Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication
Abstract
:1. Introduction
2. Overview of Selenium in Biological Function
3. Selenium in Hypothalamic Function
4. Signals from Brain to Body
4.1. Hypothalamic–Pituitary–Thyroid Axis
4.2. Hypothalamic–Pituitary–Adrenal Axis
4.3. Hypothalamic–Pituitary–Gonadal Axis
4.4. Hypothalamic–Pituitary–Prolactin Axis
4.5. Hypothalamic–Pituitary–Somatotropic Axis
4.6. Oxytocin and Vasopressin
5. Signals from Body to Brain
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Affinati, A.H.; Myers, M.G., Jr. Neuroendocrine Control of Body Energy Homeostasis; Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; South Dartmouth: Dartmouth, MA, USA, 2000. [Google Scholar]
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018. NCHS Data Brief 2020, 360, 1–8. [Google Scholar]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2019; U.S. Department of Health & Human Services, Ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. [Google Scholar]
- Vettori, A.; Pompucci, G.; Paolini, B.; Del Ciondolo, I.; Bressan, S.; Dundar, M.; Kenanoglu, S.; Unfer, V.; Bertelli, M.; Project, G. Genetic background, nutrition and obesity: A review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1751–1761. [Google Scholar]
- Hamdy, O.; Barakatun-Nisak, M.-Y. Nutrition in Diabetes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 799–817. [Google Scholar] [CrossRef]
- Walsh, J.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes 2017, 24, 389–394. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Visiedo-García, F.M.; Domínguez-Riscart, J.; González-Domínguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron Metabolism in Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef]
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zou, Y.; Shen, Z.; Xiong, Y.; Zhang, W.; Liu, C.; Chen, S. Trace Elements, PPARs, and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2612. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.C.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef] [Green Version]
- Marcum, J.A. Nutrigenetics/Nutrigenomics, Personalized Nutrition, and Precision Healthcare. Curr. Nutr. Rep. 2020, 9, 338–345. [Google Scholar] [CrossRef]
- Namazi, N.; Esmaeili, S.; Ahmadikhatir, S.; Razi, F.; Nasli-Esfahani, E.; Larijani, B. Nutrition and Diet Therapy in Diabetes Mellitus: A Roadmap based on available evidence. J. Diabetes Metab. Disord. 2021, 20, 1913–1918. [Google Scholar] [CrossRef]
- de Toro-Martín, J.; Arsenault, B.J.; Després, J.-P.; Vohl, M.-C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [Google Scholar] [CrossRef] [Green Version]
- Schweizer, U.; Bohleber, S.; Zhao, W.; Fradejas-Villar, N. The Neurobiology of Selenium: Looking Back and to the Future. Front. Neurosci. 2021, 15, 652099. [Google Scholar] [CrossRef]
- Gong, T.; Torres, D.; Berry, M.J.; Pitts, M.W. Hypothalamic redox balance and leptin signaling—Emerging role of selenoproteins. Free Radic. Biol. Med. 2018, 127, 172–181. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2019, 19, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Ogawa-Wong, A.N.; Berry, M.J.; Seale, L.A. Selenium and Metabolic Disorders: An Emphasis on Type 2 Diabetes Risk. Nutrients 2016, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.C., Jr.; Gerald, F.; Wu, T.L.; Zeng, H.; Cheng, W.H. Selenium status and type 2 diabetes risk. Arch. Biochem. Biophys. 2022, 730, 109400. [Google Scholar] [CrossRef]
- Seale, L.A.; Hashimoto, A.C.; Kurokawa, S.; Gilman, C.L.; Seyedali, A.; Bellinger, F.P.; Raman, A.V.; Berry, M.J. Disruption of the Selenocysteine Lyase-Mediated Selenium Recycling Pathway Leads to Metabolic Syndrome in Mice. Mol. Cell Biol. 2012, 32, 4141–4154. [Google Scholar] [CrossRef] [Green Version]
- Pitts, M.W.; Reeves, M.A.; Hashimoto, A.C.; Ogawa, A.; Kremer, P.; Seale, L.A.; Berry, M.J. Deletion of Selenoprotein M Leads to Obesity without Cognitive Deficits. J. Biol. Chem. 2013, 288, 26121–26134. [Google Scholar] [CrossRef] [Green Version]
- Kremer, P.M.; Torres, D.J.; Hashimoto, A.C.; Berry, M.J. Sex-Specific Metabolic Impairments in a Mouse Model of Disrupted Selenium Utilization. Front. Nutr. 2021, 8, 682700. [Google Scholar] [CrossRef]
- Marsili, A.; Aguayo-Mazzucato, C.; Chen, T.; Kumar, A.; Chung, M.; Lunsford, E.P.; Harney, J.W.; Van-Tran, T.; Gianetti, E.; Ramadan, W.; et al. Mice with a Targeted Deletion of the Type 2 Deiodinase Are Insulin Resistant and Susceptible to Diet Induced Obesity. PLoS ONE 2011, 6, e20832. [Google Scholar] [CrossRef]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A Liver-Derived Secretory Protein, Selenoprotein P, Causes Insulin Resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef] [Green Version]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive Oxygen Species Enhance Insulin Sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Torres, D.J.; Pitts, M.W.; Hashimoto, A.C.; Berry, M.J. Agrp-Specific Ablation of Scly Protects against Diet-Induced Obesity and Leptin Resistance. Nutrients 2019, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.J.; Pitts, M.W.; Seale, L.A.; Hashimoto, A.C.; An, K.J.; Hanato, A.N.; Hui, K.W.; Remigio, S.M.A.; Carlson, B.A.; Hatfield, D.L.; et al. Female Mice with Selenocysteine tRNA Deletion in Agrp Neurons Maintain Leptin Sensitivity and Resist Weight Gain While on a High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 11010. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [Green Version]
- Monsen, E.R. Dietary Reference Intakes for The Antioxidant Nutrients: Vitamin C, Vitamin E, Selenium, and Carotenoids. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2013, 6, 25–54. [Google Scholar] [CrossRef]
- Schomburg, L. Selenoprotein P—Selenium transport protein, enzyme and biomarker of selenium status. Free Radic. Biol. Med. 2022, 191, 150–163. [Google Scholar] [CrossRef]
- Saito, Y. Selenium Transport Mechanism via Selenoprotein P—Its Physiological Role and Related Diseases. Front. Nutr. 2021, 8, 685517. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry (Moscow) 2022, 87, S168–S177. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J.; Banu, L.; Chen, Y.; Mandel, S.J.; Kieffer, J.D.; Harney, J.W.; Larsen, P.R. Recognition of UGA as a selenocysteine codon in Type I deiodinase requires sequences in the 3′ untranslated region. Nature 1991, 353, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Essential trace element selenium and redox regulation-its metabolism, physiological function, and related diseases. Redox Exp. Med. 2022, 1, R149–R158. [Google Scholar] [CrossRef]
- Low, S.C.; Grundner-Culemann, E.; Harney, J.W.; Berry, M.J. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000, 19, 6882–6890. [Google Scholar] [CrossRef] [Green Version]
- Seyedali, A.; Berry, M.J. Nonsense-mediated decay factors are involved in the regulation of selenoprotein mRNA levels during selenium deficiency. RNA 2014, 20, 1248–1256. [Google Scholar] [CrossRef] [Green Version]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [Green Version]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Hoekstra, W.G. Prevention of Oxidative Damage to Rat Erythrocytes by Dietary Selenium. J. Nutr. 1972, 102, 689–696. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Ursini, F.; Travain, V.B.; Cozza, G.; Miotto, G.; Roveri, A.; Toppo, S.; Maiorino, M. A white paper on Phospholipid Hydroperoxide Glutathione Peroxidase (GPx4) forty years later. Free Radic. Biol. Med. 2022, 188, 117–133. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Gencheva, R.; Cheng, Q.; Arnér, E.S. Thioredoxin reductase selenoproteins from different organisms as potential drug targets for treatment of human diseases. Free Radic. Biol. Med. 2022, 190, 320–338. [Google Scholar] [CrossRef] [PubMed]
- Tarrago, L.; Kaya, A.; Kim, H.-Y.; Manta, B.; Lee, B.-C.; Gladyshev, V.N. The selenoprotein methionine sulfoxide reductase B1 (MSRB1). Free Radic. Biol. Med. 2022, 191, 228–240. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef]
- Sabatino, L.; Vassalle, C.; Del Seppia, C.; Iervasi, G. Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions. Endocrinol. Metab. 2021, 36, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Carlson, B.A.; Irons, R.; Mix, H.; Zhong, N.; Gladyshev, V.N.; Hatfield, D.L. Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem. J. 2007, 404, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Pitts, M.W.; Hoffmann, P.R. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium 2018, 70, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liu, M.; Ni, J.; Tian, J. Role of Selenoprotein F in Protein Folding and Secretion: Potential Involvement in Human Disease. Nutrients 2018, 10, 1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shchedrina, V.A.; Everley, R.A.; Zhang, Y.; Gygi, S.P.; Hatfield, D.L.; Gladyshev, V.N. Selenoprotein K Binds Multiprotein Complexes and Is Involved in the Regulation of Endoplasmic Reticulum Homeostasis. J. Biol. Chem. 2011, 286, 42937–42948. [Google Scholar] [CrossRef] [Green Version]
- Fredericks, G.J.; Hoffmann, F.W.; Rose, A.H.; Osterheld, H.J.; Hess, F.M.; Mercier, F.; Hoffmann, P.R. Stable expression and function of the inositol 1,4,5-triphosphate receptor requires palmitoylation by a DHHC6/selenoprotein K complex. Proc. Natl. Acad. Sci. USA 2014, 111, 16478–16483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, M.A.; Bellinger, F.P.; Berry, M.J. The Neuroprotective Functions of Selenoprotein M and its Role in Cytosolic Calcium Regulation. Antioxid. Redox Signal. 2010, 12, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Chernorudskiy, A.; Varone, E.; Colombo, S.F.; Fumagalli, S.; Cagnotto, A.; Cattaneo, A.; Briens, M.; Baltzinger, M.; Kuhn, L.; Bachi, A.; et al. Selenoprotein N is an endoplasmic reticulum calcium sensor that links luminal calcium levels to a redox activity. Proc. Natl. Acad. Sci. USA 2020, 117, 21288–21298. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, K.J.; Jang, J.K.; Jeon, Y.H.; Ko, K.Y.; Kwon, J.H.; Lee, S.-R.; Kim, I.Y. Selenoprotein S-dependent Selenoprotein K Binding to p97(VCP) Protein Is Essential for Endoplasmic Reticulum-associated Degradation. J. Biol. Chem. 2015, 290, 29941–29952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamieh, A.; Cartier, D.; Abid, H.; Calas, A.; Burel, C.; Bucharles, C.; Jehan, C.; Grumolato, L.; Landry, M.; Lerouge, P.; et al. Selenoprotein T is a novel OST subunit that regulates UPR signaling and hormone secretion. EMBO Rep. 2017, 18, 1935–1946. [Google Scholar] [CrossRef]
