Effects of β-amyloid (1-42) Administration on the Main Neurogenic Niches of the Adult Brain: Amyloid-Induced Neurodegeneration Influences Neurogenesis
Abstract
1. Introduction
2. Results
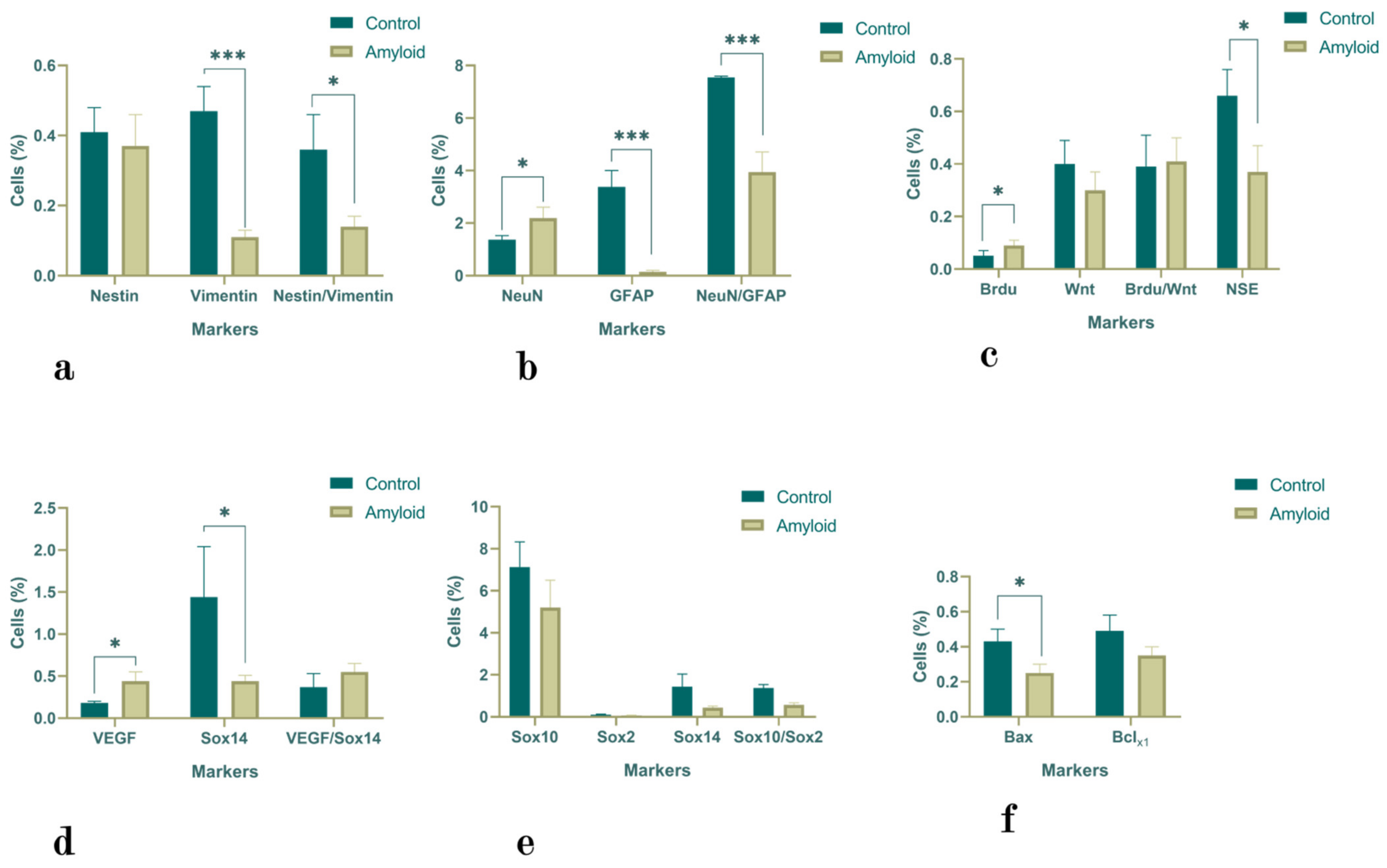
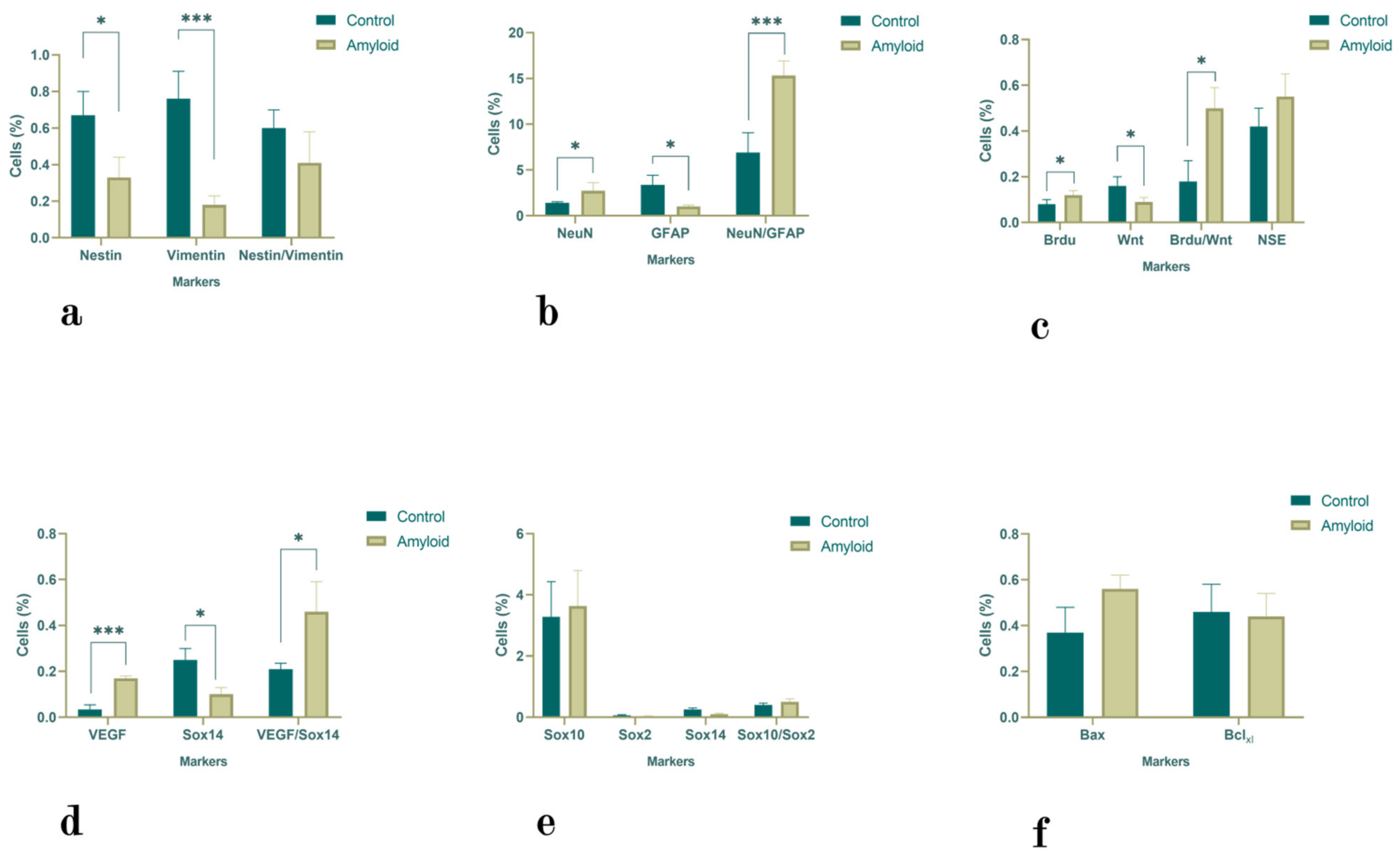
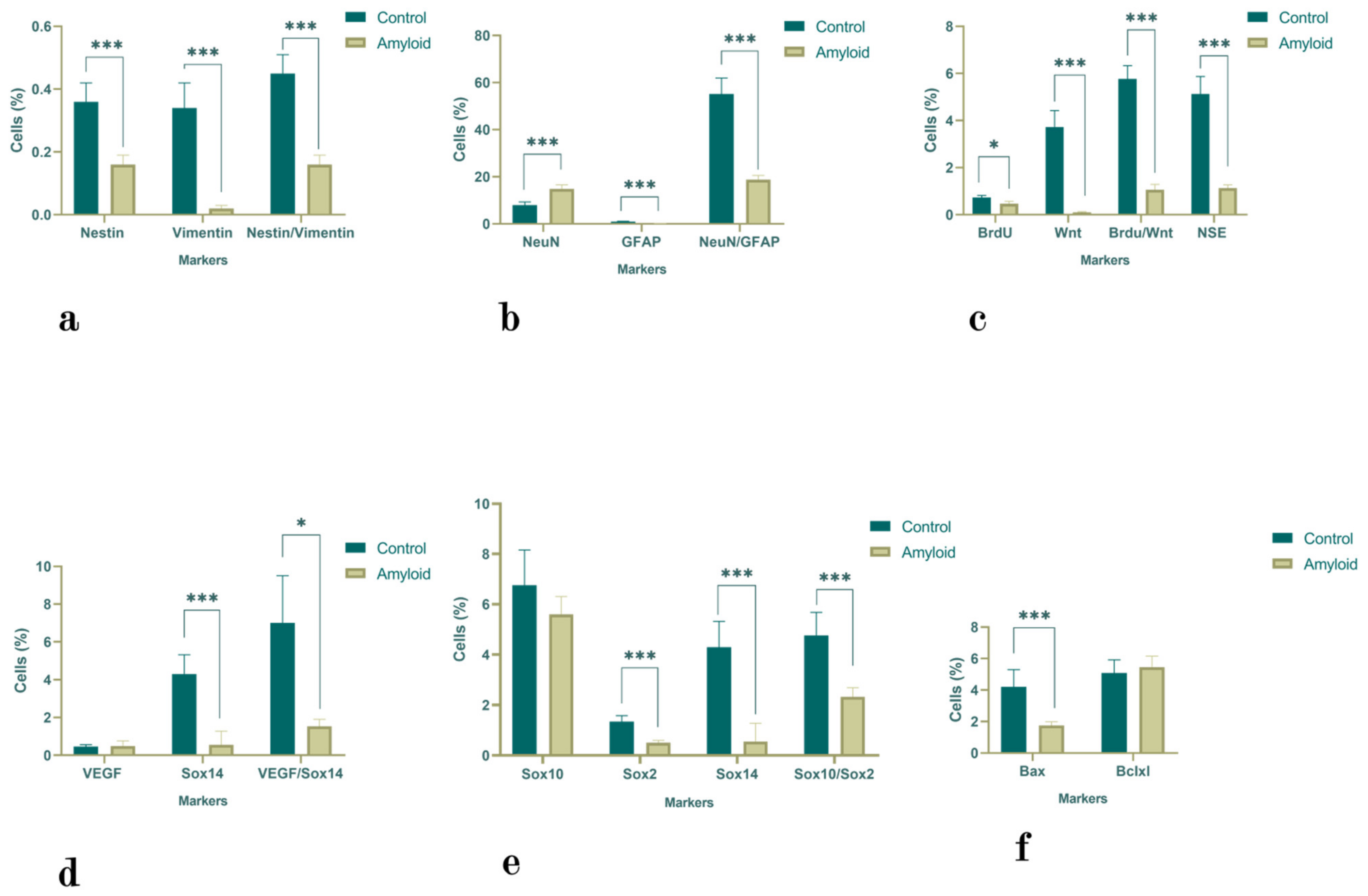
2.1. Flow Cytometry Results
2.1.1. Hippocampus
2.1.2. Subventricular Zone
2.1.3. Olfactory Bulb
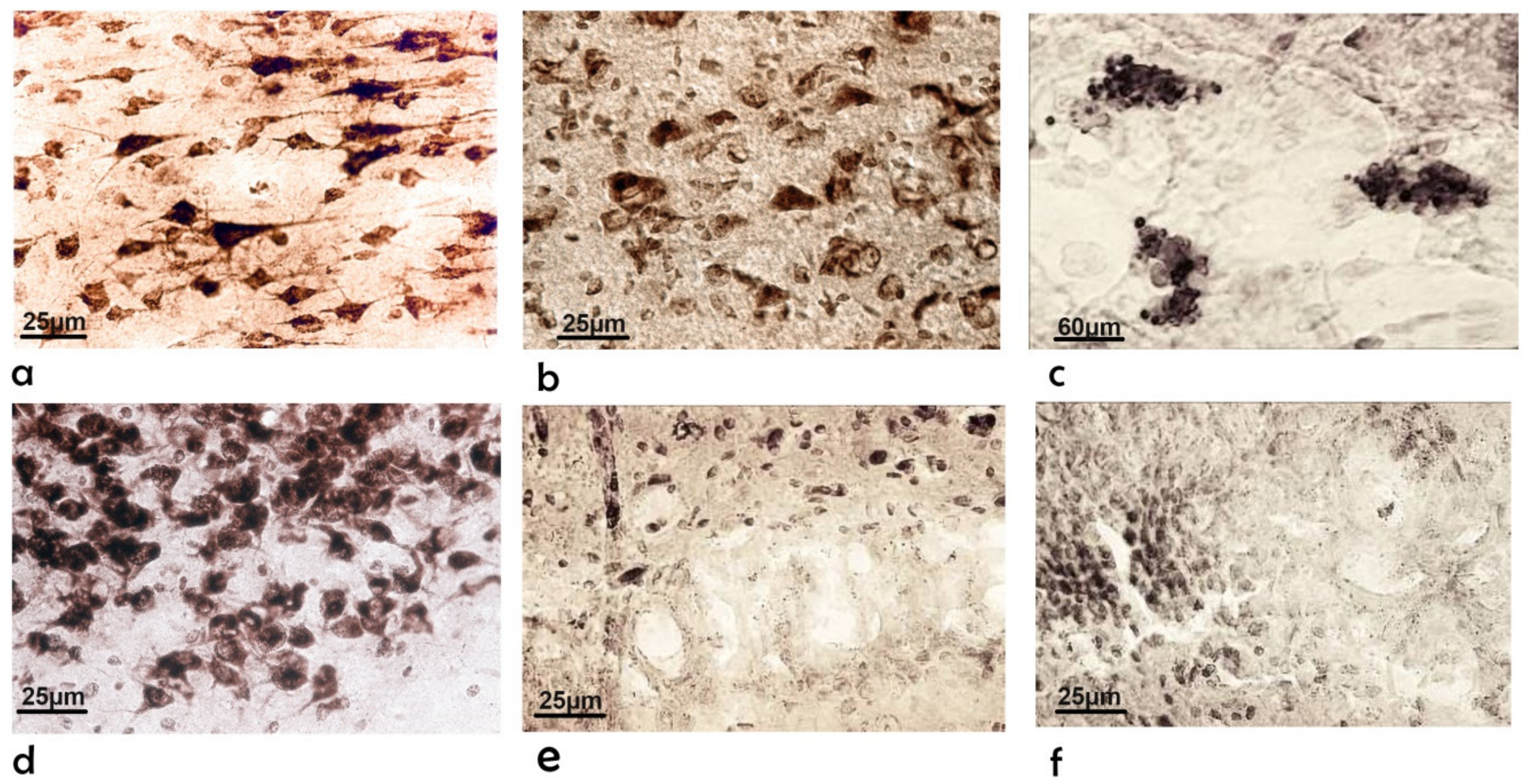
2.2. Morphological Changes
2.2.1. Subventricular Zone
2.2.2. Cerebral Cortex and Hippocampus
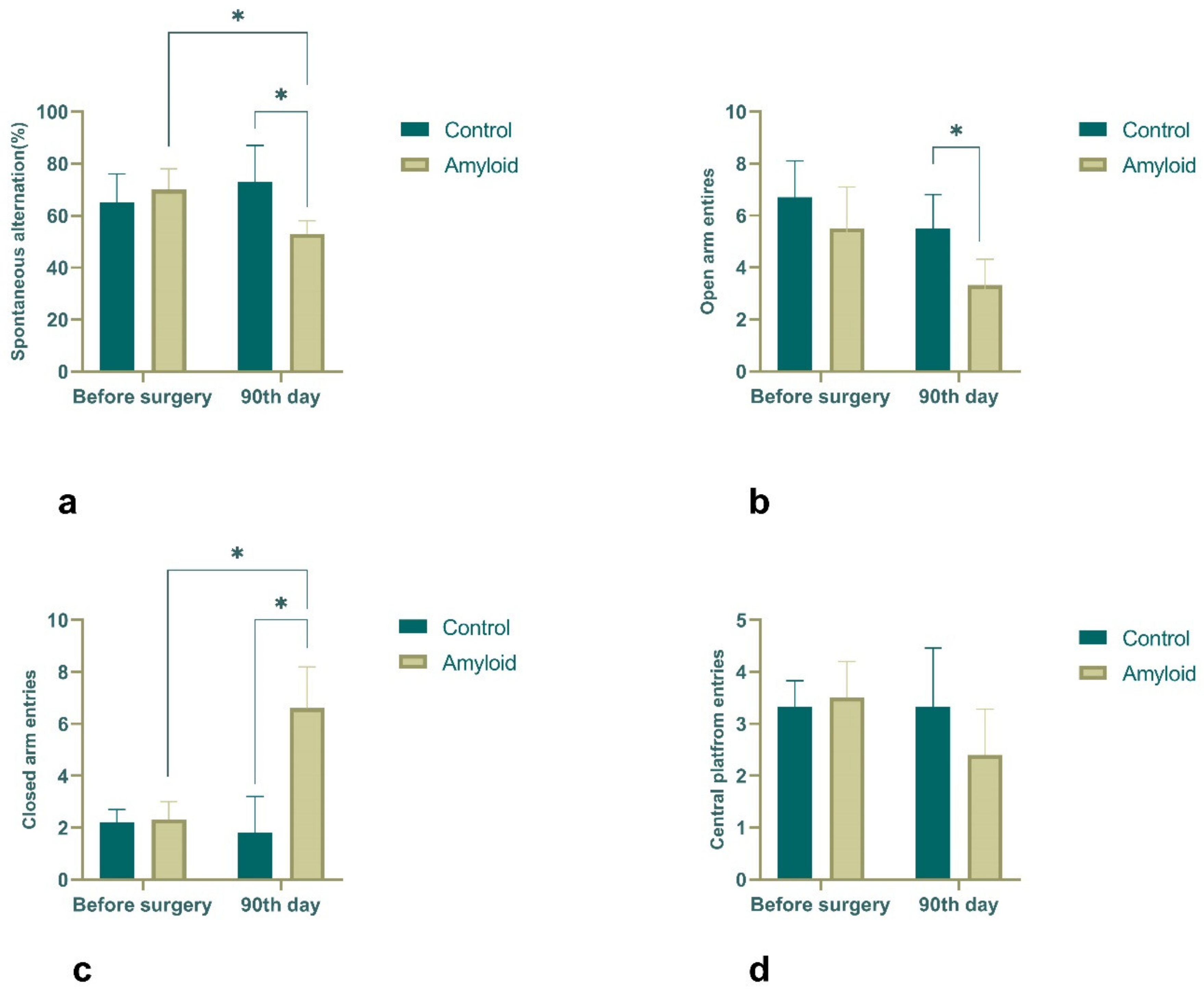
2.3. Behavioral Results
2.4. Electrophysiological Recordings
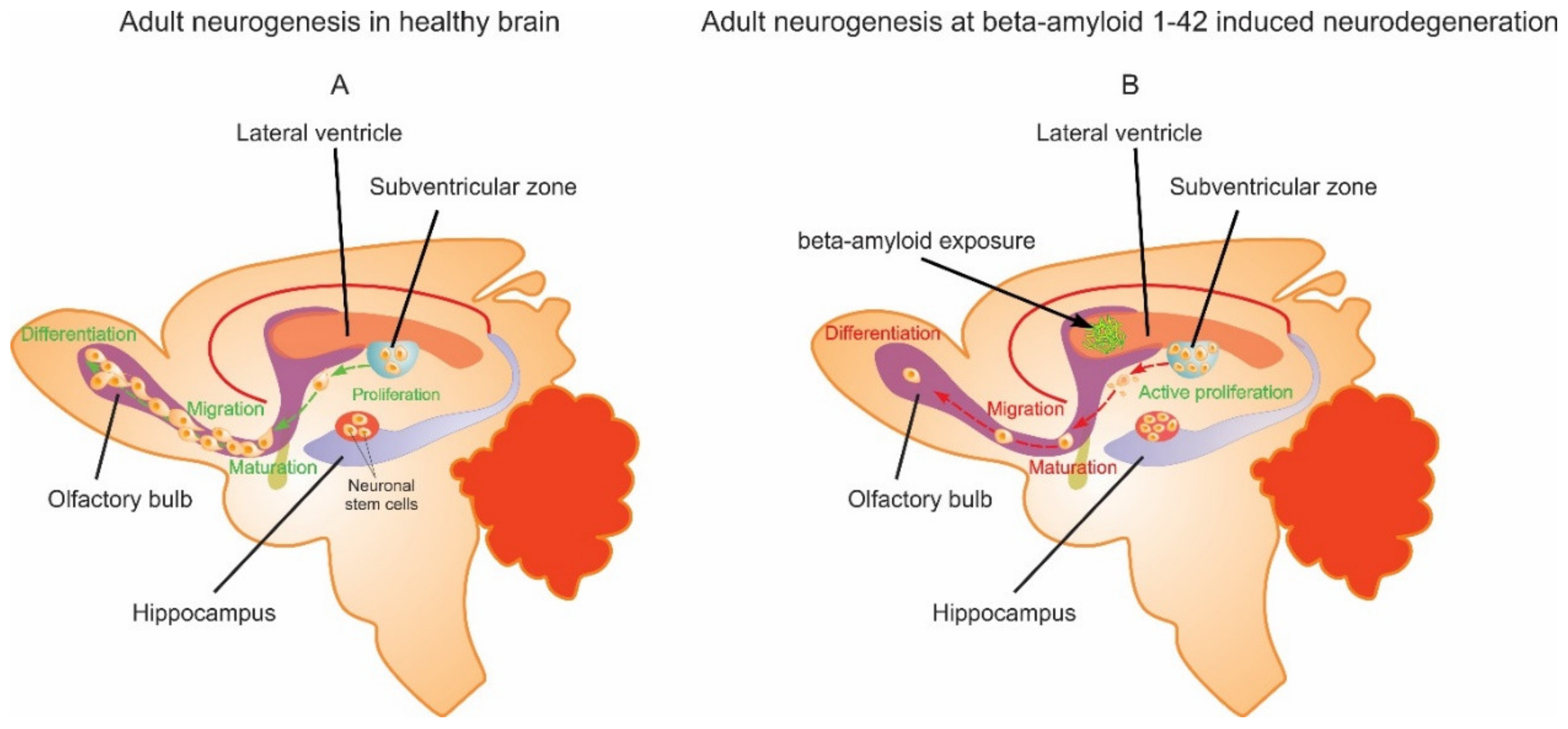
3. Discussion
Limitations of This Study
4. Methods and Materials
4.1. Animals
4.2. Experimental Protocol
4.3. Preparation of Aβ (1-42) Peptide
4.4. Surgical Procedure
4.5. Flow Cytometry of Cells
4.6. Morphological Study
4.7. Behavioral Testing
4.8. In Vivo Electrophysiology
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic Neuropathology in Alzheimer’s Disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.W.; Lane, H.Y.; Lin, C.H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 117957352090739. [Google Scholar] [CrossRef]
- Blanco-Silvente, L.; Castells, X.; Saez, M.; Barceló, M.A.; Garre-Olmo, J.; Vilalta-Franch, J.; Capellà, D. Discontinuation, Efficacy, and Safety of Cholinesterase Inhibitors for Alzheimer’s Disease: A Meta-Analysis and Meta-Regression of 43 Randomized Clinical Trials Enrolling 16 106 Patients. Int. J. Neuropsychopharmacol. 2017, 20, 519. [Google Scholar] [CrossRef]
