Inhibition of JAK1,2 Prevents Fibrotic Remodeling of Pulmonary Vascular Bed and Improves Outcomes in the Rat Model of Chronic Thromboembolic Pulmonary Hypertension
Abstract
1. Introduction
2. Results
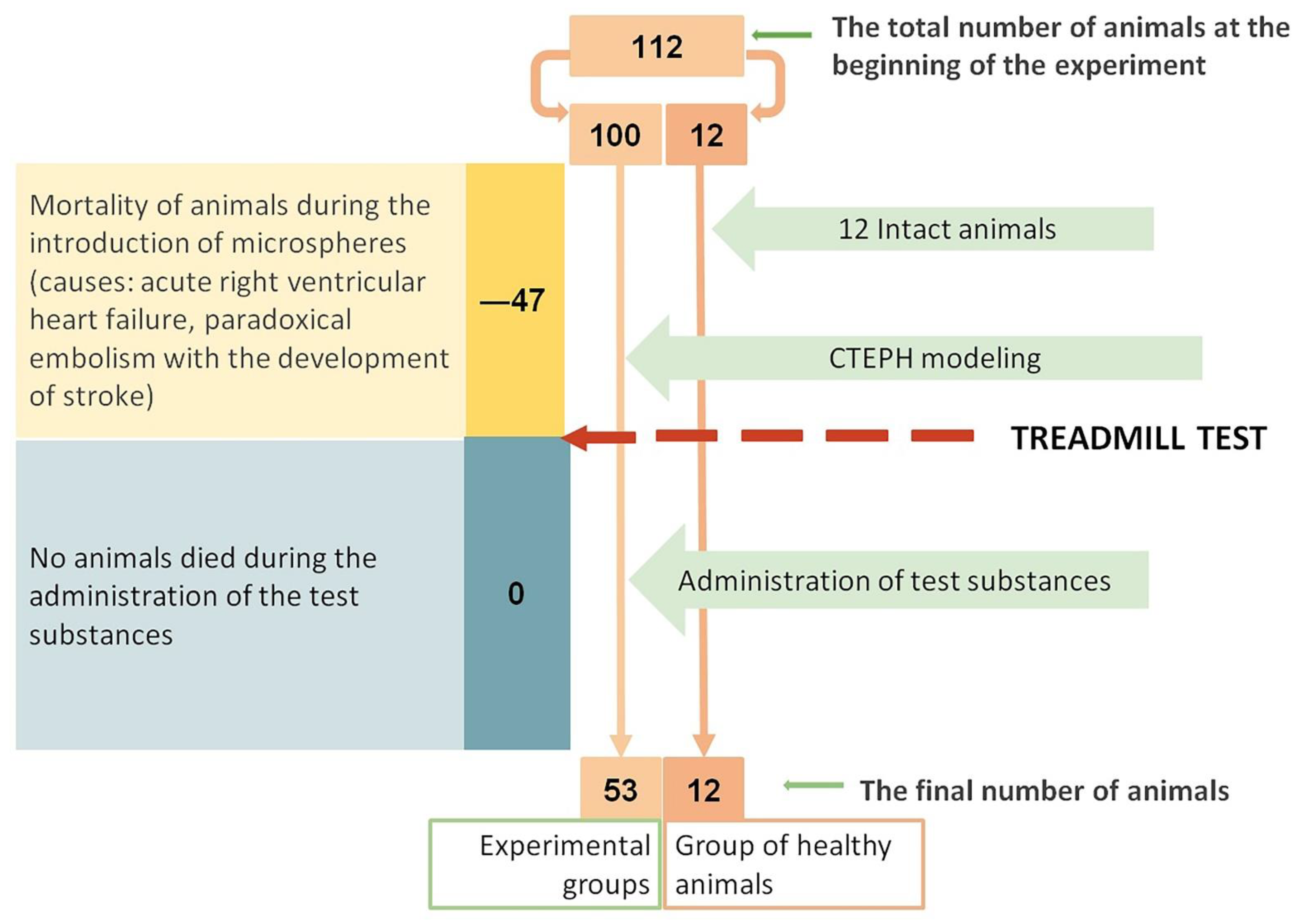
2.1. Animal Survival
2.2. Treadmill Test
2.3. Transthoracic Echocardiography
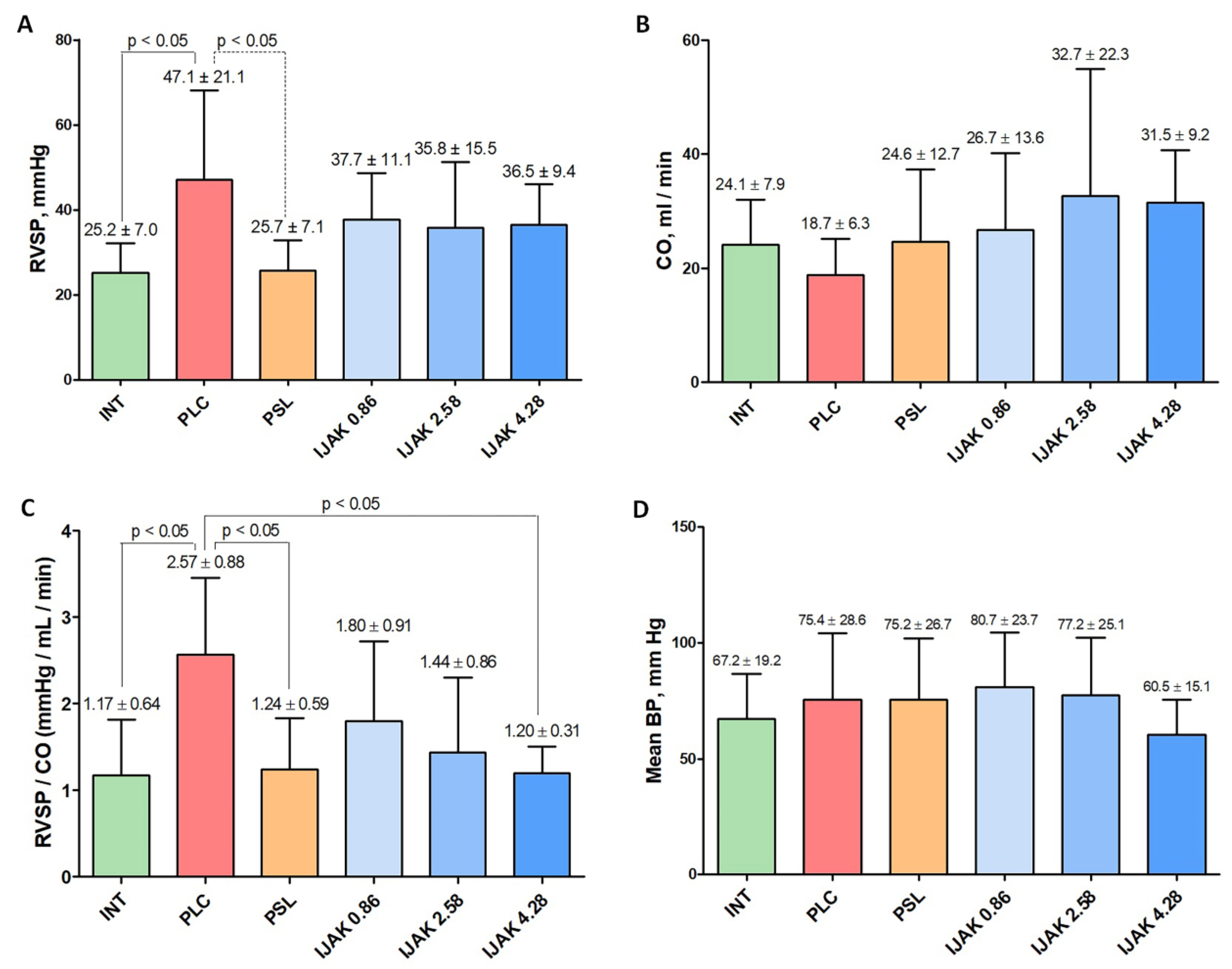
