The Binomial “Inflammation-Epigenetics” in Breast Cancer Progression and Bone Metastasis: IL-1β Actions Are Influenced by TET Inhibitor in MCF-7 Cell Line
Abstract
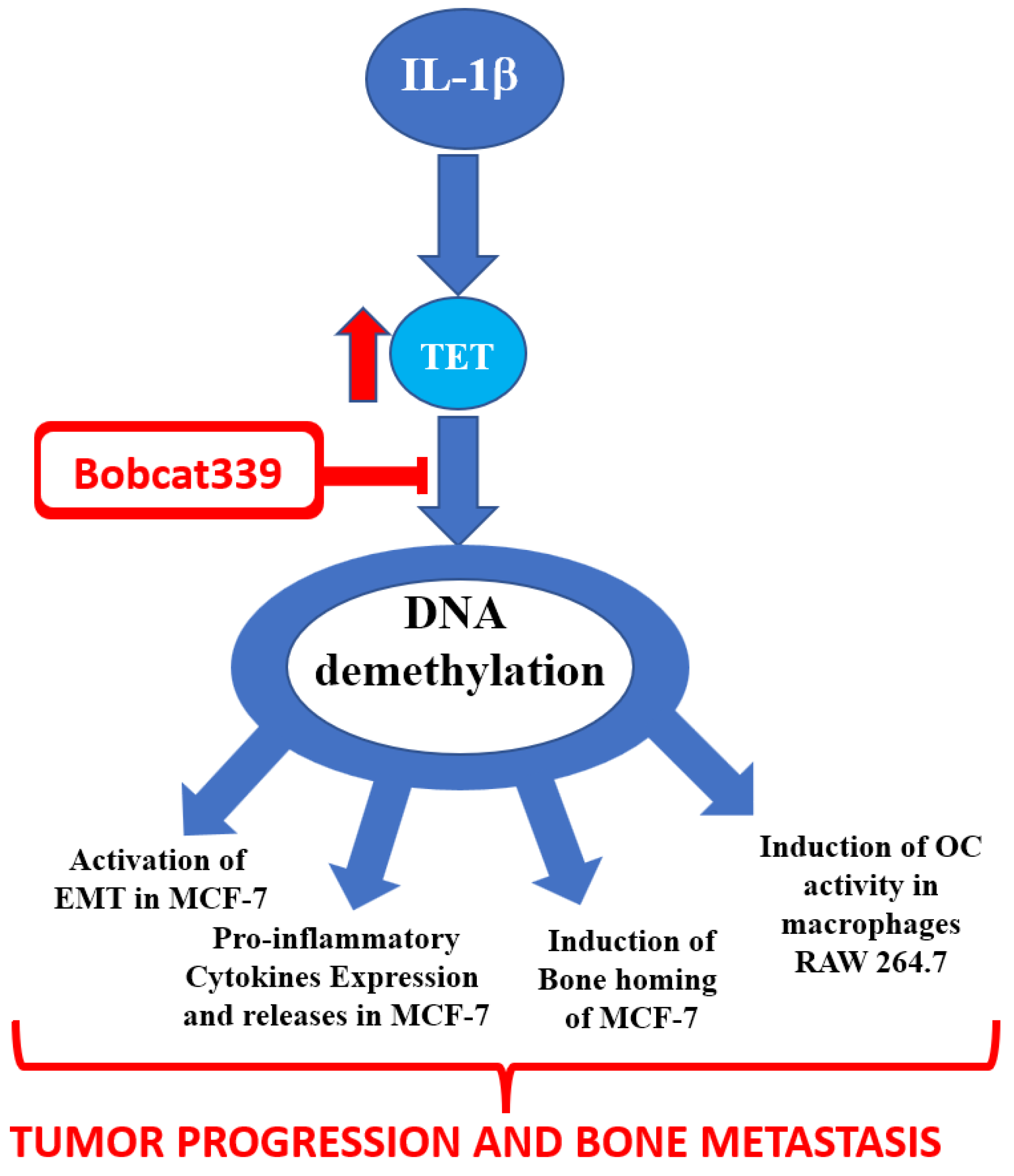
:1. Introduction
2. Results
2.1. Methylase and Demethylase Inhibitors Change Genomic DNA Methylation in MCF-7 Cell Lines Treated with IL-1β
2.2. IL-1β Regulates IL-6 and IL-8 Releases through DNA Methylation Modifications
2.3. AZA and BC Affect EMT Process induced by IL-1β Treatment
2.4. AZA and BC Affect Expression of Factors Involved in Bone Homing and Metastasis in MCF-7 Treated with IL-1β
2.5. IL-1β Treatment Induces Modification in DNMT and TET Expressions
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures
4.3. Viability Assay (MTT Assay)
4.4. Genomic DNA (gDNA) Extraction
4.5. Dot Spot Hybridization Analysis
4.6. Wound Healing Assay
4.7. MSRE–PCR Analysis
4.8. ELISA Assay
4.9. Western Blot Analysis
4.10. Bone Resorption Pit Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghoncheh, M.; Pournamdar, Z.; Salehiniya, H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac. J. Cancer Prev. 2016, 17, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyre, R.; Alférez, D.G.; Santiago-Gómez, A.; Spence, K.; McConnell, J.C.; Hart, C.; Simões, B.M.; Lefley, D.; Tulotta, C.; Storer, J.; et al. Microenvironmental IL1β promotes breast cancer metastatic colonisation in the bone via activation of Wnt signalling. Nat. Commun. 2019, 10, 5016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terceiro, L.E.L.; Edechi, C.A.; Ikeogu, N.M.; Nickel, B.E.; Hombach-Klonisch, S.; Sharif, T.; Leygue, E.; Myal, Y. The Breast Tumor Microenvironment: A Key Player in Metastatic Spread. Cancers 2021, 13, 4798. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Joshi, M.B. Comprehensive analysis of regulation of DNA methyltransferase isoforms in human breast tumors. J. Cancer Res. Clin. Oncol. 2021, 147, 937–971. [Google Scholar] [CrossRef] [PubMed]
- Reyngold, M.; Turcan, S.; Giri, D.; Kannan, K.; Walsh, L.A.; Viale, A.; Drobnjak, M.; Vahdat, L.T.; Lee, W.; Chan, T.A. Remodeling of the methylation landscape in breast cancer metastasis. PLoS ONE 2014, 9, e103896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Meng, Y.; Zhou, L.; Qiu, L.; Wang, H.; Su, D.; Zhang, B.; Chan, K.M.; Han, J. Targeting epigenetic regulators for inflammation: Mechanisms and intervention therapy. MedComm (2020) 2022, 3, e173. [Google Scholar] [CrossRef]
- Barter, M.J.; Cheung, K.; Falk, J.; Panagiotopoulos, A.C.; Cosimini, C.; O’Brien, S.; Teja-Putri, K.; Neill, G.; Deehan, D.J.; Young, D.A. Dynamic chromatin accessibility landscape changes following interleukin-1 stimulation. Epigenetics 2021, 16, 106–119. [Google Scholar] [CrossRef]
- Vezzani, B.; Carinci, M.; Previati, M.; Giacovazzi, S.; Della Sala, M.; Gafà, R.; Lanza, G.; Wieckowski, M.R.; Pinton, P.; Giorgi, C. Epigenetic Regulation: A Link between Inflammation and Carcinogenesis. Cancers 2022, 14, 1221. [Google Scholar] [CrossRef]
