CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy
Abstract
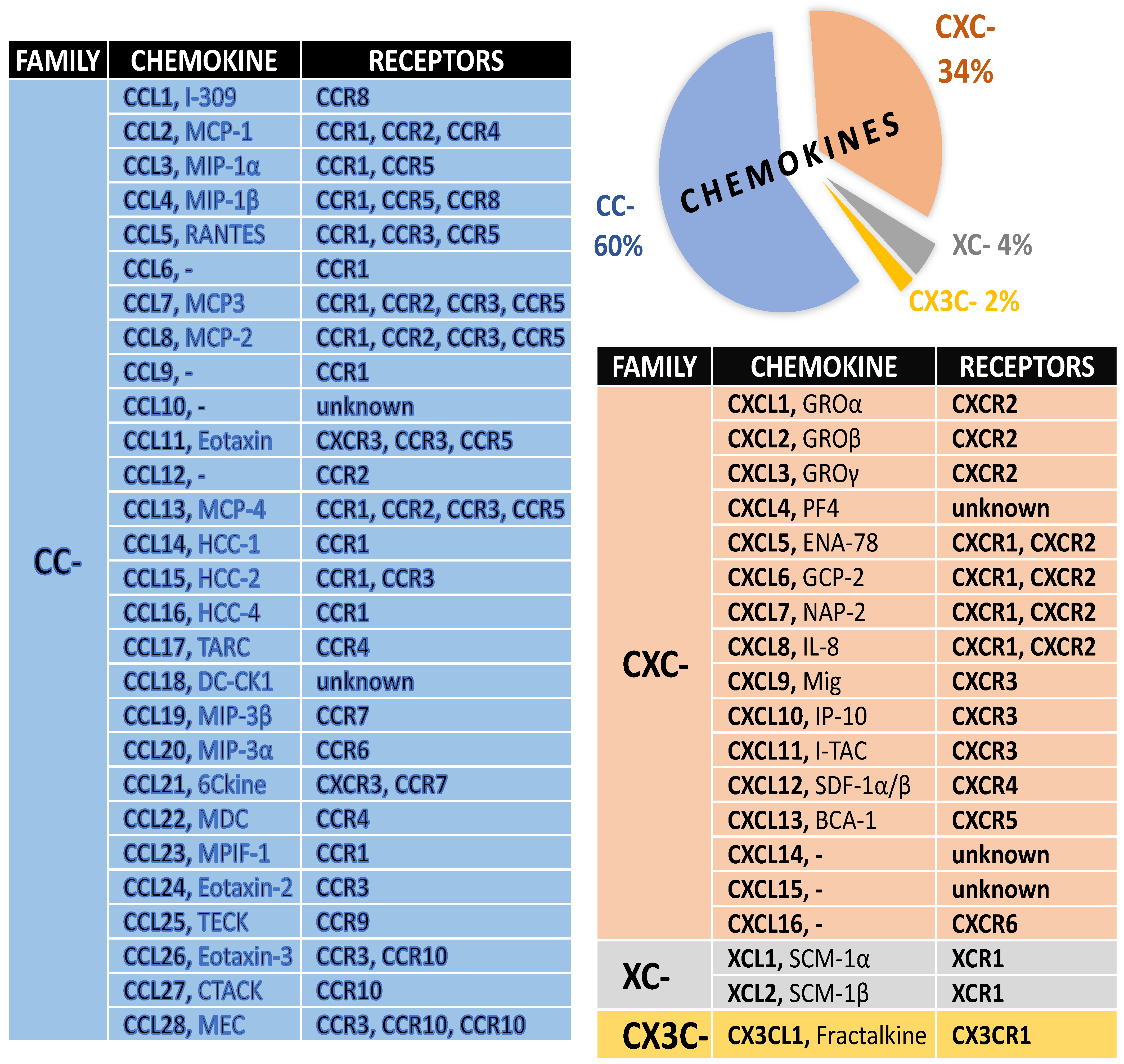
1. Chemokines and Their Receptors
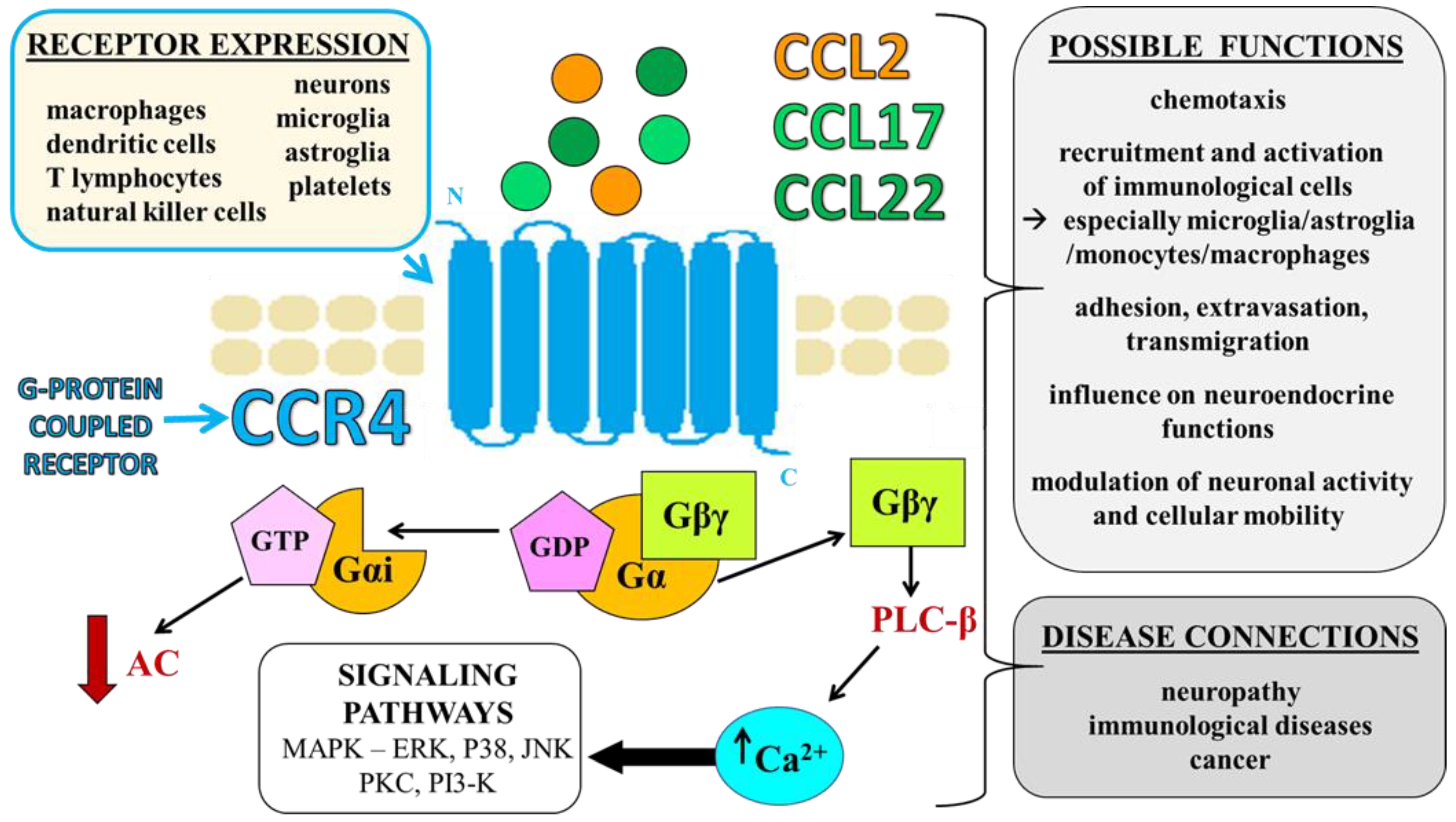
2. CCR4 and Its Ligands—Identification, Expression, and Functions
2.1. Selective Ligands of CCR4—CCL17 and CCL22
2.2. Nonselective Ligand of CCR4—CCL2
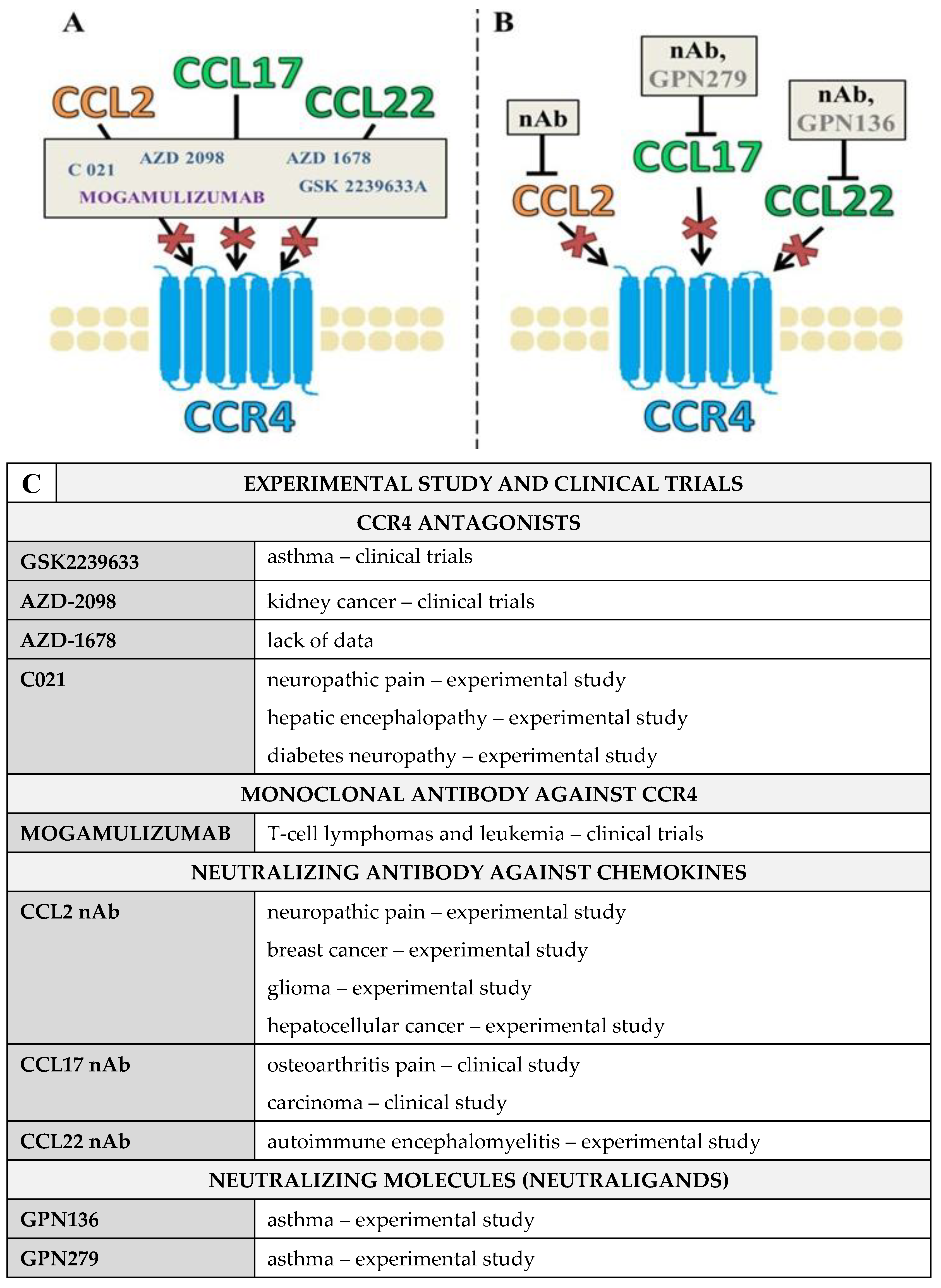
3. Pharmacological Blockade of CCR4 and Its Ligands Using CCR4 Antagonists and Blocking Antibodies
CCL2/CCL17/CCL22 Neutraligands and Neutralizing Antibodies
4. CCR4 and Nociceptive Processes
5. CCR4 and Immunological Diseases
6. CCR4 and Neoplastic Diseases
7. Future Perspectives and Treatment Strategies
| LIGAND | PAIN | IMMUNITY | TUMORS |
|---|---|---|---|
| CCL17 Thymus and Activation-Regulated Chemokine (TARC) CELL TYPES neurons, lymphocytes, basophils, mononuclear cells, dendritic cells | CLINICAL STUDY | ||
| fibromyalgia—clinical trials [71] | asthma [143] dermatitis [140] autoimmune diseases [57,59,60,61] atherosclerosis [61] | breast cancer [169] hepatocellural carcinoma [170] colon cancer– clinical trials [171] | |
| EXPERIMENTAL STUDY | |||
| neuropathic pain [33,34,35] inflammatory pain [172] | asthma [173] | pituitary adenoma [174] glioblastoma [175] gastric cancer [176] | |
| CCL22 Macrophage-Derived Chemokine-(MDC) CELL TYPES lymphocytes, basophils, NK, mononuclear cells, dendritic cells | CLINICAL STUDY | ||
| fibromyalgia [71] | atopic dermatitis [137] allergy [64] asthma [143,177] dermatitis [178] pneumonia [179] | breast cancer [169] hepatocellural carcinoma [170] colon cancer [171] gastric cancer [176] | |
| EXPERIMENTAL STUDY | |||
| neuropathic pain [33,34,35] | autoimmune encephalomyelitis [106] | melanoma [180] t-cell lymphoma [181] hepatocellular carcinoma [182] bladder cancer [183] | |
| CCL2 Monocyte Chemoattractant Protein-1 (MCP-1) CELL TYPES T lymphocytes, NK cells monocytes/macrophages, endothelial/epithelial cells, fibroblasts, smooth muscle, astroglia and microglia | CLINICAL STUDY | ||
| lumbar disk herniation [184] traumatic spinal cord injury [185] | multiple sclerosis [186] tuberculosis [187] myocardial infarction [188] aids [189] multiple sclerosis [190] nephropathy [191] inflammatory bowel disease [192] allergic asthma [193] rheumatoid arthritis [194] | colorectal cancer [195] ovarian cancer [195] esophagus cancer [195] pancreatic cancer [195] breast cancer [195] | |
| EXPERIMENTAL STUDY | |||
| neuropathic pain [15,16,109,119] arthritis bone cancer pain [196] | insulin resistance [197] asthma [198] autoimmune encephalomyelitis [199] | meningioma [200] colon cancer [201] carcinomas [202] melanoma [203] bladder cancer [204] | |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zlotnik, A.; Yoshie, O. The Chemokine Superfamily Revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Jiang, B.-C.; Gao, Y.-J. Chemokines in neuron–glial cell interaction and pathogenesis of neuropathic pain. Cell. Mol. Life Sci. 2017, 74, 3275–3291. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-J.; Ji, R.-R. Chemokines, neuronal–glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Mika, J. Chemokines under neuropathic pain. Ból 2014, 1, 19–35. [Google Scholar] [CrossRef]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.-H. Chemokine receptors in the central nervous system: Role in brain inflammation and neurodegenerative diseases. Brain Res. Rev. 2005, 48, 16–42. [Google Scholar] [CrossRef]
- Murdoch, C.; Finn, A. Chemokine receptors and their role in inflammation and infectious diseases. Blood 2000, 95, 3032–3043. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Ji, R.-R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010, 7, 482–493. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Nalepa, I.; Mika, J. Pharmacological Blockade of Spinal CXCL3/CXCR2 Signaling by NVP CXCR2 20, a Selective CXCR2 Antagonist, Reduces Neuropathic Pain Following Peripheral Nerve Injury. Front. Immunol. 2019, 10, 2198. [Google Scholar] [CrossRef]
- Sorensen, T.L.; Ransohoff, R.M.; Strieter, R.M.; Sellebjerg, F. Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur. J. Neurol. 2004, 11, 445–449. [Google Scholar] [CrossRef]
- Mines, M.; Ding, Y.; Fan, G.-H. The many roles of chemokine receptors in neurodegenerative disorders: Emerging new therapeutical strategies. Curr. Med. Chem. 2007, 14, 2456–2470. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. The Chemokine System in Neuroinflammation: An Update. J. Infect. Dis. 2002, 186, S152–S156. [Google Scholar] [CrossRef]
- Pawlik, K.; Piotrowska, A.; Kwiatkowski, K.; Ciapała, K.; Popiolek-Barczyk, K.; Makuch, W.; Mika, J. The blockade of CC chemokine receptor type 1 influences the level of nociceptive factors and enhances opioid analgesic potency in a rat model of neuropathic pain. Immunology 2020, 159, 413–428. [Google Scholar] [CrossRef]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Mika, J. The RS504393 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in Neuropathic Rats. J. Neuroimmune Pharmacol. 2017, 12, 402–419. [Google Scholar] [CrossRef]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Slusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Direct and indirect pharmacological modulation of CCL2/CCR2 pathway results in attenuation of neuropathic pain—In vivo and in vitro evidence. J. Neuroimmunol. 2016, 297, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial properties of maraviroc on neuropathic pain development and opioid effectiveness in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Makuch, W.; Mika, J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia—Evidence from in vivo and in vitro studies. Neuropharmacology 2016, 108, 207–219. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Spinal CCL1/CCR8 signaling interplay as a potential therapeutic target—Evidence from a mouse diabetic neuropathy model. Int. Immunopharmacol. 2017, 52, 261–271. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Mika, J. Dataset of (±)-NBI-74330 (CXCR3 antagonist) influence on chemokines under neuropathic pain. Data Brief 2018, 21, 1145–1150. [Google Scholar] [CrossRef]