2.4. Cardiac Catheterization with Manometry
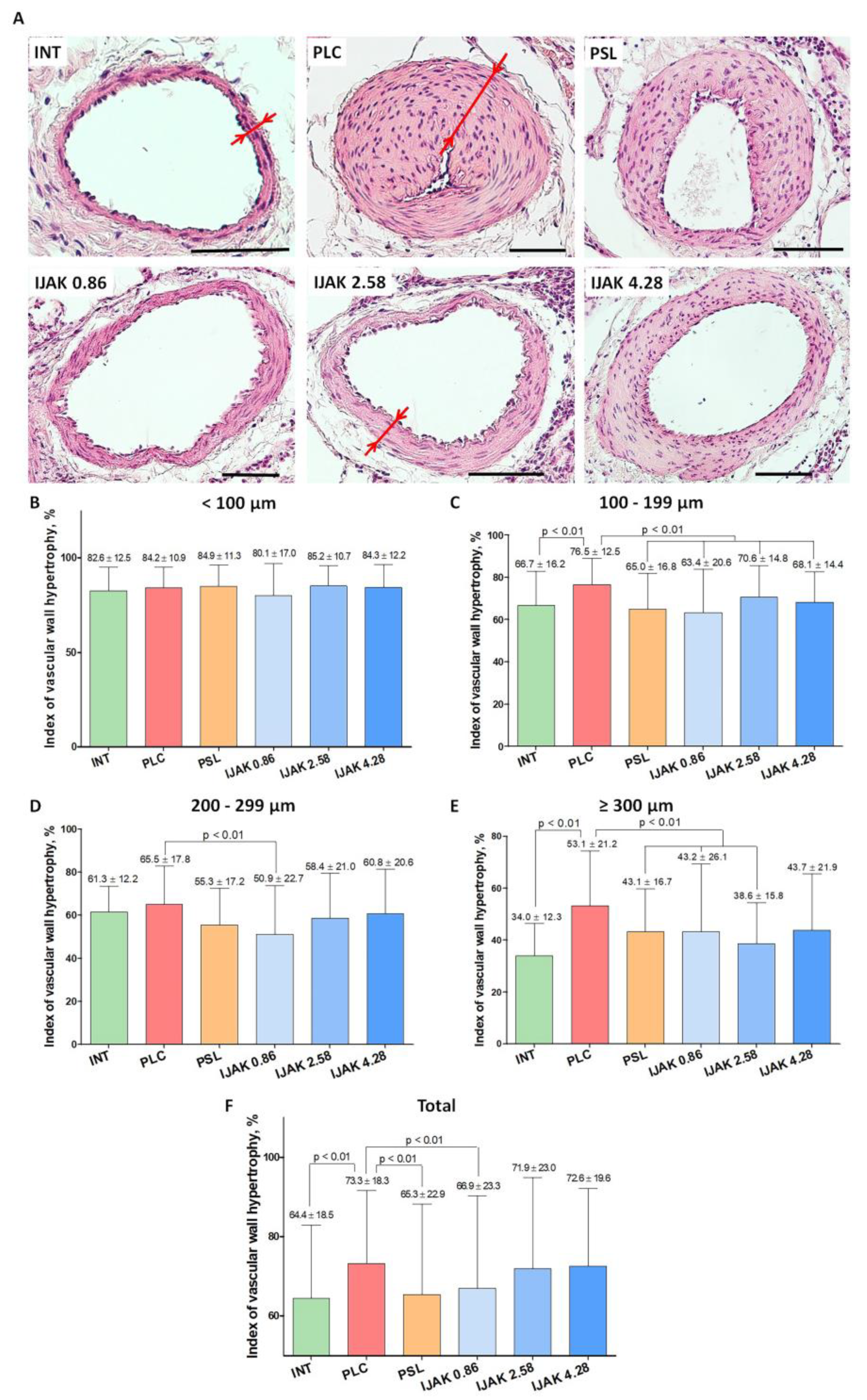
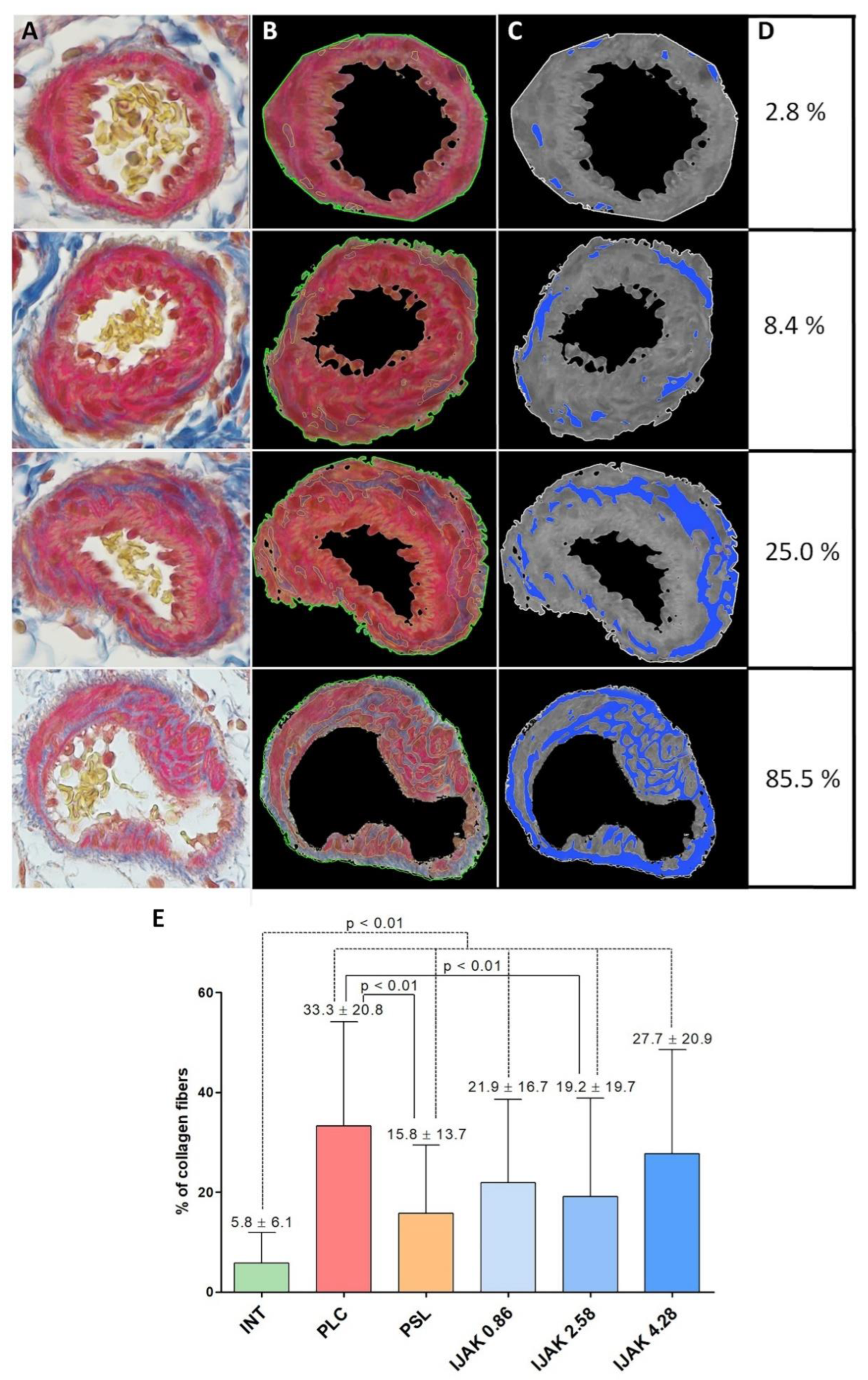
2.5. Histological Examination of the Lung Vessels
- -
- Vessels with mean outer diameter <100 µm: no significant differences were found between all studied groups (Figure 4B);
- -
- 100–199 μm: the hypertrophy index in the PLC group was significantly higher compared to both intact animals (p < 0.01) and all groups treated with the tested substances (p < 0.01) (Figure 4C);
- -
- 200–299 µm: the hypertrophy index in the IJAK 0.86 group was significantly lower compared to the PLC group (p < 0.01) (Figure 4D);
- -
- ≥300 μm: in the IJAK 0.86 and PSL groups, the hypertrophy index was significantly lower than in the PLC group (p < 0.01) (Figure 4E).
