The Phosphinate Group in the Formation of 2D Coordination Polymer with Sm(III) Nodes: X-ray Structural, Electrochemical and Mössbauer Study
Abstract
1. Introduction
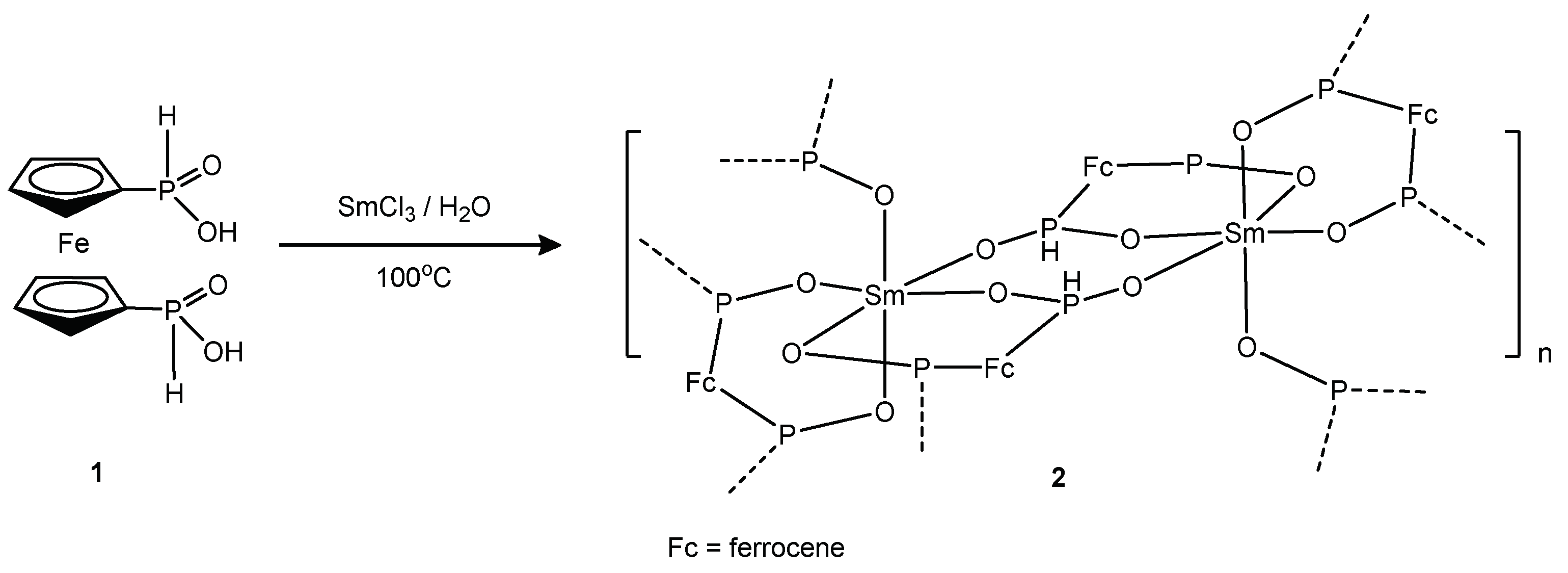
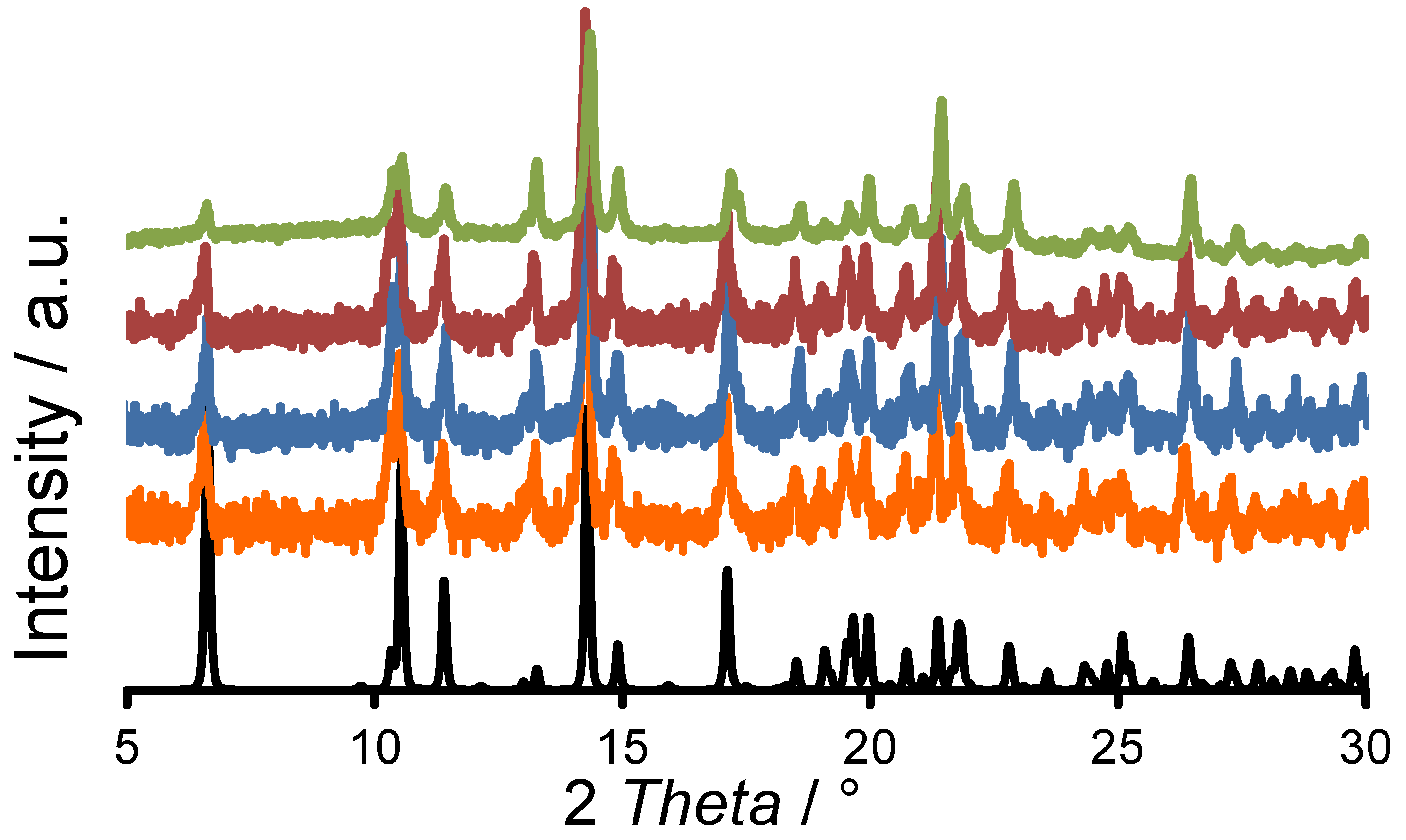
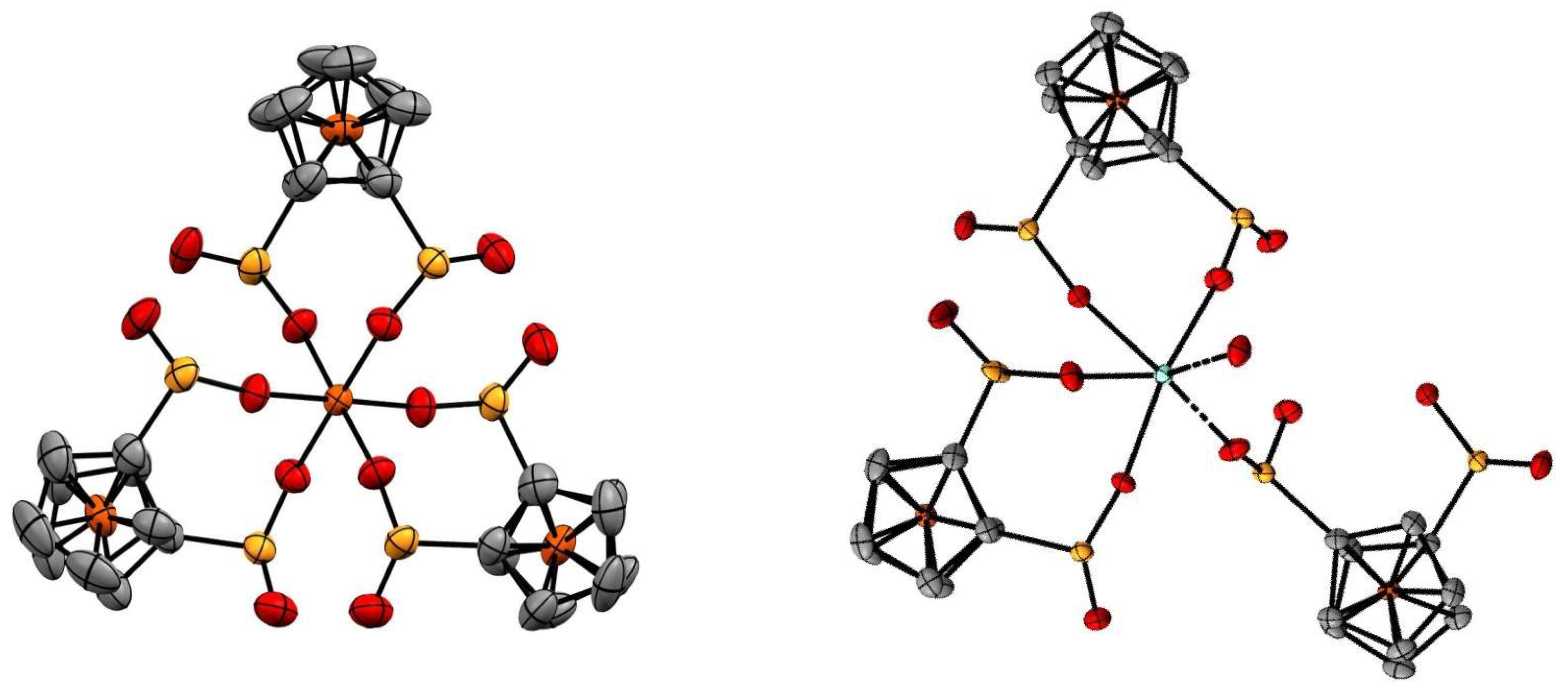
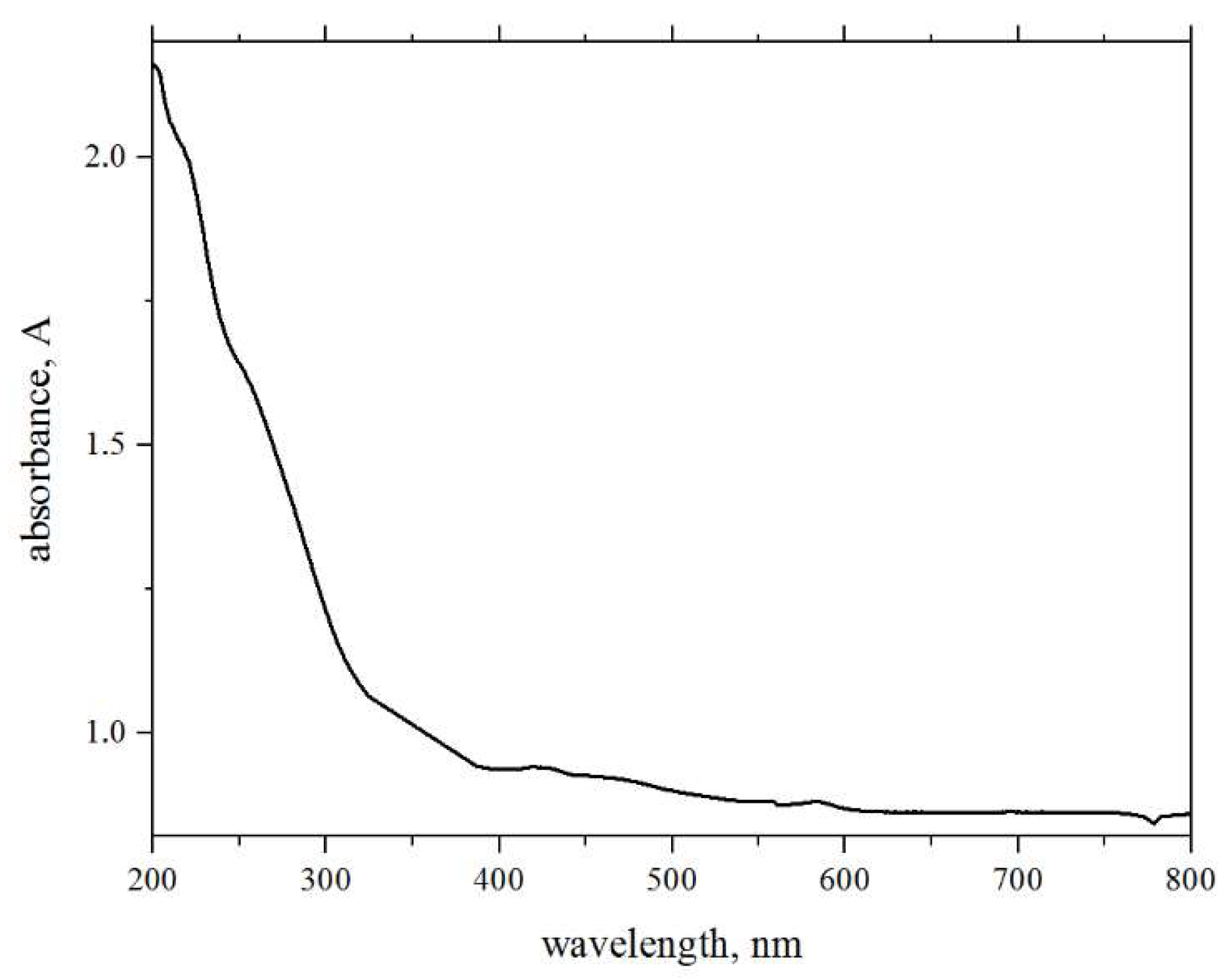
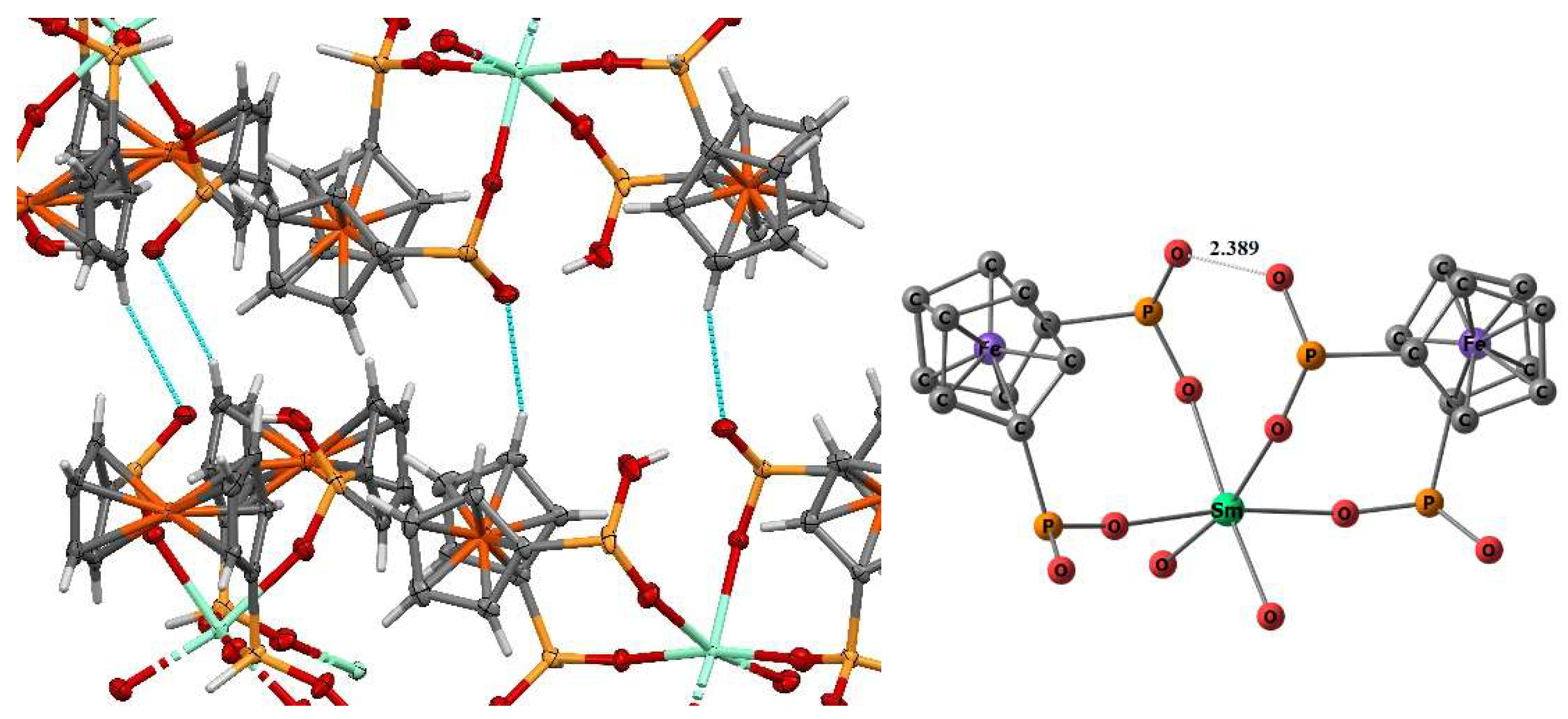
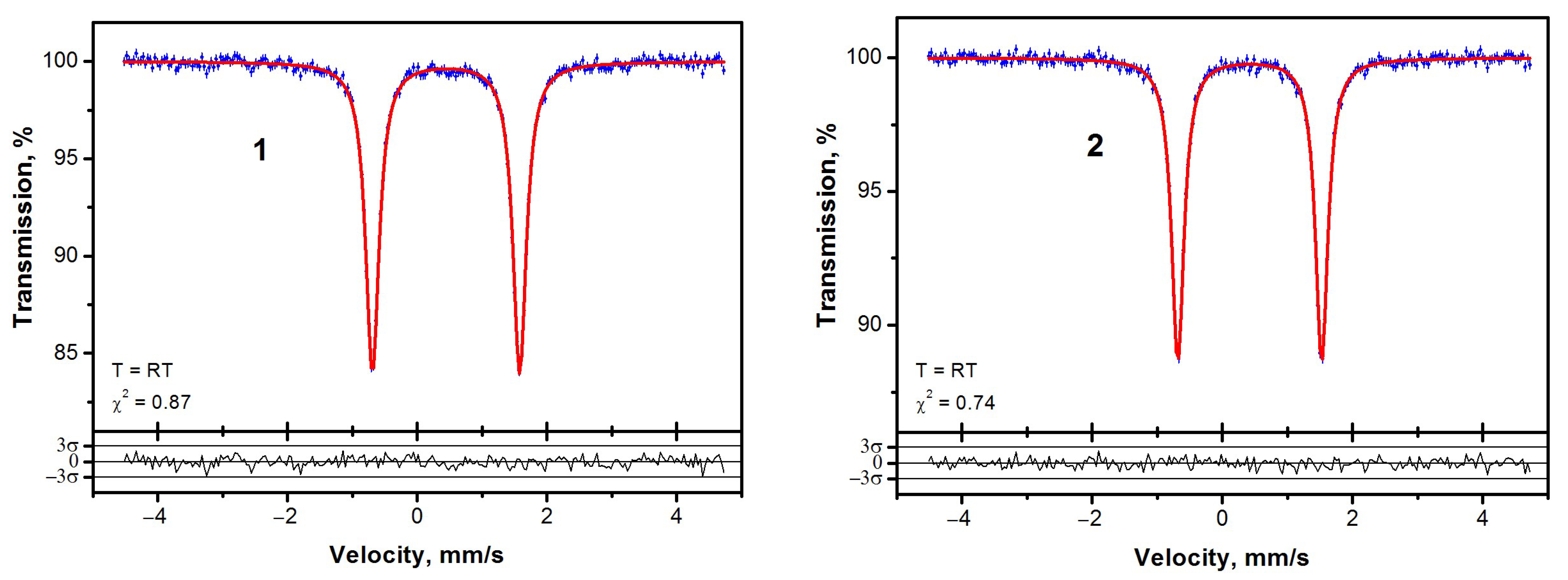
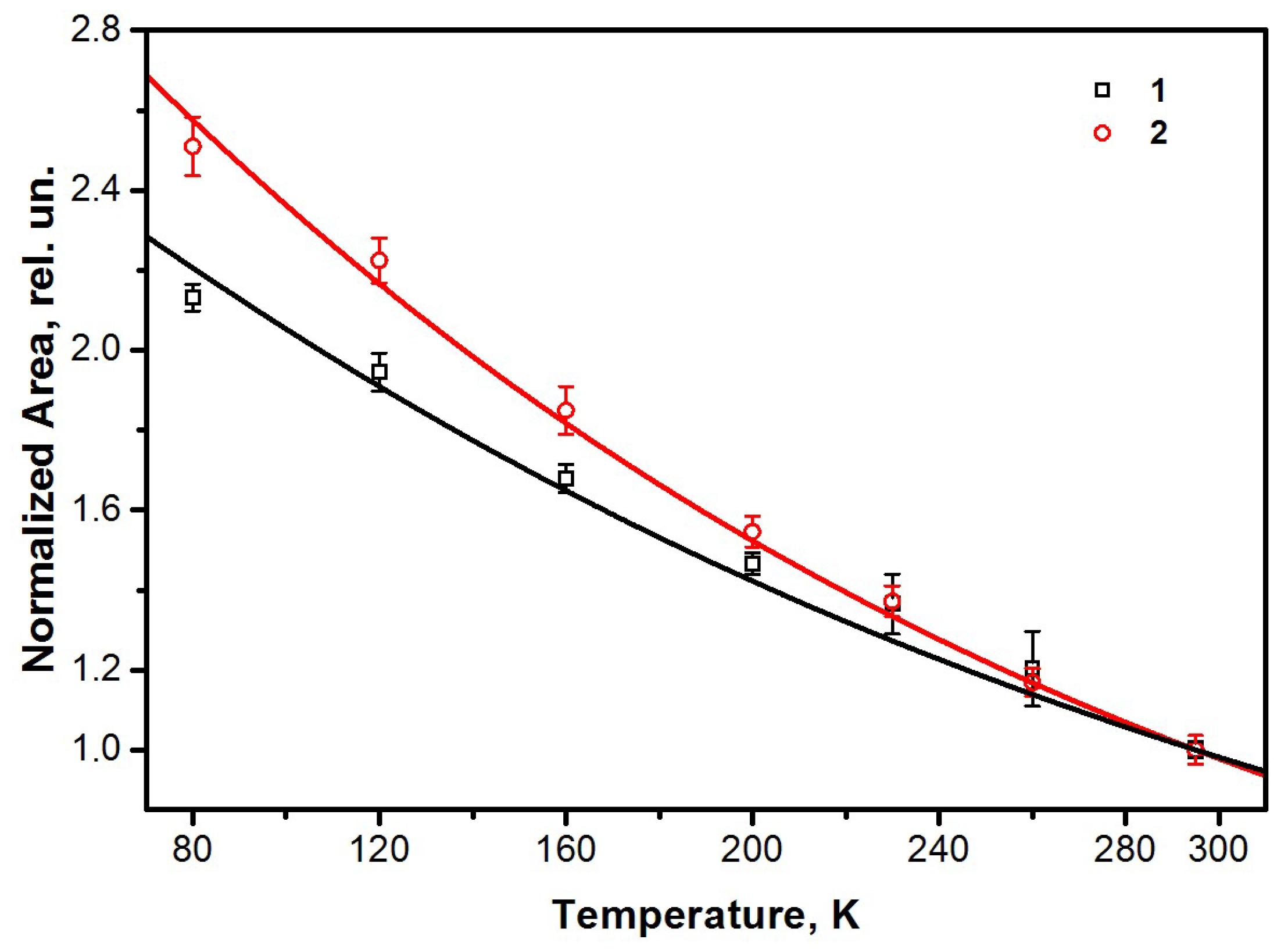
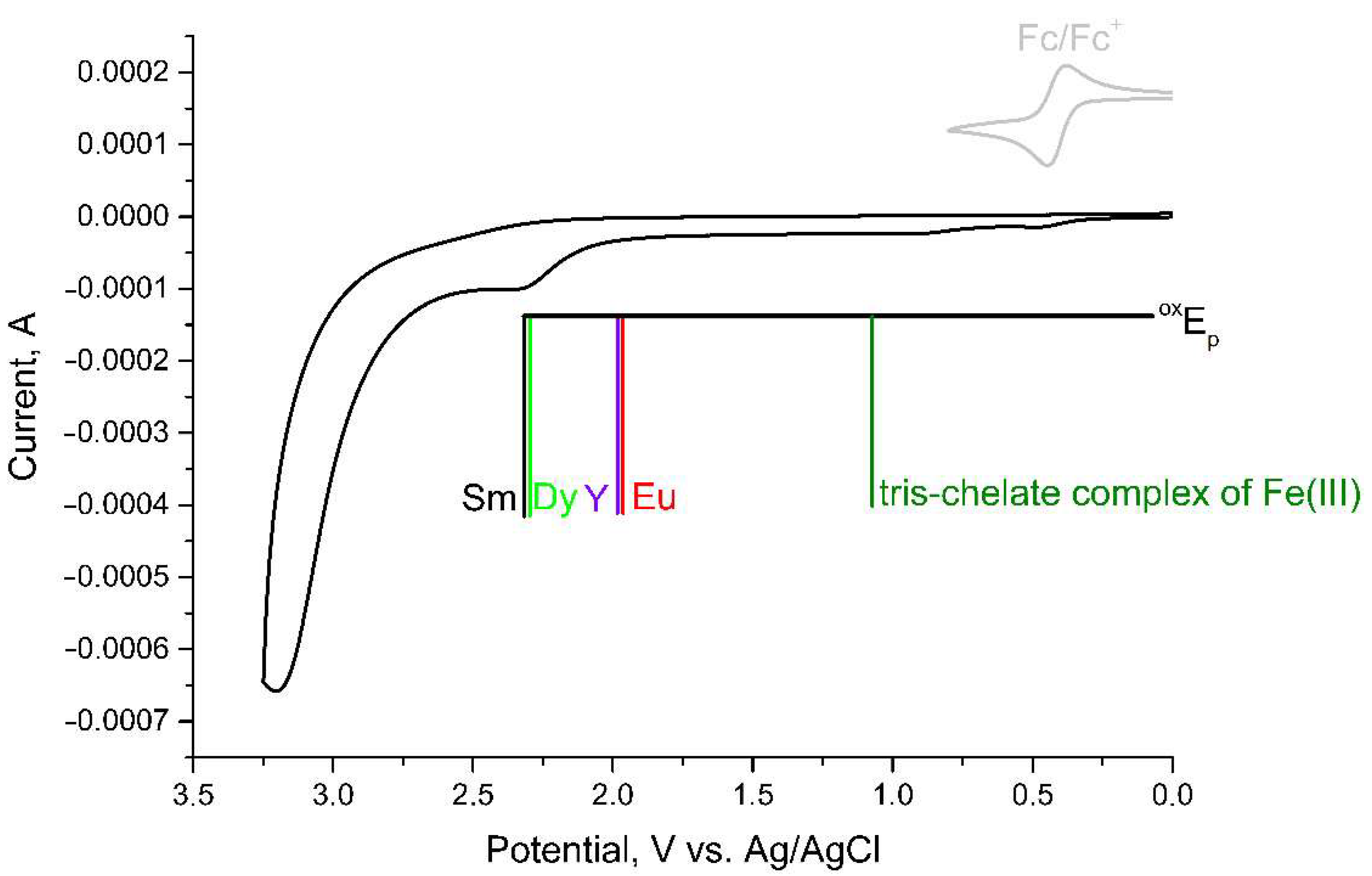
2. Results
| Sample | Center Shift, mm/s | Quadrupole Splitting, mm/s | Lamb–Mössbauer Factor |
|---|---|---|---|
| 1 | 0.444 (5) | 2.27 (1) | 0.33 |
| 2 | 0.421 (5) | 2.21 (1) | 0.27 |
| Ferrocene | 0.44 [32] | 2.40 [32] | 0.07 [33] |
3. Materials and Methods
3.1. General
3.2. X-ray Diffraction Analysis
3.3. Mössbauer Effect Studies
3.4. Electrochemical Measurements
3.5. ESR Measurements
3.6. Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Yuan, K.; Zhang, Y. Advances and prospects of rare earth metal-organic frameworks in catalytic applications. J. Rare Earths 2020, 38, 801–818. [Google Scholar] [CrossRef]
- Paderni, D.; Giorgi, L.; Fusi, V.; Formica, M.; Ambrosi, G.; Micheloni, M. Chemical sensors for rare earth metal ions. Coord. Chem. Rev. 2021, 429, 213639. [Google Scholar] [CrossRef]
- Guo, Z.; Huo, R.; Tan, Y.Q.; Blair, V.; Deacon, G.B.; Junk, P.C. Syntheses of reactive rare earth complexes by redox transmetallation/protolysis reactions—A simple and convenient method. Coord. Chem. Rev. 2020, 415, 213232. [Google Scholar] [CrossRef]