- Pothion, H.; Jehan, C.; Tostivint, H.; Cartier, D.; Bucharles, C.; Falluel-Morel, A.; Boukhzar, L.; Anouar, Y.; Lihrmann, I. Selenoprotein T: An Essential Oxidoreductase Serving as a Guardian of Endoplasmic Reticulum Homeostasis. Antioxidants Redox Signal. 2020, 33, 1257–1275. [Google Scholar] [CrossRef]
- Cakir, I.; Nillni, E.A. Endoplasmic Reticulum Stress, the Hypothalamus, and Energy Balance. Trends Endocrinol. Metab. 2019, 30, 163–176. [Google Scholar] [CrossRef]
- Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.-J.; Han, H.; Zhou, G.-B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef] [Green Version]
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.-J.; Meng, Q.; Han, H.; et al. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Flohé, L.; Garolla, A.; Roveri, A.; Ursini, F.; Maiorino, M. Male fertility is linked to the selenoprotein phospholipid hydroperoxide glutathione peroxidase. Biol. Reprod. 2002, 67, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.; Tomlinson, R. Selenium in blood and human tissues. Clin. Chim. Acta 1967, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairweather-Tait, S.J.; Filippini, T.; Vinceti, M. Selenium status and immunity. Proc. Nutr. Soc. 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Taylor, E.W.; Zhang, J. The relevance of selenium to viral disease with special reference to SARS-CoV-2 and COVID-19. Proc. Nutr. Soc. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.K.; Newton, T.D.; Hettiaratchi, M.H.; Pluth, M.D. Reactive sulfur and selenium species in the regulation of bone homeostasis. Free Radic. Biol. Med. 2022, 190, 148–157. [Google Scholar] [CrossRef]
- Shimada, B.K.; Alfulaij, N.; Seale, L.A. The Impact of Selenium Deficiency on Cardiovascular Function. Int. J. Mol. Sci. 2021, 22, 10713. [Google Scholar] [CrossRef]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium Status and Its Antioxidant Role in Metabolic Diseases. Oxid. Med. Cell Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- Rataan, A.O.; Geary, S.M.; Zakharia, Y.; Rustum, Y.M.; Salem, A.K. Potential Role of Selenium in the Treatment of Cancer and Viral Infections. Int. J. Mol. Sci. 2022, 23, 2215. [Google Scholar] [CrossRef]
- Stolwijk, J.M.; Garje, R.; Sieren, J.C.; Buettner, G.R.; Zakharia, Y. Understanding the Redox Biology of Selenium in the Search of Targeted Cancer Therapies. Antioxidants 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Arch. Biochem. Biophys. 2013, 536, 152–157. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Selenoprotein P—Expression, functions, and roles in mammals. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2009, 1790, 1441–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomburg, L.; Riese, C.; Michaelis, M.; Griebert, E.; Klein, M.; Sapin, R.; Schweizer, U.; Köhrle, J. Synthesis and Metabolism of Thyroid Hormones Is Preferentially Maintained in Selenium-Deficient Transgenic Mice. Endocrinology 2006, 147, 1306–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.P.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.M.; Hill, K.E.; Burk, R.F.; Weeber, E.J. Altered hippocampus synaptic function in selenoprotein P deficient mice. Mol. Neurodegener. 2006, 1, 12. [Google Scholar] [CrossRef] [Green Version]
- Byrns, C.N.; Pitts, M.W.; Gilman, C.A.; Hashimoto, A.C.; Berry, M.J. Mice Lacking Selenoprotein P and Selenocysteine Lyase Exhibit Severe Neurological Dysfunction, Neurodegeneration, and Audiogenic Seizures. J. Biol. Chem. 2014, 289, 9662–9674. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Yang, Y.; Zhang, Z.; Zhang, L.; Song, J.; Ping, Y.; Du, X.; Song, G.; Liu, Q.; Li, N. Loss of MsrB1 perturbs spatial learning and long-term potentiation/long-term depression in mice. Neurobiol. Learn. Mem. 2019, 166, 107104. [Google Scholar] [CrossRef]
- Schweizer, U.; Fabiano, M. Selenoproteins in brain development and function. Free Radic. Biol. Med. 2022, 190, 105–115. [Google Scholar] [CrossRef]
- Leiter, O.; Zhuo, Z.; Rust, R.; Wasielewska, J.M.; Grönnert, L.; Kowal, S.; Overall, R.W.; Adusumilli, V.S.; Blackmore, D.G.; Southon, A.; et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab. 2022, 34, 408–423.e8. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.L.; Toh, P.; Alfulaij, N.; Berry, M.J.; Torres, D.J. New insights on selenoproteins and neuronal function. Free Radic. Biol. Med. 2022, 190, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, R.-P.; Cheng, W.-H.; Zhu, J.-H. Prioritized brain selenium retention and selenoprotein expression: Nutritional insights into Parkinson’s disease. Mech. Ageing Dev. 2019, 180, 89–96. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Prasad, S.; Kothapalli, J.; Manne, M. Brain Selenium in Alzheimer’s Disease (BRAIN SEAD Study): A Systematic Review and Meta-Analysis. Biol. Trace Element Res. 2019, 189, 361–369. [Google Scholar] [CrossRef]
- Solovyev, N.; Drobyshev, E.; Bjørklund, G.; Dubrovskii, Y.; Lysiuk, R.; Rayman, M.P. Selenium, selenoprotein P, and Alzheimer’s disease: Is there a link? Free Radic. Biol. Med. 2018, 127, 124–133. [Google Scholar] [CrossRef]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Ste Marie, E.J.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279.e25. [Google Scholar] [CrossRef] [Green Version]
- Jais, A.; Brüning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism—Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2021, 43, 314–328. [Google Scholar] [CrossRef]
- Korf, H.-W.; Møller, M. Arcuate nucleus, median eminence, and hypophysial pars tuberalis. Handb. Clin. Neurol. 2021, 180, 227–251. [Google Scholar] [CrossRef]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2021, 915, 174611. [Google Scholar] [CrossRef]
- Deem, J.D.; Faber, C.L.; Morton, G.J. AgRP neurons: Regulators of feeding, energy expenditure, and behavior. FEBS J. 2021, 289, 2362–2381. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, L.E.; Unger, E.K.; Cheung, C.C.; Xu, A.W. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc. Natl. Acad. Sci. USA 2013, 110, E697–E706. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Pan, D.; Wang, Z.; Chen, Y.; Cao, J. Regulation of the central melanocortin system on energy balance in mammals and birds. Neuropeptides 2022, 95, 102267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Y. The central melanocortin system and human obesity. J. Mol. Cell Biol. 2020, 12, 785–797. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKβ/NF-κB and ER Stress Link Overnutrition to Energy Imbalance and Obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, L.; Ergin, A.S.; Lu, A.; Chung, J.; Sarkar, S.; Nie, D.; Myers, M.G., Jr.; Ozcan, U. Endoplasmic Reticulum Stress Plays a Central Role in Development of Leptin Resistance. Cell Metab. 2009, 9, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Merriam, G.R.; Nunnelley, L.L.; Trish, J.W.; Naftolin, F. Sex-related and cyclic variation of trace elements in rat hypothalamus and pituitary. Brain Res. 1979, 171, 503–510. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Behne, D.; Hilmert, H.; Scheid, S.; Gessner, H.; Elger, W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1988, 966, 12–21. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, O.; Danscher, G. Selenium in the anterior pituitary of rats exposed to sodium selenite: Light and electron microscopic localization. Toxicol. Appl. Pharmacol. 1985, 81, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius-Ussing, O.; Danscher, G.; Møller-Madsen, B.; Rungby, J. Selenium in the anterior pituitary. Sci. Total Environ. 1985, 42, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius-Ussing, O.; Flyvbjerg, A.; Esmann, J. Evidence that Selenium Induces Growth Retardation through Reduced Growth Hormone and Somatomedin C Production*. Endocrinology 1987, 120, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Thorlacius-Ussing, O.; Flyvbjerg, A.; Jørgensen, K.D.; Orskov, H. Growth hormone restores normal growth in selenium-treated rats without increase in circulating somatomedin C. Eur. J. Endocrinol. 1988, 117, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorlacius-Ussing, O.; Gregersen, M.; Hertel, N. The concentration of twelve elements in the anterior pituitary from human subjects and rats as measured by particle induced X-ray emission (PIXE). Biol. Trace Element Res. 1988, 16, 189–202. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, O.; Jensen, F.T. Selenium in the anterior pituitary of the rat after a single injection of75Se sodium selenite. Biol. Trace Element Res. 1988, 15, 277–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Schweizer, U.; Savaskan, N.E.; Hua, D.; Kipnis, J.; Hatfield, D.L.; Gladyshev, V.N. Comparative Analysis of Selenocysteine Machinery and Selenoproteome Gene Expression in Mouse Brain Identifies Neurons as Key Functional Sites of Selenium in Mammals. J. Biol. Chem. 2008, 283, 2427–2438. [Google Scholar] [CrossRef] [Green Version]
- Henry, F.E.; Sugino, K.; Tozer, A.; Branco, T.; Sternson, S.M. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 2015, 4, e09800. [Google Scholar] [CrossRef]
- Tung, Y.-C.L.; Ma, M.; Piper, S.; Coll, A.; O’Rahilly, S.; Yeo, G.S.H. Novel Leptin-Regulated Genes Revealed by Transcriptional Profiling of the Hypothalamic Paraventricular Nucleus. J. Neurosci. 2008, 28, 12419–12426. [Google Scholar] [CrossRef] [Green Version]
- Katunga, L.A.; Gudimella, P.; Efird, J.T.; Abernathy, S.; Mattox, T.A.; Beatty, C.; Darden, T.M.; Thayne, K.A.; Alwair, H.; Kypson, A.P.; et al. Obesity in a model of GPX4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Mol. Metab. 2015, 4, 493–506, Erratum in Mol. Metab. 2015, 4, 753–753. [Google Scholar] [CrossRef]