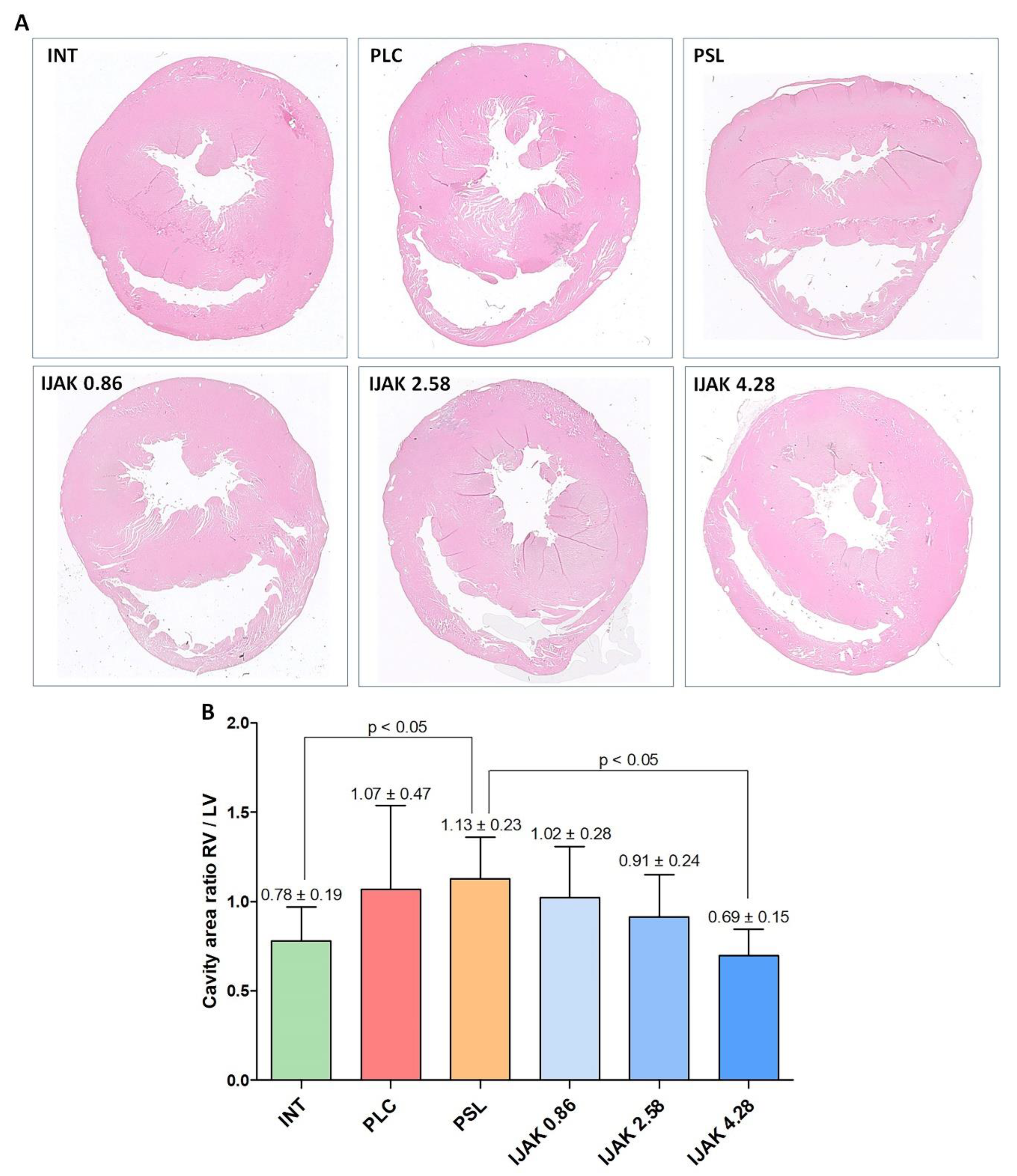
2.6. Histological Heart Examination
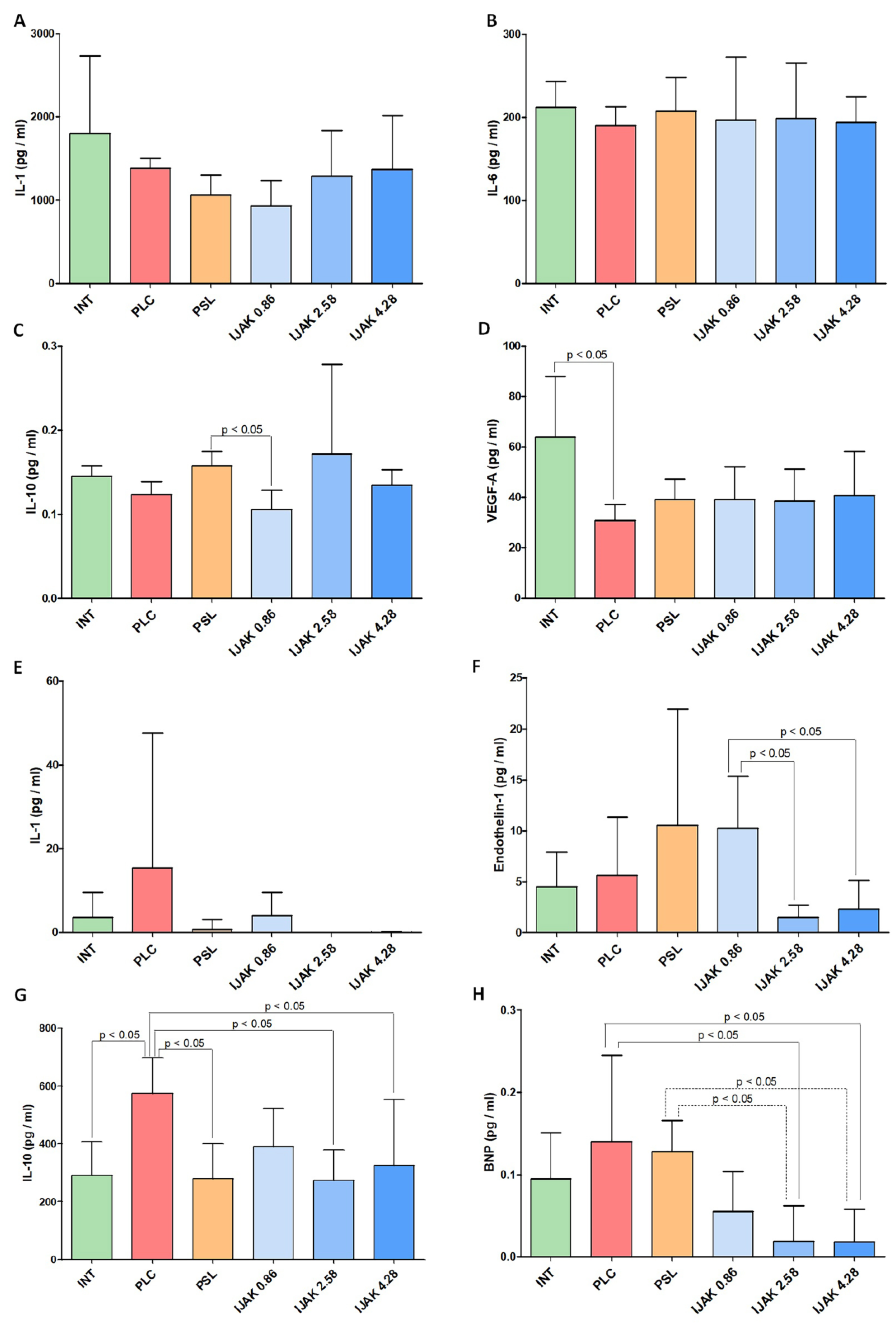
2.7. Enzyme Immunoassay
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Embolic Particles
4.3. JAK Inhibitor Dose Calculation
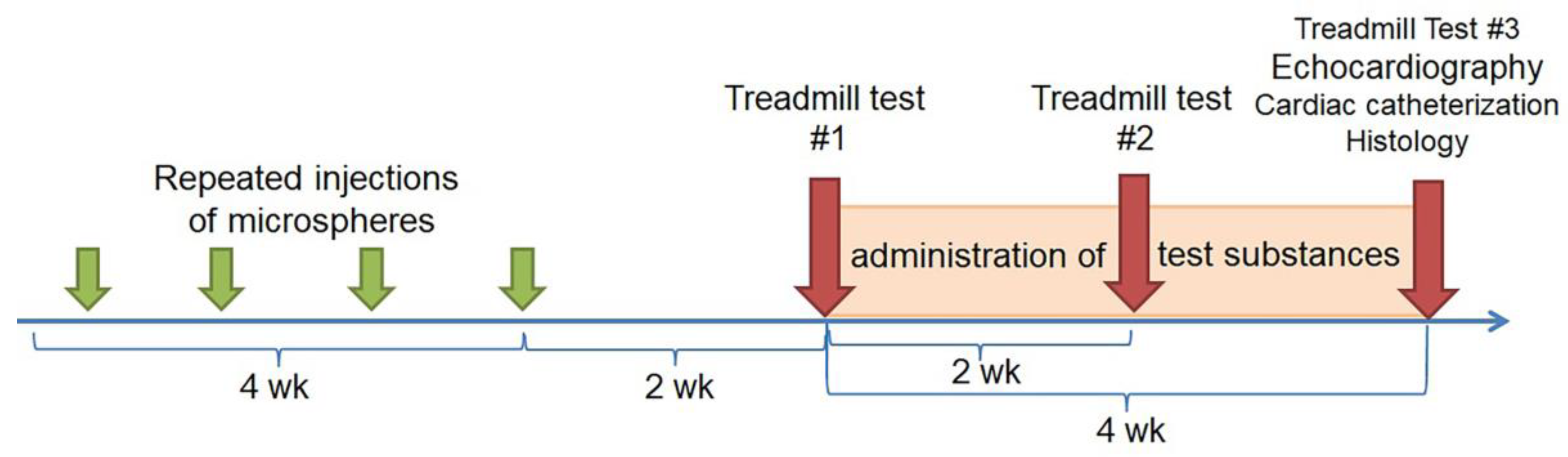
4.4. Experimental Protocol
4.5. Animal Groups
- Intact animals (INT), n = 11
- Placebo (PLC), n = 11: two weeks after the last injection of MSs, animals were injected intravenously with saline daily over 4 weeks.
- Prednisolone (PSL), n = 10: two weeks after the last administration of MSs, prednisolone at a dose of 1.475 mg/kg (which is equivalent to 0.25 mg/kg in humans) was administered intramuscularly over 4 weeks.
- Low-dose iJAK (IJAK 0.86), n = 11: two weeks after the last administration of MSs, iJAK was administered at a daily dose of 0.86 mg/kg (divided into two intakes) over 4 weeks per os (equivalent to 10 mg per day in humans).
- Middle-dose iJAK (IJAK 2.58), n = 11: two weeks after the last administration of MSs, iJAK was administered at a daily dose of 2.58 mg/kg (divided into two intakes) over 4 weeks per os (which is equivalent to 30 mg per day in humans).
- High-dose iJAK (IJAK 4.28), n = 10: two weeks after the last administration of MSs, iJAK was administered at a daily dose of 4.28 mg/kg (divided into two intakes) during 4 weeks per os (which is equivalent to 50 mg per day in humans).
4.6. Research Design
- Treadmill Test
- 2.
- Transthoracic Echocardiography
- 3.
- Cardiac Catheterization with Manometry
- 4.
- Histological Examination
4.7. Enzyme Immunoassay
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belohlavek, J.; Dytrych, V.; Linhart, A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp. Clin. Cardiol. 2013, 18, 129–138. [Google Scholar] [PubMed]
- Karpov, A.A.; Vaulina, D.D.; Smirnov, S.S.; Moiseeva, O.M.; Galagudza, M.M. Rodent models of pulmonary embolism and chronic thromboembolic pulmonary hypertension. Heliyon 2022, 8, e09014. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Nelson, R.E.; Nyarko, K.A.; Richardson, L.C.; Raskob, G.E. The economic burden of incident venous thromboembolism in the United States: A review of estimated attributable healthcare costs. Thromb. Res. 2016, 137, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Ageno, W.; Ay, C.; Back, M.; Barco, S.; Bertoletti, L.; Becattini, C.; Carlsen, J.; Delcroix, M.; van Es, N.; et al. Optimal follow-up after acute pulmonary embolism: A position paper of the European Society of Cardiology Working Group on Pulmonary Circulation and Right Ventricular Function, in collaboration with the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology, endorsed by the European Respiratory Society. Eur. Heart. J. 2022, 43, 183–189. [Google Scholar] [CrossRef]
- Lang, I.M.; Pesavento, R.; Bonderman, D.; Yuan, J.X. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: A current understanding. Eur. Resp. J. 2013, 41, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.; Stanek, V.; Widimsky, J.; Prerovsky, I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982, 81, 151–158. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart. J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Lang, I.M.; Campean, I.A.; Sadushi-Kolici, R.; Badr-Eslam, R.; Gerges, C.; Skoro-Sajer, N. Chronic Thromboembolic Disease and Chronic Thromboembolic Pulmonary Hypertension. Clin. Chest Med. 2021, 42, 81–90. [Google Scholar] [CrossRef]
- Haga, S.; Tsuchiya, H.; Hirai, T.; Hamano, T.; Mimori, A.; Ishizaka, Y. A novel ACE2 activator reduces monocrotaline-induced pulmonary hypertension by suppressing the JAK/STAT and TGF-beta cascades with restored caveolin-1 expression. Exp. Lung Res. 2015, 41, 21–31. [Google Scholar] [CrossRef]
- Dees, C.; Tomcik, M.; Palumbo-Zerr, K.; Distler, A.; Beyer, C.; Lang, V.; Horn, A.; Zerr, P.; Zwerina, J.; Gelse, K.; et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor beta in systemic sclerosis. Arthritis Rheum. 2012, 64, 3006–3015. [Google Scholar] [CrossRef]
- Yu, Y.; Sweeney, M.; Zhang, S.; Platoshyn, O.; Landsberg, J.; Rothman, A.; Yuan, J.X. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am. J. Physiol. Cell Physiol. 2003, 284, C316–C330. [Google Scholar] [CrossRef]
- Matsui, F.; Meldrum, K.K. The role of the Janus kinase family/signal transducer and activator of transcription signaling pathway in fibrotic renal disease. J. Surg. Res. 2012, 178, 339–345. [Google Scholar] [CrossRef]
