Genetic Manipulation of CB1 Cannabinoid Receptors Reveals a Role in Maintaining Proper Skeletal Muscle Morphology and Function in Mice
Abstract
1. Introduction
2. Results
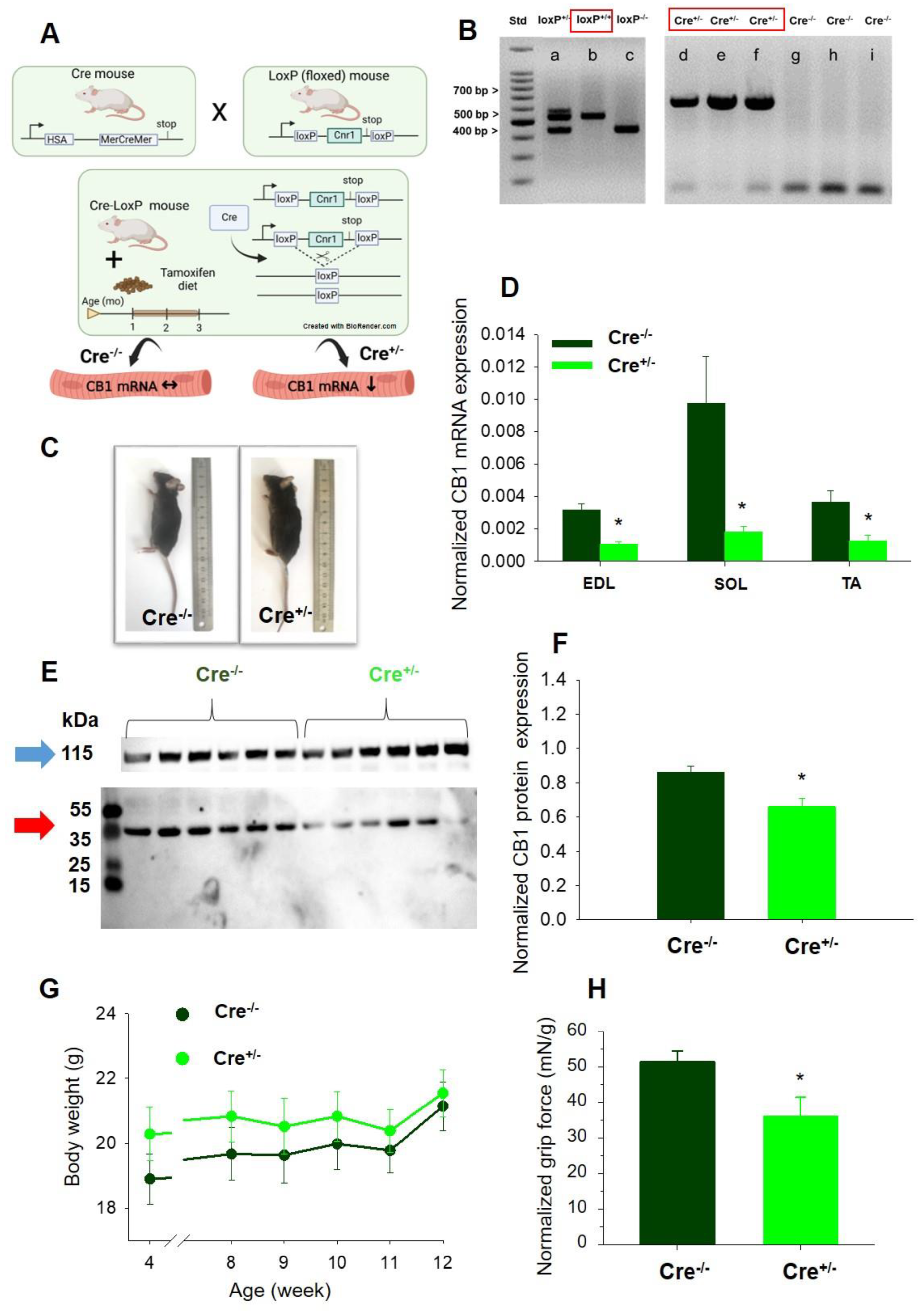
2.1. Skeletal Muscle Specific Knock-Down of CB1R Resulted in Maintained Specimen Phenotype
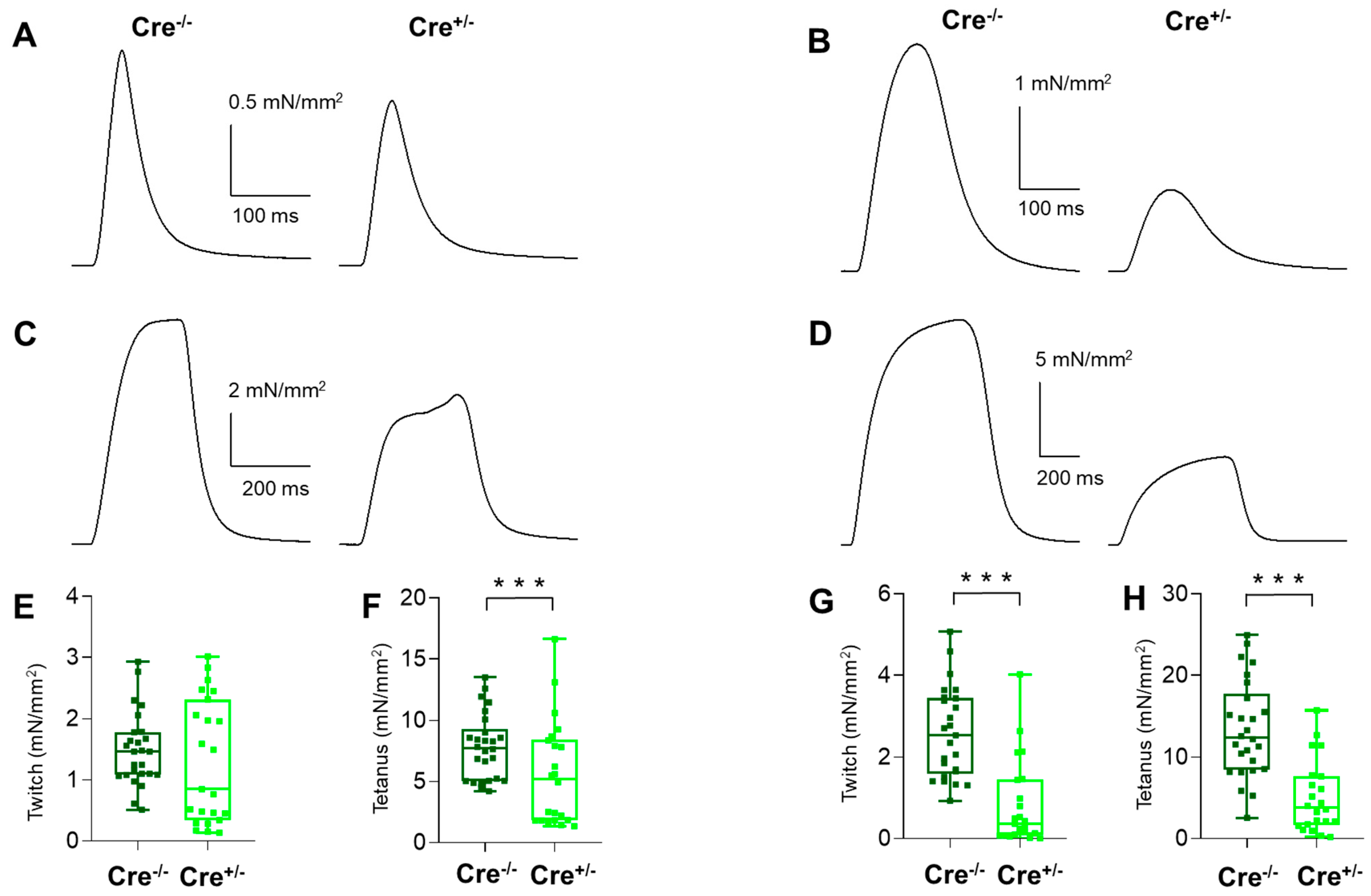
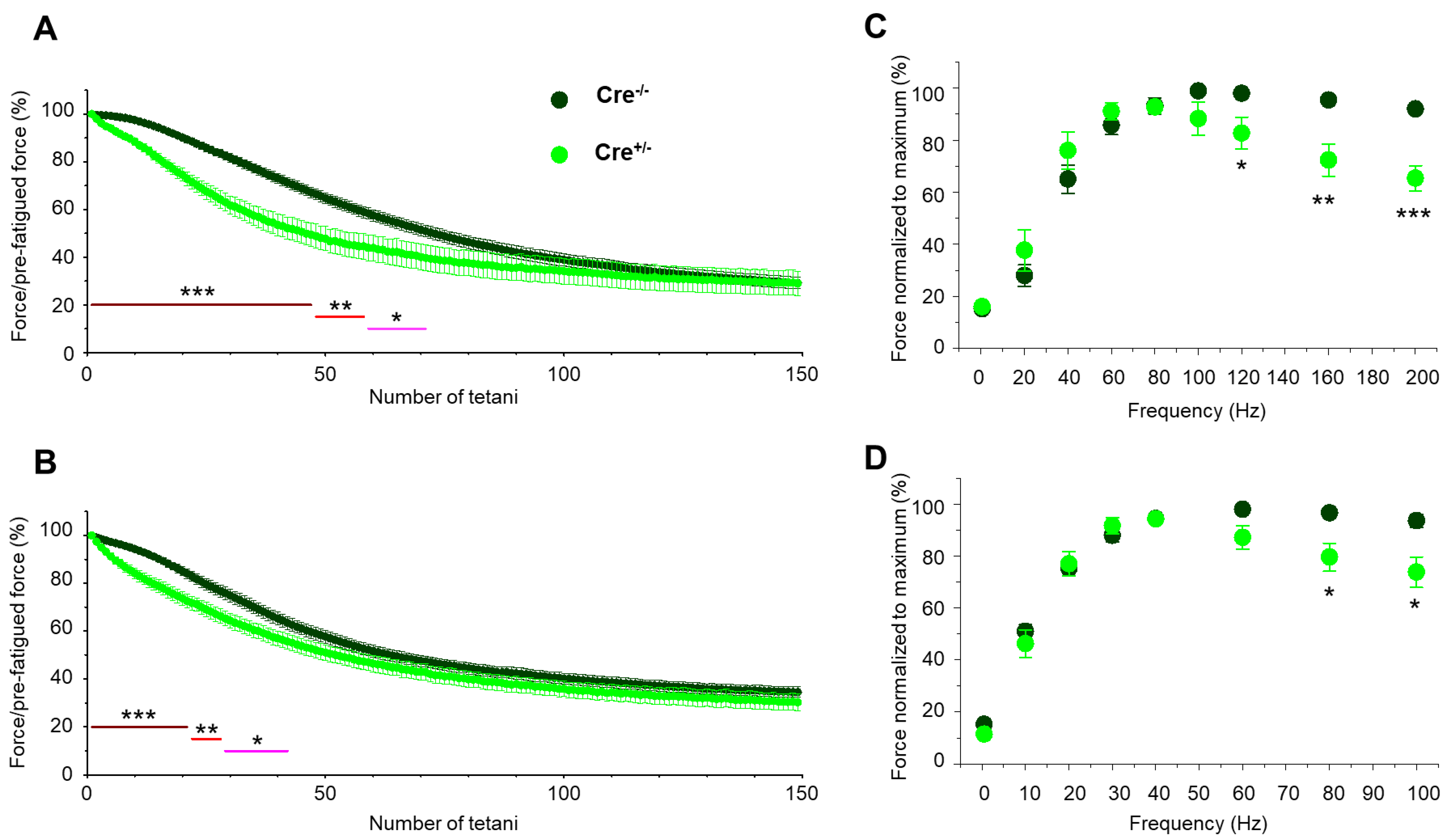
2.2. Skeletal Muscle Specific Knock-Down of CB1R Resulted in Altered Isometric Force
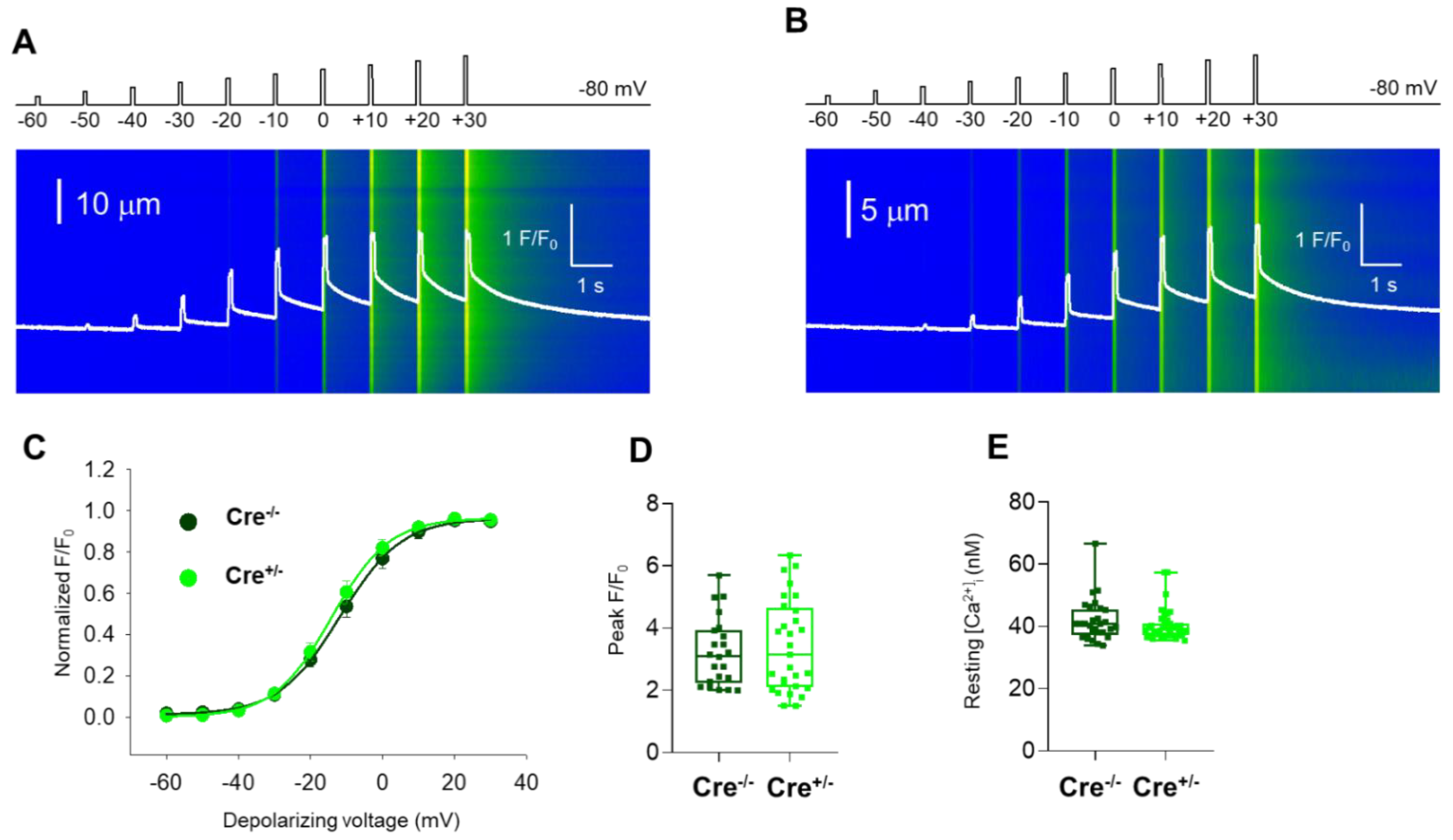
2.3. Skeletal Muscle Specific Knock-Down of CB1R Resulted in Unaffected Resting Intracellular Calcium Levels and Release Channel Sensitivity to Activation
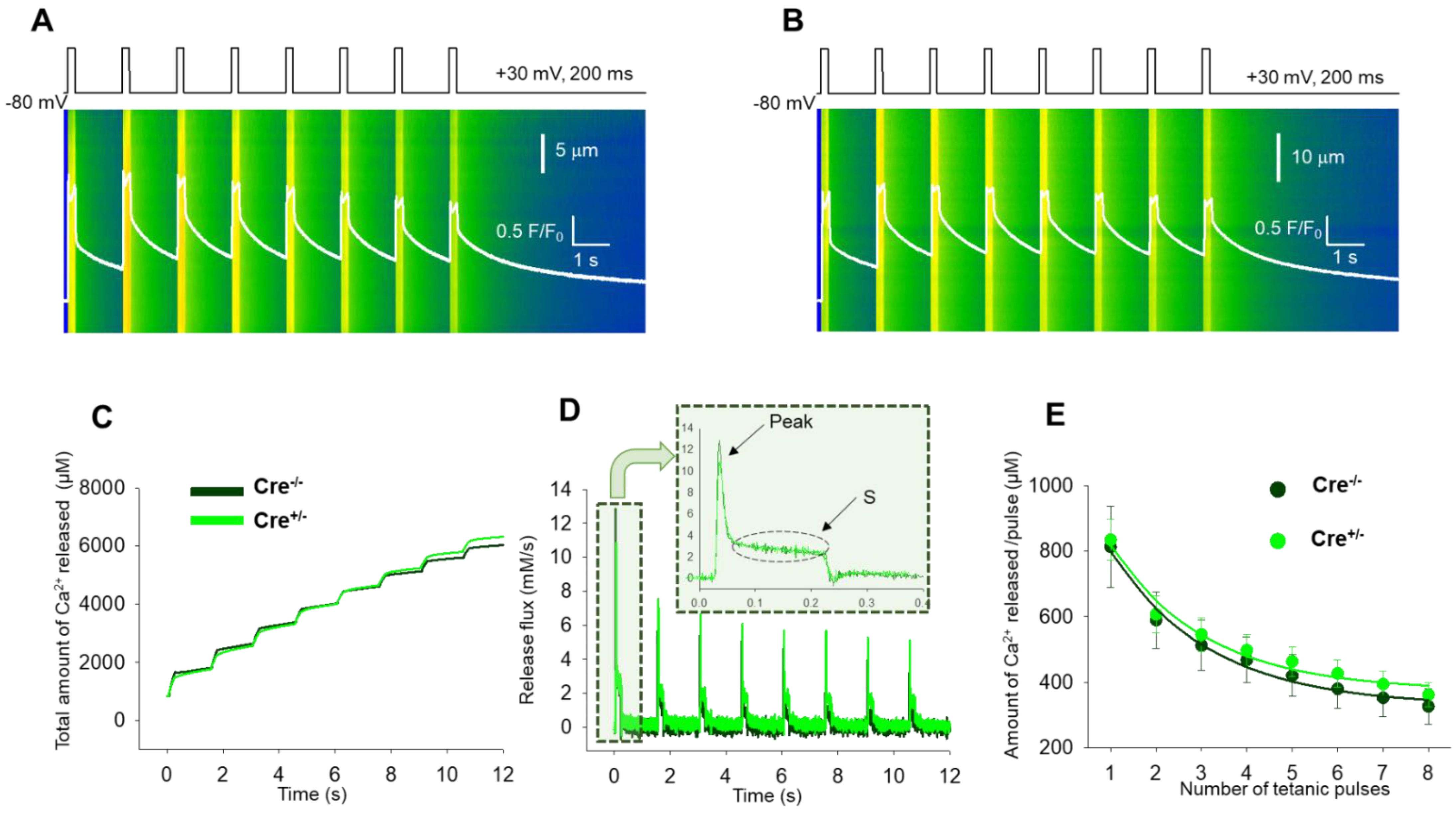
2.4. Skeletal Muscle Specific Knock-Down of CB1R Resulted in Unimpaired Fatigability of Ca2+ Release
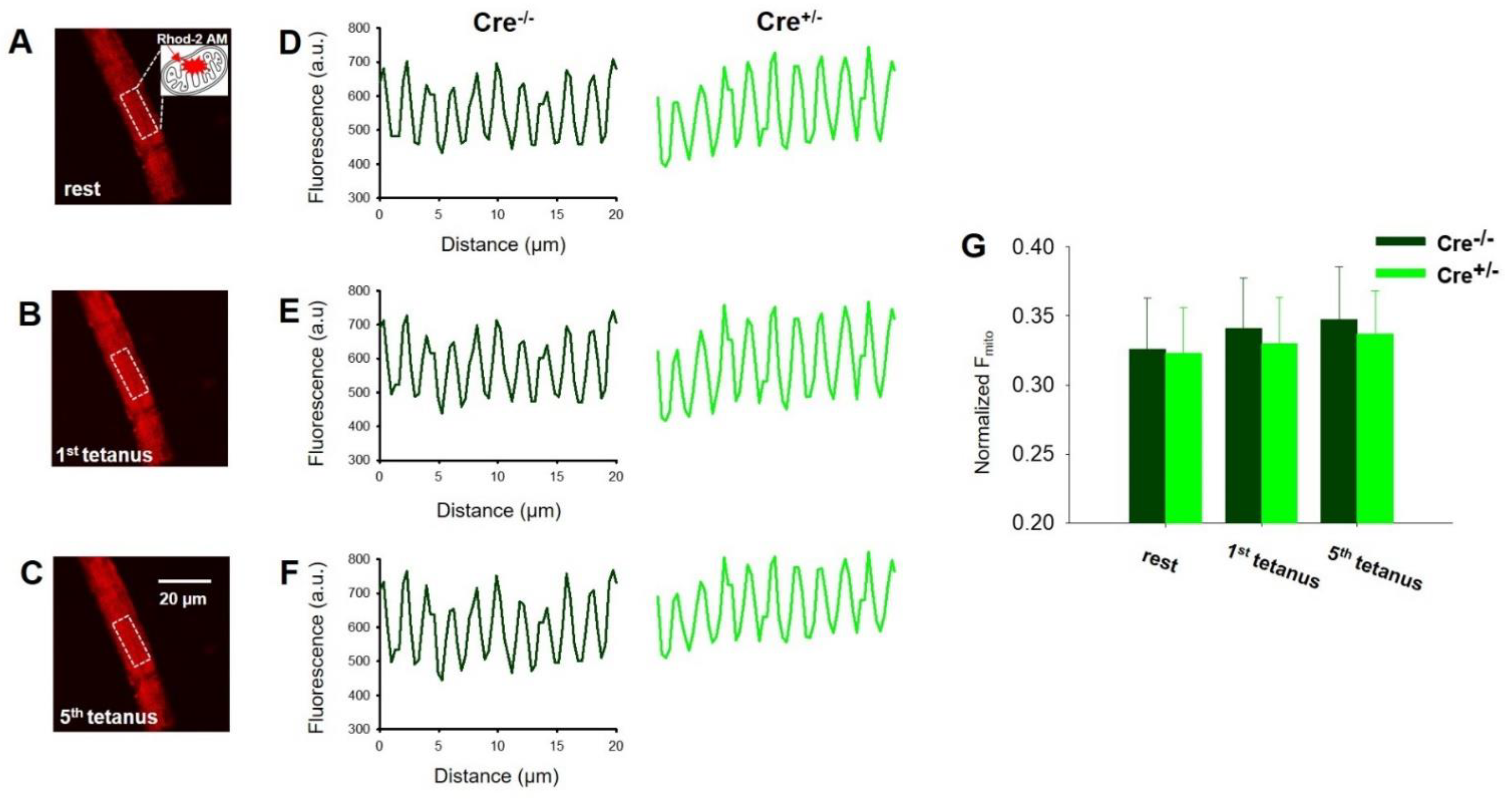
2.5. Skeletal Muscle Specific Knock-Down of CB1R Resulted in Unaltered Activity Dependent Mitochondrial Calcium Uptake
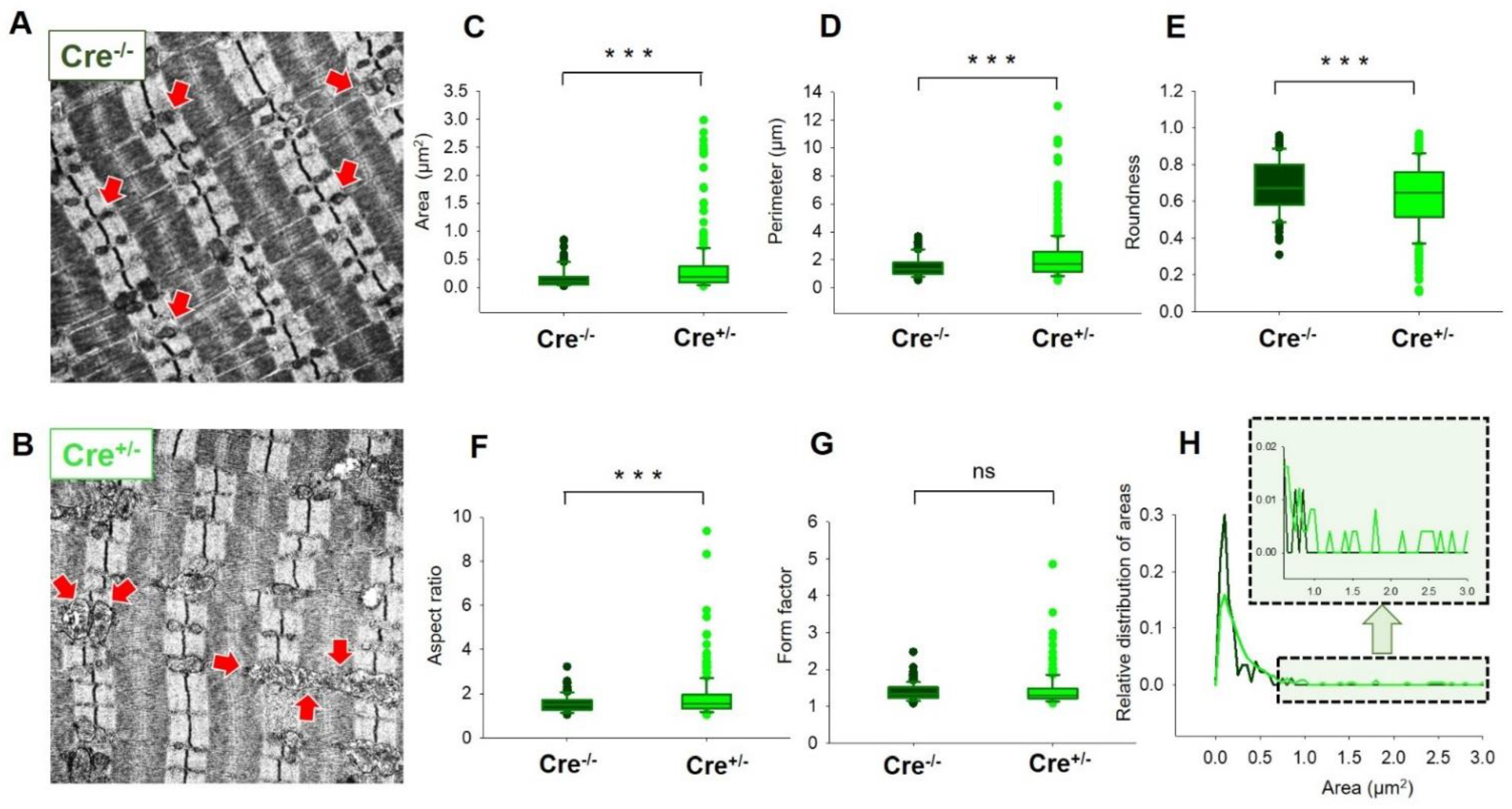
2.6. Skeletal Muscle Specific Knock-Down of CB1R Resulted in Significant Alterations of Mitochondrial Morphology
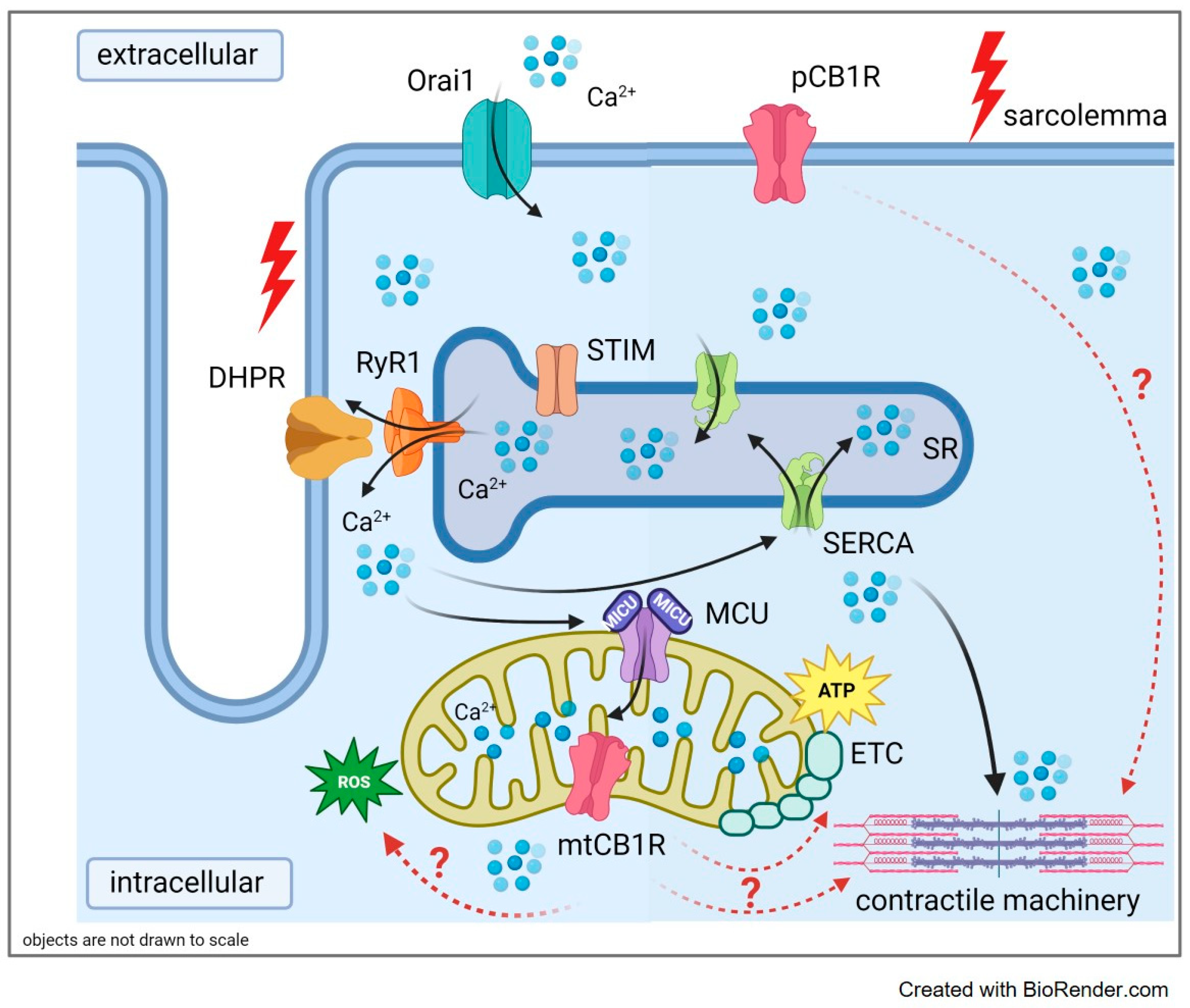
3. Discussion
4. Materials and Methods
4.1. Animal Care and Generation of the Muscle Specific CB1 Knock-Down Mouse Strain
4.2. Tamoxifen Diet
4.3. Molecular Biology
4.3.1. Genotyping
4.3.2. Quantitative PCR Analysis
4.3.3. Western Blot Analysis
4.4. In Vivo Experiments
4.4.1. Body Weight Measurement
4.4.2. Forepaw Grip Test
4.5. In Vitro Experiments
4.5.1. Measurement of Muscle Force and Fatigue
4.5.2. Transmission Electron Microscopy and Quantitative Analysis of EM Images
4.5.3. Isolation of Single FDB Fibers
4.5.4. Voltage Clamp, Confocal Microscopy, and Image Processing
4.5.5. Resting Myoplasmic [Ca2+]i Measurement
4.5.6. Mitochondrial Calcium Uptake Measurement
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellocchio, L.; Lafentre, P.; Cannich, A.; Cota, D.; Puente, N.; Grandes, P.; Chaouloff, F.; Piazza, P.V.; Marsicano, G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010, 13, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Marsicano, G.; Tschöp, M.; Grübler, Y.; Flachskamm, C.; Schubert, M.; Auer, D.; Yassouridis, A.; Thöne-Reineke, C.; Ortmann, S.; et al. The endogenous cennabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003, 112, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Guindon, J.; Lambert, C.; Beaulieu, P. The local antinociceptive effects of paracetamol in neuropathic pain are mediated by cannabinoid receptors. Eur. J. Pharmacol. 2007, 573, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 2005, 8, 585–589. [Google Scholar] [CrossRef]