- Bhangoo, S.K.; Ren, D.; Miller, R.J.; Chan, D.; Ripsch, M.S.; Weiss, C.; McGinnis, C.; White, F.A. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav. Immun. 2007, 21, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Dubový, P.; Klusáková, I.; Svizenska, I.; Brazda, V. Spatio-temporal changes of SDF1 and its CXCR4 receptor in the dorsal root ganglia following unilateral sciatic nerve injury as a model of neuropathic pain. Histochem. Cell Biol. 2010, 133, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Mika, J. Microglial Inhibition Influences XCL1/XCR1 Expression and Causes Analgesic Effects in a Mouse Model of Diabetic Neuropathy. Anesthesiology 2016, 125, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, H.; Ueha, S.; Kurachi, M.; Matsushima, K.; Moriyasu, F.; Blumberg, R.S.; Kakimi, K. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int. Immunol. 2005, 17, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Haringman, J.J.; Kraan, M.C.; Smeets, T.J.M.; Zwinderman, K.H.; Tak, P.P. Chemokine blockade and chronic inflammatory disease: Proof of concept in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. CC Chemokine Receptor Small Molecule Antagonists in the Treatment of Rheumatoid Arthritis and Other Diseases: A Current View. Curr. Top. Med. Chem. 2010, 10, 1250–1267. [Google Scholar] [CrossRef]
- Elsner, J.; Escher, S.E.; Forssmann, U. Chemokine receptor antagonists: A novel therapeutic approach in allergic diseases. Allergy 2004, 59, 1243–1258. [Google Scholar] [CrossRef]
- Vangelista, L.; Vento, S. The Expanding Therapeutic Perspective of CCR5 Blockade. Front. Immunol. 2018, 8, 1981. [Google Scholar] [CrossRef]
- Miao, M.; De Clercq, E.; Li, G. Clinical significance of chemokine receptor antagonists. Expert Opin. Drug Metab. Toxicol. 2020, 16, 11–30. [Google Scholar] [CrossRef]
- Xia, J.; Sun, S.; Jotte, M.R.; Uy, G.L.; Bohana-Kashtan, O.; Sorani, E.; Vainstein, A.; Peled, A.; Link, D.C. CXCR4 Blockade By BL-8040 in T Cell Acute Lymphoblastic Leukemia Decreases Mitochondrial Mass and Induces Non-Apoptotic Cell Death. Blood 2019, 134, 2745. [Google Scholar] [CrossRef]
- Dhaiban, S.; Al-Ani, M.; Elemam, N.M.; A Maghazachi, A. Targeting Chemokines and Chemokine Receptors in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. J. Inflamm. Res. 2020, 13, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Qi, H.; Xiao, J.; Gong, H.; Xu, E.; Li, S.; Ma, D.; Wang, Y.; Li, W.; et al. A new antagonist for CCR4 attenuates allergic lung inflammation in a mouse model of asthma. Sci. Rep. 2017, 7, 15038. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Popiolek-Barczyk, K.; Pawlik, K.; Ciechanowska, A.; Makuch, W.; Rojewska, E.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 antagonist (C021) influences the level of nociceptive factors and enhances the analgesic potency of morphine in a rat model of neuropathic pain. Eur. J. Pharmacol. 2020, 880, 173166. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. Blockade of CCR4 Diminishes Hypersensitivity and Enhances Opioid Analgesia—Evidence from a Mouse Model of Diabetic Neuropathy. Neuroscience 2020, 441, 77–92. [Google Scholar] [CrossRef]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Administration Diminishes Hypersensitivity and Enhances the Analgesic Potency of Morphine and Buprenorphine in a Mouse Model of Neuropathic Pain. Front. Immunol. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Matsuo, K.; Nagakubo, D.; Komori, Y.; Fujisato, S.; Takeda, N.; Kitamatsu, M.; Nishiwaki, K.; Quan, Y.-S.; Kamiyama, F.; Oiso, N.; et al. CCR4 Is Critically Involved in Skin Allergic Inflammation of BALB/c Mice. J. Investig. Dermatol. 2018, 138, 1764–1773. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2014, 27, 11–20. [Google Scholar] [CrossRef]
- Ishida, T.; Ito, A.; Sato, F.; Kusumoto, S.; Iida, S.; Inagaki, H.; Morita, A.; Akinaga, S.; Ueda, R. Stevens-Johnson Syndrome associated with mogamulizumab treatment of adult T-cell leukemia/lymphoma. Cancer Sci. 2013, 104, 647–650. [Google Scholar] [CrossRef]
- Olkhanud, P.B.; Baatar, D.; Bodogai, M.; Hakim, F.; Gress, R.; Anderson, R.L.; Deng, J.; Xu, M.; Briest, S.; Biragyn, A. Breast Cancer Lung Metastasis Requires Expression of Chemokine Receptor CCR4 and Regulatory T Cells. Cancer Res. 2009, 69, 5996–6004. [Google Scholar] [CrossRef]
- Berlato, C.; Khan, M.N.; Schioppa, T.; Thompson, R.; Maniati, E.; Montfort, A.; Jangani, M.; Canosa, M.; Kulbe, H.; Hagemann, U.B.; et al. A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. J. Clin. Investig. 2017, 127, 801–813. [Google Scholar] [CrossRef]
- Scheu, S.; Ali, S.; Ruland, C.; Arolt, V.; Alferink, J. The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int. J. Mol. Sci. 2017, 18, 2306. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Thompson, M.; Galindo, C.; Standeford, H.; Whittington, E.; Alpini, G.; DeMorrow, S. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J. Neuroinflammation 2014, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.; Handel, T. Chemokine oligomerization and interactions with receptors and glycosaminoglycans: The role of structural dynamics in function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Meucci, O.; Fatatis, A.; Simen, A.A.; Bushell, T.J.; Gray, P.W.; Miller, R.J. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. USA 1998, 95, 14500–14505. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.; Maru, S.; Loughlin, J.; A Romero, I.; Male, D. Regulation of chemokine receptor expression in human microglia and astrocytes. J. Neuroimmunol. 2003, 136, 84–93. [Google Scholar] [CrossRef]
- Kufareva, I.; Gustavsson, M.; Zheng, Y.; Stephens, B.S.; Handel, T.M. What Do Structures Tell Us About Chemokine Receptor Function and Antagonism? Annu. Rev. Biophys. 2017, 46, 175–198. [Google Scholar] [CrossRef]
- Scholten, D.; Canals, M.; Maussang, D.; Roumen, L.; Smit, M.; Wijtmans, M.; de Graaf, C.; Vischer, H.; Leurs, R. Pharmacological modulation of chemokine receptor function. Br. J. Cereb. Blood Flow Metab. 2012, 165, 1617–1643. [Google Scholar] [CrossRef]
- Power, C.A.; Meyer, A.; Nemeth, K.; Bacon, K.B.; Hoogewerf, A.J.; Proudfoot, A.E.; Wells, T.N. Molecular Cloning and Functional Expression of a Novel CC Chemokine Receptor cDNA from a Human Basophilic Cell Line. J. Biol. Chem. 1995, 270, 19495–19500. [Google Scholar] [CrossRef]