- Solary, E. Unplugging JAK/STAT in Chronic Myelomonocytic Leukemia. Clin. Cancer Res. 2016, 22, 3707–3709. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Yerabolu, D.; Weiss, A.; Kojonazarov, B.; Boehm, M.; Schlueter, B.C.; Ruppert, C.; Gunther, A.; Jonigk, D.; Grimminger, F.; Ghofrani, H.A.; et al. Targeting Jak-Stat Signaling in Experimental Pulmonary Hypertension. Am. J. Respir. Cell. Mol. Biol. 2021, 64, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Inhibiting Jak2 Ameliorates Pulmonary Hypertension: Fulfilling the Promise of Precision Medicine. Am. J. Respir. Cell Mol. Biol. 2021, 64, 12–13. [Google Scholar] [CrossRef]
- Karpov, A.A.; Anikin, N.A.; Mihailova, A.M.; Smirnov, S.S.; Vaulina, D.D.; Shilenko, L.A.; Ivkin, D.Y.; Bagrov, A.Y.; Moiseeva, O.M.; Galagudza, M.M. Model of Chronic Thromboembolic Pulmonary Hypertension in Rats Caused by Repeated Intravenous Administration of Partially Biodegradable Sodium Alginate Microspheres. Int. J. Mol. Sci. 2021, 22, 1149. [Google Scholar] [CrossRef]
- Chong, C.Z.; Tay, E.L.W.; Sia, C.H.; Poh, K.K. Chronic thromboembolic pulmonary hypertension: A review. Singapore Med. J. 2021, 62, 318–325. [Google Scholar] [CrossRef]
- Simonneau, G.; Torbicki, A.; Dorfmuller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26. [Google Scholar] [CrossRef]
- Gonzales, A.J.; Bowman, J.W.; Fici, G.J.; Zhang, M.; Mann, D.W.; Mitton-Fry, M. Oclacitinib (APOQUEL((R))) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Vet. Pharmacol. Ther. 2014, 37, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Giaid, A.; Yanagisawa, M.; Langleben, D.; Michel, R.P.; Levy, R.; Shennib, H.; Kimura, S.; Masaki, T.; Duguid, W.P.; Stewart, D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993, 328, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Durrington, C.; Condliffe, R.; Kiely, D.G. BNP/NT-proBNP in pulmonary arterial hypertension: Time for point-of-care testing? Eur. Respir. Rev. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, H.; Kioka, H.; Sato, K.; Kurashige, M.; Ozawa, T.; Shibayama, H.; Hikoso, S.; Morii, E.; Yamauchi-Takihara, K.; Sakata, Y. Long-term Effects of the Janus Kinase 1/2 Inhibitor Ruxolitinib on Pulmonary Hypertension and the Cardiac Function in a Patient with Myelofibrosis. Intern. Med. 2020, 59, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Johnson, T.A.; Duru, N.; Buzza, M.S.; Pawar, N.R.; Sarkar, R.; Antalis, T.M. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front. Immunol. 2019, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef]