- Gong, T.; Hashimoto, A.C.; Sasuclark, A.R.; Khadka, V.S.; Gurary, A.; Pitts, M.W. Selenoprotein M Promotes Hypothalamic Leptin Signaling and Thioredoxin Antioxidant Activity. Antioxidants Redox Signal. 2021, 35, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Lechan, R.M.; Fekete, C. Role of Thyroid Hormone Deiodination in the Hypothalamus. Thyroid 2005, 15, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Guadaño-Ferraz, A.; Obregon, M.J.; Germain, D.L.S.; Bernal, J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc. Natl. Acad. Sci. USA 1997, 94, 10391–10396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, H.M.; Kim, S.-W.; Salvatore, D.; Bartha, T.; Legradi, G.; Larsen, P.R.; Lechan, R.M. Regional Distribution of Type 2 Thyroxine Deiodinase Messenger Ribonucleic Acid in Rat Hypothalamus and Pituitary and Its Regulation by Thyroid Hormone*. Endocrinology 1997, 138, 3359–3368. [Google Scholar] [CrossRef]
- Zeöld, A.; Doleschall, M.; Haffner, M.C.; Capelo, L.; Menyhért, J.; Liposits, Z.; Da Silva, W.S.; Bianco, A.; Kacskovics, I.; Fekete, C.; et al. Characterization of the Nuclear Factor-κB Responsiveness of the Human dio2 Gene. Endocrinology 2006, 147, 4419–4429. [Google Scholar] [CrossRef]
- Barrett, P.; Ebling, F.J.P.; Schuhler, S.; Wilson, D.; Ross, A.W.; Warner, A.; Jethwa, P.; Boelen, A.; Visser, T.J.; Ozanne, D.M.; et al. Hypothalamic Thyroid Hormone Catabolism Acts as a Gatekeeper for the Seasonal Control of Body Weight and Reproduction. Endocrinology 2007, 148, 3608–3617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, A.; Liu, Z.-W.; Andrews, Z.B.; Paradis, E.; Roy, M.-C.; Friedman, J.M.; Ricquier, D.; Richard, D.; Horvath, T.L.; Gao, X.-B.; et al. A Central Thermogenic-like Mechanism in Feeding Regulation: An Interplay between Arcuate Nucleus T3 and UCP2. Cell Metab. 2007, 5, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Yagishita, Y.; Uruno, A.; Fukutomi, T.; Saito, R.; Saigusa, D.; Pi, J.; Fukamizu, A.; Sugiyama, F.; Takahashi, S.; Yamamoto, M. Nrf2 Improves Leptin and Insulin Resistance Provoked by Hypothalamic Oxidative Stress. Cell Rep. 2017, 18, 2030–2044. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Chechi, K.; Carpentier, A.C.; Richard, D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol. Metab. 2013, 24, 408–420. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R.; Pfeiffer, B.; Hamprecht, B. Synthesis of the Antioxidant Glutathione in Neurons: Supply by Astrocytes of CysGly as Precursor for Neuronal Glutathione. J. Neurosci. 1999, 19, 562–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagara, J.-I.; Makino, N.; Bannai, S. Glutathione Efflux from Cultured Astrocytes. J. Neurochem. 2002, 66, 1876–1881. [Google Scholar] [CrossRef] [PubMed]
- Zagmutt, S.; Mera, P.; Soler-Vázquez, M.C.; Herrero, L.; Serra, D. Targeting AgRP neurons to maintain energy balance: Lessons from animal models. Biochem. Pharmacol. 2018, 155, 224–232. [Google Scholar] [CrossRef] [Green Version]
- de Oca, M.A.P.-M.; Sotelo-Rivera, I.; Gutiérrez-Mata, A.; Charli, J.-L.; Joseph-Bravo, P. Sex Dimorphic Responses of the Hypothalamus-Pituitary-Thyroid Axis to Energy Demands and Stress. Front. Endocrinol. 2021, 12, 746924. [Google Scholar] [CrossRef]
- Sze, Y.; Brunton, P.J. Sex, stress and steroids. Eur. J. Neurosci. 2019, 52, 2487–2515. [Google Scholar] [CrossRef]
- Shi, Z.; Wong, J.; Brooks, V.L. Obesity: Sex and sympathetics. Biol. Sex Differ. 2020, 11, 10. [Google Scholar] [CrossRef]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A leptin–BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef]
- Seale, L.A. Selenocysteine β-Lyase: Biochemistry, Regulation and Physiological Role of the Selenocysteine Decomposition Enzyme. Antioxidants 2019, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Seale, L.A.; Gilman, C.L.; Hashimoto, A.C.; Ogawa-Wong, A.N.; Berry, M.J. Diet-Induced Obesity in the Selenocysteine Lyase Knockout Mouse. Antioxid. Redox Signal. 2015, 23, 761–774. [Google Scholar] [CrossRef] [Green Version]
- Ogawa-Wong, A.N.; Hashimoto, A.C.; Ha, H.; Pitts, M.W.; Seale, L.A.; Berry, M.J. Sexual Dimorphism in the Selenocysteine Lyase Knockout Mouse. Nutrients 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, A.W.; Kaelin, C.B.; Morton, G.J.; Ogimoto, K.; Stanhope, K.; Graham, J.; Baskin, D.G.; Havel, P.; Schwartz, M.W.; Barsh, G.S. Effects of Hypothalamic Neurodegeneration on Energy Balance. PLOS Biol. 2005, 3, e415. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP Neurons Are Essential for Feeding in Adult Mice but Can Be Ablated in Neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, A.; Xu, A.W. De Novo Neurogenesis in Adult Hypothalamus as a Compensatory Mechanism to Regulate Energy Balance. J. Neurosci. 2010, 30, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Diano, S.; Liu, Z.-W.; Jeong, J.K.; Dietrich, M.; Ruan, H.-B.; Kim, E.; Suyama, S.; Kelly, K.; Gyengesi, E.; Arbiser, J.L.; et al. Peroxisome proliferation–associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat. Med. 2011, 17, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, D.-Y.; Chen, P.-N.; Yang, S.-F.; Chu, S.-C.; Chen, C.-H.; Kuo, M.-H.; Yu, C.-H.; Hsieh, Y.-S. Role of Reactive Oxygen Species-Related Enzymes in Neuropeptide Y and Proopiomelanocortin-Mediated Appetite Control: A Study Using Atypical Protein Kinase C Knockdown. Antioxid. Redox Signal. 2011, 15, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Islinger, M.; Voelkl, A.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries 2.0. Histochemistry 2018, 150, 443–471. [Google Scholar] [CrossRef] [Green Version]
- Okumoto, K.; Tamura, S.; Honsho, M.; Fujiki, Y. Peroxisome: Metabolic Functions and Biogenesis. Adv. Exp. Med. Biol. 2020, 1299, 3–17. [Google Scholar] [CrossRef]
- Avshalumov, M.V.; Chen, B.T.; Koós, T.; Tepper, J.M.; Rice, M.E. Endogenous Hydrogen Peroxide Regulates the Excitability of Midbrain Dopamine Neurons via ATP-Sensitive Potassium Channels. J. Neurosci. 2005, 25, 4222–4231. [Google Scholar] [CrossRef] [Green Version]
- Avshalumov, M.V.; Chen, B.T.; Marshall, S.P.; Peña, D.M.; Rice, M.E. Glutamate-Dependent Inhibition of Dopamine Release in Striatum Is Mediated by a New Diffusible Messenger, H2O2. J. Neurosci. 2003, 23, 2744–2750. [Google Scholar] [CrossRef] [Green Version]
- Avshalumov, M.V.; Rice, M.E. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc. Natl. Acad. Sci. USA 2003, 100, 11729–11734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, T.; Zoga, V.; Gemes, G.; McCallum, J.B.; Wu, H.-E.; Pravdic, D.; Liang, M.-Y.; Kwok, W.-M.; Hogan, Q.; Sarantopoulos, C. Suppressed Ca2+ /CaM/CaMKII-dependent K ATP channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc. Natl. Acad. Sci. USA 2009, 106, 8725–8730. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Bosch, M.A.; Zhang, C.; Rønnekleiv, O.K.; Kelly, M.J. Estradiol Protects Neuropeptide Y/Agouti-Related Peptide Neurons against Insulin Resistance in Females. Neuroendocrinology 2019, 110, 105–118. [Google Scholar] [CrossRef]
- Dong, Y.; Carty, J.; Goldstein, N.; He, Z.; Hwang, E.; Chau, D.; Wallace, B.; Kabahizi, A.; Lieu, L.; Peng, Y.; et al. Time and metabolic state-dependent effects of GLP-1R agonists on NPY/AgRP and POMC neuronal activity in vivo. Mol. Metab. 2021, 54, 101352. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xiao, K. Electrophysiological Mechanism of Peripheral Hormones and Nutrients Regulating Energy Homeostasis. Neural Regul. Metab. 2018, 1090, 183–198. [Google Scholar] [CrossRef]
- Cowen, N.; Bhatnagar, A. The Potential Role of Activating the ATP-Sensitive Potassium Channel in the Treatment of Hyperphagic Obesity. Genes 2020, 11, 450. [Google Scholar] [CrossRef] [Green Version]
- Schriever, S.C.; Zimprich, A.; Pfuhlmann, K.; Baumann, P.; Giesert, F.; Klaus, V.; Kabra, D.G.; Hafen, U.; Romanov, A.; Tschöp, M.H.; et al. Alterations in neuronal control of body weight and anxiety behavior by glutathione peroxidase 4 deficiency. Neuroscience 2017, 357, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Jehan, C.; Cartier, D.; Bucharles, C.; Anouar, Y.; Lihrmann, I. Emerging roles of ER-resident selenoproteins in brain physiology and physiopathology. Redox Biol. 2022, 55, 102412. [Google Scholar] [CrossRef]
- Addinsall, A.B.; Wright, C.R.; Andrikopoulos, S.; Van Der Poel, C.; Stupka, N. Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem. J. 2018, 475, 1037–1057. [Google Scholar] [CrossRef]
- Yoo, S.; Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 2018, 170, 53–66. [Google Scholar] [CrossRef]
- Oo, S.M.; Oo, H.K.; Takayama, H.; Ishii, K.-A.; Takeshita, Y.; Goto, H.; Nakano, Y.; Kohno, S.; Takahashi, C.; Nakamura, H.; et al. Selenoprotein P-mediated reductive stress impairs cold-induced thermogenesis in brown fat. Cell Rep. 2022, 38, 110566. [Google Scholar] [CrossRef] [PubMed]
- Kitabayashi, N.; Nakao, S.; Mita, Y.; Arisawa, K.; Hoshi, T.; Toyama, T.; Ishii, K.-A.; Takamura, T.; Noguchi, N.; Saito, Y. Role of selenoprotein P expression in the function of pancreatic β cells: Prevention of ferroptosis-like cell death and stress-induced nascent granule degradation. Free. Radic. Biol. Med. 2022, 183, 89–103. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Saito, Y. Selenoprotein P; P for Plasma, Prognosis, Prophylaxis, and More. Biol. Pharm. Bull. 2020, 43, 366–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seale, L.A.; Ogawa-Wong, A.N.; Berry, M.J. Sexual dimorphism in selenium metabolism and selenoproteins. Free Radic. Biol. Med. 2018, 127, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, S.; Beck-Peccoz, P. Physiology of the Hypothalamic–Pituitary–Thyroid Axis; Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; South Dartmouth: Dartmouth, MA, USA, 2021. [Google Scholar]
- Walczak, K.; Sieminska, L. Obesity and Thyroid Axis. Int. J. Environ. Res. Public Health 2021, 18, 9434. [Google Scholar] [CrossRef] [PubMed]