- Bernot, K.; Daiguebonne, C.; Calvez, G.; Suffren, Y.; Guillou, O. A Journey in Lanthanide Coordination Chemistry: From Evaporable Dimers to Magnetic Materials and Luminescent Devices. Acc. Chem. Res. 2021, 54, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Saraci, F.; Quezada-Novoa, V.; Donnarumma, P.R.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Cardinaels, T.; Lunstroot, K.; Van Hecke, K.; Van Meervelt, L.; Görller-Walrand, C.; Binnemans, K.; Nockemann, P. Rare-earth complexes of ferrocene-containing ligands: Visible-light excitable luminescent materials. Inorg. Chem. 2007, 46, 5302–5309. [Google Scholar] [CrossRef]
- Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Rouzières, M.; Kiskin, M.A.; Clérac, R.; Novotortsev, V.M. Synthesis, structure, and physical properties of new rare earth ferrocenoylacetonates. Dalton Trans. 2016, 45, 6405–6417. [Google Scholar] [CrossRef]
- Hu, M.-L.; Abbasi-Azad, M.; Habibi, B.; Rouhani, F.; Moghanni-Bavil-Olyaei, H.; Liu, K.-G.; Morsali, A. Electrochemical Applications of Ferrocene-Based Coordination Polymers. ChemPlusChem 2020, 85, 2397–2418. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, H.; Wang, L.; Liu, X.; Lin, T.; Haq, F.; Vatsadze, S.Z.; Lemenovskiy, D.A. Ferrocene-contained metal organic frameworks: From synthesis to applications. Coord. Chem. Rev. 2021, 430, 213737. [Google Scholar] [CrossRef]
- Pal, A.; Bhatta, S.R.; Thakur, A. Recent advances in the development of ferrocene based electroactive small molecules for cation recognition: A comprehensive review of the years 2010–2020. Coord. Chem. Rev. 2021, 431, 213685. [Google Scholar] [CrossRef]
