Klotho Protein Decreases MMP-Mediated Degradation of Contractile Proteins during Ischaemia/Reperfusion Injury to the Cardiomyocytes
Abstract
1. Introduction
2. Results
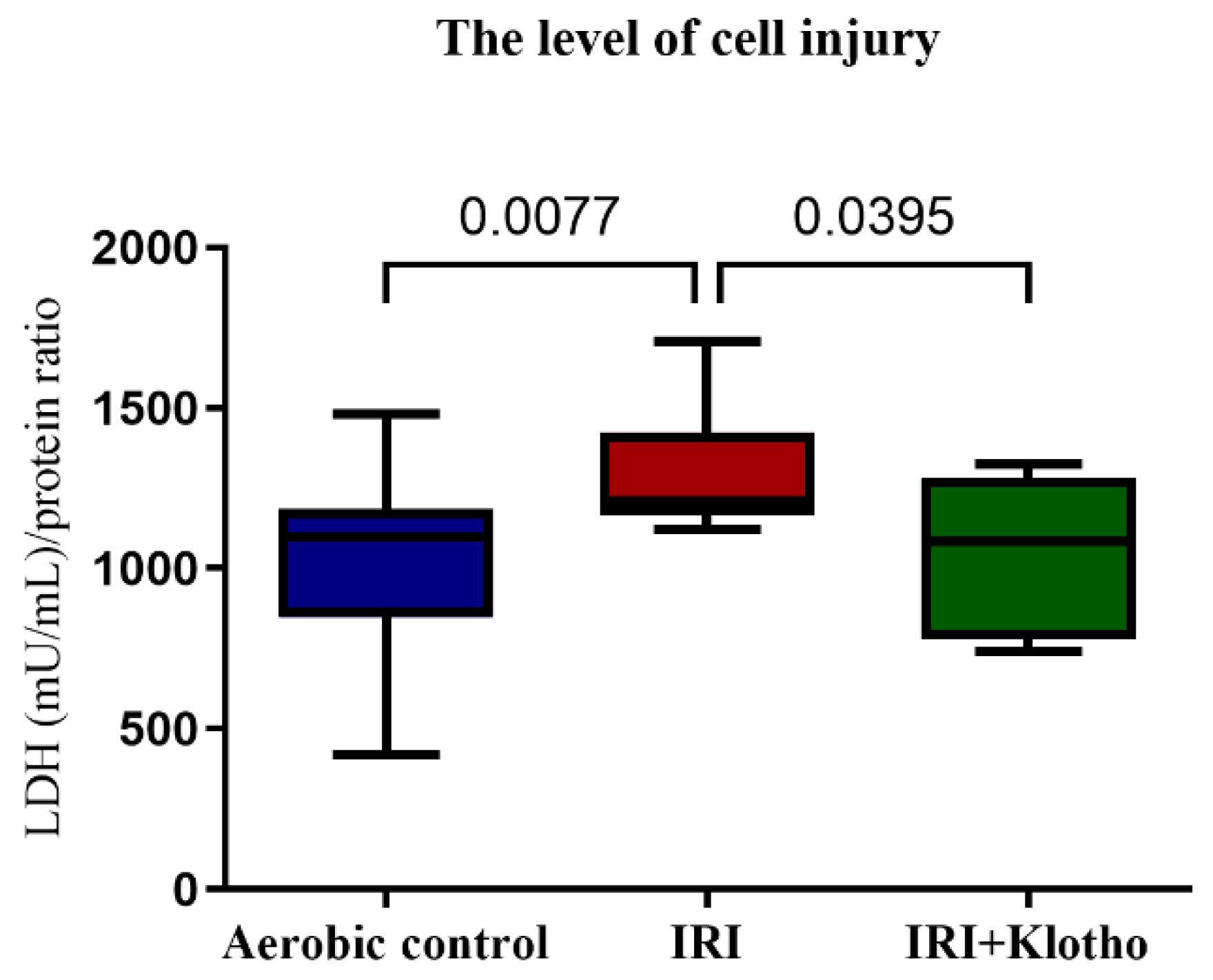
2.1. The Level of Injury to Cardiac Cells
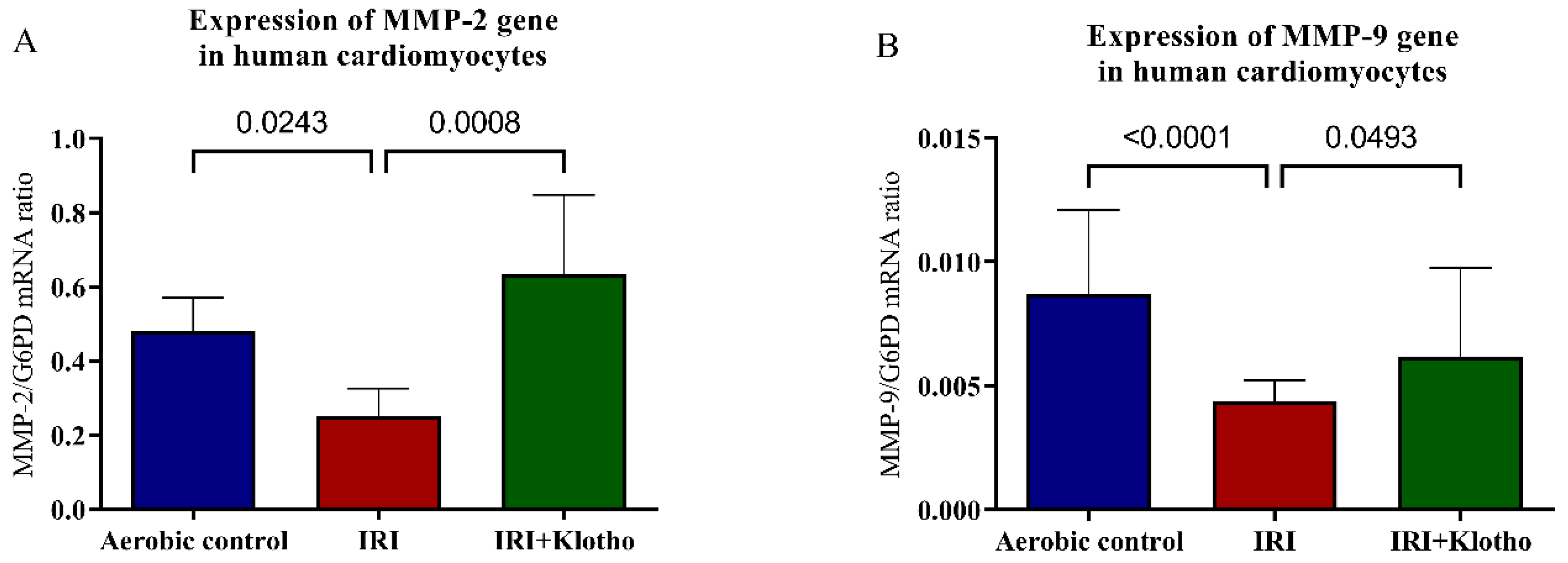
2.2. Expression of MMP Genes in Human Myocytes
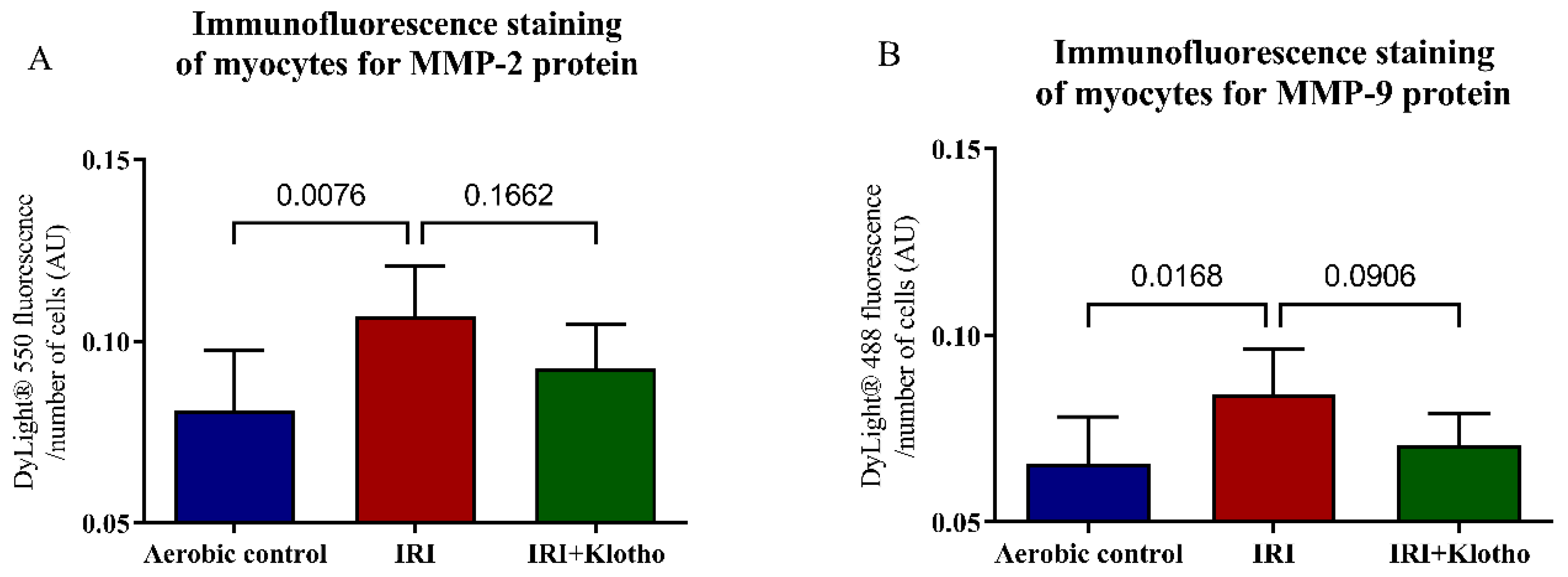
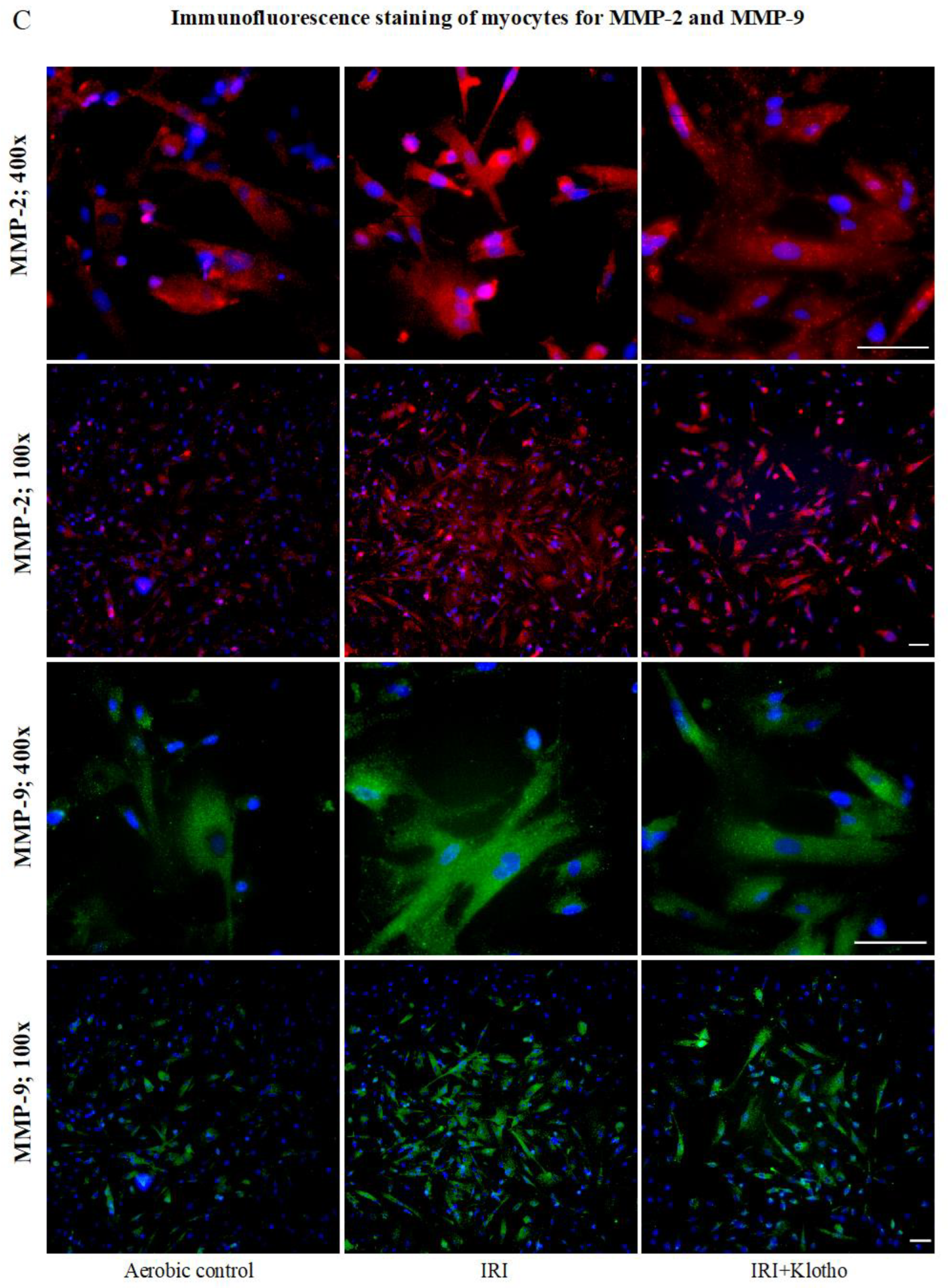
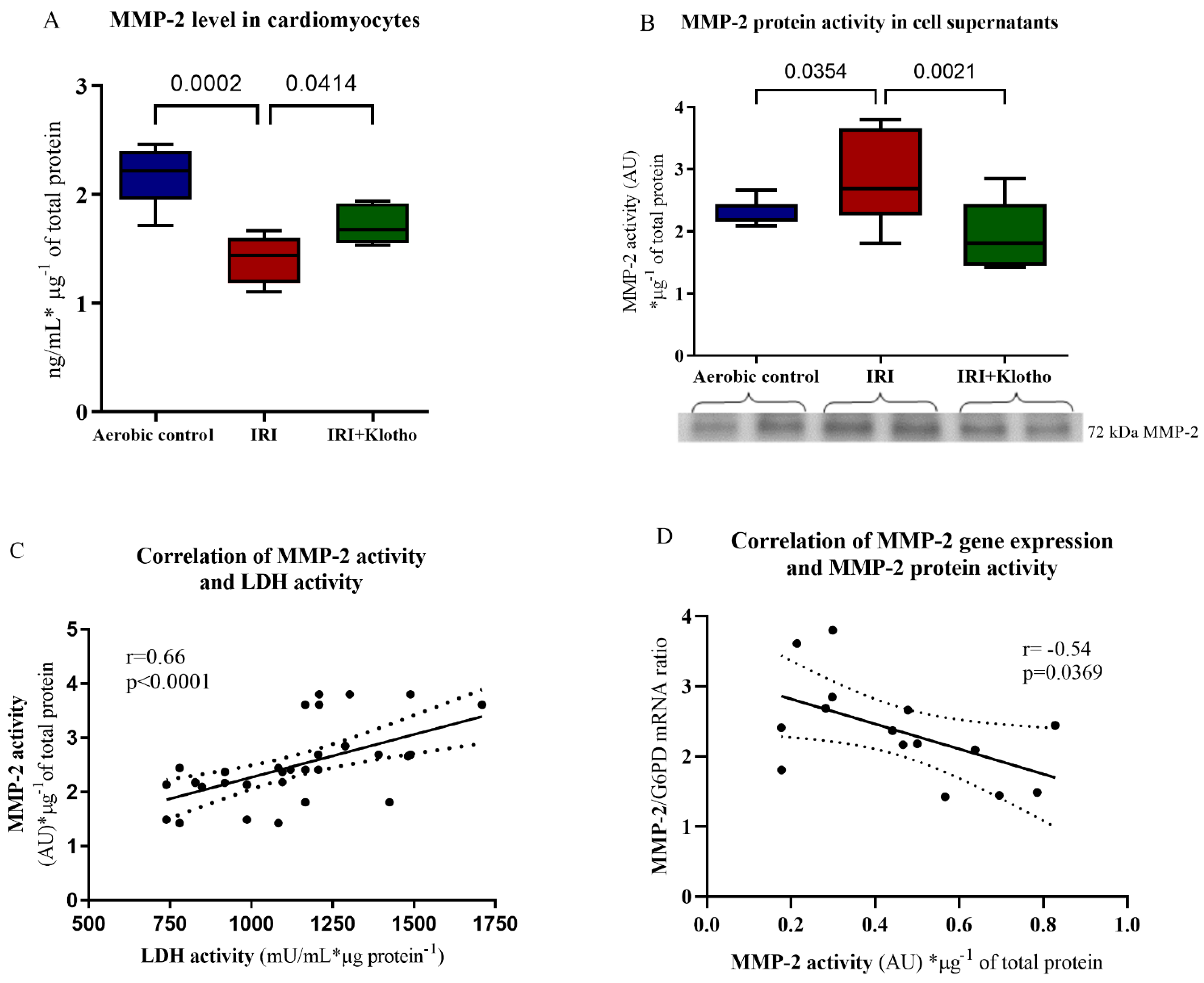
2.3. Expression of the MMP Proteins in Human Myocytes
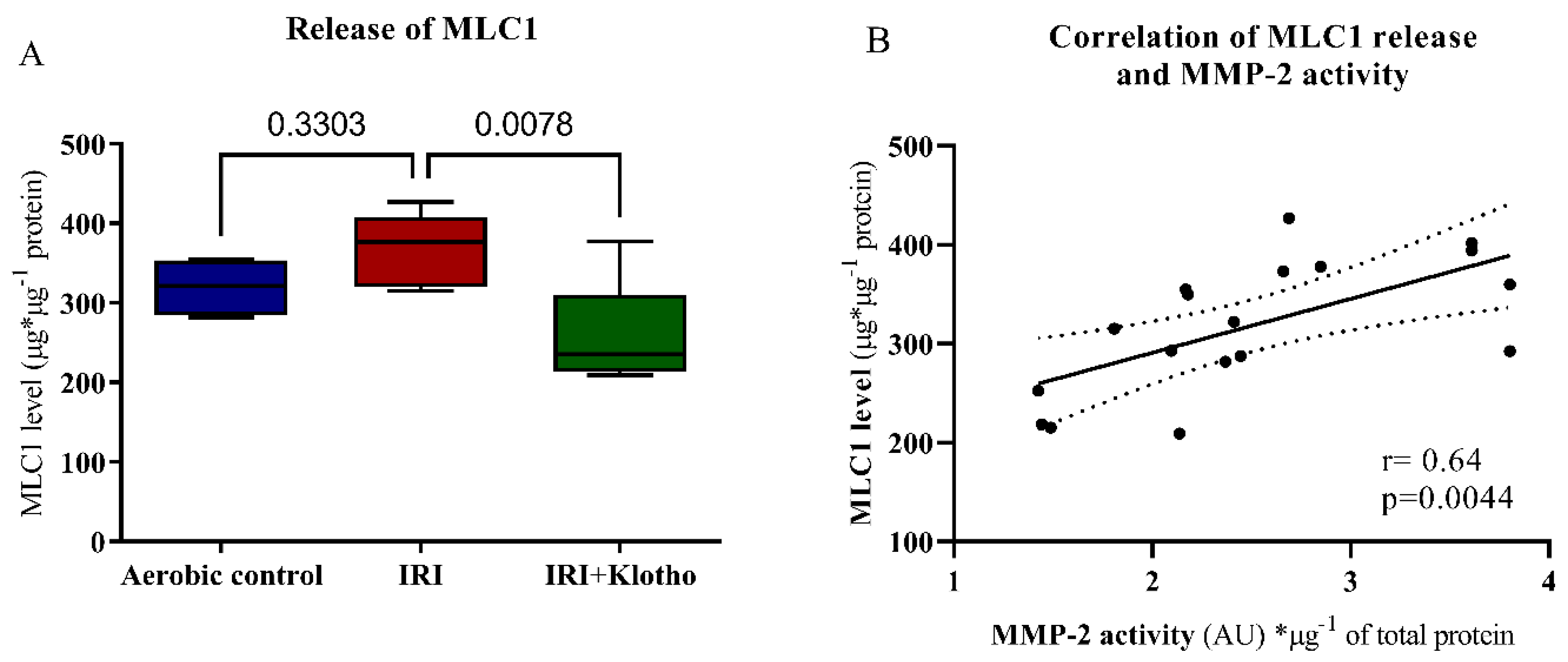
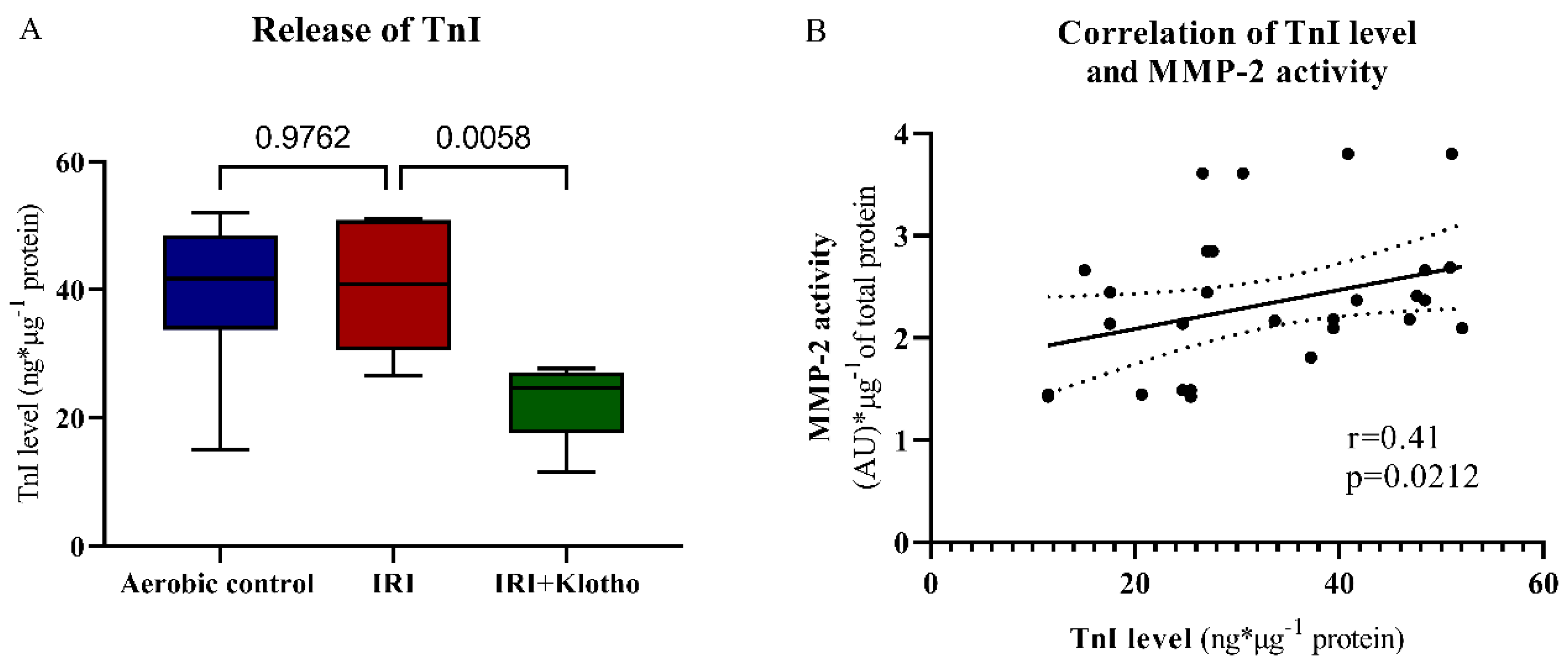
2.4. Release of Contractile Proteins from Cardiac Myocytes
3. Discussion
4. Materials and Methods
4.1. Cell Culture
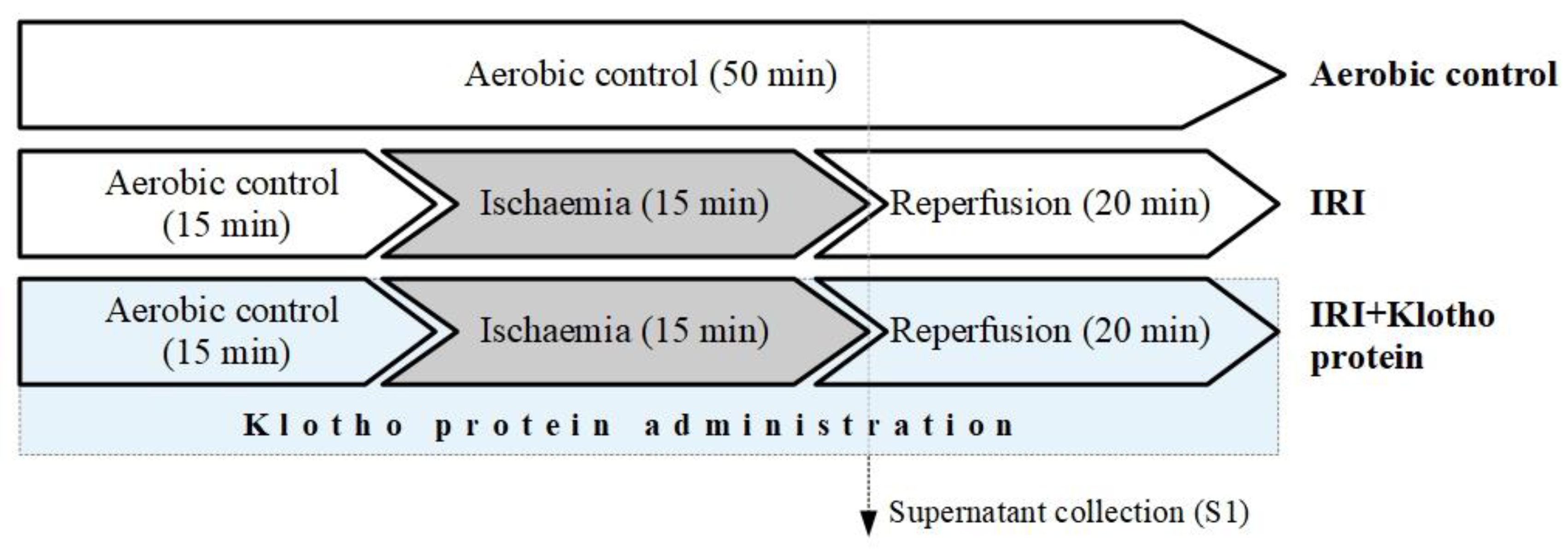
4.2. In Vitro Chemical Ischaemia/Reperfusion Injury to Human Cardiomyocytes
4.3. LDH Activity Measurement
4.4. MMP-2 and MMP-9 mRNA Expression
4.5. Immunofluorescence Staining for MMP-2 and MMP-9 Proteins
4.6. Analysis of MMP-2 Concentration
4.7. Zymography
4.8. The Measurement of Contractile Proteins Release
4.9. Determining the Total Protein Concentration
4.10. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heusch, G.; Gersh, B.J. The Pathophysiology of Acute Myocardial Infarction and Strategies of Protection beyond Reperfusion: A Continual Challenge. Eur. Heart J. 2017, 38, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.A.; Grieve, D.J.; Cave, A.C.; Shah, A.M. Oxidative Stress and Heart Failure. Arch. Mal. Coeur. Vaiss. 2003, 96, 214–221. [Google Scholar] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative Stress and Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sawicki, G.; Schulz, R. Peroxynitrite-Induced Myocardial Injury Is Mediated through Matrix Metalloproteinase-2. Cardiovasc. Res. 2002, 53, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, A.D.; Chow, A.K.; Ali, M.A.M.; Schulz, R. Matrix Metalloproteinase-2 and Myocardial Oxidative Stress Injury: Beyond the Matrix. Cardiovasc. Res. 2010, 85, 413–423. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and Function of Matrix Metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Krzywonos-Zawadzka, A.; Franczak, A.; Olejnik, A.; Radomski, M.; Gilmer, J.F.; Sawicki, G.; Woźniak, M.; Bil-Lula, I. Cardioprotective Effect of MMP-2-Inhibitor-NO-Donor Hybrid against Ischaemia/Reperfusion Injury. J. Cell Mol. Med. 2019, 23, 2836–2848. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho as a Regulator of Fibroblast Growth Factor Signaling and Phosphate/Calcium Metabolism. Curr. Opin. Nephrol. Hypertens. 2006, 15, 437–441. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the Mouse Klotho Gene Leads to a Syndrome Resembling Ageing. Nature 1997, 390, 45. [Google Scholar] [CrossRef]
- Olejnik, A.; Franczak, A.; Krzywonos-Zawadzka, A.; Kałużna-Oleksy, M.; Bil-Lula, I. The Biological Role of Klotho Protein in the Development of Cardiovascular Diseases. BioMed Res. Int. 2018, 2018, 5171945. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hwang, K.-H.; Park, K.-S.; Kong, I.D.; Cha, S.-K. Biological Role of Anti-Aging Protein Klotho. J. Lifestyle Med. 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of Oxidative Stress by the Anti-Aging Hormone Klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, H.; Yoshida, T.; Tsuchiya, K.; Mitobe, M.; Nishimura, S.; Shirota, S.; Akiba, T.; Nihei, H. Klotho Reduces Apoptosis in Experimental Ischaemic Acute Renal Failure. Nephrol. Dial. Transplant. 2005, 20, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Jin, L.; Luo, K.; Jin, J.; Shin, Y.J.; Hong, S.Y.; Yang, C.W. Klotho Enhances FoxO3-Mediated Manganese Superoxide Dismutase Expression by Negatively Regulating PI3K/AKT Pathway during Tacrolimus-Induced Oxidative Stress. Cell Death Dis 2017, 8, e2972. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Kobori, H.; Inoue, T.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Hayashi, M. [Op.4b.02] Klotho Supplementation Attenuates Blood Pressure and Oxidative Stress in Diabetes. J. Hypertens. 2017, 35, e38. [Google Scholar] [CrossRef]
