Top-Down Preparation of Nanoquartz for Toxicological Investigations
Abstract
:1. Introduction
2. Results and Discussion
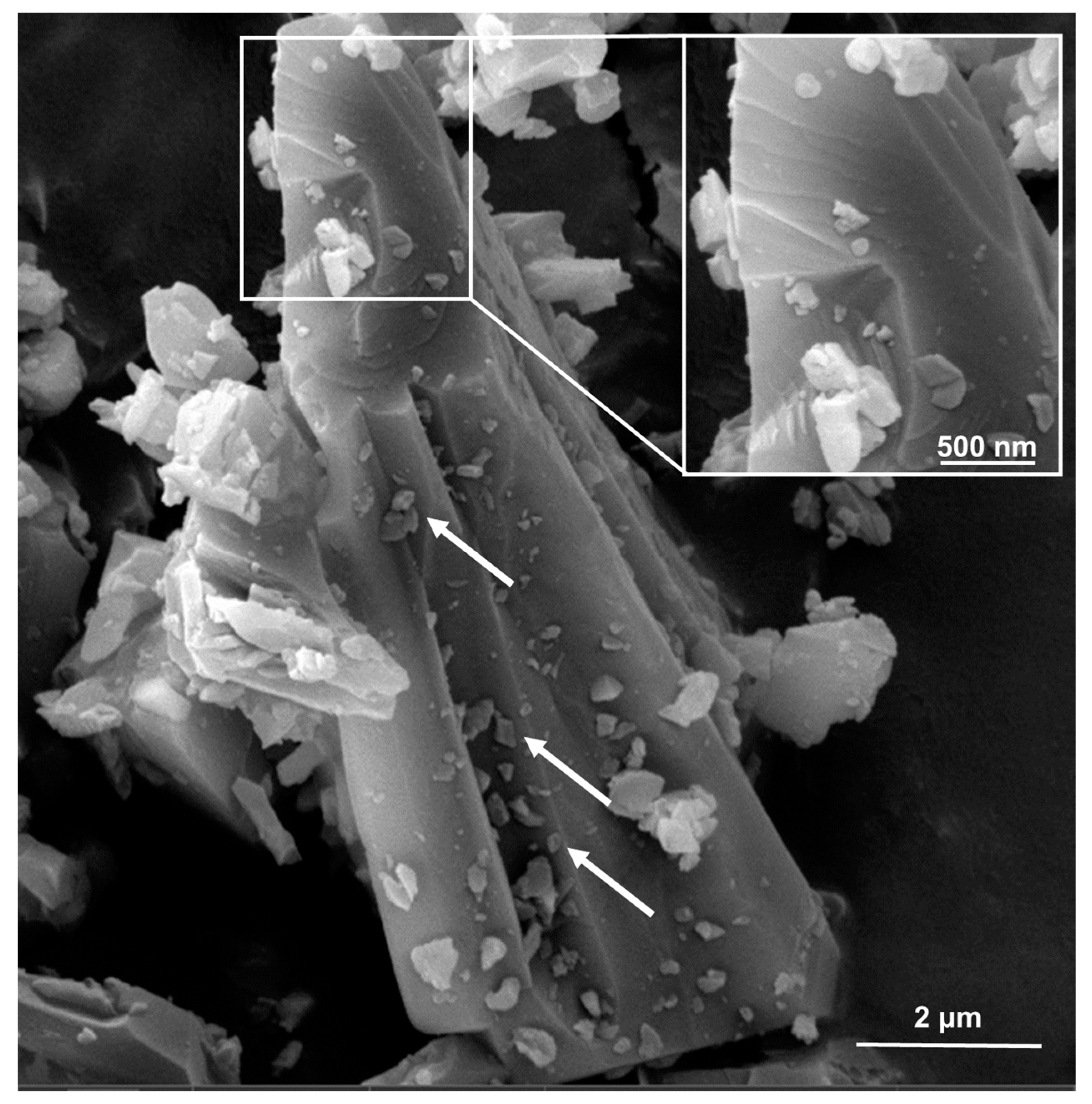
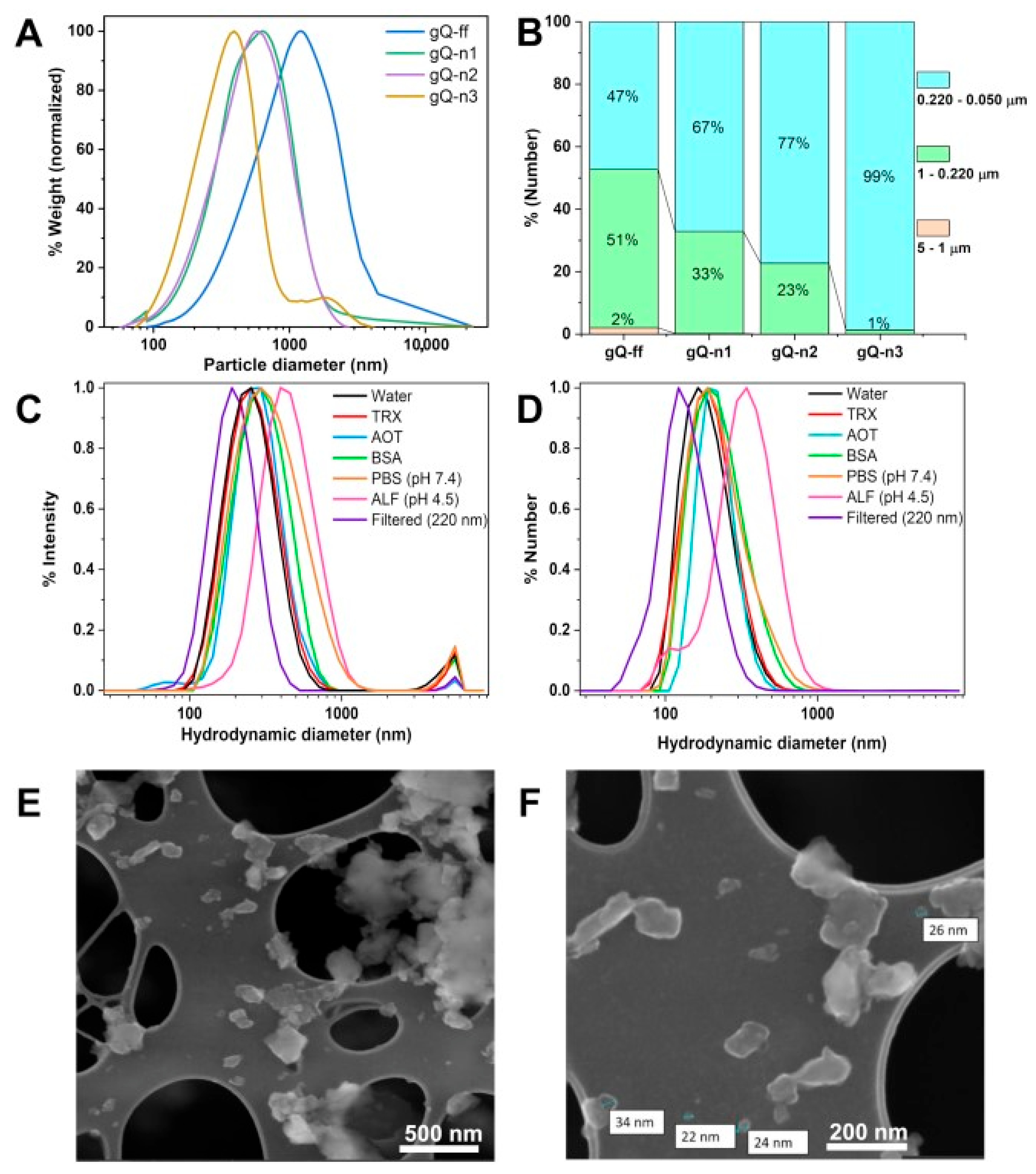
2.1. High-Energy Ball Milling Is an Effective Tool for Top-Down Generation of Nanoquartz
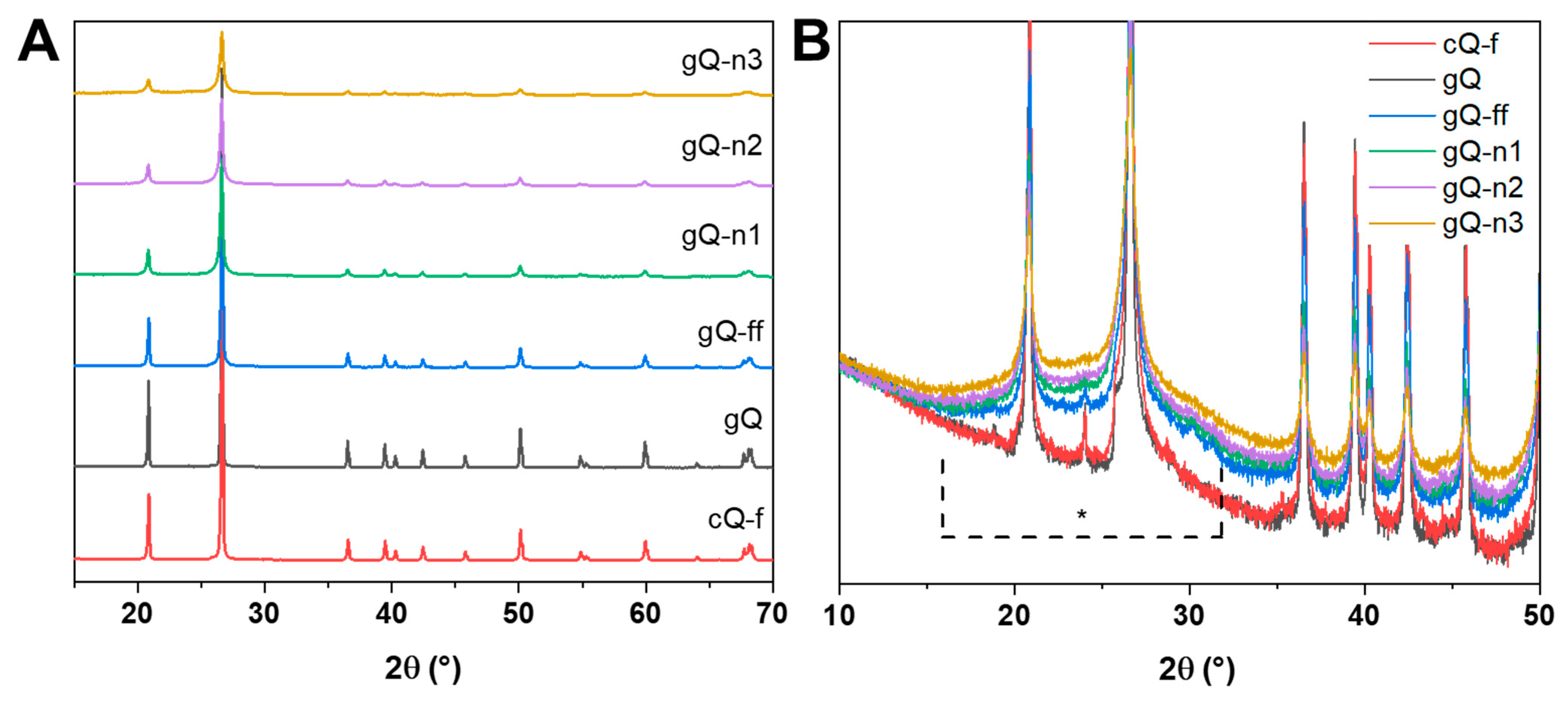
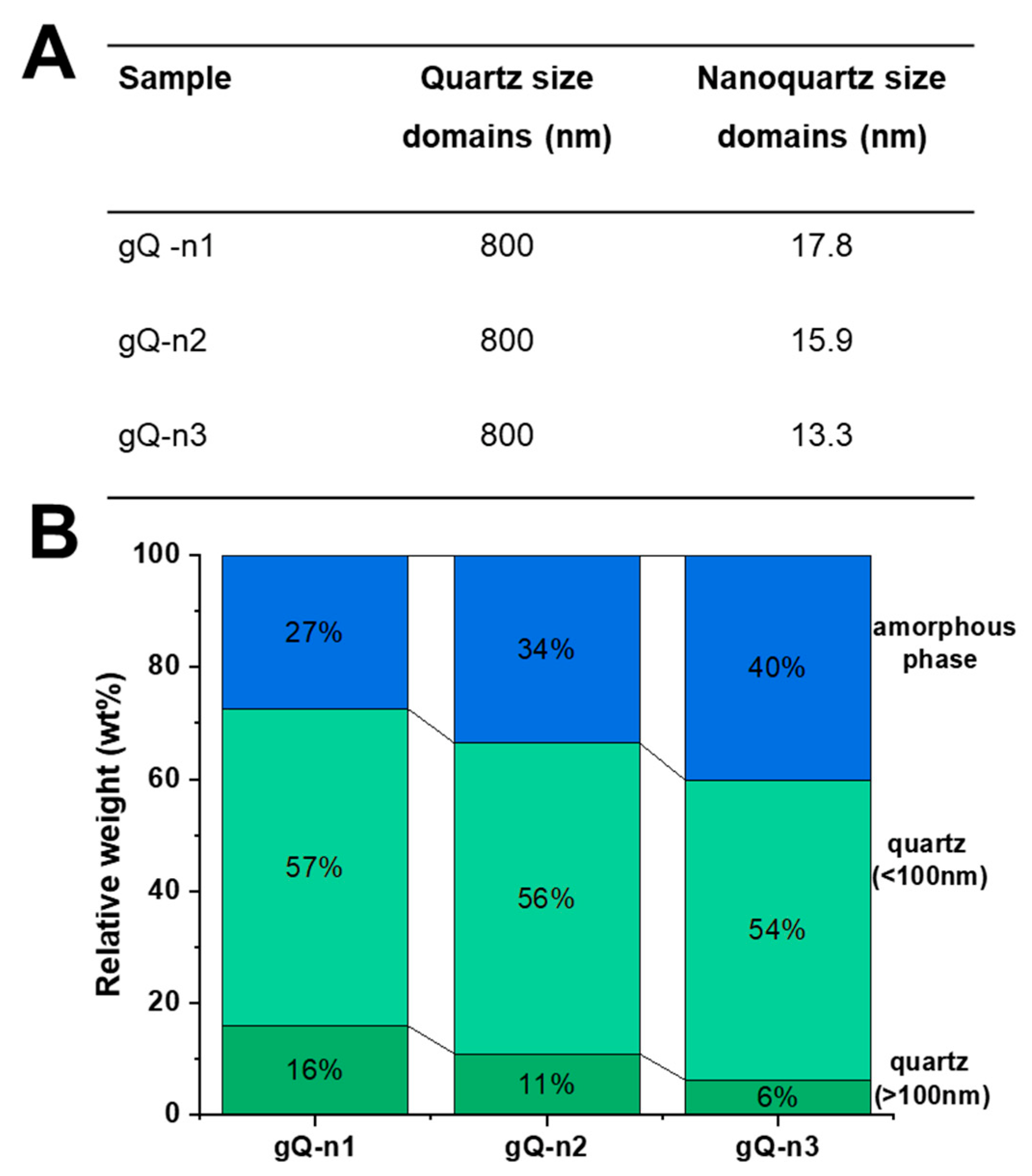
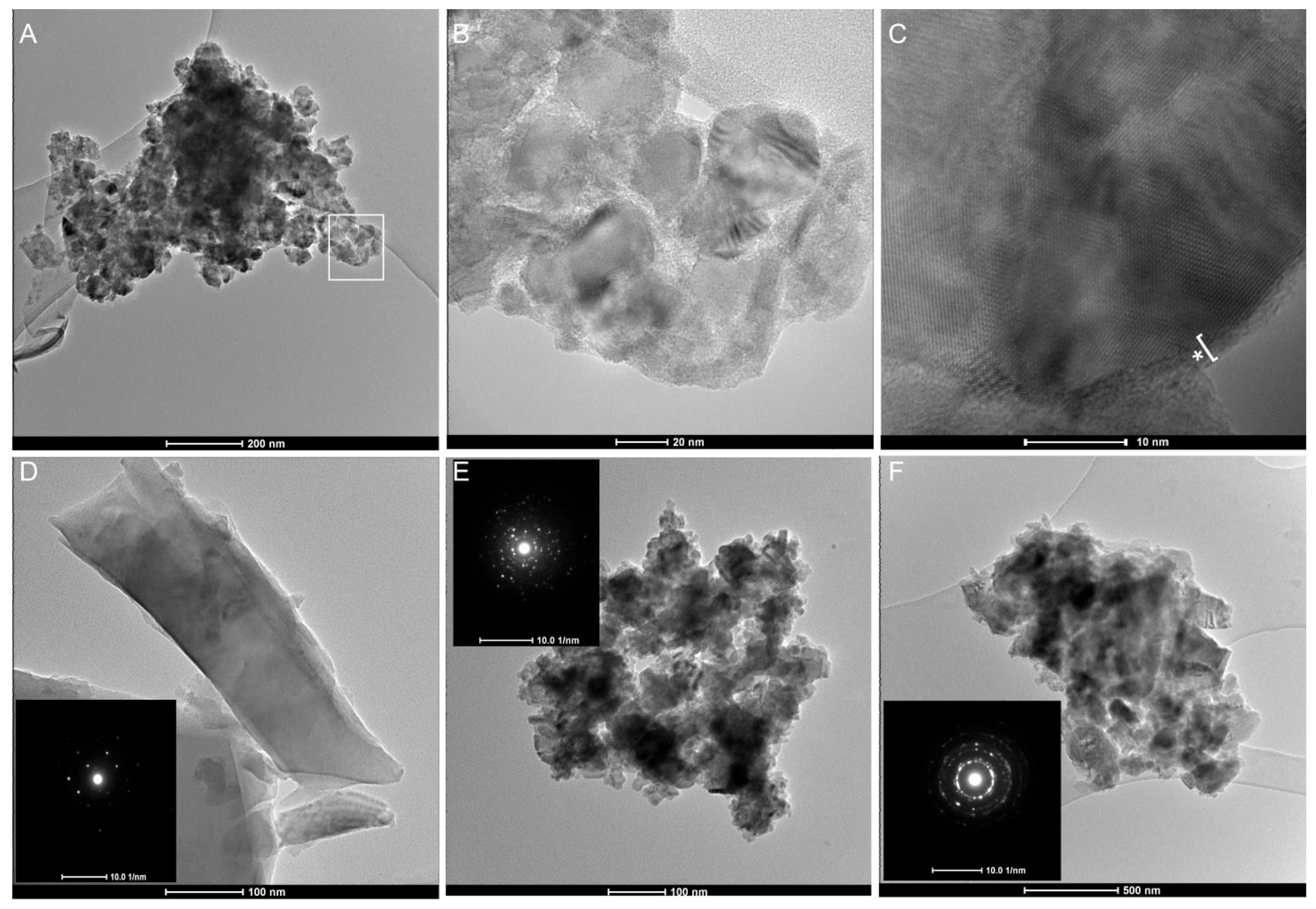
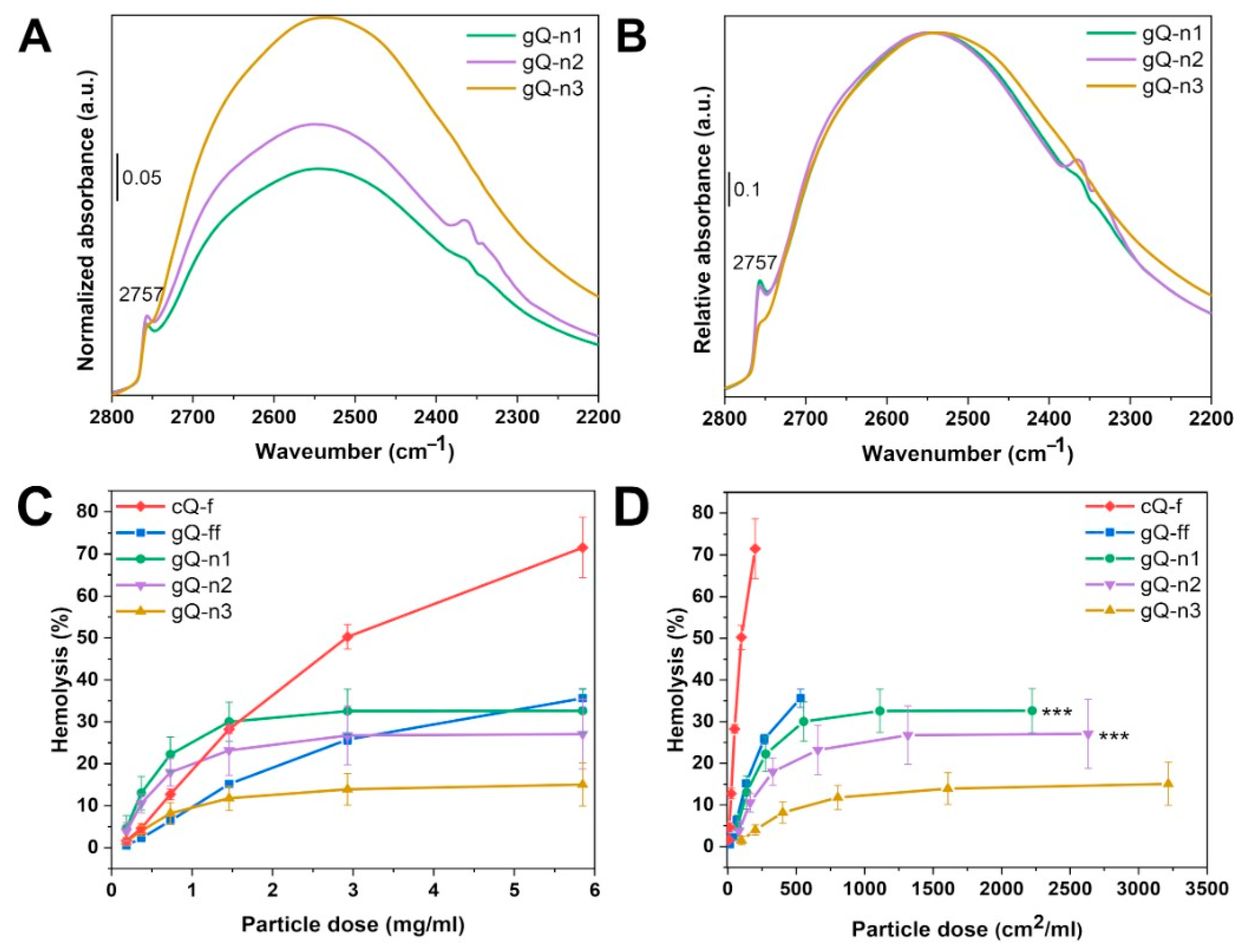
2.2. Current Top-Down Approach Mostly Preserves Crystallinity in Nanoquartz
2.3. Nanoquartz Particles Form Clusters in Biological Media
2.4. Nanoquartz Shows NFS and Membranolytic Activity
3. Materials and Methods
3.1. Quartz Particles
3.2. Morphology
3.3. Specific Surface Area (SSA)
3.4. Crystallinity
3.5. Particle Dispersion and Size Analysis
3.6. IR Spectroscopy
3.7. Membranolytic Activity
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALF | artificial phagolysosome fluid |
| AOT | dioctyl sulfosuccinate sodium salt |
| BSA | bovine serum albumin |