- Kirkham, T.; Tucci, S. Endocannabinoids in Appetite Control and the Treatment of Obesity. CNS Neurol. Disord. Drug Targets 2008, 5, 275–292. [Google Scholar] [CrossRef]
- Cavuoto, P.; McAinch, A.J.; Hatzinikolas, G.; Cameron-Smith, D.; Wittert, G.A. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell. Endocrinol. 2007, 267, 63–69. [Google Scholar] [CrossRef]
- Bisogno, T.; Di Marzo, V. Cannabinoid Receptors and Endocannabinoids: Role in Neuroinflammatory and Neurodegenerative Disorders. CNS Neurol. Disord. Drug Targets 2012, 9, 564–573. [Google Scholar] [CrossRef]
- Arrabal, S.; Lucena, M.A.; Canduela, M.J.; Ramos-Uriarte, A.; Rivera, P.; Serrano, A.; Pavón, F.J.; Decara, J.; Vargas, A.; Baixeras, E.; et al. Pharmacological blockade of cannabinoid CB1 receptors in diet-induced obesity regulates mitochondrial dihydrolipoamide dehydrogenase in muscle. PLoS ONE 2015, 10, e0145244. [Google Scholar] [CrossRef]
- Stasiulewicz, A.; Znajdek, K.; Grudzień, M.; Pawiński, T.; Sulkowska, J.I. A guide to targeting the endocannabinoid system in drug design. Int. J. Mol. Sci. 2020, 21, 2778. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005, 76, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Esposito, I.; Proto, M.C.; Gazzerro, P.; Laezza, C.; Miele, C.; Alberobello, A.T.; D’Esposito, V.; Beguinot, F.; Formisano, P.; Bifulco, M. The cannabinoid CB1 receptor antagonist rimonabant stimulates 2-deoxyglucose uptake in skeletal muscle cells by regulating the expression of phosphatidylinositol-3-kinase. Mol. Pharmacol. 2008, 74, 1678–1686. [Google Scholar] [CrossRef]
- Eckardt, K.; Sell, H.; Taube, A.; Koenen, M.; Platzbecker, B.; Cramer, A.; Horrighs, A.; Lehtonen, M.; Tennagels, N.; Eckel, J. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia 2009, 52, 664–674. [Google Scholar] [CrossRef]
- Lipina, C.; Stretton, C.; Hastings, S.; Hundal, J.S.; Mackie, K.; Irving, A.J.; Hundal, H.S. Regulation of MAP kinase-directed mitogenic and protein kinase B-mediated signaling by cannabinoid receptor type 1 in skeletal muscle cells. Diabetes 2010, 59, 375–385. [Google Scholar] [CrossRef]