- Oh, S.B.; Tran, P.B.; Gillard, S.E.; Hurley, R.; Hammond, D.; Miller, R.J. Chemokines and Glycoprotein120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. J. Neurosci. 2001, 21, 5027–5035. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Arabi, Z.; Ahangar-Parvin, R.; Mohammadi-Kordkhayli, M.; Nemati, M. Ginger Extract Modulates the Expression of Chemokines CCL20 and CCL22 and Their Receptors (CCR6 and CCR4) in the Central Nervous System of Mice with Experimental Autoimmune Encephalomyelitis. Drug Res. 2017, 67, 632–639. [Google Scholar] [CrossRef]
- Bajetto, A.; Bonavia, R.; Barbero, S.; Schettini, G. Characterization of chemokines and their receptors in the central nervous system: Physiopathological implications. J. Neurochem. 2002, 82, 1311–1329. [Google Scholar] [CrossRef] [PubMed]
- Viney, J.M.; Andrew, D.P.; Phillips, R.M.; Meiser, A.; Patel, P.; Lennartz-Walker, M.; Cousins, D.J.; Barton, N.P.; Hall, D.A.; Pease, J.E. Distinct Conformations of the Chemokine Receptor CCR4 with Implications for Its Targeting in Allergy. J. Immunol. 2014, 192, 3419–3427. [Google Scholar] [CrossRef]
- Moriguchi, K.; Miyamoto, K.; Tanaka, N.; Ueno, R.; Nakayama, T.; Yoshie, O.; Kusunoki, S. C-C chemokine receptor type 4 antagonist Compound 22 ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2015, 291, 54–58. [Google Scholar] [CrossRef]
- Pilette, C.; Francis, J.; Till, S.; Durham, S. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur. Respir. J. 2004, 23, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Ding, H.; Peters, C.M.; Kock, N.D.; Kishioka, S.; Cline, J.M.; Wagner, J.D.; Ko, M.-C. Altered expression of glial markers, chemokines, and opioid receptors in the spinal cord of type 2 diabetic monkeys. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Imaib, T.; Kusudac, J.; Miuraa, R.; Callen, D.; Yoshie, O. Assignment of the Human CC Chemokine Gene TARC (SCYA17) to Chromosome 16q13. Genomics 1997, 40, 211–213. [Google Scholar] [CrossRef]
- Alferink, J.; Lieberam, I.; Reindl, W.; Behrens, A.; Weiss, S.; Hüser, N.; Gerauer, K.; Ross, R.; Reske-Kunz, A.B.; Ahmad-Nejad, P.; et al. Compartmentalized Production of CCL17 In Vivo: Strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. J. Exp. Med. 2003, 197, 585–599. [Google Scholar] [CrossRef]
- Fülle, L.; Offermann, N.; Hansen, J.N.; Breithausen, B.; Erazo, A.B.; Schanz, O.; Radau, L.; Gondorf, F.; Knöpper, K.; Alferink, J.; et al. CCL17 exerts a neuroimmune modulatory function and is expressed in hippocampal neurons. Glia 2018, 66, 2246–2261. [Google Scholar] [CrossRef]
- Heiseke, A.F.; Faul, A.C.; Lehr, H.; Förster, I.; Schmid, R.M.; Krug, A.B.; Reindl, W. CCL17 Promotes Intestinal Inflammation in Mice and Counteracts Regulatory T Cell–Mediated Protection From Colitis. Gastroenterology 2012, 142, 335–345. [Google Scholar] [CrossRef]
- Stutte, S.; Quast, T.; Gerbitzki, N.; Savinko, T.; Novak, N.; Reifenberger, J.; Homey, B.; Kolanus, W.; Alenius, H.; Förster, I. Requirement of CCL17 for CCR7- and CXCR4-dependent migration of cutaneous dendritic cells. Proc. Natl. Acad. Sci. USA 2010, 107, 8736–8741. [Google Scholar] [CrossRef]
- Weber, C.; Meiler, S.; Döring, Y.; Koch, M.; Drechsler, M.; Megens, R.; Rowinska, Z.; Bidzhekov, K.; Fecher, C.; Ribechini, E.; et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J. Clin. Investig. 2011, 121, 2898–2910. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Yoshie, O. The T Cell-directed CC Chemokine TARC Is a Highly Specific Biological Ligand for CC Chemokine Receptor 4. J. Biol. Chem. 1997, 272, 15036–15042. [Google Scholar] [CrossRef]
- Bonecchi, R.; Galliera, E.; Borroni, E.M.; Corsi, M.M.; Locati, M.; Mantovani, A. Chemokines and chemokine receptors: An overview. Front. Biosci. 2009, 14, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, U.; Kuroda, E. Regulation of Macrophage-Derived Chemokine (MDC/CCL22) Production. Crit. Rev. Immunol. 2002, 22, 105–114. [Google Scholar] [CrossRef]
- Montane, J.; Bischoff, L.; Soukhatcheva, G.; Dai, D.L.; Hardenberg, G.; Levings, M.; Orban, P.C.; Kieffer, T.J.; Tan, R.; Verchere, C.B. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J. Clin. Investig. 2011, 121, 3024–3028. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Xu, Z.-Z.; Wang, X.; Park, J.Y.; Zhuang, Z.-Y.; Tan, P.-H.; Gao, Y.-J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Mikhak, Z.; Fukui, M.; Farsidjani, A.; Medoff, B.; Tager, A.M.; Luster, A.D. Contribution of CCR4 and CCR8 to antigen-specific TH2 cell trafficking in allergic pulmonary inflammation. J. Allergy Clin. Immunol. 2009, 123, 67–73.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Liu, F.-F.; Zhang, X.-M.; Guo, X.-J.; Ren, M.-J.; Fu, L. Tumor Secretion of CCL22 Activates Intratumoral Treg Infiltration and Is Independent Prognostic Predictor of Breast Cancer. PLoS ONE 2013, 8, e76379. [Google Scholar] [CrossRef]
- Martinenaite, E.; Ahmad, S.M.; Hansen, M.; Met, Ö.; Westergaard, M.W.; Larsen, S.K.; Klausen, T.W.; Donia, M.; Svane, I.M.; Andersen, M.H. CCL22-specific T Cells: Modulating the immunosuppressive tumor microenvironment. OncoImmunology 2016, 5, e1238541. [Google Scholar] [CrossRef]
- Imai, T.; Chantry, D.; Raport, C.J.; Wood, C.L.; Nishimura, M.; Godiska, R.; Yoshie, O.; Gray, P.W. Macrophage-derived Chemokine Is a Functional Ligand for the CC Chemokine Receptor 4. J. Biol. Chem. 1998, 273, 1764–1768. [Google Scholar] [CrossRef]
- García, J.J.; Cidoncha, A.; E Bote, M.; Hinchado, M.D.; Ortega, E. Altered profile of chemokines in fibromyalgia patients. Ann. Clin. Biochem. 2014, 51, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Frossard, J.L.; Lenglet, S.; Montecucco, F.; Steffens, S.; Galan, K.; Pelli, G.; Spahr, L.; Mach, F.; Hadengue, A. Role of CCL-2, CCR-2 and CCR-4 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. J. Clin. Pathol. 2011, 64, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Tan, J.; Guo, Y.; Chen, S.; Fan, L.; Li, Y. Notch3 Knockout Suppresses Mouse Mammary Gland Development and Inhibits the Proliferation of 4T1 Murine Mammary Carcinoma Cells via CCL2/CCR4 Axis. Front. Cell Dev. Biol. 2020, 8, 1296. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; A Robinson, E.; Tanaka, S.; Appella, E.; Leonard, E.J. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J. Immunol. 1989, 142, 1956–1962. [Google Scholar] [PubMed]