- Eissa, N.S.; Khairuddin, U.; Yusof, R. A hybrid metaheuristic-deep learning technique for the pan-classification of cancer based on DNA methylation. BMC Bioinform. 2022, 23, 273. [Google Scholar] [CrossRef]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Bhol, C.S.; Praharaj, P.P.; Panigrahi, D.P.; Patra, S.; Singh, A.; Patil, S.; Dhiman, R.; et al. Inflammasomes in cancer: Effect of epigenetic and autophagic modulations. Semin. Cancer Biol. 2022, 83, 399–412. [Google Scholar] [CrossRef]
- Li, R.; Ong, S.L.; Tran, L.M.; Jing, Z.; Liu, B.; Park, S.J.; Huang, Z.L.; Walser, T.C.; Heinrich, E.L.; Lee, G.; et al. Chronic IL-1β-induced inflammation regulates epithelial-to-mesenchymal transition memory phenotypes via epigenetic modifications in non-small cell lung cancer. Sci. Rep. 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.C.; Chen, C.W.; Yang, C.L.; Tsai, I.M.; Hou, Y.C.; Chen, C.J.; Shan, Y.S. Tumor-Associated Macrophages Promote Epigenetic Silencing of Gelsolin through DNA Methyltransferase 1 in Gastric Cancer Cells. Cancer Immunol. Res. 2017, 5, 885–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Garduño, A.M.; Mendoza-Rodríguez, M.G.; Urrutia-Cabrera, D.; Domínguez-Robles, M.C.; Pérez-Yépez, E.A.; Ayala-Sumuano, J.T.; Meza, I. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 490, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Chua, G.N.L.; Wassarman, K.L.; Sun, H.; Alp, J.A.; Jarczyk, E.I.; Kuzio, N.J.; Bennett, M.J.; Malachowsky, B.G.; Kruse, M.; Kennedy, A.J. Cytosine-Based TET Enzyme Inhibitors. ACS Med. Chem. Lett. 2019, 10, 180–185. [Google Scholar] [CrossRef]
- Caradonna, F.; Cruciata, I.; Schifano, I.; La Rosa, C.; Naselli, F.; Chiarelli, R.; Perrone, A.; Gentile, C. Methylation of cytokines gene promoters in IL-1β-treated human intestinal epithelial cells. Inflamm. Res. 2018, 67, 327–337. [Google Scholar] [CrossRef]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J.P. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. 2017, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Hou, S.; Zou, M.; Ye, K.; Xiang, L. miR-543 impairs cell proliferation, migration, and invasion in breast cancer by suppressing VCAN. Biochem. Biophys. Res. Commun. 2021, 570, 191–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, X.; Qian, W.; Weng, X.; Zhang, L.; Wang, S.; Cao, X.; Ma, L.; Wei, G.; Wu, Y.; et al. Enhanced PAPSS2/VCAN sulfation axis is essential for Snail-mediated breast cancer cell migration and metastasis. Cell Death Differ. 2019, 26, 565–579. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Gong, K.; Zhang, X.; Wu, S.; Cui, Y.; Qian, B.Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharm. Res. 2019, 144, 235–244. [Google Scholar] [CrossRef]
- Kovacheva, M.; Zepp, M.; Schraad, M.; Berger, S.; Berger, M.R. Conditional Knockdown of Osteopontin Inhibits Breast Cancer Skeletal Metastasis. Int. J. Mol. Sci. 2019, 20, 4918. [Google Scholar] [CrossRef]
- Shemanko, C.S. Prolactin receptor in breast cancer: Marker for metastatic risk. J. Mol. Endocrinol. 2016, 57, R153–R165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, A.; Forsyth, A.; Cong, Y.; Grant, L.; Juan, T.H.; Lee, J.K.; Klimowicz, A.; Petrillo, S.K.; Hu, J.; Chan, A.; et al. The Role of Prolactin in Bone Metastasis and Breast Cancer Cell-Mediated Osteoclast Differentiation. J. Natl. Cancer Inst. 2016, 108, djv338. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Fang, L.; Yang, W.; Sheng, W.; Zhang, Y.; Seth, A.; Yang, B.B.; Yee, A.J. The role of versican G3 domain in regulating breast cancer cell motility including effects on osteoblast cell growth and differentiation in vitro—Evaluation towards understanding breast cancer cell bone metastasis. BMC Cancer 2012, 12, 341. [Google Scholar] [CrossRef] [Green Version]