- Horikoshi, R.; Mochida, T. Ferrocene-Containing Coordination Polymers: Ligand Design and Assembled Structures. Eur. J. Inorg. Chem. 2010, 2010, 5355–5371. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Li, L.; Hou, H.; Fan, Y. Secondary ligand-directed assembly of CdII coordination architectures: From 0D to 3D complexes based on ferrocenyl carboxylate. Cryst. Growth Des. 2010, 10, 2490–2500. [Google Scholar] [CrossRef]
- Shearan, S.J.; Stock, N.; Emmerling, F.; Demel, J.; Wright, P.A.; Demadis, K.D.; Vassaki, M.; Costantino, F.; Vivani, R.; Sallard, S.; et al. New directions in metal phosphonate and phosphinate chemistry. Crystals 2019, 9, 270. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Shekurov, R.; Miluykov, V.; Gilmanova, L.; Kataeva, O.; Yamaleeva, Z.; Gerasimova, T.; Ermolaev, V.; Gubaidullin, A.; Laskin, A.; et al. Excellent supercapacitor and sensor performance of robust cobalt phosphinate ferrocenyl organic framework materials achieved by intrinsic redox and structure properties. Dalton Trans. 2019, 48, 16986–16992. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Shekurov, R.; Zagidullin, A.; Gerasimova, T.; Ivshin, K.; Kataeva, O.; Miluykov, V. Zwitterionic form of Ugi amine H-phosphinic acid: Structure and electrochemical properties. Electrochem. Commun. 2021, 126, 107019. [Google Scholar] [CrossRef]