- Barna, B.P.; Pettay, J.; Barnett, G.H.; Zhou, P.; Iwasaki, K.; Estes, M.L. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J. Neuroimmunol. 1994, 50, 101–107. [Google Scholar] [CrossRef]
- Brown, Z.; Strieter, R.M.; Neild, G.H.; Thompson, R.C.; Kunkel, S.L.; Westwick, J. IL-1 receptor antagonist inhibits monocyte chemotactic peptide 1 generation by human mesangial cells. Kidney Int. 1992, 42, 95–101. [Google Scholar] [CrossRef]
- A Begley, L.; Kasina, S.; Mehra, R.; Adsule, S.; Admon, A.J.; Lonigro, R.J.; Chinnaiyan, A.M.; A Macoska, J. CXCL5 Promotes Prostate Cancer Progression. Neoplasia 2008, 10, 244–254. [Google Scholar] [CrossRef]
- Singh, R.K.; Lokeshwar, B.L. Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Mol. Cancer 2009, 8, 57. [Google Scholar] [CrossRef]
- Dutta, P.; Sarkissyan, M.; Paico, K.; Wu, Y.; Vadgama, J.V. MCP-1 is overexpressed in triple-negative breast cancers and drives cancer invasiveness and metastasis. Breast Cancer Res. Treat. 2018, 170, 477–486. [Google Scholar] [CrossRef]
- Hayashida, K.; Nanki, T.; Girschick, H.; Yavuz, S.; Ochi, T.; E Lipsky, P. Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 2001, 3, 118–126. [Google Scholar] [CrossRef]
- Kusano, K.F.; Nakamura, K.; Kusano, H.; Nishii, N.; Banba, K.; Ikeda, T.; Hashimoto, K.; Yamamoto, M.; Fujio, H.; Miura, A.; et al. Significance of the Level of Monocyte Chemoattractant Protein-1 in Human Atherosclerosis-Assessment in Chronic Hemodialysis Patients. Circ. J. 2004, 68, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwinckel, J.; LE Goazigo, A.R.; Pommier, B.; Mauborgne, A.; Dansereau, M.-A.; Kitabgi, P.; Sarret, P.; Pohl, M.; Parsadaniantz, S.M. CCL2 Released from Neuronal Synaptic Vesicles in the Spinal Cord Is a Major Mediator of Local Inflammation and Pain after Peripheral Nerve Injury. J. Neurosci. 2011, 31, 5865–5875. [Google Scholar] [CrossRef]
- Bose, S.; Cho, J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharmacal Res. 2013, 36, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Jiao, H.; Cai, H. Analgesic Effect of Intrathecal Administration of Chemokine Receptor CCR2 Antagonist is Related to Change in Spinal NR2B, nNOS, and SIGIRR Expression in Rat with Bone Cancer Pain. Cell Biophys. 2015, 72, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Yang, J.-P.; Hu, J.-H.; Wang, L.-N.; Zuo, J.-L. MCP-1 stimulates spinal microglia via PI3K/Akt pathway in bone cancer pain. Brain Res. 2015, 1599, 158–167. [Google Scholar] [CrossRef]
- Nikodemova, M.; Duncan, I.D.; Watters, J.J. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IκBα degradation in a stimulus-specific manner in microglia. J. Neurochem. 2006, 96, 314–323. [Google Scholar] [CrossRef]
- Purandare, A.; Somerville, J. Antagonists of CCR4 as Immunomodulatory Agents. Curr. Top. Med. Chem. 2006, 6, 1335–1344. [Google Scholar] [CrossRef]
- Purandare, A.V.; Gao, A.; Wan, H.; Somerville, J.; Burke, C.; Seachord, C.; Vaccaro, W.; Wityak, J.; Poss, M.A. Identification of chemokine receptor CCR4 antagonist. Bioorg. Med. Chem. Lett. 2005, 15, 2669–2672. [Google Scholar] [CrossRef]
- Andrews, G.; Jones, C.; Wreggett, K.A. An Intracellular Allosteric Site for a Specific Class of Antagonists of the CC Chemokine G Protein-Coupled Receptors CCR4 and CCR5. Mol. Pharmacol. 2008, 73, 855–867. [Google Scholar] [CrossRef]
- Banfield, G.; Watanabe, H.; Scadding, G.; Jacobson, M.R.; Till, S.J.; Hall, D.A.; Robinson, D.S.; Lloyd, C.M.; Nouri-Aria, K.T.; Durham, S.R. CC Chemokine Receptor 4 (CCR4) in human allergen-induced late nasal responses. Allergy 2010, 65, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Burdi, D.F.; Chi, S.; Mattia, K.; Harrington, C.; Shi, Z.; Chen, S.; Jacutin-Porte, S.; Bennett, R.; Carson, K.; Yin, W.; et al. Small molecule antagonists of the CC chemokine receptor 4 (CCR4). Bioorg. Med. Chem. Lett. 2007, 17, 3141–3145. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Hodgson, S.; Wilson, R.; Robertson, J.; Watson, J.; Beerahee, M.; Hughes, S.C.; Young, G.; Graves, R.; Hall, D.; et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: Results from an open-label and from a randomised study. BMC Pharmacol. Toxicol. 2013, 14, 14. [Google Scholar] [CrossRef]
- Kuhn, C.F.; Bazin, M.; Philippe, L.; Zhang, J.; Tylaska, L.; Miret, J.; Bauer, P.H. Bipiperidinyl Carboxylic Acid Amides as Potent, Selective, and Functionally Active CCR4 Antagonists. Chem. Biol. Drug Des. 2007, 70, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Solari, R.; Pease, J.E. Targeting chemokine receptors in disease—A case study of CCR4. Eur. J. Pharmacol. 2015, 763, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Ishikawa, N.; Igarashi, S.; Kawano, N.; Masuda, N.; Hamaguchi, W.; Yamasaki, S.; Koganemaru, Y.; Hattori, K.; Miyazaki, T.; et al. Potent and orally bioavailable CCR4 antagonists: Synthesis and structure–activity relationship study of 2-aminoquinazolines. Bioorg. Med. Chem. 2009, 17, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Ishikawa, N.; Igarashi, S.; Kawano, N.; Masuda, N.; Hattori, K.; Miyazaki, T.; Ogino, S.-I.; Orita, M.; Matsumoto, Y.; et al. Potent CCR4 antagonists: Synthesis, evaluation, and docking study of 2,4-diaminoquinazolines. Bioorg. Med. Chem. 2008, 16, 7968–7974. [Google Scholar] [CrossRef]
- Kindon, N.; Andrews, G.; Baxter, A.; Cheshire, D.; Hemsley, P.; Johnson, T.; Liu, Y.-Z.; McGinnity, D.; McHale, M.; Mete, A.; et al. Discovery of AZD-2098 and AZD-1678, Two Potent and Bioavailable CCR4 Receptor Antagonists. ACS Med. Chem. Lett. 2017, 8, 981–986. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, M.; Ishii, T.; Urakawa, I.; Matsumoto, A.; Masaki, A.; Ito, A.; Kusumoto, S.; Suzuki, S.; Hiura, M.; et al. Mogamulizumab Treatment Elicits Autoantibodies Attacking the Skin in Patients with Adult T-Cell Leukemia-Lymphoma. Clin. Cancer Res. 2019, 25, 4388–4399. [Google Scholar] [CrossRef]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A phase 1 study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef]
- Remer, M.; Al-Shamkhani, A.; Glennie, M.; Johnson, P. Mogamulizumab and the treatment of CCR4-positive T-cell lymphomas. Immunotherapy 2014, 6, 1187–1206. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated Anti-CCR4 Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: A Multicenter Phase II Study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef]