- Diep, S.; Maddukuri, M.; Yamauchi, S.; Geshow, G.; Delk, N.A. Interleukin-1 and Nuclear Factor Kappa B Signaling Promote Breast Cancer Progression and Treatment Resistance. Cells 2022, 11, 1673. [Google Scholar] [CrossRef]
- Lu, A.; Wang, W.; Wang-Renault, S.F.; Ring, B.Z.; Tanaka, Y.; Weng, J.; Su, L. 5-Aza-2′-deoxycytidine advances the epithelial-mesenchymal transition of breast cancer cells by demethylating. J. Cell Sci. 2020, 133, jcs236125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Valdivia, N.I.; Calderón, C.C.; Díaz, J.E.; Lobos-González, L.; Sepulveda, H.; Ortíz, R.J.; Martinez, S.; Silva, V.; Maldonado, H.J.; Silva, P.; et al. Anti-neoplastic drugs increase caveolin-1-dependent migration, invasion and metastasis of cancer cells. Oncotarget 2017, 8, 111943–111965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Sarli, V.N.; Lucci, A. Inhibition of resistant triple-negative breast cancer cells with low-dose 6-mercaptopurine and 5-azacitidine. Oncotarget 2021, 12, 626–637. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; El-Shinawi, M.; Sabet, S.; Ibrahim, S.A.; Mohamed, M.M. Role of adipose tissue-derived cytokines in the progression of inflammatory breast cancer in patients with obesity. Lipids Health Dis. 2022, 21, 67. [Google Scholar] [CrossRef]
- Liu, R.; Choi, H.S.; Kim, S.L.; Kim, J.H.; Yun, B.S.; Lee, D.S. 6-Methoxymellein Isolated from Carrot ( Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules 2020, 25, 4374. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, Y.; Dai, Z.J.; Wu, C.J.; Ji, Y.H.; Xu, J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef]
- Peng, X.; Chang, H.; Gu, Y.; Chen, J.; Yi, L.; Xie, Q.; Zhu, J.; Zhang, Q.; Mi, M. 3,6-Dihydroxyflavone Suppresses Breast Carcinogenesis by Epigenetically Regulating miR-34a and miR-21. Cancer Prev. Res. 2015, 8, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Zhang, K.; Clarke, K.; Weiland, T.; Sauter, E.R. Methylation and miRNA effects of resveratrol on mammary tumors vs. normal tissue. Nutr. Cancer 2014, 66, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mayo, M.W.; Nagji, A.S.; Smith, P.W.; Ramsey, C.S.; Li, D.; Jones, D.R. Phosphorylation of RelA/p65 promotes DNMT-1 recruitment to chromatin and represses transcription of the tumor metastasis suppressor gene BRMS1. Oncogene 2012, 31, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.F.; Morin, S.; Beaulieu, N.; Gauthier, F.; Chute, I.C.; Barsalou, A.; MacLeod, A.R. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat. Genet. 2003, 33, 61–65. [Google Scholar] [CrossRef]