- Ortiga-Carvalho, T.M.; Chiamolera, M.I.; Pazos-Moura, C.C.; Wondisford, F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016, 6, 1387–1428. [Google Scholar] [CrossRef]
- Williams, G.R.; Bassett, J.H.D. Thyroid diseases and bone health. J. Endocrinol. Investig. 2017, 41, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Yau, W.W.; Yen, P.M. Thermogenesis in Adipose Tissue Activated by Thyroid Hormone. Int. J. Mol. Sci. 2020, 21, 3020. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Erampamoorthy, A.; Zybek-Kocik, A.; Kyriacou, A.; Zgorzalewicz-Stachowiak, M.; Czarnywojtek, A.; Ruchała, M. The Role of Thyroid Hormones on Skeletal Muscle Thermogenesis. Metabolites 2022, 12, 336. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.S.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes 2015, 22, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.C.; Salas-Lucia, F.; Bianco, A.C. Deiodinases and the Metabolic Code for Thyroid Hormone Action. Endocrinology 2021, 162, bqab059. [Google Scholar] [CrossRef]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Li, Y.; Gu, X.; Lei, Z. The correlation between selenium levels and autoimmune thyroid disease: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 4398–4408. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Feng, W.; Chen, H.; Shi, H.; Jiang, L.; Zheng, X.; Liu, X.; Zhang, W.; Ge, Y.; Liu, Y.; et al. Effect of selenium on thyroid autoimmunity and regulatory T cells in patients with Hashimoto’s thyroiditis: A prospective randomized-controlled trial. Clin. Transl. Sci. 2021, 14, 1390–1402. [Google Scholar] [CrossRef]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef]
- Cao, J.; Su, Y.; Chen, Z.; Ma, C.; Xiong, W. The risk factors for Graves’ ophthalmopathy. Graefes. Arch. Clin. Exp. Ophthalmol. 2021, 260, 1043–1054. [Google Scholar] [CrossRef]
- Hou, J.; Tang, Y.; Chen, Y.; Chen, D. The Role of the Microbiota in Graves’ Disease and Graves’ Orbitopathy. Front. Cell Infect. Microbiol. 2021, 11, 739707. [Google Scholar] [CrossRef]
- Benvenga, S.; Nordio, M.; Laganà, A.S.; Unfer, V. The Role of Inositol in Thyroid Physiology and in Subclinical Hypothyroidism Management. Front. Endocrinol. 2021, 12, 662582. [Google Scholar] [CrossRef]
- Nordio, M. A novel treatment for subclinical hyperthyroidism: A pilot study on the beneficial effects of l-carnitine and selenium. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2268–2273. [Google Scholar]
- Derumeaux, H.; Valeix, P.; Castetbon, K.; Bensimon, M.; Boutron-Ruault, M.-C.; Arnaud, J.; Hercberg, S. Association of selenium with thyroid volume and echostructure in 35- to 60-year-old French adults. Eur. J. Endocrinol. 2003, 148, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Coad, J.; Zhou, S.J.; Skeaff, S.; Ramilan, T.; Brough, L. Prevalence of thyroid dysfunction in postpartum women with suboptimal iodine and selenium and adequate iron status. Clin. Endocrinol. 2021, 95, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Sabatino, L.; Coi, A.; Iervasi, G.; Vassalle, C. Thyroid Dysfunction and COVID-19: The Emerging Role of Selenium in This Intermingled Relationship. Int. J. Environ. Res. Public Health 2022, 19, 6912. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders—Essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Carreras, O.; Nogales, F. The Role of Selenoprotein Tissue Homeostasis in MetS Programming: Energy Balance and Cardiometabolic Implications. Antioxidants 2022, 11, 394. [Google Scholar] [CrossRef]
- Gorini, F.; Vassalle, C. Selenium and Selenoproteins at the Intersection of Type 2 Diabetes and Thyroid Pathophysiology. Antioxidants 2022, 11, 1188. [Google Scholar] [CrossRef]
- Hofstee, P.; James-McAlpine, J.; McKeating, D.R.; Vanderlelie, J.J.; Cuffe, J.S.M.; Perkins, A.V. Low serum selenium in pregnancy is associated with reduced T3 and increased risk of GDM. J. Endocrinol. 2021, 248, 45–57. [Google Scholar] [CrossRef]
- Hogan, C.; Perkins, A.V. Selenoproteins in the Human Placenta: How Essential Is Selenium to a Healthy Start to Life? Nutrients 2022, 14, 628. [Google Scholar] [CrossRef]
- Winther, K.H.; Wichman, J.E.M.; Bonnema, S.J.; Hegedüs, L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine 2016, 55, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Benvenga, S.; Feldt-Rasmussen, U.; Bonofiglio, D.; Asamoah, E. Nutraceutical Supplements in the Thyroid Setting: Health Benefits beyond Basic Nutrition. Nutrients 2019, 11, 2214. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Xing, Z.; Xiang, Q.; Yang, Q.; Zhu, J.; Su, A. Insufficient evidence to support the clinical efficacy of selenium supplementation for patients with chronic autoimmune thyroiditis. Endocrine 2021, 73, 384–397. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium in Endocrinology—Selenoprotein-Related Diseases, Population Studies, and Epidemiological Evidence. Endocrinology 2020, 162, bqaa228. [Google Scholar] [CrossRef] [PubMed]
- Hubalewska-Dydejczyk, A.; Duntas, L.; Gilis-Januszewska, A. Pregnancy, thyroid, and the potential use of selenium. Hormones 2019, 19, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Polska 2017, 68, 440–465. [Google Scholar] [CrossRef] [Green Version]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, W.; Xu, Q.; Li, X.; Zhou, L.; Ye, L. Di(2-ethylhexyl) phthalate (DEHP) and thyroid: Biological mechanisms of interference and possible clinical implications. Environ. Sci. Pollut. Res. 2021, 29, 1634–1644. [Google Scholar] [CrossRef]
- Garberg, P.; Högberg, J. Selenium metabolism in isolated hepatocytes: Inhibition of incorporation in proteins by mono(2-ethylhexyl)phthalate, a metabolite of the peroxisome proliferator di(2-ethylhexyl)phthalate. Carcinogenesis 1991, 12, 7–12. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, X.; Yang, M.; Zeng, L.; Liu, C. Roles and potential mechanisms of selenium in countering thyrotoxicity of DEHP. Sci. Total Environ. 2017, 619–620, 732–739. [Google Scholar] [CrossRef]
- Sherif, N.A.E.-H.; El-Banna, A.; Abdel-Moneim, R.A.; Sobh, Z.K.; Balah, M.I.F. The possible thyroid disruptive effect of di-(2-ethyl hexyl) phthalate and the potential protective role of selenium and curcumin nanoparticles: A toxicological and histological study. Toxicol. Res. 2021, 11, 108–121. [Google Scholar] [CrossRef]
- Stannard, L.; Doak, S.H.; Doherty, A.; Jenkins, G.J. Is Nickel Chloride really a Non-Genotoxic Carcinogen? Basic Clin. Pharmacol. Toxicol. 2016, 121, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-Y.; Huang, Y.-L.; Lin, T.-H. Lipid peroxidation in liver of mice administrated with nickel chloride: With special reference to trace elements and antioxidants. Biol. Trace Element Res. 1998, 61, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Salah, I.; Adjroud, O.; Elwej, A. Protective Effects of Selenium and Zinc Against Nickel Chloride–Induced Hormonal Changes and Oxidative Damage in Thyroid of Pregnant Rats. Biol. Trace Element Res. 2021, 200, 2183–2194. [Google Scholar] [CrossRef] [PubMed]
- Mal’Tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, J.; Xu, J.-F.; Pi, J. The Advancing of Selenium Nanoparticles Against Infectious Diseases. Front. Pharmacol. 2021, 12, 682284. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Mal’Tseva, V.N.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V.; Plotnikov, E.Y. Features of the cytoprotective effect of selenium nanoparticles on primary cortical neurons and astrocytes during oxygen–glucose deprivation and reoxygenation. Sci. Rep. 2022, 12, 1710. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef]
- Gustin, K.; Vahter, M.; Barman, M.; Jacobsson, B.; Skröder, H.; Nyström, H.F.; Sandin, A.; Sandberg, A.-S.; Wold, A.E.; Kippler, M. Assessment of Joint Impact of Iodine, Selenium, and Zinc Status on Women’s Third-Trimester Plasma Thyroid Hormone Concentrations. J. Nutr. 2022, 152, 1737–1746. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, L.; Xu, J.; Liu, Z.; Liu, J.; Yan, C. Prenatal Maternal Low Selenium, High Thyrotropin, and Low Birth Weights. Biol. Trace Element Res. 2020, 199, 18–25. [Google Scholar] [CrossRef]
- Kobayashi, R.; Hasegawa, M.; Kawaguchi, C.; Ishikawa, N.; Tomiwa, K.; Shima, M.; Nogami, K. Thyroid function in patients with selenium deficiency exhibits high free T4 to T3 ratio. Clin. Pediatr. Endocrinol. 2021, 30, 19–26. [Google Scholar] [CrossRef]
- Lossow, K.; Renko, K.; Schwarz, M.; Schomburg, L.; Schwerdtle, T.; Kipp, A.P. The Nutritional Supply of Iodine and Selenium Affects Thyroid Hormone Axis Related Endpoints in Mice. Nutrients 2021, 13, 3773. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, M.; Mahmoudi, L.; Ostadrahimi, A.; Pourmoradian, S.; Soleimanzadeh, H.; Kafili, B. Effect of selenium-enriched yeast supplementation on serum thyroid-stimulating hormone and anti-thyroid peroxidase antibody levels in subclinical hypothyroidism: Randomized controlled trial. Adv. Biomed. Res. 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhu, M.; Li, L.; Fan, H.; Lv, F.; Zhu, D. Clinical Observation of Levothyroxine Sodium Combined with Selenium in the Treatment of Patients with Chronic Lymphocytic Thyroiditis and Hypothyroidism and the Effects on Thyroid Function, Mood, and Inflammatory Factors. Evidence-Based Complement. Altern. Med. 2021, 2021, 5471281. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Mortara, L.; Veronesi, G.; Cattaneo, S.A.; Genoni, A.; Gallazzi, M.; Peruzzo, C.; Lasalvia, P.; Moretto, P.; Bruno, A.; et al. Add-On Effect of Selenium and Vitamin D Combined Supplementation in Early Control of Graves’ Disease Hyperthyroidism During Methimazole Treatment. Front. Endocrinol. 2022, 13, 886451. [Google Scholar] [CrossRef]
- Li, Y.; Zuo, X.; Hua, C.; Zhao, Y.; Pei, X.; Tian, M. Effects of Selenium Supplement on B Lymphocyte Activity in Experimental Autoimmune Thyroiditis Rats. Int. J. Endocrinol. 2021, 2021, 9439344. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Calogero, A.E.; Bagnara, V.; Aversa, A.; Greco, E.A.; Brunetti, A.; La Vignera, S. Effects of Selenium Supplementation on Sperm Parameters and DNA-Fragmentation Rate in Patients with Chronic Autoimmune Thyroiditis. J. Clin. Med. 2021, 10, 3755. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [Green Version]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Kuo, T.; McQueen, A.; Chen, T.-C.; Wang, J.-C. Regulation of Glucose Homeostasis by Glucocorticoids. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2015; Volume 872, pp. 99–126. [Google Scholar]
