Potential Roles of Exosomes in the Development and Detection of Malignant Mesothelioma: An Update
Abstract
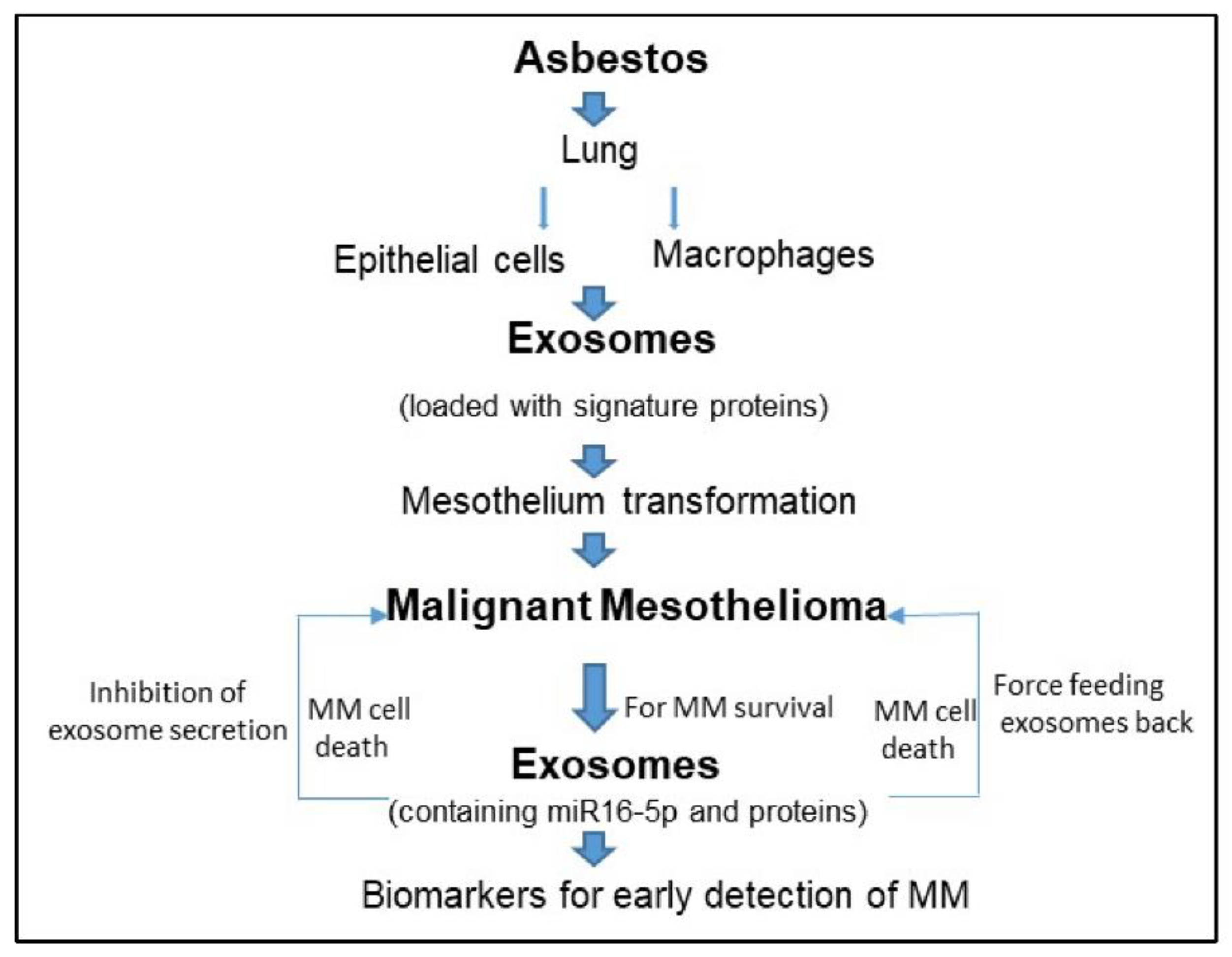
1. Introduction
2. Malignant Mesothelioma and Exosomes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Munson, P.; Shukla, A. Exosomes: Potential in cancer diagnosis and therapy. Medicines 2015, 2, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Jin, Y.; Li, Y.; Huang, Y.; Zhao, R. Recent progress of exosome isolation and peptide recognition-guided strategies for exosome research. Front. Chem. 2022, 10, 844124. [Google Scholar] [PubMed]
- Teixeira, J.H.; Silva, A.M.; Almeida, M.I.; Barbosa, M.A.; Santos, S.G. Circulating extracellular vesicles: Their role in tissue repair and regeneration. Transfus. Apher. Sci. 2016, 55, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Smalheiser, N.R. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol. Direct 2007, 2, 35. [Google Scholar]
- Ibrahim, A.G.-E.; Cheng, K.; Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Madison, M.N.; Okeoma, C.M. Exosomes: Implications in HIV-1 Pathogenesis. Viruses 2015, 7, 4093–4118. [Google Scholar]
- Properzi, F.; Logozzi, M.; Fais, S. Exosomes: The future of biomarkers in medicine. Biomark Med. 2013, 7, 769–778. [Google Scholar] [CrossRef]
- Moore, C.; Kosgodage, U.; Lange, S.; Inal, J.M. The emerging role of exosome and microvesicle- (EMV-) based cancer therapeutics and immunotherapy. Int. J. Cancer 2017, 141, 428–436. [Google Scholar] [CrossRef]
- Syn, N.L.; Wang, L.; Chow, E.K.-H.; Lim, C.T.; Goh, B.-C. Exosomes in cancer nanomedicine and immunotherapy: Prospects and challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cao, Y.; Sun, M.; Feng, H. Expression, regulation, and function of exosome-derived miRNAs in cancer progression and therapy. FASEB J. 2021, 35, e21916. [Google Scholar] [PubMed]
- Zhou, Y.; Zhang, Y.; Gong, H.; Luo, S.; Cui, Y. The role of exosomes and their applications in cancer. Int. J. Mol. Sci. 2021, 22, 12204. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Teixeira, A.F.; Zhu, H.-J.; Dijke, P.T. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol. Cancer 2021, 20, 154. [Google Scholar]
- Nafar, S.; Nouri, N.; Alipour, M.; Fallahi, J.; Zare, F.; Tabei, S.M.B. Exosome as a target for cancer treatment. J. Investig. Med. 2022, 70, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization/International Agency for Research on Cancer. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100C, pp. 219–309. [Google Scholar]
- Pira, E.; Donato, F.; Maida, L.; Discalzi, G. Exposure to asbestos: Past, present and future. J. Thorac. Dis. 2018, 10 (Suppl. 2), S237–S245. [Google Scholar]
- Frank, A.L.; Joshi, T. The global spread of asbestos. Ann. Glob. Health 2014, 80, 257–262. [Google Scholar] [CrossRef]
- Berman, D.W.; Crump, K.S. Update of potency factors for asbestos-related lung cancer and mesothelioma. Crit. Rev. Toxicol. 2008, 38 (Suppl. 1), 1–47. [Google Scholar]
- Markowitz, S.B.; Levin, S.M.; Miller, A.; Morabia, A. Asbestos, asbestosis, smoking, and lung cancer. New findings from the North American insulator cohort. Am. J. Respir. Crit. Care Med. 2013, 188, 90–96. [Google Scholar]