- Burleson, J.D.; Siniard, D.; Yadagiri, V.K.; Chen, X.; Weirauch, M.T.; Ruff, B.P.; Brandt, E.B.; Hershey, G.K.K.; Ji, H. TET1 contributes to allergic airway inflammation and regulates interferon and aryl hydrocarbon receptor signaling pathways in bronchial epithelial cells. Sci. Rep. 2019, 9, 7361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Abreu-Rodriguez, I.; Ye, S.; Gay, S.; Distler, O.; Neidhart, M.; Karouzakis, E. TET1 is an important transcriptional activator of TNFα expression in macrophages. PLoS ONE 2019, 14, e0218551. [Google Scholar] [CrossRef]
- Bellavia, D.; Raimondo, S.; Calabrese, G.; Forte, S.; Cristaldi, M.; Patinella, A.; Memeo, L.; Manno, M.; Raccosta, S.; Diana, P.; et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics 2017, 7, 1333–1345. [Google Scholar] [CrossRef]
- Jia, Z.; Liang, Y.; Ma, B.; Xu, X.; Xiong, J.; Duan, L.; Wang, D. A 5-mC Dot Blot Assay Quantifying the DNA Methylation Level of Chondrocyte Dedifferentiation In Vitro. J. Vis. Exp. 2017, 123, e55565. [Google Scholar] [CrossRef]
- De Luca, A.; Bellavia, D.; Raimondi, L.; Carina, V.; Costa, V.; Fini, M.; Giavaresi, G. Multiple Effects of Resveratrol on Osteosarcoma Cell Lines. Pharmaceuticals 2022, 15, 342. [Google Scholar] [CrossRef]
- Bellavia, D.; Dimarco, E.; Caradonna, F. Characterization of three different clusters of 18S-26S ribosomal DNA genes in the sea urchin P. lividus: Genetic and epigenetic regulation synchronous to 5S rDNA. Gene 2016, 580, 118–124. [Google Scholar] [CrossRef]
- Raimondi, L.; De Luca, A.; Fontana, S.; Amodio, N.; Costa, V.; Carina, V.; Bellavia, D.; Raimondo, S.; Siragusa, S.; Monteleone, F.; et al. Multiple Myeloma-Derived Extracellular Vesicles Induce Osteoclastogenesis through the Activation of the XBP1/IRE1α Axis. Cancers 2020, 12, 2167. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Version 4.1.2 (Bird Hippie); R Core Team: Vienna, Austria, 2021. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellavia, D.; Costa, V.; De Luca, A.; Cordaro, A.; Fini, M.; Giavaresi, G.; Caradonna, F.; Raimondi, L. The Binomial “Inflammation-Epigenetics” in Breast Cancer Progression and Bone Metastasis: IL-1β Actions Are Influenced by TET Inhibitor in MCF-7 Cell Line. Int. J. Mol. Sci. 2022, 23, 15422. https://doi.org/10.3390/ijms232315422
Bellavia D, Costa V, De Luca A, Cordaro A, Fini M, Giavaresi G, Caradonna F, Raimondi L. The Binomial “Inflammation-Epigenetics” in Breast Cancer Progression and Bone Metastasis: IL-1β Actions Are Influenced by TET Inhibitor in MCF-7 Cell Line. International Journal of Molecular Sciences. 2022; 23(23):15422. https://doi.org/10.3390/ijms232315422
Chicago/Turabian StyleBellavia, Daniele, Viviana Costa, Angela De Luca, Aurora Cordaro, Milena Fini, Gianluca Giavaresi, Fabio Caradonna, and Lavinia Raimondi. 2022. "The Binomial “Inflammation-Epigenetics” in Breast Cancer Progression and Bone Metastasis: IL-1β Actions Are Influenced by TET Inhibitor in MCF-7 Cell Line" International Journal of Molecular Sciences 23, no. 23: 15422. https://doi.org/10.3390/ijms232315422
APA StyleBellavia, D., Costa, V., De Luca, A., Cordaro, A., Fini, M., Giavaresi, G., Caradonna, F., & Raimondi, L. (2022). The Binomial “Inflammation-Epigenetics” in Breast Cancer Progression and Bone Metastasis: IL-1β Actions Are Influenced by TET Inhibitor in MCF-7 Cell Line. International Journal of Molecular Sciences, 23(23), 15422. https://doi.org/10.3390/ijms232315422