| b.t. | beam temperature |
| DCS | differential centrifugal sedimentation |
| NFS | nearly free silanols |
| RCS | respirable crystalline silica |
| SAED | single area electron diffraction |
| TRX | Triton X |
References
- Cullinan, P.; Muñoz, X.; Suojalehto, H.; Agius, R.; Jindal, S.; Sigsgaard, T.; Blomberg, A.; Charpin, D.; Annesi-Maesano, I.; Gulati, M. Occupational lung diseases: From old and novel exposures to effective preventive strategies. Lancet Respir. Med. 2017, 5, 445–455. [Google Scholar] [CrossRef]
- Hoy, R.F.; Chambers, D.C. Silica-related diseases in the modern world. Allergy 2020, 75, 2805–2817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iler, K.R. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; Wiley-Interscience: New York, NY, USA, 1979. [Google Scholar]
- Mishra, A.K.; Belgamwar, R.; Jana, R.; Datta, A.; Polshettiwar, V. Defects in nanosilica catalytically convert CO2 to methane without any metal and ligand. Proc. Natl. Acad. Sci. USA 2020, 117, 6383–6390. [Google Scholar]
- International Agency for Research on Cancer (IARC). A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts; IARC: Lyon, France, 2012; Volume 100C. [Google Scholar]
- Donaldson, K.; Borm, P.J. The quartz hazard: A variable entity. Ann. Occup. Hyg. 1998, 42, 287–294. [Google Scholar] [PubMed]
- Schins, R.P.; Duffin, R.; Höhr, D.; Knaapen, A.M.; Shi, T.; Weishaupt, C.; Stone, V.; Donaldson, K.; Borm, P.J. Surface modification of quartz inhibits toxicity, particle uptake, and oxidative DNA damage in human lung epithelial cells. Chem. Res. Toxicol. 2002, 15, 1166–1173. [Google Scholar] [PubMed]
- Pavan, C.; Fubini, B. Unveiling the variability of “quartz hazard” in light of recent toxicological findings. Chem. Res. Toxicol. 2017, 30, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Costa, D.; Sodupe, M.; Lambert, J.F.; Ugliengo, P. Silica surface features and their role in the adsorption of biomolecules: Computational modeling and experiments. Chem. Rev. 2013, 113, 4216–4313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turci, F.; Pavan, C.; Leinardi, R.; Tomatis, M.; Pastero, L.; Garry, D.; Anguissola, S.; Lison, D.; Fubini, B. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: The role of crystallinity and surface disorder. Part. Fibre Toxicol. 2016, 13, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavan, C.; Santalucia, R.; Leinardi, R.; Fabbiani, M.; Yakoub, Y.; Uwambayinema, F.; Ugliengo, P.; Tomatis, M.; Martra, G.; Turci, F. Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc. Natl. Acad. Sci. USA 2020, 117, 27836–27846. [Google Scholar] [CrossRef] [PubMed]
- Pavan, C.; Sydor, M.J.; Bellomo, C.; Leinardi, R.; Cananà, S.; Kendall, R.L.; Rebba, E.; Corno, M.; Ugliengo, P.; Mino, L.; et al. Molecular recognition between membrane epitopes and nearly free surface silanols explains silica membranolytic activity. Colloids Surf. B Biointerfaces 2022, 217, 112625. [Google Scholar] [CrossRef] [PubMed]
- Leinardi, R.; Pavan, C.; Yedavally, H.; Tomatis, M.; Salvati, A.; Turci, F. Cytotoxicity of fractured quartz on THP-1 human macrophages: Role of the membranolytic activity of quartz and phagolysosome destabilization. Arch. Toxicol. 2020, 94, 2981–2995. [Google Scholar] [CrossRef]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Merget, R.; Bauer, T.; Küpper, H.; Philippou, S.; Bauer, H.; Breitstadt, R.; Bruening, T. Health hazards due to the inhalation of amorphous silica. Arch. Toxicol. 2002, 75, 625–634. [Google Scholar] [PubMed]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Kotake, N.; Kuboki, M.; Kiya, S.; Kanda, Y. Influence of dry and wet grinding conditions on fineness and shape of particle size distribution of product in a ball mill. Adv. Powder Technol. 2011, 22, 86–92. [Google Scholar]
- Shin, H.; Lee, S.; Jung, H.S.; Kim, J.-B. Effect of ball size and powder loading on the milling efficiency of a laboratory-scale wet ball mill. Ceram. Int. 2013, 39, 8963–8968. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Silica, Some Silicates, Coal Dust and Para-Aramid Fibrils; World Health Organization (WHO): Lyon, France, 1997; Volume 68, p. 521. [Google Scholar]
- EC. Commission Recommendation of 10 June 2022 on the Definition of Nanomaterial (Text with EEA Relevance) 2022/C 229/01. 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32022H0614(01)&from=EN (accessed on 5 November 2022).
- Kim, W.; Kim, W.K.; Lee, K.; Son, M.J.; Kwak, M.; Chang, W.S.; Min, J.-K.; Song, N.W.; Lee, J.; Bae, K.-H. A reliable approach for assessing size-dependent effects of silica nanoparticles on cellular internalization behavior and cytotoxic mechanisms. Int. J. Nanomed. 2019, 14, 7375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- ANSES. OPINION of the French Agency for Food, Environmental and Occupational Health & Safety on “Updating Knowledge on the Hazards, Exposures and Risks Associated with Crystalline Silica”. Request No 2015-SA-0236—Crystalline Silica. 2019. Available online: https://www.anses.fr/en/system/files/AIR2015SA0236EN.pdf (accessed on 5 November 2022).