- Gilmanova, L.; Shekurov, R.; Khrizanforov, M.; Ivshin, K.; Kataeva, O.; Bon, V.; Senkovska, I.; Kaskel, S.; Miluykov, V. First Example of Ugi’s Amine as a Platform for Construction of Chiral Coordination Polymers: Synthesis and Properties. New J. Chem. 2021, 45, 2791–2794. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforov, M.; Islamov, D.; Gerasimova, T.; Zagidullin, A.; Budnikova, Y.; Miluykov, V. Synthesis, crystal structure and electrochemical properties of poly(cadmium 1,10-ferrocenediyl-bis(H-phosphinate)). J. Organomet. Chem. 2020, 914, 121233. [Google Scholar] [CrossRef]
- Khrizanforova, V.; Shekurov, R.; Miluykov, V.; Khrizanforov, M.; Bon, V.; Kaskel, S.; Gubaidullin, A.; Sinyashin, O.; Budnikova, Y. 3D Ni and Co Redox-Active Metal-Organic Frameworks Based on Ferrocenyl Diphosphinate and 4,4′-Bipyridine Ligands as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Dalton Trans. 2020, 49, 2794–2802. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforova, V.; Gilmanova, L.; Khrizanforov, M.; Miluykov, V.; Kataeva, O.; Yamaleeva, Z.; Burganov, T.; Gerasimova, T.; Khamatgalimov, A.; et al. Zn and Co Redox Active Coordination Polymers Based on Ferrocene-containing Diphosphinate Ligand as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Dalton Trans. 2019, 48, 3601–3609. [Google Scholar] [CrossRef]
- Shekurov, R.; Khrizanforov, M.; Ivshin, K.; Miluykov, V.; Budnikova, Y.; Kataeva, O. Supramolecular architecture of diammonium ferrocene-1,1′-diyldi(methylphosphinate). J. Organomet. Chem. 2019, 904, 121004. [Google Scholar] [CrossRef]