- Crespillo, A.; Suárez, J.; Bermúdez-Silva, F.J.; Rivera, P.; Vida, M.; Alonso, M.; Palomino, A.; Lucena, M.A.; Serrano, A.; Pérez-Martín, M.; et al. Expression of the cannabinoid system in muscle: Effects of a high-fat diet and CB1 receptor blockade. Biochem. J. 2011, 433, 175–185. [Google Scholar] [CrossRef]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef]
- Hebert-Chatelain, E.; Reguero, L.; Puente, N.; Lutz, B.; Chaouloff, F.; Rossignol, R.; Piazza, P.V.; Benard, G.; Grandes, P.; Marsicano, G. Studying mitochondrial CB1 receptors: Yes we can. Mol. Metab. 2014, 3, 339. [Google Scholar] [CrossRef] [PubMed]
- Hebert-Chatelain, E.; Desprez, T.; Serrat, R.; Bellocchio, L.; Soria-Gomez, E.; Busquets-Garcia, A.; Pagano Zottola, A.C.; Delamarre, A.; Cannich, A.; Vincent, P.; et al. A cannabinoid link between mitochondria and memory. Nature 2016, 539, 555–559. [Google Scholar] [CrossRef] [PubMed]
- SANDOW, A. Excitation-contraction coupling in muscular response. Yale J. Biol. Med. 1952, 25, 176–201. [Google Scholar] [PubMed]
- Franzini-Armstrong, C. Correction: The relationship between form and function throughout the history of excitation-contraction coupling. J. Gen. Physiol. 2018, 150, 369. [Google Scholar] [CrossRef]
- Dirksen, R.T.; Eisner, D.A.; Eduardo, R.; Sipido, K.R. Excitation–Contraction Coupling Excitation–contraction coupling in cardiac, skeletal, and smooth muscle. J. Gen. Physiol. 2022, 154, 9–11. [Google Scholar] [CrossRef]
- Eisenberg, B.R. Quantitative Ultrastructure of Mammalian Skeletal Muscle. Compr. Physiol. 1983, 73–112. [Google Scholar] [CrossRef]
- O’Rourke, B.; Blatter, L.A. Mitochondrial Ca2+ uptake: Tortoise or hare? J. Mol. Cell. Cardiol. 2009, 46, 767–774. [Google Scholar] [CrossRef]
- Gunter, T.E.; Yule, D.I.; Gunter, K.K.; Eliseev, R.A.; Salter, J.D. Calcium and mitochondria. FEBS Lett. 2004, 567, 96–102. [Google Scholar] [CrossRef]
- Pernas, L.; Scorrano, L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Oláh, T.; Bodnár, D.; Tóth, A.; Vincze, J.; Fodor, J.; Reischl, B.; Kovács, A.; Ruzsnavszky, O.; Dienes, B.; Szentesi, P.; et al. Cannabinoid signalling inhibits sarcoplasmic Ca2+ release and regulates excitation–contraction coupling in mammalian skeletal muscle. J. Physiol. 2016, 594, 7381–7398. [Google Scholar] [CrossRef]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; De Fonseca, F.R.; et al. Cannabinoid CB1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front. Physiol. 2016, 7, 476. [Google Scholar] [CrossRef]