- Marques Da Silva, V.; Benjdir, M.; Montagne, P.; Pairon, J.-C.; Lanone, S.; Andujar, P. Pulmonary Toxicity of Silica Linked to Its Micro- or Nanometric Particle Size and Crystal Structure: A Review. Nanomaterials 2022, 12, 2392. [Google Scholar] [PubMed]
- Warheit, D.B.; Webb, T.R.; Colvin, V.L.; Reed, K.L.; Sayes, C.M. Pulmonary bioassay studies with nanoscale and fine-quartz particles in rats: Toxicity is not dependent upon particle size but on surface characteristics. Toxicol. Sci. 2007, 95, 270–280. [Google Scholar] [PubMed] [Green Version]
- Mischler, S.E.; Cauda, E.G.; Di Giuseppe, M.; McWilliams, L.J.; St Croix, C.; Sun, M.; Franks, J.; Ortiz, L.A. Differential activation of RAW 264.7 macrophages by size-segregated crystalline silica. J. Occup. Med. Toxicol. 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiessner, J.H.; Mandel, N.S.; Sohnle, P.G.; Mandel, G.S. Effect of particle size on quartz-induced hemolysis and on lung inflammation and fibrosis. Exp. Lung Res. 1989, 15, 801–812. [Google Scholar] [CrossRef]
- Kajiwara, T.; Ogami, A.; Yamato, H.; Oyabu, T.; Morimoto, Y.; Tanaka, I. Effect of particle size of intratracheally instilled crystalline silica on pulmonary inflammation. J. Occup. Health 2007, 49, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.J.; Sanderson, B.J.; Wang, H. Cytotoxicity and genotoxicity of ultrafine crystalline SiO2 particulate in cultured human lymphoblastoid cells. Environ. Mol. Mutagen. 2007, 48, 151–157. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.A.; Ostovan, F.; Abdul Aziz, S.; Mamat, M.S. Effect of milling time on the microstructure, physical and mechanical properties of Al-Al2O3 nanocomposite synthesized by ball milling and powder metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mio, H.; Kano, J.; Saito, F.; Kaneko, K. Optimum revolution and rotational directions and their speeds in planetary ball milling. Int. J. Miner. Process. 2004, 74, S85–S92. [Google Scholar] [CrossRef]
- Mio, H.; Kano, J.; Saito, F.; Kaneko, K. Effects of rotational direction and rotation-to-revolution speed ratio in planetary ball milling. Mater. Sci. Eng. A 2002, 332, 75–80. [Google Scholar] [CrossRef]
- Sohoni, S.; Sridhar, R.; Mandal, G. The effect of grinding aids on the fine grinding of limestone, quartz and Portland cement clinker. Powder Technol. 1991, 67, 277–286. [Google Scholar]
- Kabezya, K.; Motjotji, H. The effect of ball size diameter on milling performance. J. Mater. Sci. Eng 2014, 4, 1–3. [Google Scholar]
- Gorrasi, G.; Sorrentino, A. Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem. 2015, 17, 2610–2625. [Google Scholar]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [PubMed]
- Hasegawa, M.; Kimata, M.; Shimane, M.; Shoji, T.; Tsuruta, M. The effect of liquid additives on dry ultrafine grinding of quartz. Powder Technol. 2001, 114, 145–151. [Google Scholar] [CrossRef]
- Glasson, D.R. Vacuum balance studies of milled material and mechanochemical reactions. Thermochim. Acta 1981, 51, 45–52. [Google Scholar] [CrossRef]
- Finch, G.I. The Beilby Layer on Non-Metals. Nature 1936, 138, 1010. [Google Scholar] [CrossRef]
- Drescher, D.; Orts-Gil, G.; Laube, G.; Natte, K.; Veh, R.W.; Österle, W.; Kneipp, J. Toxicity of amorphous silica nanoparticles on eukaryotic cell model is determined by particle agglomeration and serum protein adsorption effects. Anal. Bioanal. Chem. 2011, 400, 1367–1373. [Google Scholar] [CrossRef]
- Thomassen, L.C.; Rabolli, V.; Masschaele, K.; Alberto, G.; Tomatis, M.; Ghiazza, M.; Turci, F.; Breynaert, E.; Martra, G.; Kirschhock, C.E.; et al. Model system to study the influence of aggregation on the hemolytic potential of silica nanoparticles. Chem. Res. Toxicol. 2011, 24, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Pastero, L.; Turci, F.; Leinardi, R.; Pavan, C.; Monopoli, M. Synthesis of α-quartz with controlled properties for the investigation of the molecular determinants in silica toxicology. Cryst. Growth Des. 2016, 16, 2394–2403. [Google Scholar] [CrossRef] [Green Version]