- Mossman, B.T.; Churg, A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998, 157 Pt 1, 1666–1680. [Google Scholar] [CrossRef]
- Sen, D. Working with asbestos and the possible health risks. Occup. Med. 2015, 65, 6–14. [Google Scholar]
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [PubMed]
- Bard, M.P.; Hegmans, J.P.; Hemmes, A.; Luider, T.M.; Willemsen, R.; Severijnen, L.-A.A.; van Meerbeeck, J.P.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 114–121. [Google Scholar] [PubMed]
- Hegmans, J.P.; Bard, M.P.; Hemmes, A.; Luider, T.M.; Kleijmeer, M.J.; Prins, J.-B.; Zitvogel, L.; Burgers, S.A.; Hoogsteden, H.C.; Lambrecht, B.N. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am. J. Pathol. 2004, 164, 1807–1815. [Google Scholar]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef]
- Clayton, A.; Tabi, Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol. Dis. 2005, 34, 206–213. [Google Scholar] [CrossRef]
- Mahaweni, N.M.; Kaijen-Lambers, M.E.; Dekkers, J.; Aerts, J.G.; Hegmans, J.P. Tumour-derived exosomes as antigen delivery carriers in dendritic cell-based immunotherapy for malignant mesothelioma. J. Extracell Vesicles 2013, 2, 22492. [Google Scholar] [CrossRef] [PubMed]
- Thayanithy, V.; Babatunde, V.; Dickson, E.L.; Wong, P.; Oh, S.; Ke, X.; Barlas, A.; Fujisawa, S.; Romin, Y.; Moreira, A.L.; et al. Tumor exosomes induce tunneling nanotubes in lipid raft-enriched regions of human mesothelioma cells. Exp. Cell Res. 2014, 323, 178–188. [Google Scholar]
- Heusermann, W.; Hean, J.; Trojer, D.; Steib, E.; von Bueren, S.; Graff-Meyer, A.; Genoud, C.; Martin, K.; Pizzato, N.; Voshol, J.; et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J. Cell Biol. 2016, 213, 173–184. [Google Scholar] [CrossRef]
- Delage, E.; Cervantes, D.C.; Pénard, E.; Schmitt, C.; Syan, S.; Disanza, A.; Scita, G.; Zurzolo, C. Differential identity of Filopodia and Tunneling Nanotubes revealed by the opposite functions of actin regulatory complexes. Sci. Rep. 2016, 6, 39632. [Google Scholar]
- Greening, D.W.; Ji, H.; Chen, M.; Robinson, B.W.; Dick, I.M.; Creaney, J.; Simpson, R.J. Secreted primary human malignant mesothelioma exosome signature reflects oncogenic cargo. Sci. Rep. 2016, 6, 32643. [Google Scholar]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Creaney, J.; Dick, I.M.; Leon, J.S.; Robinson, B.W. A proteomic analysis of the malignant mesothelioma secretome using iTRAQ. Cancer Genom. Proteom. 2017, 14, 103–117. [Google Scholar]
- Javadi, J.; Görgens, A.; Vanky, H.; Gupta, D.; Hjerpe, A.; El-Andaloussi, S.; Hagey, D.; Dobra, K. Diagnostic and prognostic utility of the extracellular vesicles subpopulations present in pleural effusion. Biomolecules 2021, 11, 1606. [Google Scholar] [CrossRef]
- Cavalleri, T.; Angelici, L.; Favero, C.; Dioni, L.; Mensi, C.; Bareggi, C.; Palleschi, A.; Rimessi, A.; Consonni, D.; Bordini, L.; et al. Plasmatic extracellular vesicle microRNAs in malignant pleural mesothelioma and asbestos-exposed subjects suggest a 2-miRNA signature as potential biomarker of disease. PLoS ONE 2017, 12, e0176680. [Google Scholar]