- Kresinski, R.A.; Platt, A.W.G.; Seddon, J.A. Lanthanide complexes with O2PH2, O2P(CH2Cl)2 and O2P(CH2OH)2 ligands: A solid-state tubular micelle. CrystEngComm 2000, 2, 177–182. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Islamov, D.R.; Krivolapov, D.B.; Miluykov, V.A.; Kataeva, O.N.; Gerasimova, T.P.; Katsyuba, S.A.; Sinyashin, O.G. Synthesis and structure of the iron (III) tris-chelate complex based on 1,1′-ferrocenediylbis(phenylphosphinic acid). Russ. Chem. Bull. 2015, 64, 1819–1822. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Arkhipova, D.M.; Shekurov, R.P.; Gerasimova, T.P.; Ermolaev, V.V.; Islamov, D.R.; Miluykov, V.A.; Kataeva, O.N.; Khrizanforova, V.V.; Sinyashin, O.G.; et al. Novel paste electrodes based on phosphonium salt room temperature ionic liquids for studying the redox properties of insoluble compounds. J. Solid State Electrochem. 2015, 19, 2883–2890. [Google Scholar] [CrossRef]
- Khrizanforova, V.V.; Shekurov, R.P.; Nizameev, I.R.; Gerasimova, T.P.; Khrizanforov, M.N.; Bezkishko, I.A.; Miluykov, V.A.; Budnikova, Y.H. Aerogel based on nanoporous aluminium ferrocenyl diphosphinate metal-organic framework. Inorgan. Chim. Acta 2021, 518, 120240. [Google Scholar] [CrossRef]
- Shekurov, R.P.; Gilmanova, L.H.; Miluykov, V.A. New porous Fe(III)-based ferrocene-containing diphosphinate. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1007–1009. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Shekurov, R.P.; Gilmanova, L.H.; Gerasimova, T.P.; Miluykov, V.A.; Budnikova, Y.H. Electrochemical properties of poly ([Eu or Dy or Y] 1,1′-ferrocenediyl-bis(H-phosphinates)). Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 1010–1012. [Google Scholar] [CrossRef]