- Blanco-Silvente, L.; Capellà, D.; Garre-Olmo, J.; Vilalta-Franch, J.; Castells, X. Predictors of Discontinuation, Efficacy, and Safety of Memantine Treatment for Alzheimer’s Disease: Meta-Analysis and Meta-Regression of 18 Randomized Clinical Trials Involving 5004 Patients. BMC Geriatr. 2018, 18, 168. [Google Scholar] [CrossRef]
- FDA’s Decision to Approve New Treatment for Alzheimer’s Disease|FDA. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fdas-decision-approve-new-treatment-alzheimers-disease (accessed on 31 October 2022).
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Khachaturian, Z.S. The ‘Aducanumab Story’: Will the Last Chapter Spell the End of the ‘Amyloid Hypothesis’ or Mark a New Beginning? J. Prev. Alzheimer’s Dis. 2022, 9, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Tanzi, R.E. Is Alzheimer’s Disease a Neurogenesis Disorder? Stem Cell 2019, 25, 7–8. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; García-Verdugo, J.M. Neurogenesis in Adult Subventricular Zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef]
- Rahman, A.A.; Amruta, N.; Pinteaux, E.; Bix, G.J. Neurogenesis After Stroke: A Therapeutic Perspective. Transl. Stroke Res. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Verkhratsky, A. Neurogenesis in Alzheimer’s Disease. J. Anat. 2011, 219, 78–89. [Google Scholar] [CrossRef]