- Kerr, K.M.; Auger, W.R.; Marsh, J.J.; Devendra, G.; Spragg, R.G.; Kim, N.H.; Channick, R.N.; Jamieson, S.W.; Madani, M.M.; Manecke, G.R.; et al. Efficacy of methylprednisolone in preventing lung injury following pulmonary thromboendarterectomy. Chest 2012, 141, 27–35. [Google Scholar] [CrossRef]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.; Cai, M.; Zhang, C.; Mao, W.; Wang, Y.; Xu, Q.; Chen, M.; Wang, L.; Huang, X. Integrated bioinformatic analysis reveals the underlying molecular mechanism of and potential drugs for pulmonary arterial hypertension. Aging (Albany NY) 2021, 13, 14234–14257. [Google Scholar] [CrossRef]
- Floss, D.M.; Klocker, T.; Schroder, J.; Lamertz, L.; Mrotzek, S.; Strobl, B.; Hermanns, H.; Scheller, J. Defining the functional binding sites of interleukin 12 receptor beta1 and interleukin 23 receptor to Janus kinases. Mol. Biol. Cell. 2016, 27, 2301–2316. [Google Scholar] [CrossRef]
- Freireich, E.J.; Gehan, E.A.; Rall, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966, 50, 219–244. [Google Scholar] [PubMed]
- El-Kady, M.M.; Naggar, R.A.; Guimei, M.; Talaat, I.M.; Shaker, O.G.; Saber-Ayad, M. Early Renoprotective Effect of Ruxolitinib in a Rat Model of Diabetic Nephropathy. Pharmaceuticals 2021, 14, 608. [Google Scholar] [CrossRef] [PubMed]
- Le Ribeuz, H.; Courboulin, A.; Ghigna, M.R.; Lambert, M.; Hautefort, A.; Humbert, M.; Montani, D.; Cohen-Kaminsky, S.; Perros, F.; Antigny, F. In vivo miR-138-5p inhibition alleviates monocrotaline-induced pulmonary hypertension and normalizes pulmonary KCNK3 and SLC45A3 expression. Respir. Res. 2020, 21, 186. [Google Scholar] [CrossRef]
- Chaudhary, K.R.; Deng, Y.; Suen, C.M.; Taha, M.; Petersen, T.H.; Mei, S.H.J.; Stewart, D.J. Efficacy of treprostinil in the SU5416-hypoxia model of severe pulmonary arterial hypertension: Haemodynamic benefits are not associated with improvements in arterial remodelling. Br. J. Pharmacol. 2018, 175, 3976–3989. [Google Scholar] [CrossRef] [PubMed]

| Parameters | INT | PLC | PSL | IJAK 0.86 | IJAK 2.58 | IJAK 4.28 |
|---|---|---|---|---|---|---|
| PT diameter (mm) | 2.87 ± 0.23 | 2.98 ± 0.28 | 3.07 ± 0.32 | 2.88 ± 0.26 | 3.14 ± 0.4 | 2.96 ± 0.24 |
| RVOT diameter (mm) | 3.79 ± 0.15 | 3.95 ± 0.33 | 3.73 ± 0.26 | 3.85 ± 0.19 | 3.94 ± 0.19 | 4.0 ± 0.4 |