- Luo, T.; Zhang, R.; Zeng, W.-W.; Zhou, C.; Yang, X.; Ren, Z. Alkoxylation Reaction of Alcohol on Silica Surfaces Studied by Sum Frequency Vibrational Spectroscopy. J. Phys. Chem. C 2021, 125, 8638–8646. [Google Scholar] [CrossRef]
- Kohobhange, S.; Manoratne, C.; Pitawala, H.; Rajapakse, R. The effect of prolonged milling time on comminution of quartz. Powder Technol. 2018, 330, 266–274. [Google Scholar]
- Pavan, C.; Turci, F.; Tomatis, M.; Ghiazza, M.; Lison, D.; Fubini, B. Ζ potential evidences silanol heterogeneity induced by metal contaminants at the quartz surface: Implications in membrane damage. Colloids Surf. B Biointerfaces 2017, 157, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Carteret, C. Mid-and near-infrared study of hydroxyl groups at a silica surface: H-bond effect. J. Phys. Chem. C 2009, 113, 13300–13308. [Google Scholar] [CrossRef]
- Pavan, C.; Rabolli, V.; Tomatis, M.; Fubini, B.; Lison, D. Why does the hemolytic activity of silica predict its pro-inflammatory activity? Part. Fibre Toxicol. 2014, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organisation: Lyon, France, 2012; Volume 100C. [Google Scholar]
- Young, R.A. The Rietveld Method; International Union of Crystallography: Chester, UK, 1993; Volume 5. [Google Scholar]
- Lutterotti, L. Total pattern fitting for the combined size–strain–stress–texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Colombo, C.; Monhemius, A.J.; Plant, J.A. Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotoxicol. Environ. Saf. 2008, 71, 722–730. [Google Scholar] [CrossRef] [PubMed]


| Quartz | Origin | Milling Speed (rpm) | Milling Time (Hours) | Ball Diameter (mm) | SSA (m2/g) b | |
|---|---|---|---|---|---|---|
| A | gQ | Synthesis | / | / | / | 0.1 |
| B | gQ-ff | gQ dry milling | 450 | 1 | 5 | 10 |
| C1 | gQ-n1 a | gQ-ff wet milling | 450 | 1 | 2 | 38 |
| C2 | gQ-n2 a | gQ-ff wet milling | 550 | 1 | 2 | 45 |
| C3 | gQ-n3 a | gQ-ff wet milling | 550 | 2 | 2 | 55 |
| Quartz | Peak (µm) | Range % (22–5.0 µm) | Range % (5.0–1.0 µm) | Range % (1.0–0.22 µm) | Range % (0.22–0.050 µm) |
|---|---|---|---|---|---|
| gQ-ff | 1.45 | 23.1 | 46.37 | 24.75 | 1.17 |
| gQ-n1 | 0.635 | 0.0 | 13.42 | 73.06 | 6.97 |
| gQ-n2 | 0.574 | 0.0 | 12.27 | 76.44 | 10.49 |
| gQ-n3 | 0.392 | 0.0 | 7.42 | 72.43 | 19.93 |
| Medium | Z-Average (nm) ± s.d. a | Peak (nm) ± s.d. a Intensity | Peak (nm) ± s.d. a Number |
|---|---|---|---|
| H2O | 276.2 ± 16.1 | 264.8 ± 14.0 | 198.3 ± 6.6 |
| H2O + TRX | 280.4 ± 13.9 | 274.8 ± 20.7 | 199.0 ± 14.8 |
| H2O + AOT | 298 ± 4.5 | 274.8 ± 52.9 | 175.9 ± 81.3 |
| H2O + BSA | 321.6 ± 6.7 | 315.7 ± 16.4 | 238.0 ± 7.0 |
| PBS | 330.9 ± 3.2 | 366.9 ± 33.4 | 243.0 ± 2.0 |
| ALF | 435.0 ± 26.9 | 470.0 ± 48.8 | 345.2 ± 62.9 |
| Filtered (220 nm-pores) | 246.8 ± 20.7 | 203.0 ± 10.7 | 144.2 ± 19.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellomo, C.; Pavan, C.; Fiore, G.; Escolano-Casado, G.; Mino, L.; Turci, F. Top-Down Preparation of Nanoquartz for Toxicological Investigations. Int. J. Mol. Sci. 2022, 23, 15425. https://doi.org/10.3390/ijms232315425
Bellomo C, Pavan C, Fiore G, Escolano-Casado G, Mino L, Turci F. Top-Down Preparation of Nanoquartz for Toxicological Investigations. International Journal of Molecular Sciences. 2022; 23(23):15425. https://doi.org/10.3390/ijms232315425
Chicago/Turabian StyleBellomo, Chiara, Cristina Pavan, Gianluca Fiore, Guillermo Escolano-Casado, Lorenzo Mino, and Francesco Turci. 2022. "Top-Down Preparation of Nanoquartz for Toxicological Investigations" International Journal of Molecular Sciences 23, no. 23: 15425. https://doi.org/10.3390/ijms232315425
APA StyleBellomo, C., Pavan, C., Fiore, G., Escolano-Casado, G., Mino, L., & Turci, F. (2022). Top-Down Preparation of Nanoquartz for Toxicological Investigations. International Journal of Molecular Sciences, 23(23), 15425. https://doi.org/10.3390/ijms232315425