- Seaberg, R.M.; Van der Kooy, D. Adult Rodent Neurogenic Regions: The Ventricular Subependyma Contains Neural Stem Cells, but the Dentate Gyrus Contains Restricted Progenitors. J. Neurosci. 2002, 22, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The Role of Adult Hippocampal Neurogenesis in Brain Health and Disease. Mol. Psychiatry 2018, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; McNeil, E.; Dressler, L.; Siman, R. Long-Lasting Impairment in Hippocampal Neurogenesis Associated with Amyloid Deposition in a Knock-in Mouse Model of Familial Alzheimer’s Disease. Exp. Neurol. 2007, 204, 77–87. [Google Scholar] [CrossRef]
- Feng, R.; Rampon, C.; Tang, Y.P.; Shrom, D.; Jin, J.; Kyin, M.; Sopher, B.; Martin, G.M.; Kim, S.H.; Langdon, R.B.; et al. Deficient Neurogenesis in Forebrain-Specific Presenilin-1 Knockout Mice Is Associated with Reduced Clearance of Hippocampal Memory Traces. Neuron 2001, 32, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Sotthibundhu, A.; Li, Q.X.; Thangnipon, W.; Coulson, E.J. Abeta (1-42) Stimulates Adult SVZ Neurogenesis through the P75 Neurotrophin Receptor. Neurobiol. Aging 2009, 30, 1975–1985. [Google Scholar] [CrossRef]