- Monaco, F.; Gaetani, S.; Alessandrini, F.; Tagliabracci, A.; Bracci, M.; Valentino, M.; Neuzil, J.; Amati, M.; Bovenzi, M.; Tomasetti, M.; et al. Exosomal transfer of miR-126 promotes the anti-tumour response in malignant mesothelioma: Role of miR-126 in cancer-stroma communication. Cancer Lett. 2019, 463, 27–36. [Google Scholar]
- Monaco, F.; De Conti, L.; Vodret, S.; Zanotta, N.; Comar, M.; Manzotti, S.; Rubini, C.; Graciotti, L.; Fulgenzi, G.; Bovenzi, M.; et al. Force-feeding malignant mesothelioma stem-cell like with exosome-delivered miR-126 induces tumour cell killing. Transl. Oncol. 2022, 20, 101400. [Google Scholar] [CrossRef]
- Munson, P.; Lam, Y.; Dragon, J.; MacPherson, M.; Shukla, A. Exosomes from asbestos-exposed cells modulate gene expression in mesothelial cells. FASEB J. 2018, 32, 4328–4342. [Google Scholar]
- Bruno, R.; Poma, A.M.; Alì, G.; Giannini, R.; Puppo, G.; Melfi, F.; Lucchi, M.; Mussi, A.; Falcone, A.; Chella, A.; et al. Novel prognostic markers for epithelioid malignant pleural mesothelioma. J. Clin. Oncol. 2017, 35 (Suppl. 15), e20028. [Google Scholar] [CrossRef]
- Røe, O.D.; Anderssen, E.; Sandeck, H.; Christensen, T.; Larsson, E.; Lundgren, S. Malignant pleural mesothelioma: Genome-wide expression patterns reflecting general resistance mechanisms and a proposal of novel targets. Lung Cancer 2010, 67, 57–68. [Google Scholar]
- Munson, P.; Lam, Y.; MacPherson, M.; Beuschel, S.; Shukla, A. Mouse serum exosomal proteomic signature in response to asbestos exposure. J. Cell Biochem. 2018, 119, 6266–6273. [Google Scholar] [CrossRef]
- Ghio, A.J.; Stonehuerner, J.; Richards, J.; Devlin, R.B. Iron homeostasis in the lung following asbestos exposure. Antioxid Redox Signal. 2008, 10, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; Levin, S.M.; Harbut, M.R.; Melamed, J.; Chiriboga, L.; Donington, J.; Huflejt, M.; Carbone, M.; Chia, D.; Goodglick, L.; et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N. Engl. J. Med. 2012, 367, 1417–1427. [Google Scholar] [PubMed]
- Munson, P.B.; Hall, E.M.; Farina, N.H.; Pass, H.; Shukla, A. Exosomal miR-16-5p as a target for malignant mesothelioma. Sci. Rep. 2019, 9, 11688. [Google Scholar] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [PubMed]
- Rajagopal, C.; Harikumar, K.B. The origin and functions of exosomes in cancer. Front. Oncol. 2018, 8, 66. [Google Scholar]
- Whiteside, T.L. The emerging role of plasma exosomes in diagnosis, prognosis and therapies of patients with cancer. Contemp. Oncol. 2018, 22, 38–40. [Google Scholar] [CrossRef]
| MM Samples/Specimens | Molecules (RNA, Proteins) in Exosomes | Core Findings | Reference |
|---|---|---|---|
| Human pleural effusions | Proteins and peptides | Known and new exosomal proteins SNX25, BTG1, PEDF, identified as biomarkers. | Bard et al., 2004 [24] |
| Seven humani MM tumor cell lines | Proteomic cargo | Identified 38 proteins, confirmed 4 by immunoblot. New information on MM exosomes | Hegmans et al., 2004 [25] |
| MM cell lines | Proteins, ligands | Immunological function of exosomes via NKG2D ligand expression | Clayton et al., 2005 [27] |
