-1H-perimidine 1b and 5-(phenylethynyl)pyrimidine 5b in a yield of 196 mg (0.36 mmol, 36%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-yellow powder, m.p. 268–269 °C, Rf 0.19 (EtOAc/Hexane, 1:1). 1H NMR (400 MHz, CDCl3) δ 9.01 (d, J = 9.4 Hz, 1H), 8.87 (s, 1H), 8.68 (d, J = 7.9 Hz, 2H), 8.56 (d, J = 9.4 Hz, 1H), 8.31 (d, J = 9.4 Hz, 1H), 8.26–8.19 (m, 2H), 8.15 (s, 1H), 7.69–7.58 (m, 5H), 7.43 (d, J = 7.9 Hz, 2H), 5.19 (d, J = 6.0 Hz, 2H), 2.44 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 161.2, 161.0, 153.6, 153.5, 141.5, 140.6, 138.9, 137.7, 135.6, 134.1, 132.5, 130.5(2С), 129.4(2С), 129.3, 128.8(2С), 128.4(2С), 128.3, 127.0, 126.9, 126.0, 125.5, 122.7, 114.4, 38.5, 21.1; FTIR, vmax: 3285, 3032, 2874, 1919, 1732, 1661, 1557, 1497, 1410, 1391, 1342, 1179, 835, 758 cm−1; HRMS (ESI TOF) m/z: calc’d for C29H22N3O [M + H]+: 428.1757, found 428.1759 (0.5 ppm).
6-(Benzylamino)-2-(4-ethylphenyl)benzo[gh]perimidin-7-carbaldehyde (17c): This compound was prepared by employing 2-(4-ethylphenyl)-1H-perimidine 1c and 5-(phenylethynyl)pyrimidine 5b in a yield of 141 mg (0.32 mmol, 32%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-yellow powder, m.p. 233–234.5 °C, Rf 0.19 (EtOAc/Hexane, 1:1). 1H NMR (400 MHz, CDCl3) δ 9.04 (d, J = 9.5 Hz, 1H), 8.90 (s, 1H), 8.72 (d, J = 8.0 Hz, 2H), 8.59 (d, J = 9.5 Hz, 1H), 8.35 (d, J = 9.5 Hz, 1H), 8.28–8.23 (m, 2H), 8.17 (s, 1H), 7.73–7.61 (m, 5H), 7.48 (d, J = 8.0 Hz, 2H), 5.21 (d, J = 6.0 Hz, 2H), 2.75 (q, J = 7.5 Hz, 2H), 1.29 (t, J = 7.6 Hz, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 161.2, 161.0, 153.6 (2С), 146.8, 141.6, 138.9, 137.8, 135.8, 134.2, 132.6, 130.5(2C), 129.4, 128.9(2C), 128.5(2C), 128.3, 128.2(2C), 127.1, 127.0, 126.0, 125.5, 122.7, 114.5, 38.5, 28.1, 15.4; FTIR, vmax: 3084, 2968, 1946, 1825, 1776, 1697, 1557, 1495, 1406, 1393, 1339, 1240, 1180, 843 cm−1; HRMS (ESI TOF) m/z: calc’d for C30H24N3O [M + H]+: 442.1907, found 442.1916 (−1.6 ppm).
6-(Benzylamino)-2-(4-isopropylphenyl)benzo[gh]perimidin-7-carbaldehyde (17d): This compound was prepared by employing 2-(4-isopropylphenyl)-1H-perimidine 1d and 5-(phenylethynyl)pyrimidine 5b in a yield of 169 mg (0.37 mmol, 37%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as gray powder, m.p. 246–247.5 °C, Rf 0.19 (EtOAc/Hexane, 1:1). 1H NMR (400 MHz, CDCl3) δ 9.02 (d, J = 9.4 Hz, 1H), 8.88 (brs, 1H), 8.71 (d, J = 8.2 Hz, 2H), 8.57 (d, J = 9.4 Hz, 1H), 8.33 (d, J = 9.4 Hz, 1H), 8.27–8.20 (m, 2H), 8.16 (s, 1H), 7.69–7.60 (m, 5H), 7.50 (d, J = 8.2 Hz, 2H), 5.20 (d, J = 5.9 Hz, 2H), 3.07–2.99 (m, 1H), 1.30 (d, J = 7.0 Hz, 6H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 161.2, 161.0, 153.6 (2C), 151.4, 141.6, 138.9, 137.8, 136.0, 134.1, 132.6, 130.5(2C), 129.4, 128.9(2C), 128.6(2C), 128.3, 127.1, 127.0, 126.8(2C), 126.0, 125.5, 122.7, 114.5, 38.5, 33.5, 23.8 (2C); FTIR, vmax: 3265, 3034, 2949, 2872, 1938, 1738, 1678, 1651, 1557, 1495, 1408, 1385, 1265, 893, 845, 777 cm−1; HRMS (ESI TOF) m/z: calc’d for C31H26N3O [M + H]+: 456.2070, found 456.2072 (0.4 ppm).