- Ramírez, E.; Mendieta, L.; Flores, G.; Limón, I.D. Neurogenesis and Morphological-Neural Alterations Closely Related to Amyloid β-Peptide (25-35)-Induced Memory Impairment in Male Rats. Neuropeptides 2018, 67, 9–19. [Google Scholar] [CrossRef]
- Sung, P.S.; Lin, P.Y.; Liu, C.H.; Su, H.C.; Tsai, K.J. Neuroinflammation and Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 701. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Price, K.A.; Biron, K.E.; Fenninger, F.; Pfeifer, C.G.; Dickstein, D.L. Adjusting the Compass: New Insights into the Role of Angiogenesis in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2013, 5, 64. [Google Scholar] [CrossRef]
- Biron, K.E.; Dickstein, D.L.; Gopaul, R.; Jefferies, W.A. Amyloid Triggers Extensive Cerebral Angiogenesis Causing Blood Brain Barrier Permeability and Hypervascularity in Alzheimer’s Disease. PLoS ONE 2011, 6, e23789. [Google Scholar] [CrossRef]
- Gage, F.H.; Ray, J.; Fisher, L.J. Isolation, Characterization, and Use of Stem Cells from the CNS. Annu. Rev. Neurosci. 1995, 18, 159–192. [Google Scholar] [CrossRef]
- Rakic, P. Limits of Neurogenesis in Primates. Science 1985, 227, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Mix, E.; Rateitschak, K.; Rolfs, A. Wnt Signal Pathways and Neural Stem Cell Differentiation. Neurodegener. Dis. 2006, 3, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Heine, V.M.; Zareno, J.; Maslam, S.; Joëls, M.; Lucassen, P.J. Chronic Stress in the Adult Dentate Gyrus Reduces Cell Proliferation near the Vasculature and VEGF and Flk-1 Protein Expression. Eur. J. Neurosci. 