- Melzer, W.; Rios, E.; Schneider, M.F. Time course of calcium release and removal in skeletal muscle fibers. Biophys. J. 1984, 45, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Schuhmeier, R.P.; Melzer, W. Voltage-dependent Ca2+ Fluxes in Skeletal Myotubes Determined Using a Removal Model Analysis. J. Gen. Physiol. 2004, 123, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Royer, L.; Pouvreau, S.; Ríos, E. Evolution and modulation of intracellular calcium release during long-lasting, depleting depolarization in mouse muscle. J. Physiol. 2008, 586, 4609–4629. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Pagano, E.; Guardiola, O.; Adinolfi, S.; Saccone, V.; Consalvi, S.; Piscitelli, F.; Gazzerro, E.; Busetto, G.; Carrella, D.; et al. Genetic and pharmacological regulation of the endocannabinoid CB1 receptor in Duchenne muscular dystrophy. Nat. Commun. 2018, 9, 3950. [Google Scholar] [CrossRef]
- González-Mariscal, I.; Montoro, R.A.; Doyle, M.E.; Liu, Q.R.; Rouse, M.; O’Connell, J.F.; Santa-Cruz Calvo, S.; Krzysik-Walker, S.M.; Ghosh, S.; Carlson, O.D.; et al. Absence of cannabinoid 1 receptor in beta cells protects against high-fat/high-sugar diet-induced beta cell dysfunction and inflammation in murine islets. Diabetologia 2018, 61, 1470–1483. [Google Scholar] [CrossRef]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of cannabinoids in obesity. Int. J. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef]
- Dalle, S.; Schouten, M.; Meeus, G.; Slagmolen, L.; Koppo, K. Molecular networks underlying cannabinoid signaling in skeletal muscle plasticity. J. Cell. Physiol. 2022, 237, 3517–3540. [Google Scholar] [CrossRef]
- Dalle, S.; Koppo, K. Cannabinoid receptor 1 expression is higher in muscle of old vs. young males, and increases upon resistance exercise in older adults. Sci. Rep. 2021, 11, 18349. [Google Scholar] [CrossRef]
- Schartner, V.; Laporte, J.; Böhm, J. Abnormal Excitation-Contraction Coupling and Calcium Homeostasis in Myopathies and Cardiomyopathies. J. Neuromuscul. Dis. 2019, 6, 289–305. [Google Scholar] [CrossRef]
