COVID-19 Vaccines, Effectiveness, and Immune Responses
Abstract
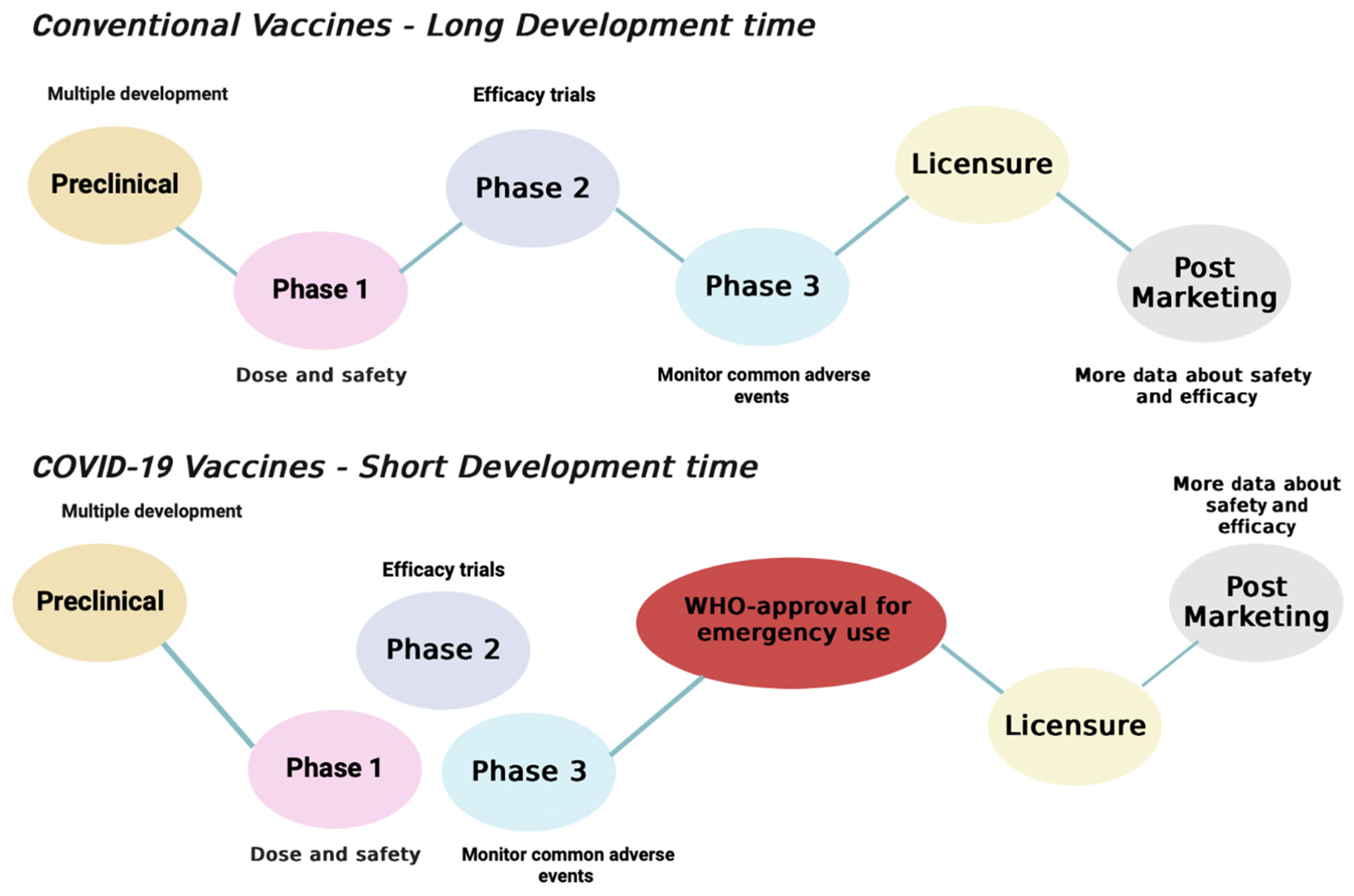
:1. Introduction
1.1. SARS-CoV-2 Vaccines
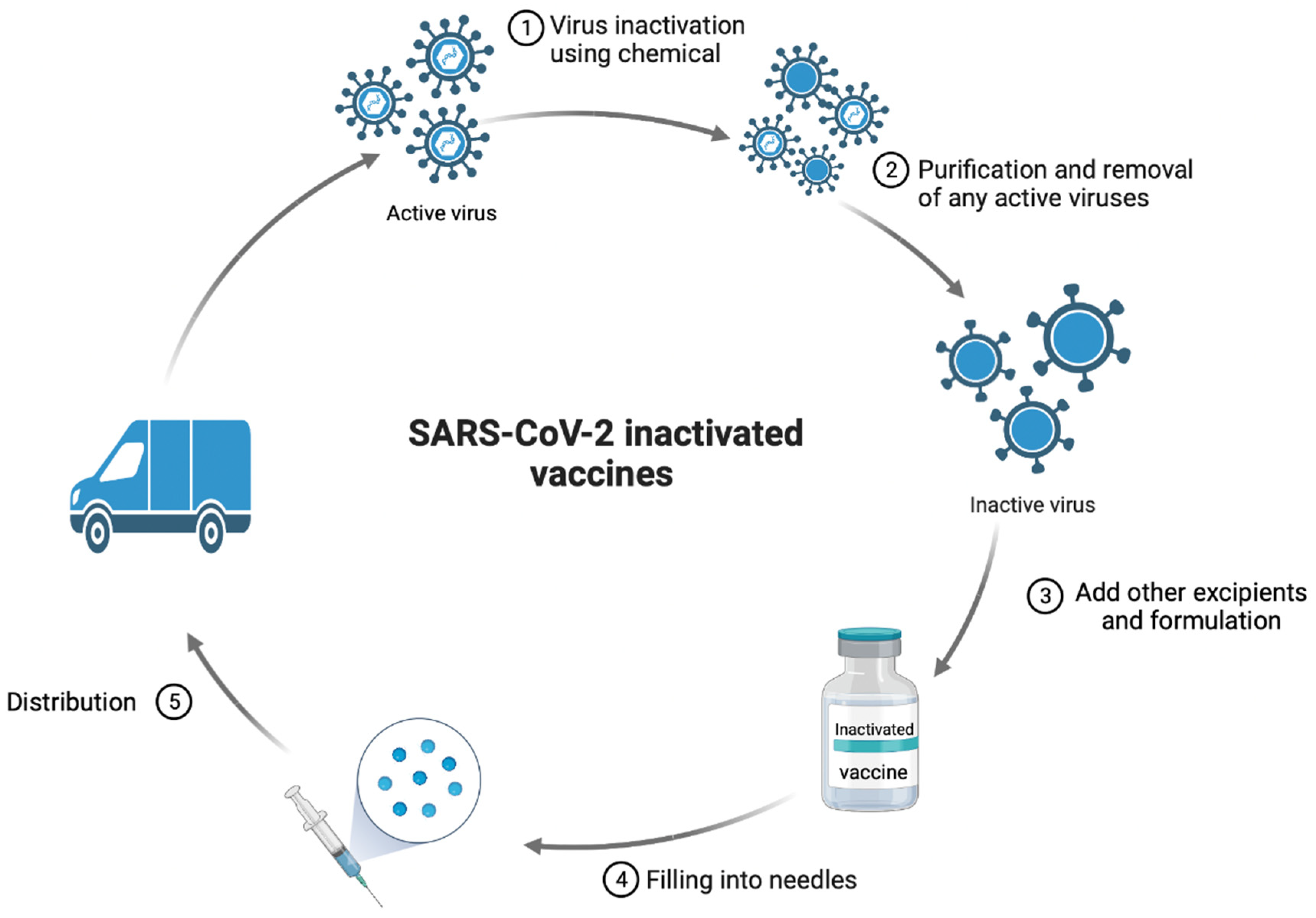
1.2. Whole Virus Vaccines
| WHO-Approved Inactivated Whole SARS-CoV-2 Vaccines | |||||
|---|---|---|---|---|---|
| The Vaccine | Efficacy | Advantages | Disadvantages | Usage | References |
| Sinopharm | 78.10% in preventing symptomatic COVID-19 infection. | Easy to preserve, manufacture, and transport. | Reduction in the efficacy of vaccines over time can lead to lower immunogenicity. | Sinopharm inactivated vaccines are given in 2 doses for 18 years and older individuals. | [19] |
| Sinovac | 51% in preventing symptomatic COVID-19 infection. | Easy to preserve, manufacture, and transport. | The need of batch control to prevent any impairment that can lead to infection. | Sinovac inactivated vaccines are given in 2 doses for 18 years and older individuals. | [19] |