- Juszczak, G.R.; Stankiewicz, A.M. Glucocorticoids, genes and brain function. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 136–168. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.J.; Alfulaij, N.; Berry, M.J. Stress and the Brain: An Emerging Role for Selenium. Front. Neurosci. 2021, 15, 666601. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.G.; Jardim, N.S.; Trindade, M.A.; Nogueira, C.W. Opioid System Contributes to the Trifluoromethyl-Substituted Diselenide Effectiveness in a Lifestyle-Induced Depression Mouse Model. Mol. Neurobiol. 2021, 58, 2231–2241. [Google Scholar] [CrossRef]
- Segat, H.; Martini, F.; Barcelos, R.; Brüning, C.; Nogueira, C.; Burger, M. m-Trifluoromethyl-diphenyldiselenide as a pharmacological tool to treat preference symptoms related to AMPH-induced dependence in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; Voss, G.T.; Rodrigues, K.d.C.; Pinz, M.P.; Biondi, J.V.; Becker, N.P.; Blodorn, E.; Domingues, W.B.; Larroza, A.; Campos, V.F.; et al. Prospecting for a quinoline containing selenium for comorbidities depression and memory impairment induced by restriction stress in mice. Psychopharmacology 2022, 239, 59–81. [Google Scholar] [CrossRef]
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127. [Google Scholar] [CrossRef] [Green Version]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; Lourenço, D.D.A.; Padilha, N.B.; Lenardão, E.J.; Sonego, M.; Seixas, F.K.; Collares, T.; Nogueira, C.W.; et al. The selenium-containing compound 3-((4-chlorophenyl)selanyl)-1-methyl-1H-indole reverses depressive-like behavior induced by acute restraint stress in mice: Modulation of oxido-nitrosative stress and inflammatory pathway. Psychopharmacology 2019, 236, 2867–2880. [Google Scholar] [CrossRef]
- Pesarico, A.P.; Birmann, P.T.; Pinto, R.; Padilha, N.B.; Lenardão, E.J.; Savegnago, L. Short- and Long-Term Repeated Forced Swim Stress Induce Depressive-Like Phenotype in Mice: Effectiveness of 3-[(4-Chlorophenyl)Selanyl]-1-Methyl-1H-Indole. Front. Behav. Neurosci. 2020, 14, 140. [Google Scholar] [CrossRef]
- Casaril, A.M.; Lourenço, D.D.A.; Domingues, M.; Smaniotto, T.; Birmann, P.T.; Vieira, B.; Sonego, M.S.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Anhedonic- and anxiogenic-like behaviors and neurochemical alterations are abolished by a single administration of a selenium-containing compound in chronically stressed mice. Compr. Psychoneuroendocrinol. 2021, 6, 100054. [Google Scholar] [CrossRef]
- Joëls, M.; Karst, H.; Sarabdjitsingh, R.A. The stressed brain of humans and rodents. Acta Physiol. 2018, 223, e13066. [Google Scholar] [CrossRef]
- Torres, R.C.; Magalhães, N.S.; e Silva, P.M.; Martins, M.A.; Carvalho, V.F. Activation of PPAR-γ reduces HPA axis activity in diabetic rats by up-regulating PI3K expression. Exp. Mol. Pathol. 2016, 101, 290–301. [Google Scholar] [CrossRef]
- Sominsky, L.; Jasoni, C.L.; Twigg, H.R.; Spencer, S.J. Hormonal and nutritional regulation of postnatal hypothalamic development. J. Endocrinol. 2018, 237, R47–R64. [Google Scholar] [CrossRef]
- Pijanowski, L.; Jurecka, P.; Irnazarow, I.; Kepka, M.; Szwejser, E.; Kemenade, B.M.L.V.-V.; Chadzinska, M. Activity of the hypothalamus–pituitary–interrenal axis (HPI axis) and immune response in carp lines with different susceptibility to disease. Fish Physiol. Biochem. 2015, 41, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Mancera, J.M.; Costas, B. The Use of Dietary Additives in Fish Stress Mitigation: Comparative Endocrine and Physiological Responses. Front. Endocrinol. 2019, 10, 447. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, D.K.; Bhushan, S.; Jamwal, A. Mitigating multiple stresses in Pangasianodon hypophthalmus with a novel dietary mixture of selenium nanoparticles and Omega-3-fatty acid. Sci. Rep. 2021, 11, 19429. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.; Dominguez, D.; Bravo, J.; Acosta, F.; Robaina, L.; Geraert, P.-A.; Kaushik, S.; Izquierdo, M. Organic Selenium (OH-MetSe) Effect on Whole Body Fatty Acids and Mx Gene Expression against Viral Infection in Gilthead Seabream (Sparus aurata) Juveniles. Animals 2021, 11, 2877. [Google Scholar] [CrossRef]
- Patterson, S.; Zee, J.; Wiseman, S.; Hecker, M. Effects of chronic exposure to dietary selenomethionine on the physiological stress response in juvenile white sturgeon (Acipenser transmontanus). Aquat. Toxicol. 2017, 186, 77–86. [Google Scholar] [CrossRef]
- Miller, L.; Hontela, A. Species-specific sensitivity to selenium-induced impairment of cortisol secretion in adrenocortical cells of rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis). Toxicol. Appl. Pharmacol. 2011, 253, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-J.; Hsu, N.-C.; Hu, M.-C.; Chung, B.-C. Steroidogenesis in zebrafish and mouse models. Mol. Cell Endocrinol. 2006, 248, 160–163. [Google Scholar] [CrossRef]
- Zhao, M.; Luo, T.; Zhao, Z.; Rong, H.; Zhao, G.; Lei, L. Food Chemistry of Selenium and Controversial Roles of Selenium in Affecting Blood Cholesterol Concentrations. J. Agric. Food Chem. 2021, 69, 4935–4945. [Google Scholar] [CrossRef] [PubMed]
- May, P.; Woldt, E.; Matz, R.L.; Boucher, P. The LDL receptor-related protein (LRP) family: An old family of proteins with new physiological functions. Ann. Med. 2007, 39, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Kaixin, Z.; Xuedie, G.; Jing, L.; Yiming, Z.; Khoso, P.A.; Zhaoyi, L.; Shu, L. Selenium-deficient diet induces inflammatory response in the pig adrenal glands by activating TLR4/NF-κB pathway via miR-30d-R. Metallomics 2021, 13, mfab037. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Luo, J.; Ding, H.; Liu, S.; Amer, S.; Xie, L.; Lyv, W.; Su, W.; Li, M.; et al. Identification of miRNomes reveals ssc-miR-30d-R_1 as a potential therapeutic target for PRRS viral infection. Sci. Rep. 2016, 6, 24854. [Google Scholar] [CrossRef] [PubMed]
- Kanczkowski, W.; Tymoszuk, P.; Chavakis, T.; Janitzky, V.; Weirich, T.; Zacharowski, K.; Ehrhart-Bornstein, M.; Bornstein, S.R. Upregulation of TLR2 and TLR4 in the human adrenocortical cells differentially modulates adrenal steroidogenesis. Mol. Cell Endocrinol. 2011, 336, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chanoine, J.-P.; Wong, A.C.; Lavoie, J.-C. Selenium deficiency impairs corticosterone and leptin responses to adrenocorticotropin in the rat. BioFactors 2004, 20, 109–118. [Google Scholar] [CrossRef]
- Abid, H.; Cartier, D.; Hamieh, A.; François-Bellan, A.-M.; Bucharles, C.; Pothion, H.; Manecka, D.-L.; Leprince, J.; Adriouch, S.; Boyer, O.; et al. AMPK Activation of PGC-1α/NRF-1-Dependent SELENOT Gene Transcription Promotes PACAP-Induced Neuroendocrine Cell Differentiation Through Tolerance to Oxidative Stress. Mol. Neurobiol. 2018, 56, 4086–4101. [Google Scholar] [CrossRef]
- Rock, C.; Moos, P.J. Selenoprotein P regulation by the glucocorticoid receptor. BioMetals 2009, 22, 995–1009. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.Y.; Kim, K.-H. Dexamethasone-induced selenoprotein S degradation is required for adipogenesis. J. Lipid Res. 2013, 54, 2069–2082. [Google Scholar] [CrossRef] [Green Version]
- Wray, J.R.; Davies, A.; Sefton, C.; Allen, T.-J.; Adamson, A.; Chapman, P.; Lam, B.; Yeo, G.S.; Coll, A.P.; Harno, E.; et al. Global transcriptomic analysis of the arcuate nucleus following chronic glucocorticoid treatment. Mol. Metab. 2019, 26, 5–17. [Google Scholar] [CrossRef]
- Marano, G.; Fischioni, P.; Graziano, C.; Iannone, M.; Morisi, G. Increased Serum Selenium Levels in Patients under Corticosteroid Treatment. Pharmacol. Toxicol. 1990, 67, 120–122. [Google Scholar] [CrossRef]
- Voĭtsekhovskaia, I.G.; Orlikov, G.A.; Voskresenskaia, N.; Umnova, L.M.; Ivanov, I.V.; Gredzena, P.; Karpov, I.G.; Ianovskaia, I.; Voĭtsekhovskiĭ, V.V.; Maulinysh, E. Experience with selenium used to recover adrenocortical function in patients taking glucocorticosteroids long. Ter. Arkhiv 2013, 85, 76–78. [Google Scholar]
- Feldt-Rasmussen, U.; Effraimidis, G.; Klose, M. The hypothalamus-pituitary-thyroid (HPT)-axis and its role in physiology and pathophysiology of other hypothalamus-pituitary functions. Mol. Cell Endocrinol. 2021, 525, 111173. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.A.; McGee, H.; Bhatta, S.; Palm, R.; Casadesus, G. Hypothalamic–pituitary–gonadal axis involvement in learning and memory and Alzheimer’s disease: More than “just” estrogen. Front. Endocrinol. 2015, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2021, 11, 604000. [Google Scholar] [CrossRef]
- Hornung, J.; Lewis, C.A.; Derntl, B. Sex hormones and human brain function. Handb. Clin. Neurol. 2020, 175, 195–207. [Google Scholar] [CrossRef]
- Rehbein, E.; Hornung, J.; Poromaa, I.S.; Derntl, B. Shaping of the Female Human Brain by Sex Hormones: A Review. Neuroendocrinology 2020, 111, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Willemars, M.M.A.; Nabben, M.; Verdonschot, J.A.J.; Hoes, M.F. Evaluation of the Interaction of Sex Hormones and Cardiovascular Function and Health. Curr. Heart Fail. Rep. 2022, 19, 200–212. [Google Scholar] [CrossRef]
- Sumien, N.; Cunningham, J.T.; Davis, D.L.; Engelland, R.; Fadeyibi, O.; Farmer, G.E.; Mabry, S.; Mensah-Kane, P.; Trinh, O.T.P.; Vann, P.H.; et al. Neurodegenerative Disease: Roles for Sex, Hormones, and Oxidative Stress. Endocrinology 2021, 162, bqab185. [Google Scholar] [CrossRef]
- Kheradmand, N.; Kamkar, R.; Moshajjari, M.; Baazm, M. Effect of selenium and pentoxifylline on expression of CATSPER1 and 2 genes and FSH/LH levels in treated mice by dexamethasone. Andrologia 2019, 51, e13279. [Google Scholar] [CrossRef]