2005, 21, 1304–1314. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ahlenius, H.; Thored, P.; Kobayashi, R.; Kokaia, Z.; Lindvall, O. Intracerebral Infusion of Glial Cell Line-Derived Neurotrophic Factor Promotes Striatal Neurogenesis after Stroke in Adult Rats. Stroke 2006, 37, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Mirzadeh, Z.; Alvarez-Buylla, A. Mosaic Organization of Neural Stem Cells in the Adult Brain. Science 2007, 317, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Aghajanov, M.I.; Yenkoyan, K.B.; Chavushyan, V.A.; Sarkissian, J.S. The Proline-Rich Hypothalamic Peptide Is a Modulator of Functions of Neurotrophins and Neuronal Activity in Amyloid-Induced Neurodegeneration. Neurochem. J. 2014, 8, 47–57. [Google Scholar] [CrossRef]
- Aghajanov, M.; Matinyan, S.; Chavushyan, V.; Danielyan, M.; Karapetyan, G.; Mirumyan, M.; Fereshetyan, K.; Harutyunyan, H.; Yenkoyan, K. The Involvement of Insulin-Like Growth Factor 1 and Nerve Growth Factor in Alzheimer’s Disease-Like Pathology and Survival Role of the Mix of Embryonic Proteoglycans: Electrophysiological Fingerprint, Structural Changes and Regulatory Effects on Neurotrophins. Int. J. Mol. Sci. 2021, 22, 7084. [Google Scholar] [CrossRef]