- Olejnik, A.; Krzywonos-Zawadzka, A.; Banaszkiewicz, M.; Bil-Lula, I. Klotho Protein Contributes to Cardioprotection during Ischaemia/Reperfusion Injury. J. Cell. Mol. Med. 2020, 24, 6448–6458. [Google Scholar] [CrossRef]
- Olejnik, A.; Banaszkiewicz, M.; Krzywonos-Zawadzka, A.; Bil-Lula, I. The Klotho Protein Supports Redox Balance and Metabolic Functions of Cardiomyocytes during Ischemia/Reperfusion Injury. Cardiol. J. 2022, 29, 836–849. [Google Scholar] [CrossRef]
- Sawicki, G.; Leon, H.; Sawicka, J.; Sariahmetoglu, M.; Schulze, C.J.; Scott, P.G.; Szczesna-Cordary, D.; Schulz, R. Degradation of Myosin Light Chain in Isolated Rat Hearts Subjected to Ischemia-Reperfusion Injury: A New Intracellular Target for Matrix Metalloproteinase-2. Circulation 2005, 112, 544–552. [Google Scholar] [CrossRef]
- Hu, M.-C.; Shi, M.; Zhang, J.; Quiñones, H.; Kuro-o, M.; Moe, O.W. Klotho Deficiency Is an Early Biomarker of Renal Ischemia–Reperfusion Injury and Its Replacement Is Protective. Kidney Int. 2010, 78, 1240–1251. [Google Scholar] [CrossRef]
- Qian, Y.; Guo, X.; Che, L.; Guan, X.; Wu, B.; Lu, R.; Zhu, M.; Pang, H.; Yan, Y.; Ni, Z.; et al. Klotho Reduces Necroptosis by Targeting Oxidative Stress Involved in Renal Ischemic-Reperfusion Injury. CPB 2018, 45, 2268–2282. [Google Scholar] [CrossRef]
- Oh, H.J.; Nam, B.Y.; Lee, M.J.; Kim, C.H.; Koo, H.M.; Doh, F.M.; Han, J.H.; Kim, E.J.; Han, J.S.; Park, J.T.; et al. Decreased Circulating Klotho Levels in Patients Undergoing Dialysis and Relationship to Oxidative Stress and Inflammation. Perit. Dial. Int. 2015, 35, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Y.; Zhang, Y.; Liu, C. Klotho Ameliorates Oxidized Low Density Lipoprotein (Ox-LDL)-Induced Oxidative Stress via Regulating LOX-1 and PI3K/Akt/ENOS Pathways. Lipids Health Dis. 2017, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Zeldich, E.; Chen, C.