- Lukusa, K.; Lehloenya, K. Selenium supplementation improves testicular characteristics and semen quality of Saanen bucks. Small Rumin. Res. 2017, 151, 52–58. [Google Scholar] [CrossRef]
- Alves, J.; Barrientos, G.; Toro, V.; Grijota, F.J.; Muñoz, D.; Maynar, M. Correlations between Basal Trace Minerals and Hormones in Middle and Long-Distance High-Level Male Runners. Int. J. Environ. Res. Public Health 2020, 17, 9473. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.; Arikan, T.; Kilinc, M.; Arikan, D.C.; Ekerbiçer, H. Plasma selenium levels in Turkish women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Vidlar, A.; Vostálová, J.; Ulrichova, J.; Student, V.; Krajicek, M.; Vrbkova, J.; Simanek, V. The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy—a six month placebo-controlled double-blind clinical trial. Biomed. Pap. 2010, 154, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Vostalova, J.; Vidlar, A.; Ulrichova, J.; Vrbkova, J.; Simanek, V.; Student, V. Use of selenium–silymarin mix reduces lower urinary tract symptoms and prostate specific antigen in men. Phytomedicine 2013, 21, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Behne, D.; Weiler, H.; Kyriakopoulos, A. Effects of selenium deficiency on testicular morphology and function in rats. Reproduction 1996, 106, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Song, R.; Yao, X.; Ren, Y. Effects of selenium on the proliferation, apoptosis and testosterone production of sheep Leydig cells in vitro. Theriogenology 2017, 93, 24–32. [Google Scholar] [CrossRef]
- Asadi, N. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Pitts, M.W.; Kremer, P.M.; Hashimoto, A.C.; Torres, D.J.; Byrns, C.; Williams, C.; Berry, M.J. Competition between the Brain and Testes under Selenium-Compromised Conditions: Insight into Sex Differences in Selenium Metabolism and Risk of Neurodevelopmental Disease. J. Neurosci. 2015, 35, 15326–15338. [Google Scholar] [CrossRef] [Green Version]
- Safarinejad, M.R.; Safarinejad, S. Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2009, 181, 741–751. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Alkan, Z.; Wong, K. Selenium Supplementation Does Not Affect Testicular Selenium Status or Semen Quality in North American Men. J. Androl. 2009, 30, 525–533. [Google Scholar] [CrossRef]
- Ahsan, U.; Kamran, Z.; Raza, I.; Ahmad, S.; Babar, W.; Riaz, M.; Iqbal, Z. Role of selenium in male reproduction—A review. Anim. Reprod. Sci. 2014, 146, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Zeybek, N.D.; Giray, B.; Asan, E.; Arnaud, J.; Hincal, F. Reproductive toxicity of di(2-ethylhexyl) phthalate in selenium-supplemented and selenium-deficient rats. Drug Chem. Toxicol. 2011, 34, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Rashad, M.M.; Galal, M.K.; Abou-El-Sherbini, K.S.; El-Behairy, A.M.; Gouda, E.M.; Moussa, S.Z. Nano-sized selenium attenuates the developmental testicular toxicity induced by di-n-butyl phthalate in pre-pubertal male rats. Biomed. Pharmacother. 2018, 107, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Aliyu-Banjo, N.O.; Odunola, O.A. Selenium attenuates diclofenac-induced testicular and epididymal toxicity in rats. Andrologia 2020, 52, e13669. [Google Scholar] [CrossRef]
- El-Fakharany, Y.M.; Mohamed, E.M.; Etewa, R.L.; Hamid, O.I.A. Selenium nanoparticles alleviate lead acetate-induced toxicological and morphological changes in rat testes through modulation of calmodulin-related genes expression. J. Biochem. Mol. Toxicol. 2022, 36, e23017. [Google Scholar] [CrossRef]
- Adedara, I.A.; Awogbindin, I.O.; Mohammed, K.A.; Da-Silva, O.F.; Farombi, E.O. Abatement of the dysfunctional hypothalamic–pituitary–gonadal axis due to ciprofloxacin administration by selenium in male rats. J. Biochem. Mol. Toxicol. 2021, 35, e22741. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, Y.; Wang, Y.; Wu, Q.; Cheng, B.; Li, Y.; Wang, Z.; Chai, X.; Ren, A.; Li, G. Role of Endoplasmic Reticulum Stress in Nano-Selenium Alleviating Prehierarchical Follicular Atresia Induced by Mercury in Laying Hens. Biol. Trace Element Res. 2022, 200, 5205–5217. [Google Scholar] [CrossRef]
- Xie, C.; Bian, Y.; Feng, H.; Zhao, Y.; Wang, L.; Li, Y.; Zhang, D.; Tian, Y.; Li, L.; Chang, S.; et al. Reversal of ciprofloxacin-induced testosterone reduction by probiotic microbes in mouse testes. Gen. Comp. Endocrinol. 2019, 284, 113268. [Google Scholar] [CrossRef]
- Derar, D.; Ali, A.; Almundarij, T.; Abd-Elmoniem, E.; Alhassun, T.; Zeitoun, M. Association between Serum Trace Elements Levels, Steroid Concentrations, and Reproductive Disorders in Ewes and Does. Veter-Med. Int. 2022, 2022, 8525089. [Google Scholar] [CrossRef]
- Cerny, K.L.; Anderson, L.; Burris, W.R.; Rhoads, M.; Matthews, J.C.; Bridges, P.J. Form of supplemental selenium fed to cycling cows affects systemic concentrations of progesterone but not those of estradiol. Theriogenology 2016, 85, 800–806. [Google Scholar] [CrossRef]
- Carr, S.; Jia, Y.; Crites, B.; Hamilton, C.; Burris, W.; Edwards, J.L.; Matthews, J.; Bridges, P.J. Form of Supplemental Selenium in Vitamin-Mineral Premixes Differentially Affects Early Luteal and Gestational Concentrations of Progesterone, and Postpartum Concentrations of Prolactin in Beef Cows. Animals 2020, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Lekatz, L.A.; Caton, J.S.; Taylor, J.B.; Reynolds, L.P.; Redmer, D.A.; Vonnahme, K.A. Maternal selenium supplementation and timing of nutrient restriction in pregnant sheep: Effects on maternal endocrine status and placental characteristics1. J. Anim. Sci. 2010, 88, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Kamada, H.; Nonaka, I.; Takenouchi, N.; Amari, M. Effects of selenium supplementation on plasma progesterone concentrations in pregnant heifers. Anim. Sci. J. 2013, 85, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.N.; Crites, B.R.; Pate, J.L.; Hughes, C.H.K.; Matthews, J.C.; Bridges, P.J. Form of Supplemental Selenium Affects the Expression of mRNA Transcripts Encoding Selenoproteins, and Proteins Regulating Cholesterol Uptake, in the Corpus Luteum of Grazing Beef Cows. Animals 2022, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Lane-Donovan, C.; Herz, J. The ApoE receptors Vldlr and Apoer2 in central nervous system function and disease. J. Lipid Res. 2017, 58, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Mo, A.; Wang, X.; Yuan, Y.; Liu, C.; Wang, J. Effects of waterborne exposure to environmentally relevant concentrations of selenite on reproductive function of female zebrafish: A life cycle assessment. Environ. Pollut. 2020, 270, 116237. [Google Scholar] [CrossRef]
- Wiseman, S.; Thomas, J.K.; Higley, E.; Hursky, O.; Pietrock, M.; Raine, J.C.; Giesy, J.P.; Janz, D.M.; Hecker, M. Chronic exposure to dietary selenomethionine increases gonadal steroidogenesis in female rainbow trout. Aquat. Toxicol. 2011, 105, 218–226. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenomethionine: A Review of Its Nutritional Significance, Metabolism and Toxicity. J. Nutr. 2000, 130, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Seeher, S.; Atassi, T.; Mahdi, Y.; Carlson, B.A.; Braun, D.; Wirth, E.K.; Klein, M.O.; Reix, N.; Miniard, A.C.; Schomburg, L.; et al. Secisbp2 Is Essential for Embryonic Development and Enhances Selenoprotein Expression. Antioxid. Redox Signal. 2014, 21, 835–849. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cheng, W.; Luo, J.; Hu, X.; Nie, T.; Lai, H.; Zheng, X.; Li, F.; Li, H. Loss of selenocysteine insertion sequence binding protein 2 suppresses the proliferation, migration/invasion and hormone secretion of human trophoblast cells via the PI3K/Akt and ERK signaling pathway. Placenta 2017, 55, 81–89. [Google Scholar] [CrossRef]
- El-Naby, A.-S.A.-H.H.; Ibrahim, S.; Hozyen, H.F.; Sosa, A.S.A.; Mahmoud, K.G.M.; Farghali, A.A. Impact of nano-selenium on nuclear maturation and genes expression profile of buffalo oocytes matured in vitro. Mol. Biol. Rep. 2020, 47, 8593–8603. [Google Scholar] [CrossRef] [PubMed]
- Starkl, P.; Marichal, T.; Galli, S.J. PLA2G3 promotes mast cell maturation and function. Nat. Immunol. 2013, 14, 527–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Ei-Samahy, M.; Fan, L.; Zheng, L.; Jin, Y.; Pang, J.; Zhang, G.; Liu, Z.; Wang, F. In vitro influence of selenium on the proliferation of and steroidogenesis in goat luteinized granulosa cells. Theriogenology 2018, 114, 70–80. [Google Scholar] [CrossRef]
- Grattan, D.R. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-prolactin axis. J. Endocrinol. 2015, 226, T101–T122. [Google Scholar] [CrossRef]
- Shen, W.; Chen, J.; Yin, J.; Wang, S.-L. Selenium protects reproductive system and foetus development in a rat model of gestational lead exposure. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 773–780. [Google Scholar] [PubMed]
- Ebokaiwe, A.P.; Obeten, K.E.; Okori, S.O.; David, E.E.; Olusanya, O.; Chukwu, C.J.; Okoro, N.; Ehiri, R.C. Co-administration of Selenium Nanoparticles and Metformin Abrogate Testicular Oxidative Injury by Suppressing Redox Imbalance, Augmenting Sperm Quality and Nrf2 Protein Expression in Streptozotocin-Induced Diabetic Rats. Biol. Trace Elem. Res. 2020, 198, 544–556. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Q.; Burris, W.R.; Aiken, G.E.; Bridges, P.J.; Matthews, J.C. Forms of selenium in vitamin-mineral mixes differentially affect serum prolactin concentration and hepatic glutamine synthetase activity of steers grazing endophyte-infected tall fescue. J. Anim. Sci. 2018, 96, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jia, Y.; Burris, W.R.; Bridges, P.J.; Matthews, J.C. Forms of selenium in vitamin–mineral mixes differentially affect the expression of genes responsible for prolactin, ACTH, and α-MSH synthesis and mitochondrial dysfunction in pituitaries of steers grazing endophyte-infected tall fescue1. J. Anim. Sci. 2018, 97, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Fox, S.R.; Jong, M.T.C.; Casanova, J.; Ye, Z.-S.; Stanley, F.; Samuels, H.H. The Homeodomain Protein, Pit-1/GHF-1, Is Capable of Binding to and Activating Cell-Specific Elements of Both the Growth Hormone and Prolactin Gene Promoters. Mol. Endocrinol. 1990, 4, 1069–1080. [Google Scholar] [CrossRef] [Green Version]