- Abboud, D.; Daubeuf, F.; Do, Q.T.; Utard, V.; Villa, P.; Haiech, J.; Bonnet, D.; Hibert, M.; Bernard, P.; Galzi, J.-L.; et al. A strategy to discover decoy chemokine ligands with an anti-inflammatory activity. Sci. Rep. 2015, 5, 14746. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.-Y.; Han, J.; Zhang, X.; Hsu, S.-H.; He, S.; Wani, N.A.; Barajas, J.M.; Snyder, L.A.; Frankel, W.L.; Caligiuri, M.A.; et al. Blocking the CCL2–CCR2 Axis Using CCL2-Neutralizing Antibody Is an Effective Therapy for Hepatocellular Cancer in a Mouse Model. Mol. Cancer Ther. 2017, 16, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-B.; Xie, F.; Chang, K.-K.; Shang, W.-Q.; Meng, Y.-H.; Yu, J.-J.; Li, H.; Sun, Q.; Yuan, M.-M.; Jin, L.-P.; et al. Chemokine CCL17 induced by hypoxia promotes the proliferation of cervical cancer cell. Am. J. Cancer Res. 2015, 5, 3072–3084. [Google Scholar]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapała, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but not CCL12, play a significant role in the development of pain-related behavior and opioid-induced analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef]
- Fang, W.B.; Yao, M.; Brummer, G.; Acevedo, D.; Alhakamy, N.; Berkland, C.; Cheng, N. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget 2016, 7, 49349–49367. [Google Scholar] [CrossRef]
- Zhu, X.; Fujita, M.; Snyder, L.A.; Okada, H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J. Neuro-Oncol. 2011, 104, 83–92. [Google Scholar] [CrossRef]
- Lee, M.-C.; Saleh, R.; Achuthan, A.; Fleetwood, A.J.; Förster, I.; Hamilton, J.A.; Cook, A.D. CCL17 blockade as a therapy for osteoarthritis pain and disease. Arthritis Res. Ther. 2018, 20, 62. [Google Scholar] [CrossRef]
- Lee, K.-C.; Prasad, V.; Achuthan, A.; Fleetwood, A.; Hamilton, J.; Cook, A. Targeting GM-CSF for collagenase-induced osteoarthritis pain and disease in mice. Osteoarthr. Cartil. 2020, 28, 486–491. [Google Scholar] [CrossRef]
- Dogan, R.-N.E.; Long, N.; Forde, E.; Dennis, K.; Kohm, A.P.; Miller, S.D.; Karpus, W.J. CCL22 regulates experimental autoimmune encephalomyelitis by controlling inflammatory macrophage accumulation and effector function. J. Leukoc. Biol. 2010, 89, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Raghavendra, V.; Rutkowski, M.D.; DeLeo, J.A. The Role of Spinal Neuroimmune Activation in Morphine Tolerance/Hyperalgesia in Neuropathic and Sham-Operated Rats. J. Neurosci. 2002, 22, 9980–9989. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. Chemokines and Chemokine Receptors: Standing at the Crossroads of Immunobiology and Neurobiology. Immunity 2009, 31, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Dansereau, M.-A.; Gosselin, R.-D.; Pohl, M.; Pommier, B.; Mechighel, P.; Mauborgne, A.; Rostene, W.; Kitabgi, P.; Beaudet, N.; Sarret, P.; et al. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J. Neurochem. 2008, 106, 757–769. [Google Scholar] [CrossRef]
- Pfützner, J.; Hellhammer, J.; Musholt, P.; Pfützner, A.H.; Böhnke, J.; Hero, T.; Amann-Zalan, I.; Ganz, M.; Forst, T.; Pfützner, A. Evaluation of Dexterity in Insulin-Treated Patients with Type 1 and Type 2 Diabetes Mellitus. J. Diabetes Sci. Technol. 2011, 5, 158–165. [Google Scholar] [CrossRef]
- Mueller, M.J.; Minor, S.D.; A Sahrmann, S.; A Schaaf, J.; Strube, M.J. Differences in the Gait Characteristics of Patients With Diabetes and Peripheral Neuropathy Compared With Age-Matched Controls. Phys. Ther. 1994, 74, 299–308. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Ciapała, K.; Rojewska, E.; Makuch, W.; Mika, J. Comparison of the beneficial effects of RS504393, maraviroc and cenicriviroc on neuropathic pain-related symptoms in rodents: Behavioral and biochemical analyses. Int. Immunopharmacol. 2020, 84, 106540. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Makuch, W.; Rojewska, E.; Pilat, D.; Mika, J. Inhibition of intracellular signaling pathways NF-κB and MEK1/2 attenuates neuropathic pain development and enhances morphine analgesia. Pharmacol. Rep. 2014, 66, 845–851. [Google Scholar] [CrossRef]
- Hung, A.L.; Lim, M.; Doshi, T.L. Targeting cytokines for treatment of neuropathic pain. Scand. J. Pain 2017, 17, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.; et al. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 16, 17002. [Google Scholar] [CrossRef] [PubMed]
- Zychowska, M.; Rojewska, E.; Kreiner, G.; Nalepa, I.; Przewlocka, B.; Mika, J. Minocycline influences the anti-inflammatory interleukins and enhances the effectiveness of morphine under mice diabetic neuropathy. J. Neuroimmunol. 2013, 262, 35–45. [Google Scholar] [CrossRef]
- Mika, J.; Osikowicz, M.; Rojewska, E.; Korostynski, M.; Wawrzczak-Bargiela, A.; Przewlocki, R.; Przewlocka, B. Differential activation of spinal microglial and astroglial cells in a mouse model of peripheral neuropathic pain. Eur. J. Pharmacol. 2009, 623, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33. [Google Scholar] [CrossRef]
- Colvin, L.A.; Bull, F.; Hales, T.G. Perioperative opioid analgesia—when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019, 393, 1558–1568. [Google Scholar] [CrossRef]
- Lin, C.-P.; Lu, D.-H. Role of Neuroinflammation in Opioid Tolerance: Translational Evidence from Human-to-Rodent Studies. In Advances in Pain Research: Mechanisms and Modulation of Chronic Pain; Springer: Singapore, 2018; Volume 1099, pp. 125–139. [Google Scholar] [CrossRef]
- Mika, J.; Wawrzczak-Bargiela, A.; Osikowicz, M.; Makuch, W.; Przewlocka, B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav. Immun. 2009, 23, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Pilat, D.; Piotrowska, A.; Rojewska, E.; Jurga, A.; Ślusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Blockade of IL-18 signaling diminished neuropathic pain and enhanced the efficacy of morphine and buprenorphine. Mol. Cell. Neurosci. 2016, 71, 114–124. [Google Scholar] [CrossRef]
- Pilat, D.; Rojewska, E.; Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. IL-1 receptor antagonist improves morphine and buprenorphine efficacy in a rat neuropathic pain model. Eur. J. Pharmacol. 2015, 764, 240–248. [Google Scholar] [CrossRef]