- De Azua, I.R.; Mancini, G.; Srivastava, R.K.; Rey, A.A.; Cardinal, P.; Tedesco, L.; Zingaretti, C.M.; Sassmann, A.; Quarta, C.; Schwitter, C.; et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Investig. 2017, 127, 4148–4162. [Google Scholar] [CrossRef] [PubMed]
- Pagano Zottola, A.; Soria-Gomez, E.; Bonilla-del-Río, I.; Muguruza, C.; Terral, G.; Robin, L.; da Cruz, J.; Redon, B.; Lesté-Lasserre, T.; Tolentino-Cortes, T.; et al. A new mutant mouse model lacking mitochondrial-associated CB 1 receptor. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhao, D.; Pond, A.; Watkins, B.; Gerrard, D.; Wen, Y.; Kuang, S.; Hannon, K. Peripheral endocannabinoids regulate skeletal muscle development and maintenance. Eur. J. Transl. Myol. 2010, 20, 167. [Google Scholar] [CrossRef]
- Lipina, C.; Vaanholt, L.M.; Davidova, A.; Mitchell, S.E.; Storey-Gordon, E.; Hambly, C.; Irving, A.J.; Speakman, J.R.; Hundal, H.S. CB1 receptor blockade counters age-induced insulin resistance and metabolic dysfunction. Aging Cell 2016, 15, 325–335. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase—An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Baylor, S.M.; Hollingworth, S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J. Physiol. 2003, 551, 125–138. [Google Scholar] [CrossRef]
- Carroll, S.L.; Klein, M.G.; Schneider, M.F. Decay of calcium transients after electrical stimulation in rat fast- and slow-twitch skeletal muscle fibres. J. Physiol. 1997, 501, 573–588. [Google Scholar] [CrossRef]
- Hijikata, T.; Wakisaka, H.; Yohro, T. Architectural design, fiber-type composition, and innervation of the rat rectus abdominis muscle. Anat. Rec. 1992, 234, 500–512. [Google Scholar] [CrossRef]
- Picard, M.; Hepple, R.T.; Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: Tailoring the organelle for optimal function. Am. J. Physiol.-Cell Physiol. 2012, 302, C629–C641. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Huo, J.; Bround, M.J.; Boyer, J.G.; Schwanekamp, J.A.; Ghazal, N.; Maxwell, J.T.; Jang, Y.C.; Khuchua, Z.; Shi, K.; et al. The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle. JCI Insight 2018, 3, e121689. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Bátkai, S.; Patel, V.; Kashiwaya, Y.; Liaudet, L.; Evgenov, O.V.; MacKie, K.; Haskó, G.; Pacher, P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc. Res. 2010, 85, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Lipina, C.; Hundal, H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016, 6, 150276. [Google Scholar] [CrossRef] [PubMed]