- Gu, X.-Y.; Han, X.-Z.; Yao, Y.-M.; Zhang, Y.; Shen, Q. Synthesis and characterization of lanthanide complexes bearing a ferrocene-containing N-aryloxo-β-ketoiminate ligand. J. Organomet. Chem. 2010, 695, 2726–2731. [Google Scholar] [CrossRef]
- Shekurov, R.; Miluykov, V.; Kataeva, O.; Krivolapov, D.; Sinyashin, O.; Gerasimova, T.; Katsyuba, S.; Kovalenko, V.; Krupskaya, Y.; Kataev, V.; et al. Reversible water-induced structural and magnetic transformations and selective water adsorption properties of poly(manganese 1,1′-ferrocenediyl-bis(H-phosphinate)). Cryst. Growth Des. 2016, 16, 5084–5090. [Google Scholar] [CrossRef]
- Gerasimova, T.; Shekurov, R.; Gilmanova, L.; Laskin, A.; Katsyuba, S.; Kovalenko, V.; Khrizanforov, M.; Milyukov, V.; Sinyashin, O. IR and UV study of reversible water-induced structural transformations of poly(manganese 1,1′-ferrocenediyl-bis(H-phosphinate)) and poly(cobalt 1,1′-ferrocenediyl-bis(H-phosphinate)). J. Mol. Struct. 2018, 1166, 237–242. [Google Scholar] [CrossRef]
- Collins, R.L. Mössbauer Studies of Iron Organometallic Complexes. IV. Sign of the Electric-Field Gradient in Ferrocene. J. Chem. Phys. 1965, 42, 1072–1080. [Google Scholar] [CrossRef]
- Goldanskii, V.I.; Herber, R.H. Chemical Applications of Mössbauer Spectroscopy; Academic Press: Cambridge, MA, USA, 1968. [Google Scholar]
- Stukan, R.A.; Gubin, S.P.; Nesmeyanov, A.N.; Gol’danskii, V.I.; Makarov, E.F. A Mössbauer study of some ferrocene derivatives. Theor. Exp. Chem. 1966, 2, 581–584. [Google Scholar] [CrossRef]
- Kohn, V.G.; Chumakov, A.I.; Rüffer, R. Combined nuclear-molecular resonance inelastic scattering of X-rays. Phys. Rev. Lett. 2004, 92, 243001. [Google Scholar] [CrossRef] [PubMed]