- Mika, J. Review paper The opioid systems and the role of glial cells in the effects of opioids The opioid systems. Adv. Pall. Med. 2008, 7, 185–196. [Google Scholar]
- Liu, Z.; Song, Z.; Guo, S.; He, J.; Wang, S.; Zhu, J.; Yang, H.; Liu, J. CXCL12/CXCR4 signaling contributes to neuropathic pain via central sensitization mechanisms in a rat spinal nerve ligation model. CNS Neurosci. Ther. 2019, 25, 922–936. [Google Scholar] [CrossRef]
- Pan, Y.; Sun, X.; Jiang, L.; Hu, L.; Kong, H.; Han, Y.; Qian, C.; Song, C.; Qian, Y.; Liu, W. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J. Neuroinflamm. 2016, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, M.; Deng, J.; Lv, X.; Liu, J.; Xiao, Y.; Yang, W.; Zhang, Y.; Li, C. Chemokine Signaling Pathway Involved in CCL2 Expression in Patients with Rheumatoid Arthritis. Yonsei Med. J. 2015, 56, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-M.; Guo, R.-X.; Hu, F.; Chen, P.-X.; Cui, Y.; Feng, J.-Q.; Meng, J.-L.; Mo, L.-Q.; Liao, X.-X. Spinal MCP-1 Contributes to the Development of Morphine Antinociceptive Tolerance in Rats. Am. J. Med. Sci. 2012, 344, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-P.; Kang, K.-H.; Tu, H.-J.; Wu, M.-Y.; Lin, T.-H.; Liou, H.-C.; Sun, W.-Z.; Fu, W.-M. CXCL12/CXCR4 Signaling Contributes to the Pathogenesis of Opioid Tolerance: A Translational Study. Anesthesia Analg. 2017, 124, 972–979. [Google Scholar] [CrossRef]
- Iellem, A.; Mariani, M.; Lang, R.; Recalde, H.; Panina-Bordignon, P.; Sinigaglia, F.; D’Ambrosio, D. Unique Chemotactic Response Profile and Specific Expression of Chemokine Receptors Ccr4 and Ccr8 by Cd4+Cd25+ Regulatory T Cells. J. Exp. Med. 2001, 194, 847–854. [Google Scholar] [CrossRef]
- Juremalm, M.; Olsson, N.; Nilsson, G. Selective CCL5/RANTES-induced mast cell migration through interactions with chemokine receptors CCR1 and CCR4. Biochem. Biophys. Res. Commun. 2002, 297, 480–485. [Google Scholar] [CrossRef]
- Campbell, J.J.; Haraldsen, G.; Pan, J.; Rottman, J.; Qin, S.; Ponath, P.; Andrew, D.P.; Warnke, R.; Ruffing, N.; Kassam, N.; et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999, 400, 776–780. [Google Scholar] [CrossRef]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Asahina, A.; Onai, N.; Matsushima, K.; et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001, 107, 535–541. [Google Scholar] [CrossRef]
- Vestergaard, C.; Bang, K.; Gesser, B.; Yoneyama, H.; Matsushima, K.; Larsen, C.G. A Th2 Chemokine, TARC, Produced by Keratinocytes May Recruit CLA+CCR4+ Lymphocytes into Lesional Atopic Dermatitis Skin. J. Investig. Dermatol. 2000, 115, 640–646. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Perros, F.; Hoogsteden, H.C.; Coyle, A.J.; Lambrecht, B.N.; Hammad, H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy 2009, 64, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Conroy, D.M.; Jopling, L.A.; Lloyd, C.M.; Hodge, M.R.; Andrew, D.P.; Williams, T.J.; Pease, J.E.; Sabroe, I. CCR4 blockade does not inhibit allergic airways inflammation. J. Leukoc. Biol. 2003, 74, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Chvatchko, Y.; Hoogewerf, A.J.; Meyer, A.; Alouani, S.; Juillard, P.; Buser, R.; Conquet, F.; Proudfoot, A.E.; Wells, T.; Power, C.A. A Key Role for Cc Chemokine Receptor 4 in Lipopolysaccharide-Induced Endotoxic Shock. J. Exp. Med. 2000, 191, 1755–1764. [Google Scholar] [CrossRef]
- Lloyd, C.M. Chemokines in allergic airway disease. Curr. Opin. Pharmacol. 2003, 3, 443–448. [Google Scholar] [CrossRef]
- Baatar, D.; Olkhanud, P.; Sumitomo, K.; Taub, D.; Gress, R.; Biragyn, A. Human Peripheral Blood T Regulatory Cells (Tregs), Functionally Primed CCR4+Tregs and Unprimed CCR4−Tregs, Regulate Effector T Cells Using FasL. J. Immunol. 2007, 178, 4891–4900. [Google Scholar] [CrossRef]
- Forde, E.A.; Dogan, R.-N.E.; Karpus, W.J. CCR4 contributes to the pathogenesis of experimental autoimmune encephalomyelitis by regulating inflammatory macrophage function. J. Neuroimmunol. 2011, 236, 17–26. [Google Scholar] [CrossRef]
- Abdul-Majid, K.-B.; Wefer, J.; Stadelmann, C.; Stefferl, A.; Lassmann, H.; Olsson, T.; Harris, R.A. Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4−/− and CD8−/− DBA/1 mice defines qualitative roles of different T cell subsets. J. Neuroimmunol. 2003, 141, 10–19. [Google Scholar] [CrossRef]
- Galimberti, D.; Fenoglio, C.; Comi, C.; Scalabrini, D.; De Riz, M.; Leone, M.; Venturelli, E.; Cortini, F.; Piola, M.; Monaco, F.; et al. MDC/CCL22 intrathecal levels in patients with multiple sclerosis. Mult. Scler. J. 2008, 14, 547–549. [Google Scholar] [CrossRef]
- Franciotta, D.; Zardini, E.; Ravaglia, S.; Piccolo, G.; Andreoni, L.; Bergamaschi, R.; Romani, A.; Tavazzi, E.; Naldi, P.; Ceroni, M.; et al. Cytokines and chemokines in cerebrospinal fluid and serum of adult patients with acute disseminated encephalomyelitis. J. Neurol. Sci. 2006, 247, 202–207. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Li, X.; Kong, Y.; Sun, W. CCL17-CCR4 axis contributes to the onset of vitiligo in mice. Immun. Inflamm. Dis. 2021, 9, 702–709. [Google Scholar] [CrossRef]
- Shan, J.; Shen, C.; Fang, J.; Li, S.; Fan, Y. Potential roles of the CCL17-CCR4 axis in immunopathogenesis of oral lichen planus. J. Oral Pathol. Med. 2019, 49, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Baay-Guzman, G.J.; Bebenek, I.G.; Zeidler, M.; Hernandez-Pando, R.; I Vega, M.; A Garcia-Zepeda, E.; Antonio-Andres, G.; Bonavida, B.; Riedl, M.; Kleerup, E.; et al. HIF-1 expression is associated with CCL2 chemokine expression in airway inflammatory cells: Implications in allergic airway inflammation. Respir. Res. 2012, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, Q.; Chen, M.; Shi, J.; Guo, Y.; Liu, S.; Pan, R.; Yuan, X.; Jiang, S. Mas receptor activation attenuates allergic airway inflammation via inhibiting JNK/CCL2-induced macrophage recruitment. Biomed. Pharmacother. 2021, 137, 111365. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.-Y. The Role of Regulatory T Cells in Cancer. Immune Netw. 2009, 9, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Nishikawa, H. Roles of regulatory T cells in cancer immunity. Int. Immunol. 2016, 28, 401–409. [Google Scholar] [CrossRef]