- Castaño, A.; Ayala, A.; Rodríguez-Gómez, J.A.; De La Cruz, C.P.; Revilla, E.; Cano, J.; Machado, A. Increase in dopamine turnover and tyrosine hydroxylase enzyme in hippocampus of rats fed on low selenium diet. J. Neurosci. Res. 1995, 42, 684–691. [Google Scholar] [CrossRef]
- Castano, A.; Ayala, A.; Rodriguez-Gomez, J.A.; Herrera, A.J.; Cano, J.; Machado, A. Low selenium diet increases the dopamine turnover in prefrontal cortex of the rat. Neurochem. Int. 1997, 30, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Castaño, A.; Cano, J.; Machado, A. Low Selenium Diet Affects Monoamine Turnover Differentially in Substantia Nigra and Striatum. J. Neurochem. 1993, 61, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramos, M.; Venero, J.L.; Cano, J.; Machado, A. Low selenium diet induces tyrosine hydroxylase enzyme in nigrostriatal system of the rat. Mol. Brain Res. 2000, 84, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yamamoto, S.; Takahata, M.; Sakaguchi, H.; Tanaka, H.; Stadtman, T.C.; Inagaki, K. Selenophosphate synthetase genes from lung adenocarcinoma cells: Sps1 for recycling l -selenocysteine and Sps2 for selenite assimilation. Proc. Natl. Acad. Sci. USA 2004, 101, 16162–16167. [Google Scholar] [CrossRef]
- Tobe, R.; Carlson, B.A.; Huh, J.H.; Castro, N.P.; Xu, X.-M.; Tsuji, P.A.; Lee, S.-G.; Bang, J.; Na, J.-W.; Kong, Y.-Y.; et al. Selenophosphate synthetase 1 is an essential protein with roles in regulation of redox homoeostasis in mammals. Biochem. J. 2016, 473, 2141–2154. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.; Han, M.; Yoo, T.-J.; Qiao, L.; Jung, J.; Na, J.; Carlson, B.A.; Gladyshev, V.N.; Hatfield, D.L.; Kim, J.-H.; et al. Identification of Signaling Pathways for Early Embryonic Lethality and Developmental Retardation in Sephs1−/− Mice. Int. J. Mol. Sci. 2021, 22, 11647. [Google Scholar] [CrossRef]
- Na, J.; Jung, J.; Bang, J.; Lu, Q.; Carlson, B.A.; Guo, X.; Gladyshev, V.N.; Kim, J.; Hatfield, D.L.; Lee, B.J. Selenophosphate synthetase 1 and its role in redox homeostasis, defense and proliferation. Free Radic. Biol. Med. 2018, 127, 190–197. [Google Scholar] [CrossRef]
- Adeniyi, M.J.; Agoreyo, F.O.; Olorunnisola, O.L.; Olaniyan, O.T.; Seriki, S.A.; Ozolua, O.P.; Odetola, A.A. Photo-pollution disrupts reproductive homeostasis in female rats: The duration-dependent role of selenium administrations. Chin. J. Physiol. 2020, 63, 235–243. [Google Scholar] [CrossRef]
- Lemley, C.; Meyer, A.; Neville, T.; Hallford, D.; Camacho, L.; Maddock-Carlin, K.; Wilmoth, T.; Wilson, M.; Perry, G.; Redmer, D.; et al. Dietary selenium and nutritional plane alter specific aspects of maternal endocrine status during pregnancy and lactation. Domest. Anim. Endocrinol. 2014, 46, 1–11. [Google Scholar] [CrossRef]
- Rana, M.; Jain, S.; Choubey, P. Prolactin and its significance in the placenta. Hormones 2022, 21, 209–219. [Google Scholar] [CrossRef]
- Santos, C.A.; Costa-Brito, A.; Gonçalves, I. The brain as a source and a target of prolactin in mammals. Neural Regen. Res. 2022, 17, 1695. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, O.A.; Al Wakeel, R.A.; Hemeda, S.A.; Abdel-Daim, M.M.; Albadrani, G.M.; El Askary, A.; Fadl, S.E.; Elgendey, F. The impact of vitamin E and/or selenium dietary supplementation on growth parameters and expression levels of the growth-related genes in broilers. BMC Veter- Res. 2021, 17, 251. [Google Scholar] [CrossRef]
- Xia, W.; Huang, Z.; Chen, W.; Fouad, A.; Abouelezz, K.; Li, K.; Huang, X.; Wang, S.; Ruan, D.; Zhang, Y.; et al. Effects of maternal and progeny dietary selenium supplementation on growth performance and antioxidant capacity in ducklings. Poult. Sci. 2021, 101, 101574. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, E.M.; El-Kader, M.F.A.; Hassan, M.M.; El-Bab, A.F.F.; Omar, A.; Farrag, F.; Gewida, A.G.; Abd-Elghany, M.F.; Shukry, M.; Alwakeel, R.A. Trial for use nanoselenium particle with different dietary regime in Oreochromis niloticus and Mugil cephalus polyculture ponds: Growth efficiency, haematological, antioxidant, immunity and transcriptional analysis. Veter-Med. Sci. 2021, 7, 1575–1586. [Google Scholar] [CrossRef]
- Knight, R.; Marlatt, V.L.; Baker, J.A.; Lo, B.P.; Debruyn, A.M.; Elphick, J.R.; Martyniuk, C.J. Dietary selenium disrupts hepatic triglyceride stores and transcriptional networks associated with growth and Notch signaling in juvenile rainbow trout. Aquat. Toxicol. 2016, 180, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Ren, T.; Han, Y.; Rahman, M.; Hu, Y.; Li, Z.; Jiang, Z. Influences of dietary selenomethionine exposure on tissue accumulation, blood biochemical profiles, gene expression and intestinal microbiota of Carassius auratus. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2018, 218, 21–29. [Google Scholar] [CrossRef]
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Bandinelli, S.; Dall’Aglio, E.; Guralnik, J.M.; Paolisso, G.; Semba, R.D.; Nouvenne, A.; Borghi, L.; et al. Association of plasma selenium concentrations with total IGF-1 among older community-dwelling adults: The InCHIANTI study. Clin. Nutr. 2010, 29, 674–677. [Google Scholar] [CrossRef] [Green Version]
- Karl, J.P.; Alemany, J.A.; Koenig, C.; Kraemer, W.J.; Frystyk, J.; Flyvbjerg, A.; Young, A.J.; Nindl, B.C. Diet, body composition, and physical fitness influences on IGF-I bioactivity in women. Growth Horm. IGF Res. 2009, 19, 491–496. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef]
- Sparapani, S.; Millet-Boureima, C.; Oliver, J.; Mu, K.; Hadavi, P.; Kalostian, T.; Ali, N.; Avelar, C.M.; Bardies, M.; Barrow, B.; et al. The Biology of Vasopressin. Biomedicines 2021, 9, 89. [Google Scholar] [CrossRef]
- Busse, N.I.; Uberti, B. Uterine Inertia due to Severe Selenium Deficiency in a Parturient Mare. J. Equine Veter- Sci. 2019, 85, 102845. [Google Scholar] [CrossRef]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress During Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Boldižárova, K.; Faix, Š.; Kováč, G. The urinary excretion of selenium in sheep treated with a vasopressin analogue. Veter- Res. 2000, 31, 499–505. [Google Scholar] [CrossRef]
- Neal, E.S.; Hofstee, P.; Askew, M.R.; Kent, N.L.; Bartho, L.A.; Perkins, A.V.; Cuffe, J.S.M. Maternal selenium deficiency in mice promotes sex-specific changes to urine flow and renal expression of mitochondrial proteins in adult offspring. Physiol. Rep. 2021, 9, e14785. [Google Scholar] [CrossRef] [PubMed]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free. Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Kowalczyk, J.C.; Meimaridou, E.; Storr, H.L.; Metherell, L.A. Oxidative stress and adrenocortical insufficiency. J. Endocrinol. 2014, 221, R63–R73. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Sengupta, P.; Das, S.; Slama, P.; Roychoudhury, S. Reactive Nitrogen Species and Male Reproduction: Physiological and Pathological Aspects. Int. J. Mol. Sci. 2022, 23, 10574. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [Green Version]
- Solovyev, N.; Vanhaecke, F.; Michalke, B. Selenium and iodine in diabetes mellitus with a focus on the interplay and speciation of the elements. J. Trace Elements Med. Biol. 2019, 56, 69–80. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Støy, J.; Edghill, E.L.; Flanagan, S.E.; Ye, H.; Paz, V.P.; Pluzhnikov, A.; Below, J.E.; Hayes, M.G.; Cox, N.J.; Lipkind, G.M.; et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15040–15044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrenner, H.; Hotze, A.L.; Speckmann, B.; Pinto, A.; Sies, H.; Schott, M.; Ehlers, M.; Scherbaum, W.A.; Schinner, S. Localization and regulation of pancreatic selenoprotein P. J. Mol. Endocrinol. 2012, 50, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y. Selenoprotein P as a significant regulator of pancreatic β cell function. J. Biochem. 2019, 167, 119–124. [Google Scholar] [CrossRef]
- Mita, Y.; Nakayama, K.; Toshinari, T.; Nishito, Y.; Yoshioka, Y.; Sakai, N.; Sotani, K.; Nagamura, T.; Kuzuhara, Y.; Inagaki, K.; et al. Selenoprotein P-neutralizing antibodies improve insulin secretion and glucose sensitivity in type 2 diabetes mouse models. Nat. Commun. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, H.; Shimizu, R.; Okuno, T.; Ogino, H.; Arakawa, T.; Murano, K.; Nakamuro, K. Effect of Seleno-L-methionine on Oxidative Stress in the Pancreatic Islets of a Short-Term Induced Diabetic Mouse Model in Insufficient Selenium Status. Biol. Pharm. Bull. 2018, 41, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Seth, M.; Biswas, R.; Ganguly, S.; Chakrabarti, N.; Chaudhuri, A. Leptin and obesity. Physiol. Int. 2021, 107, 455–468. [Google Scholar] [CrossRef] [PubMed]
- De Vos, P.; Lefebvre, A.-M.; Miller, S.G.; Guerre-Millo, M.; Wong, K.; Saladin, R.; Hamann, L.G.; Staels, B.; Briggs, M.R.; Auwerx, J. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor gamma. J. Clin. Investig. 1996, 98, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, A.N.; Susulic, V.S.; Madura, J.P.; Zhang, B.; Moller, D.E.; Tontonoz, P.; Sarraf, P.; Spiegelman, B.M.; Lowell, B.B. Functional Antagonism between CCAAT/Enhancer Binding Protein-α and Peroxisome Proliferator-activated Receptor-γ on the Leptin Promoter. J. Biol. Chem. 1997, 272, 5283–5290. [Google Scholar] [CrossRef] [Green Version]
- Sinha, D.; Addya, S.; Murer, E.; Boden, G. 15-deoxy-Δ12,14 prostaglandin J2: A putative endogenous promoter of adipogenesis suppresses the ob gene. Metabolism 1999, 48, 786–791. [Google Scholar] [CrossRef]