- Dalton, G.; Bass, C.; Van Horn, C.; Howlett, A. Signal Transduction via Cannabinoid Receptors. CNS Neurol. Disord.-Drug Targets 2012, 8, 422–431. [Google Scholar] [CrossRef]
- Garcia, G.C.; Bartol, T.M.; Phan, S.; Bushong, E.A.; Perkins, G.; Sejnowski, T.J.; Ellisman, M.H.; Skupin, A. Mitochondrial morphology provides a mechanism for energy buffering at synapses. Sci. Rep. 2019, 9, 18306. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Picard, M.; Pelletier, F.S.J.; Sgarioto, N.; Auger, M.J.; Vallée, J.; Robitaille, R.; St-Pierre, D.H.; Gouspillou, G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget 2015, 6, 17923–17937. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, B.J.; Park, D.R.; Kim, U.H. Exercise induces muscle fiber type switching via transient receptor potential melastatin 2-dependent Ca2+ signaling. J. Appl. Physiol. 2018, 124, 364–373. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Roberts, M.D.; Haun, C.T.; Schoenfeld, B.J. Muscle fiber type transitions with exercise training: Shifting perspectives. Sports 2021, 9, 127. [Google Scholar] [CrossRef]
- Gönczi, M.; Ráduly, Z.; Szabó, L.; Fodor, J.; Telek, A.; Dobrosi, N.; Balogh, N.; Szentesi, P.; Kis, G.; Antal, M.; et al. Septin7 is indispensable for proper skeletal muscle architecture and function. Elife 2022, 11, e75863. [Google Scholar] [CrossRef]
- Koitabashi, N.; Bedja, D.; Zaiman, A.L.; Pinto, Y.M.; Zhang, M.; Gabrielson, K.L.; Takimoto, E.; Kassz, D.A. Avoidance of transient cardiomyopathy in Cardiomyocyte-targeted Tamoxifen-induced mercremer gene deletion models. Circ. Res. 2009, 105, 12–15. [Google Scholar] [CrossRef]
- Andersson, K.B.; Winer, L.H.; Mørk, H.K.; Molkentin, J.D.; Jaisser, F. Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res. 2010, 19, 715–725. [Google Scholar] [CrossRef]