| Covaxin | 77.80% in preventing symptomatic COVID-19 infection. | Easy to preserve, manufacture, and transport. | The need of adjuvant to boost the immunity. | Covaxin inactivated vaccines are given in 2 doses for 18 years and older individuals. | [20] |
| Nucleic acid based WHO-approved COVID-19 vaccines | |||||
| The vaccine | Efficacy | Advantages | Disadvantages | Usage | References |
| Pfizer/BioNTech | 91.10% in reducing symptomatic COVID-19 | Safe for children along with long-term protection against COVID-19 infection. High immunogenicity | The need of ultra-low temperature for transportation and storage. They are expensive | Vaccines are given in 2 doses for 6 months and older individuals, with dose modification in younger patients. | [21,22] |
| Moderna | 94.1% in reducing symptomatic COVID-19 | High immunogenicity | The need of low temperature for transportation and storage. | Vaccines are given in 2 doses for 6 months and older individuals, with dose modification in younger patients | [23,24] |
| Viral vector WHO-approved COVID-19 vaccines | |||||
| The vaccine | Efficacy | Advantages | Disadvantages | Usage | References |
| AstraZeneca | 79% in preventing symptomatic COVID-19 infection | Stable and easier to distribute | The need for adjuvant and the limited immunogenicity | AstraZeneca is given in 2 doses for 18 years and older individuals. | [25,26] |
| Johnson and Johnson | 67% in preventing symptomatic COVID-19 infection | Stable and easier to distribute as well as it is given in a single shot | The need for adjuvant and the limited immunogenicity | Johnson and Johnson vaccine is given in 1 dose for 18 years and older individuals. | [27] |
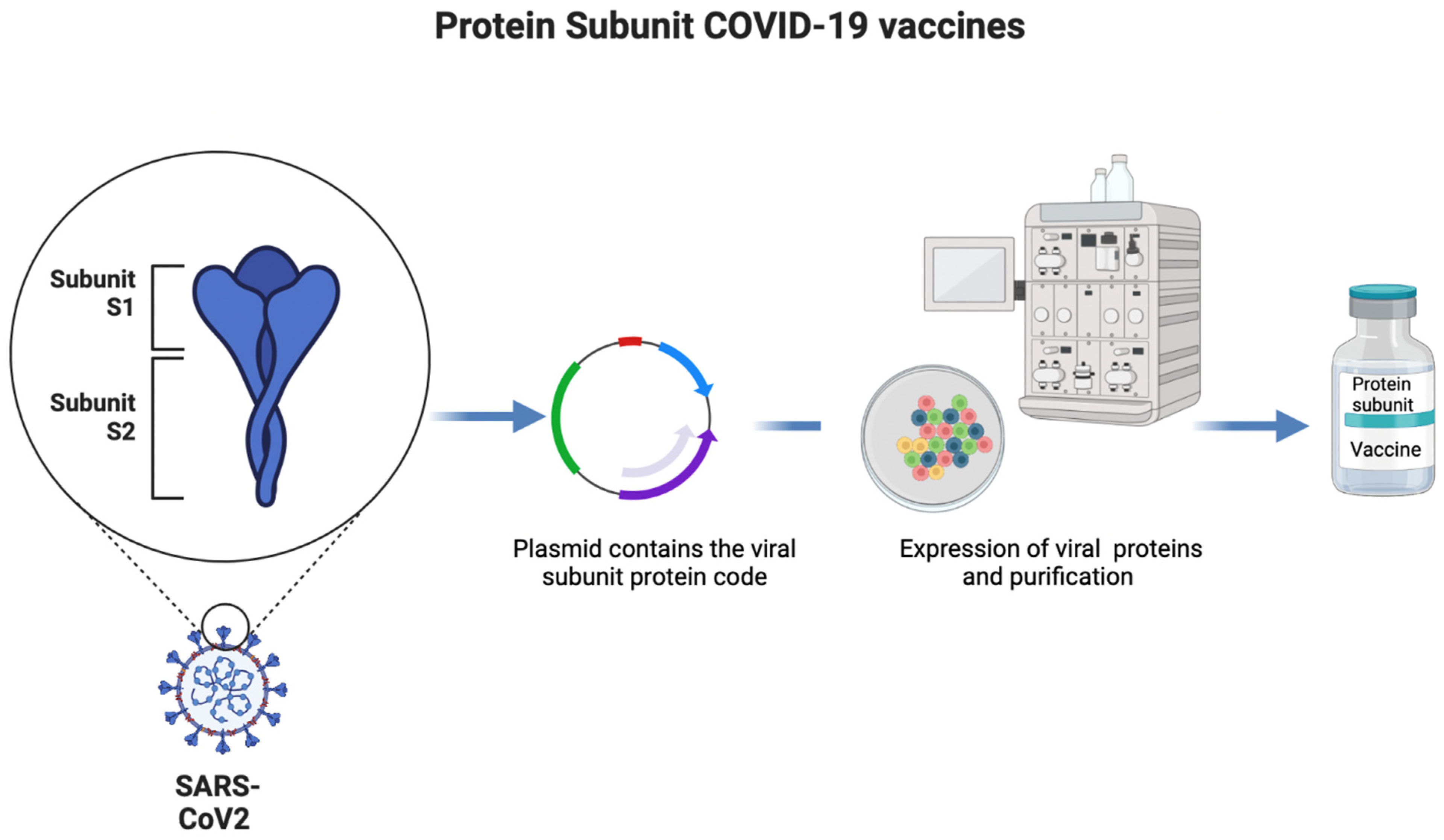
1.3. Protein-Based Vaccines
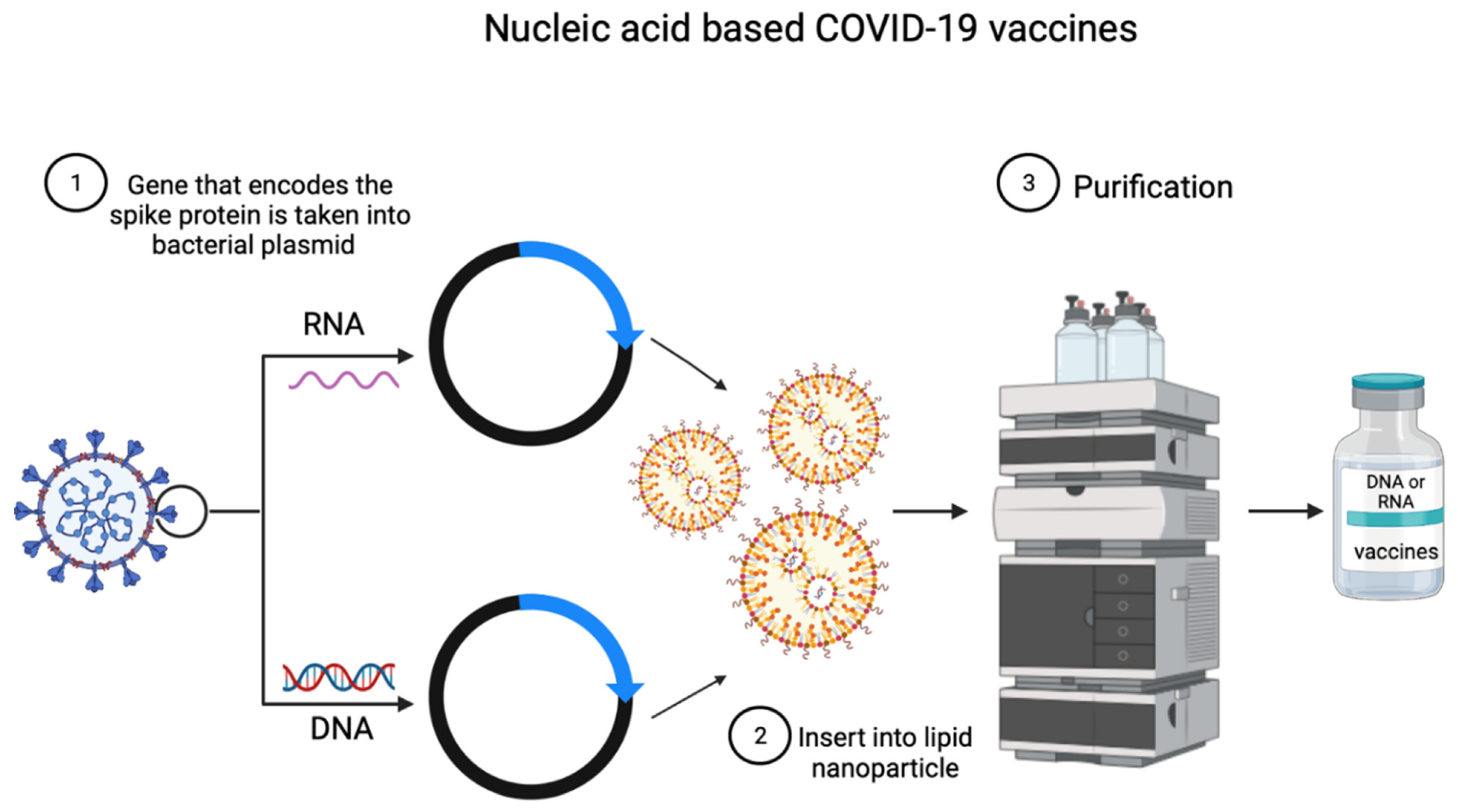
1.4. Nucleic Acid-Based Vaccines
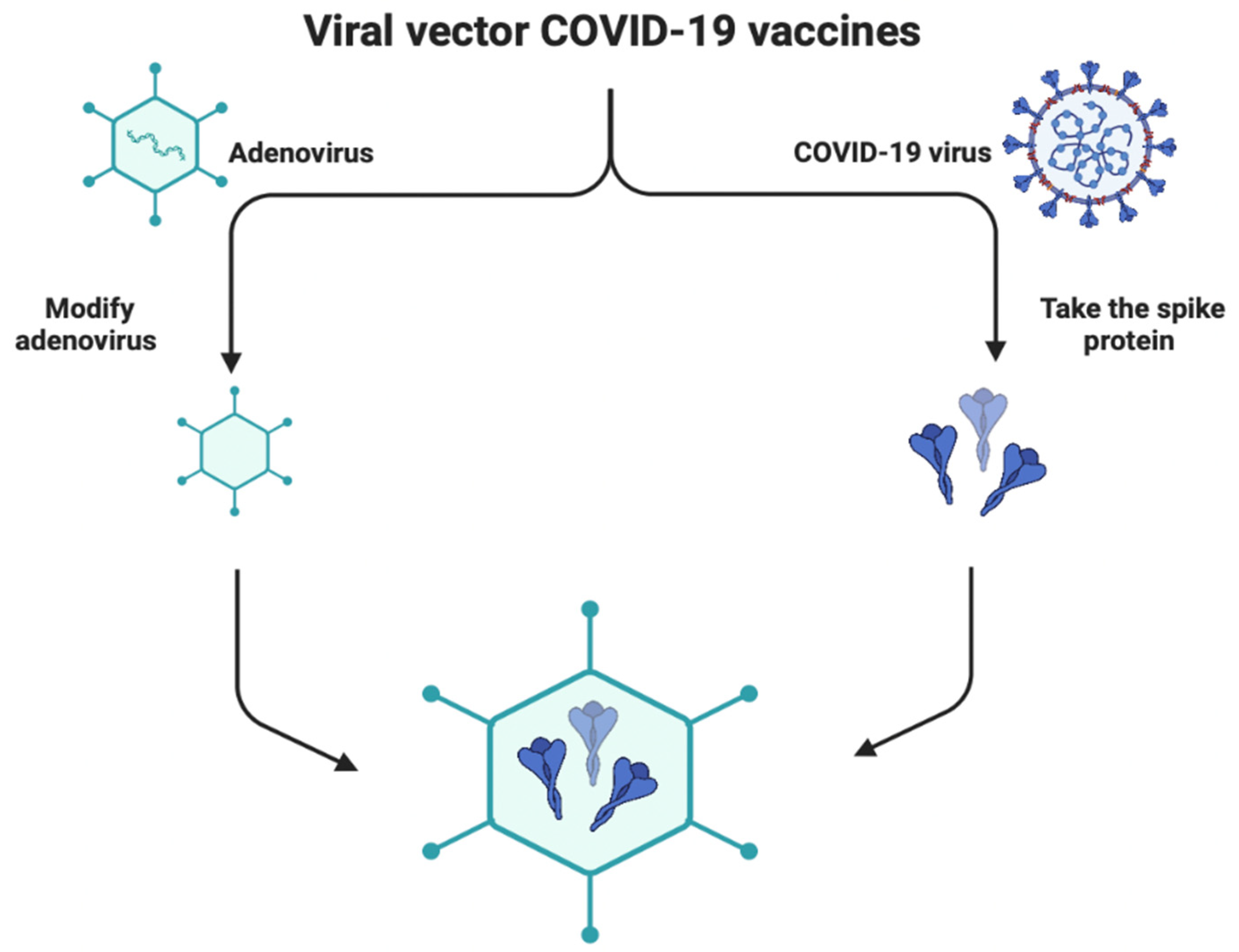
1.5. Viral Vector-Based Vaccines
2. Vaccine Formulation and Their Complication
| Vaccine | Storage and Transport | References |