- Yang, P.; Li, Q.-J.; Feng, Y.; Zhang, Y.; Markowitz, G.J.; Ning, S.; Deng, Y.; Zhao, J.; Jiang, S.; Yuan, Y.; et al. TGF-β-miR-34a-CCL22 Signaling-Induced Treg Cell Recruitment Promotes Venous Metastases of HBV-Positive Hepatocellular Carcinoma. Cancer Cell 2012, 22, 291–303. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, H.; Jin, Z.-J.; Ma, D.; Yuen, S.; Jing, X.-Q.; Shi, M.-M.; Shen, B.-Y.; Peng, C.-H.; Zhao, R.; et al. Up-regulation of chemokine receptor CCR4 is associated with Human Hepatocellular Carcinoma malignant behavior. Sci. Rep. 2017, 7, 12362. [Google Scholar] [CrossRef]
- Gao, Y.; You, M.; Fu, J.; Tian, M.; Zhong, X.; Du, C.; Zhu, Z.; Liu, J.; Markowitz, G.J.; Wang, F.-S.; et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J. Hepatol. 2021, 76, 148–159. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Beyer, M.; Kochanek, M.; Darabi, K.; Popov, A.; Jensen, M.; Endl, E.; Knolle, P.A.; Thomas, R.K.; von Bergwelt-Baildon, M.; Debey, S.; et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005, 106, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wei, X.; Li, L.; Wu, X.; Yan, J.; Yang, H.; Song, F. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-β pathway in human non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017, 488, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Karasaki, T.; Qiang, G.; Anraku, M.; Sun, Y.; Shinozaki-Ushiku, A.; Sato, E.; Kashiwabara, K.; Nagayama, K.; Nitadori, J.-I.; Sato, M.; et al. High CCR4 expression in the tumor microenvironment is a poor prognostic indicator in lung adenocarcinoma. J. Thorac. Dis. 2018, 10, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Rexiati, M.; Yang, Y.; Wang, W.-G.; Azhati, B.; Saimaiti, W.; Wang, Y.-J. Expression of chemokine receptor 4 was associated with poor survival in renal cell carcinoma. Med. Oncol. 2014, 31, 882. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Xia, R.; Wei, Y.; Wei, X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021, 54, e13115. [Google Scholar] [CrossRef]
- Ling, Z.; Li, W.; Hu, J.; Li, Y.; Deng, M.; Zhang, S.; Ren, X.; Wu, T.; Xia, J.; Bin Cheng, B.; et al. Targeting CCL2-CCR4 axis suppress cell migration of head and neck squamous cell carcinoma. Cell Death Dis. 2022, 13, 158. [Google Scholar] [CrossRef]
- Thomas, J.K.; Mir, H.; Kapur, N.; Bae, S.; Singh, S. CC chemokines are differentially expressed in Breast Cancer and are associated with disparity in overall survival. Sci. Rep. 2019, 9, 4014. [Google Scholar] [CrossRef]
- Zhu, F.; Li, X.; Chen, S.; Zeng, Q.; Zhao, Y.; Luo, F. Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med. Oncol. 2016, 33, 17. [Google Scholar] [CrossRef]
- Al-Haidari, A.A.; Syk, I.; Jirström, K.; Thorlacius, H. CCR4 mediates CCL17 (TARC)-induced migration of human colon cancer cells via RhoA/Rho-kinase signaling. Int. J. Color. Dis. 2013, 28, 1479–1487. [Google Scholar] [CrossRef]
- Silva, J.R.; Iftinca, M.; Gomes, F.I.F.; Segal, J.P.; Smith, O.M.A.; Bannerman, C.A.; Mendes, A.S.; Defaye, M.; Robinson, M.E.C.; Gilron, I.; et al. Skin-resident dendritic cells mediate postoperative pain via CCR4 on sensory neurons. Proc. Natl. Acad. Sci. USA 2022, 119. [Google Scholar] [CrossRef]
- Williams, T.C.; Jackson, D.J.; Maltby, S.; Walton, R.P.; Ching, Y.-M.; Glanville, N.; Singanayagam, A.; Brewins, J.J.; Clarke, D.; Hirsman, A.G.; et al. Rhinovirus-induced CCL17 and CCL22 in Asthma Exacerbations and Differential Regulation by STAT6. Am. J. Respir. Cell Mol. Biol. 2021, 64, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xu, Y.; Xu, H.; Ren, J.; Meng, T.; Ni, Y.; Zhu, Q.; Zhang, W.-B.; Pan, Y.-B.; Jin, J.; et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics 2021, 11, 3839–3852. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Singh, A.; Sharma, K.C.; Saxena, S. Gene Expression Profiling of Chemokines and Their Receptors in Low and High Grade Astrocytoma. Asian Pac. J. Cancer Prev. 2017, 18, 1307–1313. [Google Scholar] [PubMed]
- Mizukami, Y.; Kono, K.; Kawaguchi, Y.; Akaike, H.; Kamimura, K.; Sugai, H.; Fujii, H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer 2008, 122, 2286–2293. [Google Scholar] [CrossRef]
- Panina-Bordignon, P.; Papi, A.; Mariani, M.; Di Lucia, P.; Casoni, G.L.; Bellettato, C.; Buonsanti, C.; Miotto, D.; Mapp, C.; Villa, A.; et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Investig. 2001, 107, 1357–1364. [Google Scholar] [CrossRef]
- Sebastiani, S.; Albanesi, C.; Nasorri, F.; Girolomoni, G.; Cavani, A. Nickel-Specific CD4+ and CD8+ T Cells Display Distinct Migratory Responses to Chemokines Produced During Allergic Contact Dermatitis. J. Investig. Dermatol. 2002, 118, 1052–1058. [Google Scholar] [CrossRef]
- Katoh, S.; Fukushima, K.; Matsumoto, N.; Matsumoto, K.; Abe, K.; Onai, N.; Matsushima, K.; Matsukura, S. Accumulation of CCR4-expressing CD4+ T cells and high concentration of its ligands (TARC and MDC) in bronchoalveolar lavage fluid of patients with eosinophilic pneumonia. Allergy 2003, 58, 518–523. [Google Scholar] [CrossRef]
- Furudate, S.; Fujimura, T.; Kambayashi, Y.; Kakizaki, A.; Hidaka, T.; Aiba, S. Immunomodulatory Effect of Imiquimod Through CCL22 Produced by Tumor-associated Macrophages in B16F10 Melanomas. Anticancer Res. 2017, 37, 3461–3471. [Google Scholar] [CrossRef]
- Tanita, K.; Fujimura, T.; Sato, Y.; Lyu, C.; Kambayashi, Y.; Ogata, D.; Fukushima, S.; Miyashita, A.; Nakajima, H.; Nakamura, M.; et al. Bexarotene Reduces Production of CCL22 From Tumor-Associated Macrophages in Cutaneous T-Cell Lymphoma. Front. Oncol. 2019, 9, 907. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, X.; Li, N.; Du, C.; Yang, B.; Qin, W.; Fu, J.; Markowitz, G.J.; Wang, H.; Ma, J.; et al. CCL22 signaling contributes to sorafenib resistance in hepatitis B virus-associated hepatocellular carcinoma. Pharmacol. Res. 2020, 157, 104800. [Google Scholar] [CrossRef] [PubMed]