6-(benzylamino)-2-(4-methoxyphenyl)benzo[gh]perimidine-7-carbaldehyde (17e): This compound was prepared by employing 2-(4-methoxyphenyl)-1H-perimidine 1e and 5-(phenylethynyl)pyrimidine 5a in a yield of 124 mg (0.28 mmol, 28%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as yellow powder, m.p. 269–270,5 °C, Rf 0.15 (EtOAc/Hexane, 1:1). 1H NMR (400 MHz, DMSO-d6) δ 9.02 (d, J = 9.4 Hz, 1H), 8.88 (s, 1H), 8.74 (d, J = 8.5 Hz, 2H), 8.56 (d, J = 9.4 Hz, 1H), 8.32 (d, J = 9.4 Hz, 1H), 8.26–8.20 (m, 2H), 8.15 (s, 1H), 7.70–7.60 (m, 5H), 7.17 (d, J = 8.5 Hz, 2H), 5.20 (d, J = 6.0 Hz, 2H), 3.89 (s, 3H). 13C{1H} NMR (101 MHz, DMSO-d6) δ 161.6, 161.2, 160.9, 153.7, 153.6, 141.5, 139.0, 138.9, 137.8, 137.1, 134.1, 132.6, 130.7, 130.5, 130.1(2С), 129.3, 128.8, 128.3, 127.0 (2С), 125.9, 125.4, 125.2, 114.3, 114.2(2С), 55.4, 38.5. FTIR, vmax: 3118, 2831, 2357, 2058, 1926, 1734, 1693, 1602, 1556, 1494, 1408, 1392, 1338, 1253, 1166, 1031, 844, 771 cm−1; HRMS (ESI TOF) m/z: calc’d for C29H22N3O2 [M + H]+: 444.1705, found 444.1707 (0.4 ppm).
6-(Benzylamino)-2-(o-tolyl)benzo[gh]perimidine-7-carbaldehyde (17f): This compound was prepared by employing 2-(o-tolyl)-1H-perimidine 1f and 5-(phenylethynyl)pyrimidine 5b in a yield of 158 mg (0.37 mmol, 37%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-green powder, m.p. 218–219 °C, Rf 0.19 (EtOAc/Hexane, 1:1). 1H NMR (400 MHz, CDCl3) δ 9.05 (d, J = 9.5 Hz, 1H), 8.90 (brs, 1H), 8.60 (d, J = 9.5 Hz, 1H), 8.33 (d, J = 9.5 Hz, 1H), 8.27–8.17 (m, 3H), 8.07–8.02 (m, 1H), 7.72–7.60 (m, 5H), 7.49–7.35 (m, 3H), 5.22 (d, J = 6.0 Hz, 2H), 2.66 (s, 3H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 164.2, 161.3, 153.2, 153.2, 141.5, 139.1, 138.9, 137.8, 137.1, 134.1, 132.6, 131.2, 131.1, 130.6(2C), 129.4, 129.2, 128.7(2C), 128.3, 127.0, 126.9, 126.1, 125.9, 125.6, 122.5, 113.8, 38.5, 21.1; FTIR, vmax: 3260, 3053, 2889, 2729, 1954, 1829, 1649, 1501, 1410, 1267, 1146, 899, 854, 768 cm−1; HRMS (ESI TOF) m/z: calc’d for C29H22N3O [M + H]+: 428.1757, found 428.1762 (−1.0 ppm).