-D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Tucker Zhou, T.B.; Harris, D.A.; Abraham, C.R. The Neuroprotective Effect of Klotho Is Mediated via Regulation of Members of the Redox System. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-J.; Li, H.; Shi, M.-Q.; Mao, X.-N.; Liu, D.-L.; Chang, Y.-R.; Gan, Y.-M.; Kuang, X.; Du, J.-R. Protective Effect of Klotho against Ischemic Brain Injury Is Associated with Inhibition of RIG-I/NF-ΚB Signaling. Front. Pharmacol. 2018, 8, 950. [Google Scholar] [CrossRef] [PubMed]
- Peppin, G.J.; Weiss, S.J. Activation of the Endogenous Metalloproteinase, Gelatinase, by Triggered Human Neutrophils. Proc. Natl. Acad. Sci. USA 1986, 83, 4322–4326. [Google Scholar] [CrossRef]
- Gu, Z.; Kaul, M.; Yan, B.; Kridel, S.J.; Cui, J.; Strongin, A.; Smith, J.W.; Liddington, R.C.; Lipton, S.A. S-Nitrosylation of Matrix Metalloproteinases: Signaling Pathway to Neuronal Cell Death. Science 2002, 297, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Schulze, C.J.; Suarez-Pinzon, W.L.; Dyck, J.R.; Sawicki, G.; Schulz, R. Intracellular Action of Matrix Metalloproteinase-2 Accounts for Acute Myocardial Ischemia and Reperfusion Injury. Circulation 2002, 106, 1543–1549. [Google Scholar] [CrossRef]
- Ali Mohammad, A.M.; Cho, W.J.; Hudson, B.; Kassiri, Z.; Granzier, H.; Schulz, R. Titin Is a Target of Matrix Metalloproteinase-2. Circulation 2010, 122, 2039–2047. [Google Scholar] [CrossRef]
- Rouet-Benzineb, P.; Buhler, J.-M.; Dreyfus, P.; Delcourt, A.; Dorent, R.; Perennec, J.; Crozatier, B.; Harf, A.; Lafuma, C. Altered Balance between Matrix Gelatinases (MMP-2 and MMP-9) and Their Tissue Inhibitors in Human Dilated Cardiomyopathy: Potential Role of MMP-9 in Myosin-Heavy Chain Degradation. Eur. J. Heart Fail. 1999, 1, 337–352. [Google Scholar] [CrossRef]