- Nguyen, N.; Lee, S.B.; Lee, Y.S.; Lee, K.H.; Ahn, J.Y. Neuroprotection by NGF and BDNF against Neurotoxin-Exerted Apoptotic Death in Neural Stem Cells Are Mediated through Trk Receptors, Activating PI3-Kinase and MAPK Pathways. Neurochem. Res. 2009, 34, 942–951. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Safaryan, K.; Navasardyan, G.; Mkrtchyan, L.; Aghajanov, M. Effects of Beta-Amyloid on Behavioral and Amino Acids Spectrum in Rats’ Brain and Their Modulation by Embryonic Proteins. Neurochem. Int. 2009, 54, 292–298. [Google Scholar] [CrossRef]
- Yenkoyan, K.; Safaryan, K.; Chavushyan, V.; Meliksetyan, I.; Navasardyan, G.; Sarkissian, J.; Galoyan, A.; Aghajanov, M. Neuroprotective Action of Proline-Rich Polypeptide-1 in β-Amyloid Induced Neurodegeneration in Rats. Brain Res. Bull. 2011, 86, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Yenkoyan, K.; Fereshetyan, K.; Matinyan, S.; Chavushyan, V.; Aghajanov, M. The Role of Monoamines in the Development of Alzheimer’s Disease and Neuroprotective Effect of a Proline Rich Polypeptide. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Aghajanov, M.; Chavushyan, V.; Matinyan, S.; Danielyan, M.; Yenkoyan, K. Alzheimer’s Disease-like Pathology-Triggered Oxidative Stress, Alterations in Monoamines Levels, and Structural Damage of Locus Coeruleus Neurons Are Partially Recovered by a Mix of Proteoglycans of Embryonic Genesis. Neurochem. Int. 2019, 131, 104531. [Google Scholar] [CrossRef] [PubMed]