6-(Benzylamino)-2-(3-hydroxyphenyl)benzo[gh]perimidin-7-carbaldehyde (17h): This compound was prepared by employing 3-(1H-perimidin-2-yl)phenol 1h and 5-(phenylethynyl)pyrimidine 5b in a yield of 163 mg (0.38 mmol, 38%). Purification was performed by column chromatography (EtOAc/Hexane = 1:3). The titled compound was obtained as light-yellow powder, m.p. 264–265.5 °C, Rf 0.16 (EtOAc/Hexane, 1:1). 1H NMR (400 MHz, CDCl3) δ 9.69 (s, 1H), 9.01 (d, J = 9.5 Hz, 1H), 8.87 (s, 1H), 8.56 (d, J = 9.4 Hz, 1H), 8.30 (d, J = 9.5 Hz, 1H), 8.27–8.19 (m, 4H), 8.16 (s, 1H), 7.76–7.56 (m, 5H), 7.42 (t, J = 8.0 Hz, 1H), 6.98 (d, J = 8.0 Hz, 1H), 5.19 (d, J = 5.9 Hz, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 161.7, 161.3, 158.3, 154.0, 153.9, 142.0, 140.1, 139.3, 138.2, 134.6, 133.0, 131.0(2С), 130.2, 129.9, 129.3(2С), 128.7, 127.5, 127.4, 126.5, 126.0, 123.1, 119.8, 118.3, 115.6, 115.0, 39.0; FTIR, vmax: 3374, 3273, 3053, 2864, 2741, 1933, 1792, 1680, 1622, 1582, 1558, 1516, 1410, 1385, 1271, 1238, 1215, 1148, 1078, 1030, 995, 966, 887, 849, 835, 820, 797, 762 cm−1; HRMS (ESI TOF) m/z: calc’d for C28H20N3O2 [M + H]+: 430.1550, found 430.1541 (−2.1 ppm).
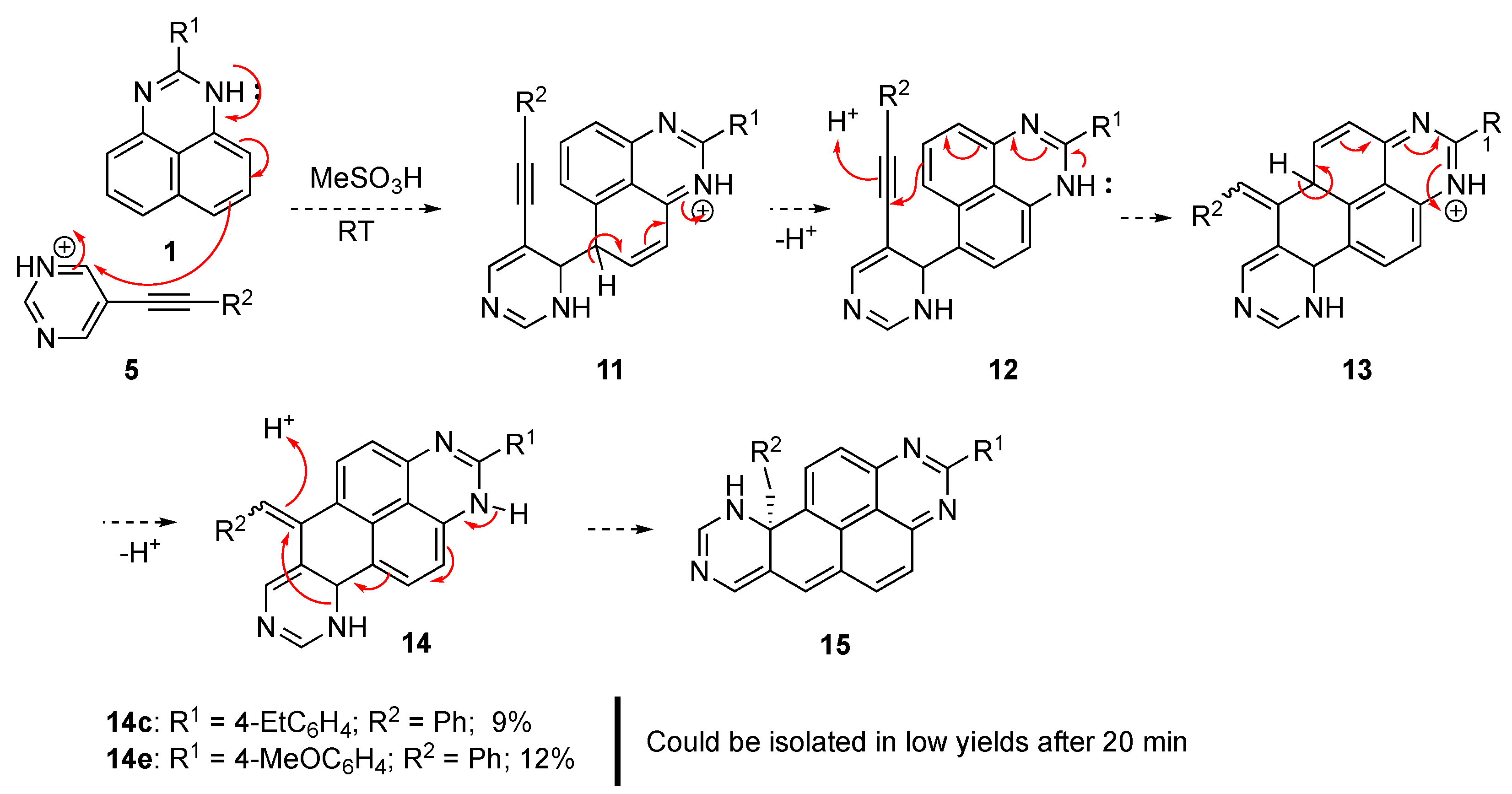
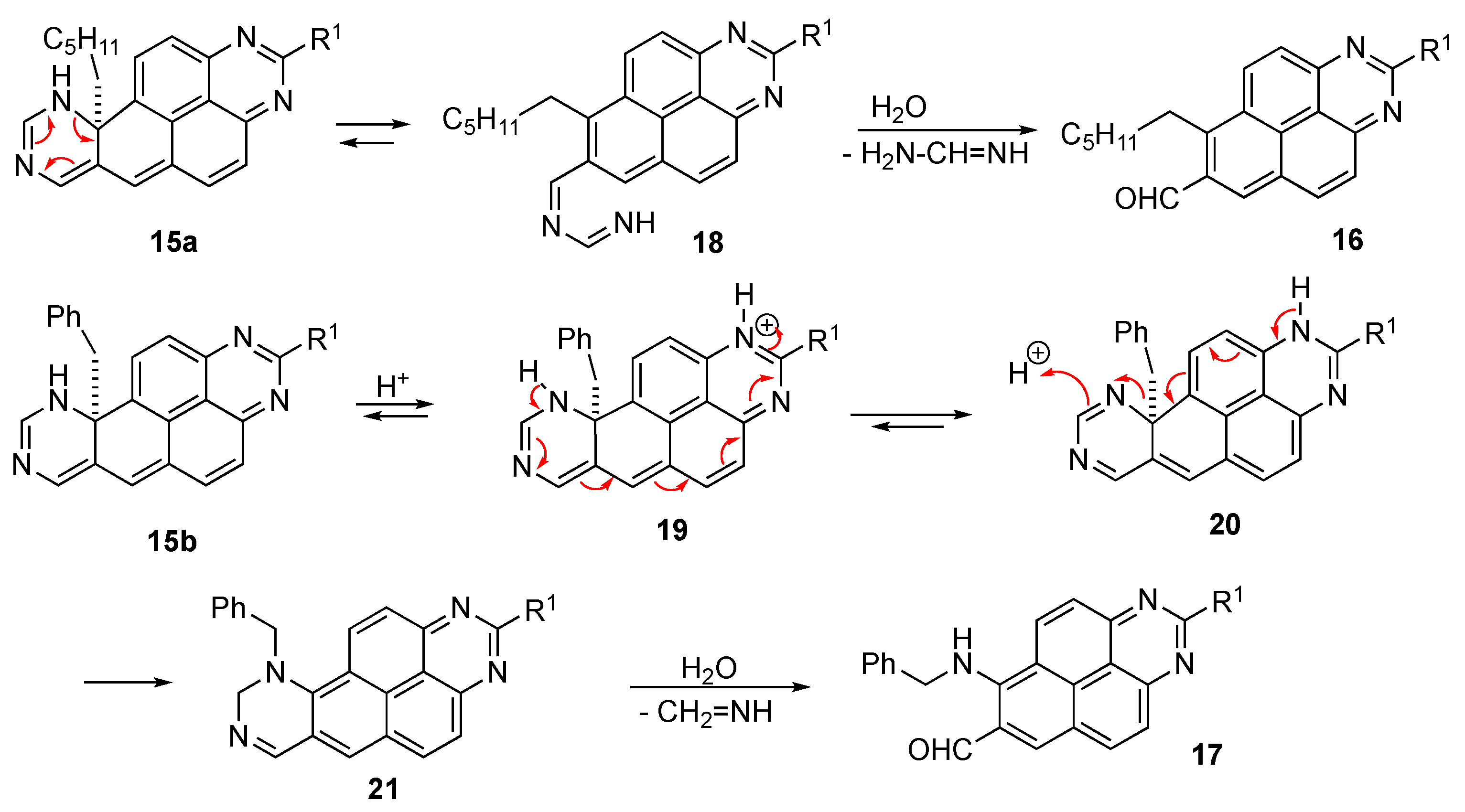
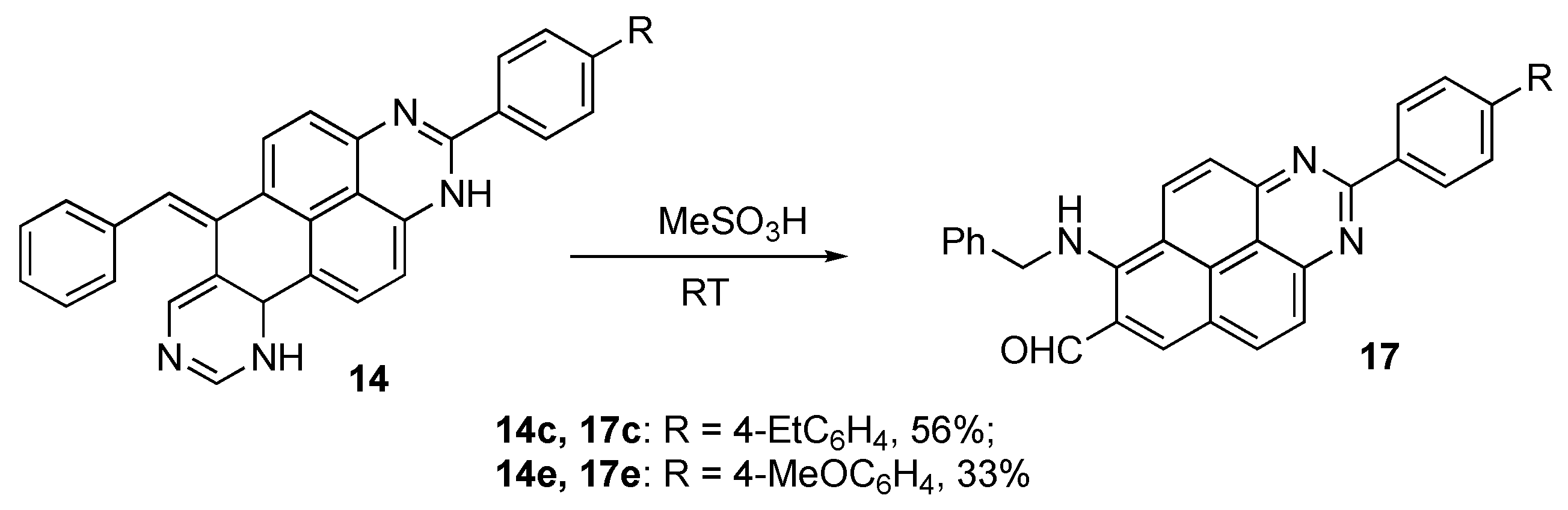
Isolation of intermediate 1,6,10,10a-tetrahydroquinazolino[6,7,8-gh]perimidines 14
A round bottomed 10 mL flask was charged with 5-(phenylethynyl)pyrimidine (5b, 1.00 mmol) and 1H-perimidine (1c or 1e, 1.00 mmol) dissolved in methanesulfonic acid was added in one portion. The mixture was stirred at room temperature, and the reaction progress was closely monitored by TLC (eluent ethyl acetate/petroleum ether, 1:3). After about 20 min, the mixture was poured into cold water and neutralized with aqueous ammonia. The formed precipitate was filtered and washed with small portions of water to remove excess ammonia. The crude material was fractionated by preparative column chromatography to isolate compounds 14c or 14e in low yields. Attempts to isolate the analogous pentacyclic products from reactions with 5-(heptynyl)pyrimidine (5a) were not successful.