- Tham, S.M.; Ng, K.H.; Pook, S.H.; Esuvaranathan, K.; Mahendran, R. Tumor and Microenvironment Modification during Progression of Murine Orthotopic Bladder Cancer. Clin. Dev. Immunol. 2011, 2011, 865684. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-Y.; Chen, R.; Fang, Z.-Z.; Chen, B.; Wang, Z.-H.; Wang, X.-Y. Increased local expressions of CX3CL1 and CCL2 are related to clinical severity in lumbar disk herniation patients with sciatic pain. J. Pain Res. 2017, 10, 157–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mordillo-Mateos, L.; Sánchez-Ramos, A.; Coperchini, F.; Bustos-Guadamillas, I.; Alonso-Bonilla, C.; Vargas-Baquero, E.; Rodriguez-Carrión, I.; Rotondi, M.; Oliviero, A. Development of chronic pain in males with traumatic spinal cord injury: Role of circulating levels of the chemokines CCL2 and CXCL10 in subacute stage. Spinal Cord 2019, 57, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Jiang, Y.; Liu, J.; Lin, Z.; Jin, Y. Study on plasma CC chemokine ligand 2 level and its promoter region 2518A/G polymorphism in MS patients. Eur. J. Inflamm. 2020, 18. [Google Scholar] [CrossRef]
- Flores-Villanueva, P.; Ruiz-Morales, J.A.; Song, C.-H.; Flores, L.M.; Jo, E.-K.; Montaño, M.; Barnes, P.F.; Selman, M.; Granados, J. A functional promoter polymorphism in monocyte chemoattractant protein–1 is associated with increased susceptibility to pulmonary tuberculosis. J. Exp. Med. 2005, 202, 1649–1658. [Google Scholar] [CrossRef]
- Tucci, M.; Barnes, E.V.; Sobel, E.S.; Croker, B.P.; Segal, M.S.; Reeves, W.H.; Richards, H.B. Strong association of a functional polymorphism in the monocyte chemoattractant protein 1 promoter gene with lupus nephritis. Arthritis Rheum. 2004, 50, 1842–1849. [Google Scholar] [CrossRef]
- Gonzalez, E.; Rovin, B.H.; Sen, L.; Cooke, G.; Dhanda, R.; Mummidi, S.; Kulkarni, H.; Bamshad, M.J.; Telles, V.; Anderson, S.A.; et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc. Natl. Acad. Sci. USA 2002, 99, 13795–13800. [Google Scholar] [CrossRef]
- Tanuma, N.; Sakuma, H.; Sasaki, A.; Matsumoto, Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006, 112, 195–204. [Google Scholar] [CrossRef]
- Granata, F.; Frattini, A.; Loffredo, S.; Del Prete, A.; Sozzani, S.; Marone, G.; Triggiani, M. Signaling events involved in cytokine and chemokine production induced by secretory phospholipase A2 in human lung macrophages. Eur. J. Immunol. 2006, 36, 1938–1950. [Google Scholar] [CrossRef]
- Spoettl, T.; Hausmann, M.; Herlyn, M.; Gunckel, M.; Dirmeier, A.; Falk, W.; Herfarth, H.; Schoelmerich, J.; Rogler, G. Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes. Clin. Exp. Immunol. 2006, 145, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.; Wong, C.K.; Lam, C.W.K. Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: Involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin. Exp. Immunol. 2006, 145, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Rantapaa-Dahlqvist, S.; Boman, K.; Tarkowski, A.; Hallmans, G. Up regulation of monocyte chemoattractant protein-1 expression in anti-citrulline antibody and immunoglobulin M rheumatoid factor positive subjects precedes onset of inflammatory response and development of overt rheumatoid arthritis. Ann. Rheum. Dis. 2006, 66, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Kong, H.; Zeng, Y.; Hao, M.; Yu, T.; Peng, J.; Xu, Z.; Chen, J.; Shi, H. Monocyte chemotactic protein-1 expression as a prognosic biomarker in patients with solid tumor: A meta analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 3876–3886. [Google Scholar] [PubMed]
- Wang, K.; Gu, Y.; Liao, Y.; Bang, S.; Donnelly, C.; Chen, O.; Tao, X.; Mirando, A.J.; Hilton, M.J.; Ji, R.-R. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J. Clin. Investig. 2020, 130, 3603–3620. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M.; et al. Overexpression of Monocyte Chemoattractant Protein-1 in Adipose Tissues Causes Macrophage Recruitment and Insulin Resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef]
- Gonzalo, J.-A.; Lloyd, C.; Wen, D.; Albar, J.P.; Wells, T.; Proudfoot, A.; Martinez-A, C.; Dorf, M.; Bjerke, T.; Coyle, A.J.; et al. The Coordinated Action of CC Chemokines in the Lung Orchestrates Allergic Inflammation and Airway Hyperresponsiveness. J. Exp. Med. 1998, 188, 157–167. [Google Scholar] [CrossRef]
- Mahad, D.J.; Ransohoff, R.M. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Semin. Immunol. 2003, 15, 23–32. [Google Scholar] [CrossRef]
- Sato, K.; Kuratsu, J.-I.; Takeshima, H.; Yoshimura, T.; Ushio, Y. Expression of monocyte chemoattractant protein-1 in meningioma. J. Neurosurg. 1995, 82, 874–878. [Google Scholar] [CrossRef]
- Connolly, K.A.; Belt, B.A.; Figueroa, N.M.; Murthy, A.; Patel, A.; Kim, M.; Lord, E.M.; Linehan, D.C.; Gerber, S.A. Increasing the efficacy of radiotherapy by modulating the CCR2/CCR5 chemokine axes. Oncotarget 2016, 7, 86522–86535. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Nelson, D.; Tian, S.; Mulvey, E.; Patel, B.; Conti, I.; Jaen, J.; Rollins, B.J. CCL2/CCR2 Regulates the Tumor Microenvironment in HER-2/neu-Driven Mammary Carcinomas in Mice. PLoS ONE 2016, 11, e0165595. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, K.J.; Bailey, M.T.; Gross, A.C.; Sumner, L.A.; Voorhees, J.L.; Crouser, N.; Curry, J.M.; Wang, Y.; DeVries, A.C.; Marsh, C.B.; et al. Stress-induced Norepinephrine Downregulates CCL2 in Macrophages to Suppress Tumor Growth in a Model of Malignant Melanoma. Cancer Prev. Res. 2020, 13, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-Y.; Sun, K.-H.; Chen, S.-Y.; Wang, H.-H.; Lee, M.-Y.; Tsou, Y.-C.; Jwo, S.-C.; Sun, G.-H.; Tang, S.-J. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine 2012, 59, 423–432. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogacka, J.; Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Mika, J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. Int. J. Mol. Sci. 2022, 23, 15638. https://doi.org/10.3390/ijms232415638
Bogacka J, Pawlik K, Ciapała K, Ciechanowska A, Mika J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. International Journal of Molecular Sciences. 2022; 23(24):15638. https://doi.org/10.3390/ijms232415638
Chicago/Turabian StyleBogacka, Joanna, Katarzyna Pawlik, Katarzyna Ciapała, Agata Ciechanowska, and Joanna Mika. 2022. "CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy" International Journal of Molecular Sciences 23, no. 24: 15638. https://doi.org/10.3390/ijms232415638
APA StyleBogacka, J., Pawlik, K., Ciapała, K., Ciechanowska, A., & Mika, J. (2022). CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. International Journal of Molecular Sciences, 23(24), 15638. https://doi.org/10.3390/ijms232415638