| MM tumor bearing mice | Tumor associated antigens (TAA) | MM exosome show some promise in immunotherapy | Mahaweni et al., 2013 [28] |
| MM cell lines and MM exosomes | Tunneling nanotubes (TnTs) | MM tumor cells form more TnTs in presence of exogenous exosomes. TnTs help in intercellular cargo transport | Thayanithy et al., 2014 [29] |
| Four human MM cell lines | Proteins | Identified 2073 unique proteins in MM exosomes. Proteins could play important roles in cancer angiogenesis, metastasis, migration and immune regulation | Greening et al., 2016 [32] |
| Six MM cell lines and 3 primary mesothelial cell lines | MM secretome proteins | MM secretome contained high abundance of exosomal proteins | Creaney et al., 2017 [34] |
| Small cohort | microRNA | Specific exosomal miRNA signature can discriminate MM from past asbestos exposure subjects | Cavalleri et al., 2017 [36] |
| Asbestos exposed lung epithelial cells, macrophages and mesothelial cells | Proteins and genes | Exosomes from asbestos exposed lung epithelial cells and macrophages can cause gene expression changes in mesothelial cells. | Munson et al., 2018 [39,42] |
| Blood/serum from asbestos exposed mice | Proteins | Asbestos exposure caused increased abundance of certain proteins in serum exosomes. | Munson et al., 2019 [45] |
| Human mesothelioma and mesothelial cells | microRNA | MM cells preferentially secrete tumor suppressor miRNA, miR16-5p via exosomes. Force feeding exosomes or inhibition of secretion of exosome is beneficial for death of MM tumor cells. | Munson et al., 2019 [45] |
| Plasma from human MM patients | proteins | Exosome isolated were enriched with immunoglobulins. Study demonstrated that plasma-derived exosomal protein signature could be beneficial for cancer type detection | Hoshino et al., 2020 [46] |
| Small number of MM patient sample | Proteins | Utility of exosomal protein signature in diagnosis of benign vs. malignant MM. | Javadi et al., 2021 [35] |
| MM tumor stromal model. MM spheroids | microRNA | Exosomal transfer of miR-126 plays an anti-tumor response in MM. | Monaco et al., 2019 [37] Monaco et al., 2022 [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munson, P.; Shukla, A. Potential Roles of Exosomes in the Development and Detection of Malignant Mesothelioma: An Update. Int. J. Mol. Sci. 2022, 23, 15438. https://doi.org/10.3390/ijms232315438
Munson P, Shukla A. Potential Roles of Exosomes in the Development and Detection of Malignant Mesothelioma: An Update. International Journal of Molecular Sciences. 2022; 23(23):15438. https://doi.org/10.3390/ijms232315438
Chicago/Turabian StyleMunson, Phillip, and Arti Shukla. 2022. "Potential Roles of Exosomes in the Development and Detection of Malignant Mesothelioma: An Update" International Journal of Molecular Sciences 23, no. 23: 15438. https://doi.org/10.3390/ijms232315438
APA StyleMunson, P., & Shukla, A. (2022). Potential Roles of Exosomes in the Development and Detection of Malignant Mesothelioma: An Update. International Journal of Molecular Sciences, 23(23), 15438. https://doi.org/10.3390/ijms232315438