| Pfizer/BioNTech (12 + formulation) | The vials can be stored between −90 °C and −60 °C until the expiration date and shipped thermally using dry ice as they are stable for 30 days. They can be kept in the freezer for up to 2 weeks and in the refrigerator for up to 1 month (31 days). | [53] |
| Moderna | This vaccine should be stored at −20 °C. It is stable for around 1 month between 2 and 8 °C Unpunctured vials can be stored in a refrigerator from 2 to 8 °C for up to 30 days. Punctured vials can be held between 8 and 25 °C for 24 h. | [54] |
| AstraZeneca | This vaccine is stored, carried, and handled at normal refrigerated conditions between 2 and 8 °C for at least 6 months. | [55] |
| Sinopharm | The vials should be stored at a normal fridge temperature from 2 to 8 °C. | [54] |
| Sinovac | The vials should be stored at a normal fridge temperature from 2 to 8 °C for 12 months, and at room temperature not to exceed +25. | [54] |
| Covaxin | The vials should be stored at a normal fridge temperature from 2 to 8 °C for 6 months. | [54] |
| Convidecia | The vials should be stored at a normal fridge temperature from 2 to 8 °C for 12 months. | [56] |
| Johnson and Johnson | Opened vials should be discarded after 6 h. The vials should be stored at a normal fridge temperature between 2 and 8 °C for 3 months. | [54] |
| Covovax (Novavax) | The vials should be stored between 2 and 8 °C until the expiration date. They should be discarded 6 h after puncture. | [57] |
3. Effectiveness of SARS-CoV-2 Vaccines
3.1. Pfizer/BioNTech
3.2. Moderna
3.3. AstraZeneca
3.4. Johnson & Johnson
3.5. Convidecia
3.6. Sinovac-CoronaVac
3.7. Sinopharm
3.8. Covaxin
3.9. Covovax (Novovax)
4. COVID-19 Vaccines Booster Dose
5. Adverse Effects Due to SARS-CoV-2 Vaccines
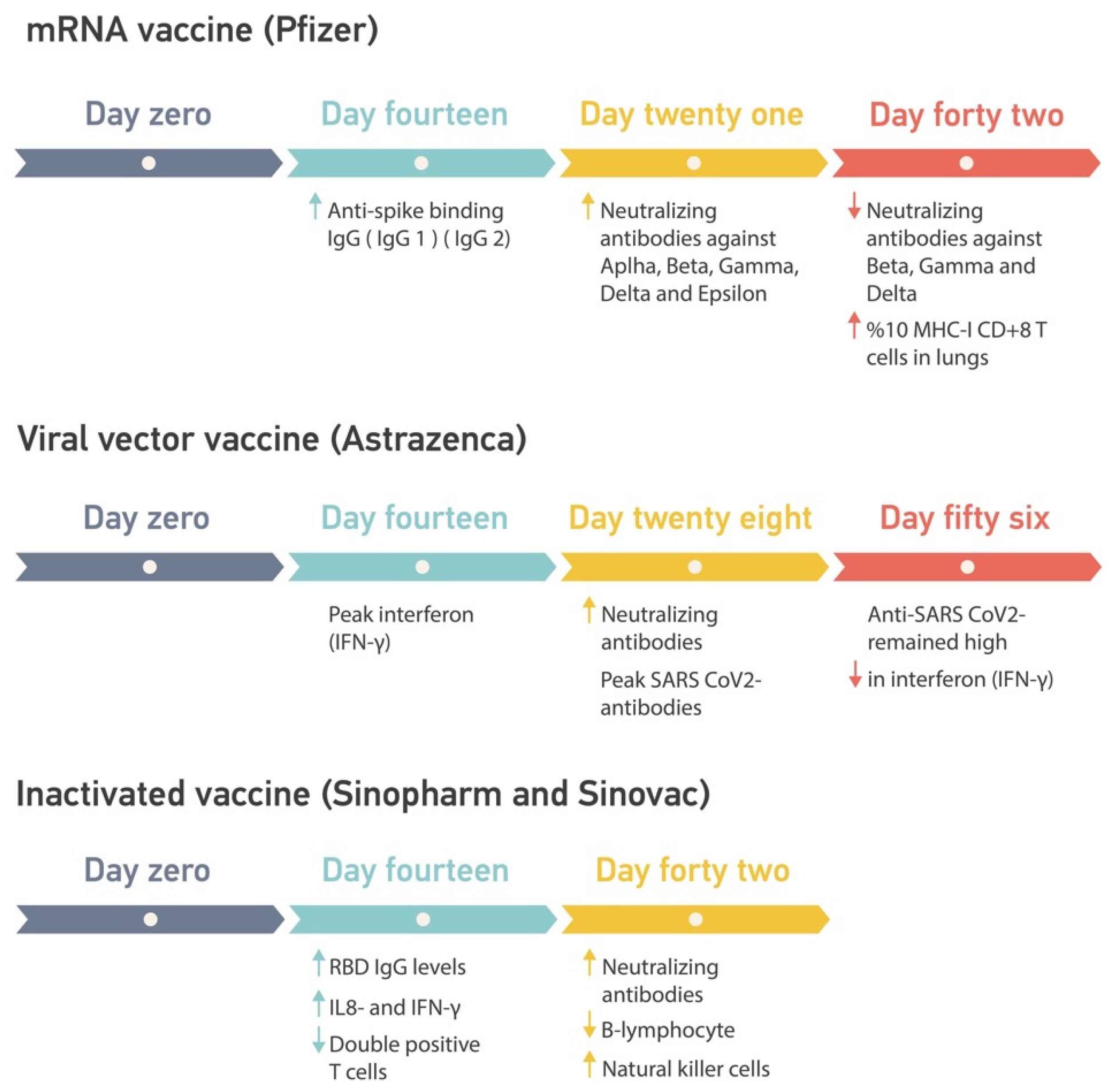
6. Vaccine Immune Responses
6.1. Beneficial Responses in Healthy Individuals
| IgG Anti-RBD Levels BAU/mL | |||
|---|---|---|---|
| mRNA Vaccines | |||
| Data | Pfizer | Moderna | References |
| Self et al. | 2950 | 4274 | [123] |
| Kanokudom et al., (Pfizer); Al-Sadeq (Moderna) et al. | 2584 | 2272 | [124,125] |
| Median | 2767 | 3273 | |
| Standard deviation | 258.801082 | 1415.62778 | |