- Törüner, F.; Akbay, E.; Cakir, N.; Sancak, B.; Elbeg, S.; Taneri, F.; Aktürk, M.; Karakoç, A.; Ayvaz, G.; Arslan, M. Effects of PPARγ and PPARα Agonists on Serum Leptin Levels in Diet-induced Obese Rats. Horm. Metab. Res. 2004, 36, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Nido, S.A.; Shituleni, S.A.; Mengistu, B.M.; Liu, Y.; Khan, A.Z.; Gan, F.; Kumbhar, S.; Huang, K. Effects of Selenium-Enriched Probiotics on Lipid Metabolism, Antioxidative Status, Histopathological Lesions, and Related Gene Expression in Mice Fed a High-Fat Diet. Biol. Trace Element Res. 2015, 171, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.S.; Pallauf, J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J. Nutr. Biochem. 2006, 17, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, D.; Chen, M.; Jiang, L.; Liu, X.; Li, Q.; Geng, C.; Sun, X.; Yang, G.; Zhang, L.; et al. Citreoviridin induces myocardial apoptosis through PPAR-γ-mTORC2-mediated autophagic pathway and the protective effect of thiamine and selenium. Chem. Interact. 2019, 311, 108795. [Google Scholar] [CrossRef]
- Shon, M.-S.; Song, J.-H.; Kim, G.-N. Anti-obese function of selenate, an essential micronutrient, by regulation of adipogenesis in C3H10T1/2 cells. Kor. J. Aesthet. Cosmetol. 2013, 11, 447–452. [Google Scholar]
- Kim, C.Y.; Kim, K.-H. Selenate Prevents Adipogenesis through Induction of Selenoprotein S and Attenuation of Endoplasmic Reticulum Stress. Molecules 2018, 23, 2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinkov, A.A.; Ajsuvakova, O.P.; Filippini, T.; Zhou, J.-C.; Lei, X.G.; Gatiatulina, E.; Michalke, B.; Skalnaya, M.G.; Vinceti, M.; Aschner, M.; et al. Selenium and Selenoproteins in Adipose Tissue Physiology and Obesity. Biomolecules 2020, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Fried, S.K. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am. J. Physiol. Metab. 2009, 296, E1230–E1238. [Google Scholar] [CrossRef]
- Ezaki, O. The insulin-like effects of selenate in rat adipocytes. J. Biol. Chem. 1990, 265, 1124–1128. [Google Scholar] [CrossRef]
- Hwang, D.; Seo, S.; Kim, Y.; Kim, C.; Shim, S.; Jee, S.; Lee, S.; Jang, M.; Kim, M.; Yim, S.; et al. Selenium acts as an insulin-like molecule for the down-regulation of diabetic symptoms via endoplasmic reticulum stress and insulin signalling proteins in diabetes-induced non-obese diabetic mice. J. Biosci. 2007, 32, 723–735. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 2018, 234, 8152–8161. [Google Scholar] [CrossRef]
- Shyu, K.-G.; Chen, S.-C.; Wang, B.-W.; Cheng, W.-P.; Hung, H.-F. Mechanism of the inhibitory effect of atorvastatin on leptin expression induced by angiotensin II in cultured human coronary artery smooth muscle cells. Clin. Sci. 2011, 122, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghantous, C.M.; Kobeissy, F.H.; Soudani, N.; Rahman, F.A.; Al-Hariri, M.; Itani, H.A.; Sabra, R.; Zeidan, A. Mechanical stretch-induced vascular hypertrophy occurs through modulation of leptin synthesis-mediated ROS formation and GATA-4 nuclear translocation. Front. Pharmacol. 2015, 6, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aritaa, Y.; Kiharaa, S.; Ouchia, N.; Takahashia, M.; Maedaa, K.; Ichiromiyagawaa, J.; Hottaa, K.; Shimomuraa, I.; Nakamuraa, T.; Miyaokaa, K.; et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Horikoshi, M.; Yamauchi, T.; Yago, H.; Miyazaki, O.; Ebinuma, H.; Imai, Y.; Nagai, R.; Kadowaki, T. Measurement of the High–Molecular Weight Form of Adiponectin in Plasma Is Useful for the Prediction of Insulin Resistance and Metabolic Syndrome. Diabetes Care 2006, 29, 1357–1362. [Google Scholar] [CrossRef]
- Diep Nguyen, T.M. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef]
- Dobrzyn, K.; Smolińska, N.; Kiezun, M.; Szeszko, K.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Kaminski, T. Adiponectin: A New Regulator of Female Reproductive System. Int. J. Endocrinol. 2018, 2018, 7965071. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Reszka, E.; Gromadzinska, J.; Wieczorek, E.; Krol, M.B.; Raimondi, S.; Socha, K.; Borawska, M.H.; Wasowicz, W. The Effect of Selenium Supplementation on Glucose Homeostasis and the Expression of Genes Related to Glucose Metabolism. Nutrients 2016, 8, 772. [Google Scholar] [CrossRef] [Green Version]
- An, S.; Yu, J.; Choi, H.; Ko, H.; Ahn, S.; Shin, J.C.; Pyo, J.J.; Jeong, L.S.; Noh, M. Selenium bioisosteric replacement of adenosine derivatives promoting adiponectin secretion increases the binding affinity to peroxisome proliferator-activated receptor δ. Bioorganic Med. Chem. 2019, 28, 115226. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, S.I.; Lee, H.R.; Hwang, I.S.; Lee, Y.J.; An, B.S.; Lee, S.H.; Kim, H.J.; Kang, B.C.; Hwang, D.Y. Selenium Significantly Inhibits Adipocyte Hypertrophy and Abdominal Fat Accumulation in OLETF Rats via Induction of Fatty Acid β-Oxidation. Biol. Trace Element Res. 2012, 150, 360–370. [Google Scholar] [CrossRef]
- Pascoal, G.D.F.L.; Novaes, G.M.; Sobrinho, M.D.P.; Hirayama, A.B.; Castro, I.A.; Ong, T.P. Selenium Supplementation during Puberty and Young Adulthood Mitigates Obesity-Induced Metabolic, Cellular and Epigenetic Alterations in Male Rat Physiology. Antioxidants 2022, 11, 895. [Google Scholar] [CrossRef]
- Deschaine, S.L.; Leggio, L. From “Hunger Hormone” to “It’s Complicated”: Ghrelin Beyond Feeding Control. Physiology 2022, 37, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Sadeghpour, S.; Ghasemnejad-Berenji, H.; Pashapour, S.; Ghasemnejad-Berenji, M. Potential Antioxidative, Anti-inflammatory and Immunomodulatory Effects of Ghrelin, an Endogenous Peptide from the Stomach in SARS-CoV2 Infection. Int. J. Pept. Res. Ther. 2021, 27, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dou, S.; Zhu, J.; Shao, Z.; Wang, C.; Cheng, B. Regulatory effects of ghrelin on endoplasmic reticulum stress, oxidative stress, and autophagy: Therapeutic potential. Neuropeptides 2020, 85, 102112. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.K.; Osborne-Lawrence, S.; Metzger, N.; Zigman, J.M. Lowering oxidative stress in ghrelin cells stimulates ghrelin secretion. Am. J. Physiol. Metab. 2020, 319, E330–E337. [Google Scholar] [CrossRef] [PubMed]
- Nettleford, S.K.; Prabhu, K.S. Selenium and Selenoproteins in Gut Inflammation—A Review. Antioxidants 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieliszek, M.; Bano, I. Selenium as an important factor in various disease states—A review. EXCLI J. 2022, 21, 948–966. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y. Systems Biology of Selenium and Complex Disease. Biol. Trace Element Res. 2019, 192, 38–50. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Liu, Q. Potential Roles of Selenium and Selenoproteins in the Prevention of Alzheimer’s Disease. Curr. Top. Med. Chem. 2015, 16, 835–848. [Google Scholar] [CrossRef]
- Wandt, V.K.; Winkelbeiner, N.; Bornhorst, J.; Witt, B.; Raschke, S.; Simon, L.; Ebert, F.; Kipp, A.P.; Schwerdtle, T. A matter of concern—Trace element dyshomeostasis and genomic stability in neurons. Redox Biol. 2021, 41, 101877. [Google Scholar] [CrossRef] [PubMed]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Merino, J. Precision nutrition in diabetes: When population-based dietary advice gets personal. Diabetologia 2022, 65, 1839–1848. [Google Scholar] [CrossRef]
- Takamura, T. Hepatokine Selenoprotein P-Mediated Reductive Stress Causes Resistance to Intracellular Signal Transduction. Antioxid. Redox Signal. 2020, 33, 517–524. [Google Scholar] [CrossRef] [PubMed]
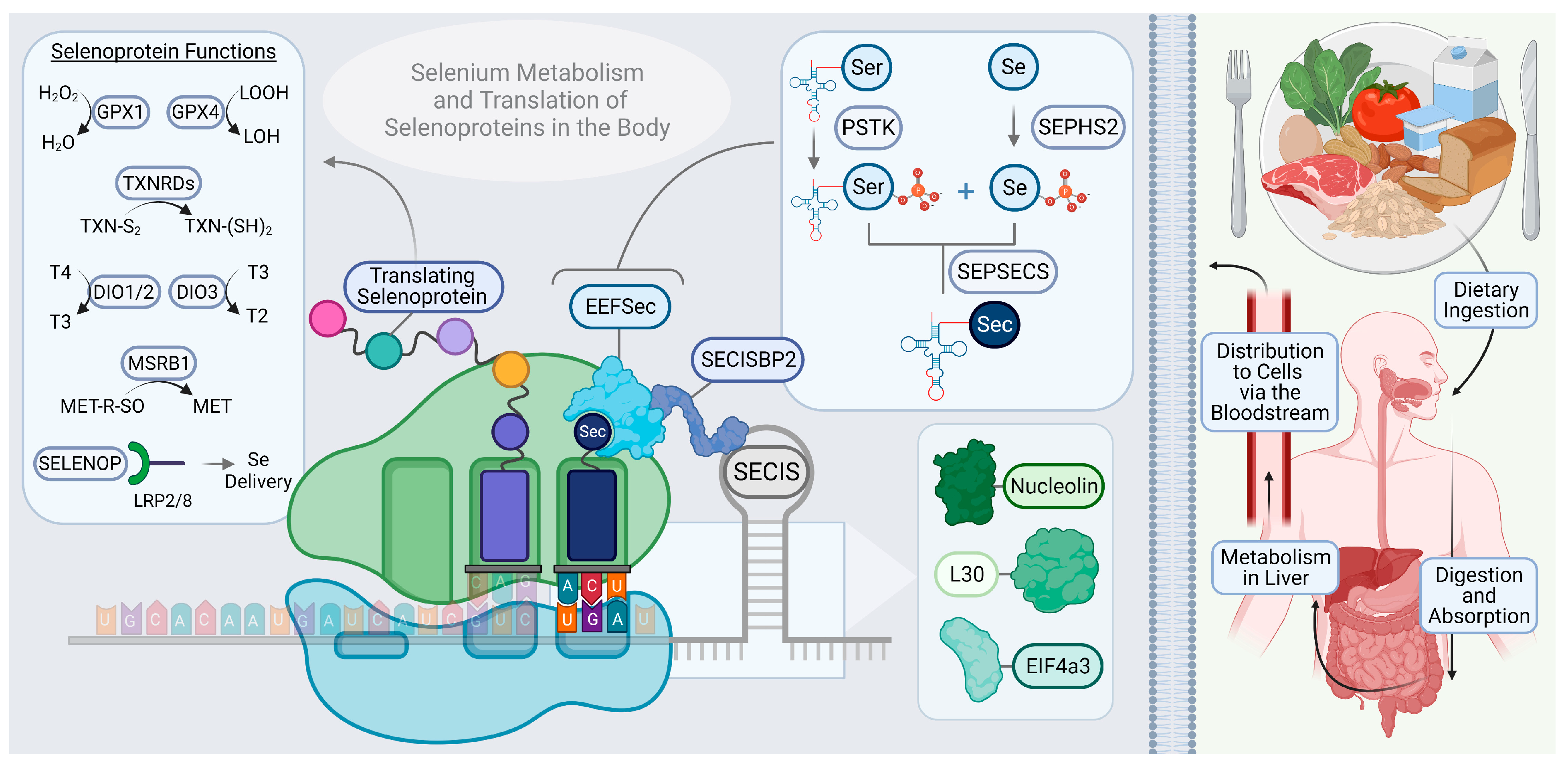

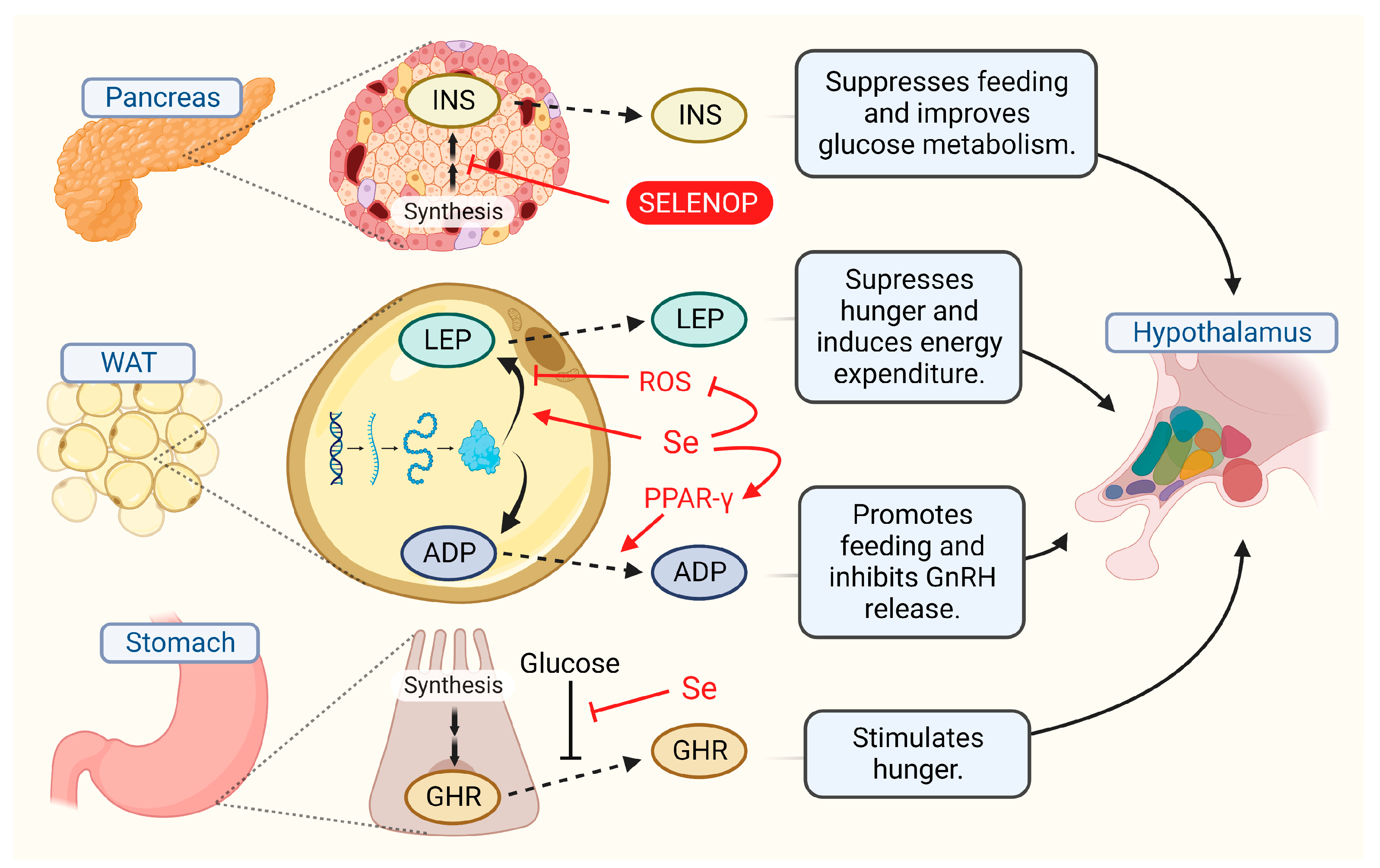
| Selenoprotein | Abbreviation(s) | Function/Reactions Catalyzed |
|---|---|---|
| lodothyronine deiodinases (1–3) | DIO (1–3) | Types 1 and 2 activate thyroid hormones (T4 to T3) and type 3 deactivates (T3 to T2, or T4 to rT3). Type 2 localizes to the ER. |
| Glutathione peroxidases (1–4, 6) | GPX (1–4, 6) | Reduces hydrogen peroxide species. Type 1 is present in cytosol. Type 4 reduces phospholipid hydroperoxides. |
| Methionine sulfoxide reductase B1 | MSRB1 | Reduces sulfoxidated methionipes. |
| Selenophosphatase synthetase 2 | SEPHS2 | Synthesis of selenophosphate to support selenoprotein synthesis. |
| Selenoprotein F | SELENOF | Thiol-disulfide oxidoreductase in the ER. |
| Selenoprotein H | SELENOH | Localized to the nucleus. Thought to conduct redox sensing to support transcription. |
| Selenoprotein I | SELENOI | Ethanolamine phosphotransferase to support the synthesis of phosphatidylethanolamine. |
| Selenoprotein K | SELENOK | Palmitoylation of inositol triphosphate receptors to facilitate store-operated Ca2+ entry from the ER. |
| Selenoprotein M | SELENOM | Thio-disulfide oxidoreductase in the ER. |
| Selenoprotein N | SELENON | Oxidoreductase that senses ER luminal Ca2+ levels. |
| Selenoprotein O | SELENOO | Localized to the mitochondrion. |
| Selenoprotein P | SELENOP | Secretory glycoprotein that delivers selenium to cells throughout the body. |
| Selenoprotein S | SELENOS | Participates in ER associated protein degradation. |
| Selenoprotein T | SELENOT | Thought to regulate Ca2+ homeostasis in the ER. |
| Selenoprotein V | SELENOV | Regulates O-GlcNAcylation. |
| Selenoprotein W | SELENOW | Proposed to have antioxidant function. |
| Thioredoxin reductases (1–3) | TXNRD (1–3) | Reduction of oxidized thioredoxin. Type 1 localizes to cytoplasm, type 2 to mitochondria. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toh, P.; Nicholson, J.L.; Vetter, A.M.; Berry, M.J.; Torres, D.J. Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication. Int. J. Mol. Sci. 2022, 23, 15445. https://doi.org/10.3390/ijms232315445
Toh P, Nicholson JL, Vetter AM, Berry MJ, Torres DJ. Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication. International Journal of Molecular Sciences. 2022; 23(23):15445. https://doi.org/10.3390/ijms232315445
Chicago/Turabian StyleToh, Pamela, Jessica L. Nicholson, Alyssa M. Vetter, Marla J. Berry, and Daniel J. Torres. 2022. "Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication" International Journal of Molecular Sciences 23, no. 23: 15445. https://doi.org/10.3390/ijms232315445
APA StyleToh, P., Nicholson, J. L., Vetter, A. M., Berry, M. J., & Torres, D. J. (2022). Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication. International Journal of Molecular Sciences, 23(23), 15445. https://doi.org/10.3390/ijms232315445