6-benzylidene-2-(4-methoxyphenyl)-6,10,10a,10b-tetrahydroquinazolino[6,7,8-gh]perimidine (14c): The titled compound was obtained as orange powder, m.p. 254.5–256.1 °C, Rf 0.33 (EtOAc/Hexane, 1:1). Yield 40.7 mg (0.09 mmol, 9%). 1H NMR (400 MHz, DMSO-d6) δ 8.78 (s, 1H), 8.43 (d, J = 8.4 Hz, 1H), 8.11 (s, 1H), 8.04 (d, J = 8.2 Hz, 2H), 7.43 (d, J = 8.4 Hz, 2H), 7.29 (s, 1H), 7.03 (s, 2H), 6.99–6.92 (m, 2H), 6.90–6.83 (m, 1H), 6.76 (s, 2H), 4.95 (s, 1H), 3.48–3.43 (m, 1H), 3.21–3.11 (m, 1H), 2.71 (q, J = 7.5 Hz, 2H), 1.23 (t, J = 7.6 Hz, 4H). 13C{1H} NMR (101 MHz, DMSO-d6) δ 163.0, 157.1, 156.2, 152.7, 148.2, 144.3, 136.5, 132.8, 131.5, 130.3, 128.4(3С), 127.7(3С), 126.1, 124.7, 122.9, 51.7, 35.9(2С), 28.6, 15.9. FTIR, vmax: 3260, 2962, 2353, 1733, 1552, 1397, 1268, 1268, 1120, 1043, 824, 784, 665 cm−1; HRMS (ESI TOF) m/z: calc’d for C31H25N4 [M + H]+: 453.2074, found 453.2073 (−0.2 ppm).
6-benzylidene-2-(4-methoxyphenyl)-6,10,10a,10b-tetrahydroquinazolino[6,7,8-gh]perimidine (14e): The titled compound was obtained as orange powder, m.p. 220.9–223.7 °C, Rf 0.23 (EtOAc/Hexane, 1:1). Yield 54.5 mg (0.12 mmol, 12%). 1H NMR (400 MHz, DMSO-d6) δ 8.76 (s, 1H), 8.41 (d, J = 8.4 Hz, 1H), 8.11–8.05 (m, 3H), 7.26 (s, 1H), 7.12 (d, J = 8.9 Hz, 2H), 7.05–6.98 (m, 2H), 6.99–6.91 (m, 2H), 6.84 (s, 1H), 6.78–6.70 (m, 2H), 4.93 (s, 1H), 3.85 (s, 3H), 3.41–3.37 (m, 1H), 3.17–3.11 (m, 1H). 13C{1H} NMR (101 MHz, DMSO) δ 163.1, 162.3, 157.0, 156.1, 153.1, 151.4, 150.1, 145.2, 144.3, 136.9, 136.2, 132.8, 129.3, 128.5(2С), 127.7, 126.1, 125.2, 124.5, 124.0, 122.6, 115.1(2С), 114.4, 104.7, 104.0, 55.9, 51.7, 35.9(2С). FTIR, vmax: 3201, 2357, 1735, 1564, 1395, 1258, 1174, 1045, 834, 647 cm−1; HRMS (ESI TOF) m/z: calc’d for C30H23N4O [M + H]+: 455.1866, found 455.1867 (0.1 ppm).