| Fold change | 1.14164087 | 1.88116197 | |
6.2. Harmful Responses in Healthy Individuals
6.3. Beneficial Responses in Unhealthy Individuals
| Solid Tumor Cases | ||||
|---|---|---|---|---|
| Study | Number of Cases | Average | Standard Deviation | Median |
| Ehmsen [142] | 139 | 152.5 | 19.091 | 152.5 |
| Mencoboni [143] | 166 | |||
| Solid tumor antibodies | ||||
| Study | Anti-spike SARS-CoV-2 IgG in BAU/mL after mRNA vaccine | Average | Standard deviation | Median |
| Ehmsen (three doses of mRNA vaccine) | 2464 | 1787.65 | 956.503343 | 1787.65 |
| Mencoboni (two doses of mRNA vaccine) | 1111.3 | |||
| Study | HIV Patients CD4+ Counts | Antibody Titer (S-RBD-IgG Titers) U/mL in Inactivated Vaccine | Average | Standard Deviation | Median |
|---|---|---|---|---|---|
| Liu et al. [144] | ≥350 cells/µL | 22.4 | 16.8 | 7.919595949 | 16.8 |
| Liu et al. [144] | <350 cells/µL) | 11.2 | |||
| Netto et al. [145] | ≥500 cells/μL | 53.3 | 47.95 | 7.566042559 | 47.95 |
| Netto et al. [145] | <500 cells/μL | 42.6 |
| Study 1 [138] | Study 2 [139] | ||