| Vmax in PT (sm/s) | 346 ± 58 | 313 ± 41 | 314 ± 19 | 331 ± 39 | 340 ± 26 | 335 ± 40 |
| Vmax RVOT (sm/s) | 255 ± 49 | 224 ± 35 | 221 ± 36 | 232 ± 21 | 235 ± 30 | 231 ± 29 |
| HR (beats/min) | 286 ± 28 | 248 ± 66 | 245 ± 25 | 272 ± 52 | 259 ± 24 | 251 ± 62 |
| LV EF (%) | 59 ± 11 | 58 ± 11 | 57 ± 7 | 50 ± 7 | 52 ± 9 | 47 ± 7 * |
| TAPSE (mm) | 2.34 ± 0.39 | 2.11 ± 0.63 | 1.87 ± 0.46 | 2.22 ± 0.58 | 2.06 ± 0.37 | 2.42 ± 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpov, A.A.; Mihailova, A.M.; Shilenko, L.A.; Vaulina, D.D.; Sidorova, E.E.; Akhmetova, A.A.; Docshin, P.M.; Krasichkov, A.S.; Sanarova, K.E.; Moiseeva, O.M.; et al. Inhibition of JAK1,2 Prevents Fibrotic Remodeling of Pulmonary Vascular Bed and Improves Outcomes in the Rat Model of Chronic Thromboembolic Pulmonary Hypertension. Int. J. Mol. Sci. 2022, 23, 15646. https://doi.org/10.3390/ijms232415646
Karpov AA, Mihailova AM, Shilenko LA, Vaulina DD, Sidorova EE, Akhmetova AA, Docshin PM, Krasichkov AS, Sanarova KE, Moiseeva OM, et al. Inhibition of JAK1,2 Prevents Fibrotic Remodeling of Pulmonary Vascular Bed and Improves Outcomes in the Rat Model of Chronic Thromboembolic Pulmonary Hypertension. International Journal of Molecular Sciences. 2022; 23(24):15646. https://doi.org/10.3390/ijms232415646
Chicago/Turabian StyleKarpov, Andrei A., Aleksandra M. Mihailova, Leonid A. Shilenko, Dariya D. Vaulina, Elizaveta E. Sidorova, Anna A. Akhmetova, Pavel M. Docshin, Alexander S. Krasichkov, Kseniia E. Sanarova, Olga M. Moiseeva, and et al. 2022. "Inhibition of JAK1,2 Prevents Fibrotic Remodeling of Pulmonary Vascular Bed and Improves Outcomes in the Rat Model of Chronic Thromboembolic Pulmonary Hypertension" International Journal of Molecular Sciences 23, no. 24: 15646. https://doi.org/10.3390/ijms232415646
APA StyleKarpov, A. A., Mihailova, A. M., Shilenko, L. A., Vaulina, D. D., Sidorova, E. E., Akhmetova, A. A., Docshin, P. M., Krasichkov, A. S., Sanarova, K. E., Moiseeva, O. M., & Galagudza, M. M. (2022). Inhibition of JAK1,2 Prevents Fibrotic Remodeling of Pulmonary Vascular Bed and Improves Outcomes in the Rat Model of Chronic Thromboembolic Pulmonary Hypertension. International Journal of Molecular Sciences, 23(24), 15646. https://doi.org/10.3390/ijms232415646