- Bodnár, D.; Geyer, N.; Ruzsnavszky, O.; Oláh, T.; Hegyi, B.; Sztretye, M.; Fodor, J.; Dienes, B.; Balogh, Á.; Papp, Z.; et al. Hypermuscular mice with mutation in the myostatin gene display altered calcium signalling. J. Physiol. 2014, 592, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Bodnár, D.; Ruzsnavszky, O.; Oláh, T.; Dienes, B.; Balatoni, I.; Ungvári, É.; Benkő, I.; Babka, B.; Prokisch, J.; Csernoch, L.; et al. Dietary selenium augments sarcoplasmic calcium release and mechanical performance in mice. Nutr. Metab. 2016, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Singlár, Z.; Szabó, L.; Angyal, Á.; Balogh, N.; Vakilzadeh, F.; Szentesi, P.; Dienes, B.; Csernoch, L. Improved tetanic force and mitochondrial calcium homeostasis by astaxanthin treatment in mouse skeletal muscle. Antioxidants 2020, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Yi, J.; Figueroa, L.; Zhou, J.; Royer, L.; Ríos, E. D4cpv-calsequestrin: A sensitive ratiometric biosensor accurately targeted to the calcium store of skeletal muscle. J. Gen. Physiol. 2011, 138, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Sztretye, M.; Geyer, N.; Vincze, J.; Al-Gaadi, D.; Oláh, T.; Szentesi, P.; Kis, G.; Antal, M.; Balatoni, I.; Csernoch, L.; et al. SOCE Is Important for Maintaining Sarcoplasmic Calcium Content and Release in Skeletal Muscle Fibers. Biophys. J. 2017, 113, 2496–2507. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tisen, R.Y. New generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1984, 260, 3440–3450. [Google Scholar] [CrossRef]
- Ainbinder, A.; Boncompagni, S.; Protasi, F.; Dirksen, R.T. Role of Mitofusin-2 in mitochondrial localization and calcium uptake in skeletal muscle. Cell Calcium 2015, 57, 14–24. [Google Scholar] [CrossRef]
| EDL | SOL | |||||||
|---|---|---|---|---|---|---|---|---|
| Twitch | Tetanus | Twitch | Tetanus | |||||
| Cre−/− | Cre+/− | Cre−/− | Cre+/− | Cre−/− | Cre+/− | Cre−/− | Cre+/− | |
| Number of muscles | 26 | 22 | 26 | 22 | 25 | 22 | 25 | 22 |
| Peak force (mN) | 1.53 ± 0.09 | 0.80 ± 0.10 *** | 8.08 ± 0.43 | 3.49 ± 0.42 *** | 1.83 ± 0.12 | 0.57 ± 0.13 *** | 9.41 ± 0.49 | 4.09 ± 0.69 *** |
| Force (mN/mm2) | 1.49 ± 0.12 | 1.29 ± 0.21 | 7.84 ± 0.52 | 5.61 ± 0.91 * | 2.60 ± 0.22 | 0.81 ± 0.22 *** | 13.38 ± 1.16 | 5.09 ± 0.94 *** |
| TTP (ms) | 37.1 ± 2.8 | 40.8 ± 1.8 | 209.9 ± 12.9 | 147.8 ± 13.4 ** | 109.1 ± 7.2 | 88.9 ± 4.2 * | 517.3 ± 12.7 | 526.0 ± 2.6 |
| HRT (ms) | 32.5 ± 2.7 | 33.1 ± 1.3 | 69.2 ± 3.7 | 118.8 ± 11.1 *** | 102.7 ± 10.9 | 110.6 ± 8.5 | 142.5 ± 5.9 | 98.9 ± 3.2 *** |