- Cadete, V.J.J.; Sawicka, J.; Jaswal, J.S.; Lopaschuk, G.D.; Schulz, R.; Szczesna-Cordary, D.; Sawicki, G. Ischemia/Reperfusion-Induced Myosin Light Chain 1 Phosphorylation Increases Its Degradation by Matrix Metalloproteinase 2: MLC1 Phosphorylation and Its Degradation by MMP-2. FEBS J. 2012, 279, 2444–2454. [Google Scholar] [CrossRef]
- Sawicki, G. Synergistic Effect of Inhibitors of MMPs and ROS-Dependent Modifications of Contractile Proteins on Protection Hearts Subjected to Oxidative Stress. Curr. Pharm. Des. 2014, 20, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Cadete, V.J.J.; Sawicka, J.; Bekar, L.K.; Sawicki, G. Combined Subthreshold Dose Inhibition of Myosin Light Chain Phosphorylation and MMP-2 Activity Provides Cardioprotection from Ischaemic/Reperfusion Injury in Isolated Rat Heart. Br. J. Pharmacol. 2013, 170, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Doroszko, A.; Polewicz, D.; Sawicka, J.; Richardson, J.S.; Cheung, P.-Y.; Sawicki, G. Cardiac Dysfunction in an Animal Model of Neonatal Asphyxia Is Associated with Increased Degradation of MLC1 by MMP-2. Basic Res. Cardiol. 2009, 104, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Bil-Lula, I.; Lin, H.; Biały, D.; Wawrzyńska, M.; Diebel, L.; Sawicka, J.; Woźniak, M.; Sawicki, G. Subthreshold Nitric Oxide Synthase Inhibition Improves Synergistic Effects of Subthreshold MMP-2/MLCK-mediated Cardiomyocyte Protection from Hypoxic Injury. J. Cell Mol. Med. 2016, 20, 1086–1094. [Google Scholar] [CrossRef]
- Bil-Lula, I.; Krzywonos-Zawadzka, A.; Sawicka, J.; Bialy, D.; Wawrzynska, M.; Wozniak, M.; Sawicki, G. L-NAME Improves Doxycycline and ML-7 Cardioprotection from Oxidative Stress. Front. Biosci. (Landmark Ed.) 2018, 23, 298–309. [Google Scholar] [PubMed]
- Krzywonos-Zawadzka, A.; Wozniak, M.; Sawicki, G.; Bil-Lula, I. A Drug Cocktail for Protecting against Ischemia-Reperfusion Injury. Front. Biosci. (Landmark Ed.) 2020, 25, 722–735. [Google Scholar]