- Chavushyan, V.; Matinyan, S.; Danielyan, M.; Aghajanov, M.; Yenkoyan, K. Embryonic Proteoglycans Regulate Monoamines in the Rat Frontal Cortex and Hippocampus in Alzheimer’s Disease-like Pathology. Neurochem. Int. 2020, 140, 104838. [Google Scholar] [CrossRef]
- Guzmán, B.C.F.; Kim, S.H.; Chawdhary, B.; Peppercorn, K.; Tate, W.P.; Waldvogel, H.J.; Faull, R.L.M.; Montgomery, J.; Kwakowsky, A. Amyloid-Beta1-42 -Induced Increase in GABAergic Tonic Conductance in Mouse Hippocampal CA1 Pyramidal Cells. Molecules 2020, 25, 693. [Google Scholar] [CrossRef]
- Yeung, J.H.Y.; Palpagama, T.H.; Tate, W.P.; Peppercorn, K.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Acute Effects of Amyloid-Beta1-42 on Glutamatergic Receptor and Transporter Expression in the Mouse Hippocampus. Front. Neurosci. 2020, 13, 1427. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Potapov, K.; Kim, S.; Peppercorn, K.; Tate, W.P.; Ábrahám, I.M. Treatment of Beta Amyloid 1-42 (Aβ1-42)-Induced Basal Forebrain Cholinergic Damage by a Non-Classical Estrogen Signaling Activator in Vivo. Sci. Rep. 2016, 6, 21101. [Google Scholar] [CrossRef] [PubMed]
- Simone Ruggeri, F.; Habchi, J.; Cerreta, A.; Dietler, G. AFM-Based Single Molecule Techniques: Unraveling the Amyloid Pathogenic Species. Curr. Pharm. Des. 2016, 22, 3950–3970. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2007; ISBN 9780123741219. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yenkoyan, K.; Margaryan, T.; Matinyan, S.; Chavushyan, V.; Danielyan, M.; Davtyan, T.; Aghajanov, M. Effects of β-amyloid (1-42) Administration on the Main Neurogenic Niches of the Adult Brain: Amyloid-Induced Neurodegeneration Influences Neurogenesis. Int. J. Mol. Sci. 2022, 23, 15444. https://doi.org/10.3390/ijms232315444
Yenkoyan K, Margaryan T, Matinyan S, Chavushyan V, Danielyan M, Davtyan T, Aghajanov M. Effects of β-amyloid (1-42) Administration on the Main Neurogenic Niches of the Adult Brain: Amyloid-Induced Neurodegeneration Influences Neurogenesis. International Journal of Molecular Sciences. 2022; 23(23):15444. https://doi.org/10.3390/ijms232315444
Chicago/Turabian StyleYenkoyan, Konstantin, Tigran Margaryan, Senik Matinyan, Vergine Chavushyan, Margarita Danielyan, Tigran Davtyan, and Michail Aghajanov. 2022. "Effects of β-amyloid (1-42) Administration on the Main Neurogenic Niches of the Adult Brain: Amyloid-Induced Neurodegeneration Influences Neurogenesis" International Journal of Molecular Sciences 23, no. 23: 15444. https://doi.org/10.3390/ijms232315444
APA StyleYenkoyan, K., Margaryan, T., Matinyan, S., Chavushyan, V., Danielyan, M., Davtyan, T., & Aghajanov, M. (2022). Effects of β-amyloid (1-42) Administration on the Main Neurogenic Niches of the Adult Brain: Amyloid-Induced Neurodegeneration Influences Neurogenesis. International Journal of Molecular Sciences, 23(23), 15444. https://doi.org/10.3390/ijms232315444