| Duration (ms) | 246.1 ± 30.5 | 233.1 ± 14.4 | 376.4 ± 18.0 | 376.9 ± 14.2 | 401.2 ± 35.2 | 479.9 ± 46.4 | 834.0 ± 29.0 | 764.5 ± 13.6 * |
| Fatigue at 50th (%) | 34.4 ± 2.3 | 52.0 ± 4.9 ** | 41.8 ± 2.6 | 48.6 ± 3.2 | ||||
| Fatigue at 100th (%) | 60.9 ± 2.6 | 65.7 ± 5.4 | 59.5 ± 2.3 | 64.1 ± 3.3 | ||||
| Fatigue at 150th (%) | 70.8 ± 2.5 | 71.0 ± 5.1 | 65.4 ± 2.2 | 69.7 ± 3.5 | ||||
| CSA (mm2) | 1.07 ± 0.04 | 0.81 ± 0.06 ** | 0.80 ± 0.06 | 0.96 ± 0.07 | ||||
| Muscle weight (mg) | 12.8 ± 0.4 | 11.5 ± 0.3 * | 11.0 ± 0.6 | 14.3 ± 0.5 *** | ||||
| Cre | CB1-loxP | |
|---|---|---|
| Forward primer | 5′-GCATGGTGGAGATCTTTGA-3′ | 5′-GCTGTCTCTGGTCCTCTTAAA-3′ |
| Reverse primer | 5′-CGACCGGCAAACGGACAGAAGC-3′ | 5′-GGTGTCACCTCTGAAAACAGA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singlár, Z.; Ganbat, N.; Szentesi, P.; Osgonsandag, N.; Szabó, L.; Telek, A.; Fodor, J.; Dienes, B.; Gönczi, M.; Csernoch, L.; et al. Genetic Manipulation of CB1 Cannabinoid Receptors Reveals a Role in Maintaining Proper Skeletal Muscle Morphology and Function in Mice. Int. J. Mol. Sci. 2022, 23, 15653. https://doi.org/10.3390/ijms232415653
Singlár Z, Ganbat N, Szentesi P, Osgonsandag N, Szabó L, Telek A, Fodor J, Dienes B, Gönczi M, Csernoch L, et al. Genetic Manipulation of CB1 Cannabinoid Receptors Reveals a Role in Maintaining Proper Skeletal Muscle Morphology and Function in Mice. International Journal of Molecular Sciences. 2022; 23(24):15653. https://doi.org/10.3390/ijms232415653
Chicago/Turabian StyleSinglár, Zoltán, Nyamkhuu Ganbat, Péter Szentesi, Nomin Osgonsandag, László Szabó, Andrea Telek, János Fodor, Beatrix Dienes, Mónika Gönczi, László Csernoch, and et al. 2022. "Genetic Manipulation of CB1 Cannabinoid Receptors Reveals a Role in Maintaining Proper Skeletal Muscle Morphology and Function in Mice" International Journal of Molecular Sciences 23, no. 24: 15653. https://doi.org/10.3390/ijms232415653
APA StyleSinglár, Z., Ganbat, N., Szentesi, P., Osgonsandag, N., Szabó, L., Telek, A., Fodor, J., Dienes, B., Gönczi, M., Csernoch, L., & Sztretye, M. (2022). Genetic Manipulation of CB1 Cannabinoid Receptors Reveals a Role in Maintaining Proper Skeletal Muscle Morphology and Function in Mice. International Journal of Molecular Sciences, 23(24), 15653. https://doi.org/10.3390/ijms232415653