- Ries, C.; Egea, V.; Karow, M.; Kolb, H.; Jochum, M.; Neth, P. MMP-2, MT1-MMP, and TIMP-2 Are Essential for the Invasive Capacity of Human Mesenchymal Stem Cells: Differential Regulation by Inflammatory Cytokines. Blood 2007, 109, 4055–4063. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial Ischemia/Reperfusion Injury: Mechanisms of Injury and Implications for Management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Cheung, P.Y.; Sawicki, G.; Wozniak, M.; Wang, W.; Radomski, M.W.; Schulz, R. Matrix Metalloproteinase-2 Contributes to Ischemia-Reperfusion Injury in the Heart. Circulation 2000, 101, 1833–1839. [Google Scholar] [CrossRef]
- Lalu, M.M.; Pasini, E.; Schulze, C.J.; Ferrari-Vivaldi, M.; Ferrari-Vivaldi, G.; Bachetti, T.; Schulz, R. Ischaemia–Reperfusion Injury Activates Matrix Metalloproteinases in the Human Heart. Eur. Heart J. 2005, 26, 27–35. [Google Scholar] [CrossRef]
- Baudrimont, A.; Voegeli, S.; Viloria, E.C.; Stritt, F.; Lenon, M.; Wada, T.; Jaquet, V.; Becskei, A. Multiplexed Gene Control Reveals Rapid MRNA Turnover. Sci. Adv. 2017, 3, e1700006. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.; Delgado-Ramos, L.; Ayala, G.; Pelechano, V.; Medina, D.A.; Carrasco, F.; González, R.; Andrés-León, E.; Steinmetz, L.; Warringer, J.; et al. The Cellular Growth Rate Controls Overall MRNA Turnover, and Modulates Either Transcription or Degradation Rates of Particular Gene Regulons. Nucleic Acids Res. 2016, 44, 3643–3658. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gharib, T.G.; Huang, C.-C.; Taylor, J.M.G.; Misek, D.E.; Kardia, S.L.R.; Giordano, T.J.; Iannettoni, M.D.; Orringer, M.B.; Hanash, S.M.; et al. Discordant Protein and MRNA Expression in Lung Adenocarcinomas. Mol. Cell Proteomics 2002, 1, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing Protein Abundance and MRNA Expression Levels on a Genomic Scale. Genome Biol. 2003, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Lindner, A.B. Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid. Med. Cell Longev. 2017, 2017, 5716409. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of Translation Initiation in Eukaryotes: Mechanisms and Biological Targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef]