- Wagener, W.; Hillberg, M.; Feyerherm, R.; Stieler, W.; Litterst, F.; Pohlmann, T.; Nuyken, O. Mixed valence behaviour in polymers containing ferrocene. J. Phys. Condens. Matter 1994, 6, L391. [Google Scholar] [CrossRef]
- Dietzsch, W.; Duffy, N.V.; Gelerinter, E.; Sinn, E. Powder EPR of tris(diethylthioselenocarbamato)-and tris(diethyldiselenocarbamato) iron(III) diluted in the analogous cobalt(III) and indium(III) complex matrixes. Inorg. Chem. 1989, 28, 3079–3081. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Clentsmith, G.K.; Gibson, V.C.; Goodgame, D.M.; McTavish, S.J.; Pankhurst, Q.A. The nature of the active site in bis(imino) pyridine iron ethylene polymerisation catalysts. Catal. Commun. 2002, 3, 207–211. [Google Scholar] [CrossRef]
- Bruker. APEX3 Crystallography Software Suite; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Bruker. SAINT Crystallography Software Suite; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An application for Mössbauer spectra modeling and fitting. AIP Conf. Proc. 2012, 1489, 178–185. [Google Scholar]
- Shekurov, R.; Miluykov, V.; Islamov, D.; Krivolapov, D.; Kataeva, O.; Gerasimova, T.; Katsyuba, S.; Nasybullina, G.; Yanilkin, V.; Sinyashin, O. Synthesis and structure of ferrocenylphosphinic acids. J. Organomet. Chem. 2014, 766, 40–48. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekurov, R.P.; Khrizanforov, M.N.; Zagidullin, A.A.; Zinnatullin, A.L.; Kholin, K.V.; Ivshin, K.A.; Gerasimova, T.P.; Sirazieva, A.R.; Kataeva, O.N.; Vagizov, F.G.; et al. The Phosphinate Group in the Formation of 2D Coordination Polymer with Sm(III) Nodes: X-ray Structural, Electrochemical and Mössbauer Study. Int. J. Mol. Sci. 2022, 23, 15569. https://doi.org/10.3390/ijms232415569
Shekurov RP, Khrizanforov MN, Zagidullin AA, Zinnatullin AL, Kholin KV, Ivshin KA, Gerasimova TP, Sirazieva AR, Kataeva ON, Vagizov FG, et al. The Phosphinate Group in the Formation of 2D Coordination Polymer with Sm(III) Nodes: X-ray Structural, Electrochemical and Mössbauer Study. International Journal of Molecular Sciences. 2022; 23(24):15569. https://doi.org/10.3390/ijms232415569
Chicago/Turabian StyleShekurov, Ruslan P., Mikhail N. Khrizanforov, Almaz A. Zagidullin, Almaz L. Zinnatullin, Kirill V. Kholin, Kamil A. Ivshin, Tatiana P. Gerasimova, Aisylu R. Sirazieva, Olga N. Kataeva, Farit G. Vagizov, and et al. 2022. "The Phosphinate Group in the Formation of 2D Coordination Polymer with Sm(III) Nodes: X-ray Structural, Electrochemical and Mössbauer Study" International Journal of Molecular Sciences 23, no. 24: 15569. https://doi.org/10.3390/ijms232415569
APA StyleShekurov, R. P., Khrizanforov, M. N., Zagidullin, A. A., Zinnatullin, A. L., Kholin, K. V., Ivshin, K. A., Gerasimova, T. P., Sirazieva, A. R., Kataeva, O. N., Vagizov, F. G., & Miluykov, V. A. (2022). The Phosphinate Group in the Formation of 2D Coordination Polymer with Sm(III) Nodes: X-ray Structural, Electrochemical and Mössbauer Study. International Journal of Molecular Sciences, 23(24), 15569. https://doi.org/10.3390/ijms232415569