- Ho, J.J.D.; Balukoff, N.C.; Theodoridis, P.R.; Wang, M.; Krieger, J.R.; Schatz, J.H.; Lee, S. A Network of RNA-Binding Proteins Controls Translation Efficiency to Activate Anaerobic Metabolism. Nat. Commun. 2020, 11, 2677. [Google Scholar] [CrossRef]
- Zhou, H.-Z.; Ma, X.; Gray, M.O.; Zhu, B.; Nguyen, A.P.; Baker, A.J.; Simonis, U.; Cecchini, G.; Lovett, D.H.; Karliner, J.S. Transgenic MMP-2 Expression Induces Latent Cardiac Mitochondrial Dysfunction. Biochem. Biophys. Res. Commun. 2007, 358, 189–195. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patel, K.D. Matrix Metalloproteinase-2 (Mmp-2) and Mmp-9 in Pulmonary Pathology. Exp. Lung Res. 2005, 31, 599–621. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, Q.; Lin, S.; Wu, R. Fosinopril and Valsartan Intervention in Gene Expression of Klotho, MMP-9, TIMP-1, and PAI-1 in the Kidney of Spontaneously Hypertensive Rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2010, 35, 1048–1056. [Google Scholar] [CrossRef]
- Chen Kai; Zhou Xiaoli; Sun Zhongjie Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension 2015, 66, 1006–1013. [CrossRef]
- Funada, Y.; Nishimura, Y.; Yokoyama, M. Imbalance of Matrix Metalloproteinase-9 and Tissue Inhibitor of Matrix Metalloproteinase-1 Is Associated with Pulmonary Emphysema in Klotho Mice. Kobe J. Med. Sci. 2004, 50, 59–67. [Google Scholar] [PubMed]
- Chang, B.; Kim, J.; Jeong, D.; Jeong, Y.; Jeon, S.; Jung, S.-I.; Yang, Y.; Kim, K.I.; Lim, J.-S.; Kim, C.; et al. Klotho Inhibits the Capacity of Cell Migration and Invasion in Cervical Cancer. Oncol. Rep. 2012, 28, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho Inhibits Transforming Growth Factor-Β1 (TGF-Β1) Signaling and Suppresses Renal Fibrosis and Cancer Metastasis in Mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Xie, J.; An, S.-W.; Oliver, N.; Barrezueta, N.X.; Lin, M.-H.; Birnbaumer, L.; Huang, C.-L. Inhibition of TRPC6 Channels Ameliorates Renal Fibrosis and Contributes to Renal Protection by Soluble Klotho. Kidney Int. 2017, 91, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Krzywonos-Zawadzka, A.; Franczak, A.; Sawicki, G.; Bil-Lula, I. Mixture of MMP-2, MLC, and NOS Inhibitors Affects NO Metabolism and Protects Heart from Cardiac I/R Injury. Available online: https://www.hindawi.com/journals/crp/2020/1561478/ (accessed on 20 April 2020).
- Lindsey, M.L.; Bolli, R.; Canty, J.M.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for Experimental Models of Myocardial Ischemia and Infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, A.; Krzywonos-Zawadzka, A.; Banaszkiewicz, M.; Bil-Lula, I. Klotho Protein Decreases MMP-Mediated Degradation of Contractile Proteins during Ischaemia/Reperfusion Injury to the Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 15450. https://doi.org/10.3390/ijms232415450
Olejnik A, Krzywonos-Zawadzka A, Banaszkiewicz M, Bil-Lula I. Klotho Protein Decreases MMP-Mediated Degradation of Contractile Proteins during Ischaemia/Reperfusion Injury to the Cardiomyocytes. International Journal of Molecular Sciences. 2022; 23(24):15450. https://doi.org/10.3390/ijms232415450
Chicago/Turabian StyleOlejnik, Agnieszka, Anna Krzywonos-Zawadzka, Marta Banaszkiewicz, and Iwona Bil-Lula. 2022. "Klotho Protein Decreases MMP-Mediated Degradation of Contractile Proteins during Ischaemia/Reperfusion Injury to the Cardiomyocytes" International Journal of Molecular Sciences 23, no. 24: 15450. https://doi.org/10.3390/ijms232415450
APA StyleOlejnik, A., Krzywonos-Zawadzka, A., Banaszkiewicz, M., & Bil-Lula, I. (2022). Klotho Protein Decreases MMP-Mediated Degradation of Contractile Proteins during Ischaemia/Reperfusion Injury to the Cardiomyocytes. International Journal of Molecular Sciences, 23(24), 15450. https://doi.org/10.3390/ijms232415450