|---|---|---|---|
| Multiple Sclerosis | Healthy Individuals | Multiple Sclerosis | Healthy Individuals |
| Untreated N = 32 | n = 47 | Untreated N = 76 | n = 89 |
| Cladribine N = 26 | Cladribine N = 48 | ||
| Ocrelizumab N = 44 | Ocrelizumab N = 114 | ||
| Fingolimod N = 26 | Fingolimod N = 42 | ||
6.4. Harmful Immune Response in Unhealthy Individuals
7. Future Perspective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salata, C.; Calistri, A.; Parolin, C.; Palù, G. Coronaviruses: A Paradigm of New Emerging Zoonotic Diseases. Pathog. Dis. 2019, 77, ftaa006. [Google Scholar] [CrossRef] [Green Version]
- Focosi, D.; Maggi, F. Neutralising Antibody Escape of SARS-CoV-2 Spike Protein: Risk Assessment for Antibody-Based COVID-19 Therapeutics and Vaccines. Rev. Med. Virol. 2021, 31, e2231. [Google Scholar] [CrossRef] [PubMed]
- Baric, R.S. SARS-CoV: Lessons for Global Health. Virus Res. 2008, 133, 1–3. [Google Scholar] [CrossRef]
- Khan, G. A Novel Coronavirus Capable of Lethal Human Infections: An Emerging Picture. Virol. J. 2013, 10, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-NCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, X.; Li, T.; Zhang, S.; Wang, L.; Wu, X.; Liu, J. The Genetic Sequence, Origin, and Diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1629–1635. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary Trajectory for the Emergence of Novel Coronavirus SARS-CoV-2. Pathogens 2020, 9, 240. [Google Scholar] [CrossRef] [Green Version]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- WHO. Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 5 August 2022).
- Richards, F.; Kodjamanova, P.; Chen, X.; Li, N.; Atanasov, P.; Bennetts, L.; Patterson, B.J.; Yektashenas, B.; Mesa-Frias, M.; Tronczynski, K.; et al. Economic Burden of COVID-19: A Systematic Review. Clin. Outcomes Res. 2022, 14, 293–307. [Google Scholar] [CrossRef]
- COVID-19 Vaccines Advice. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 6 August 2022).
- Martínez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Reséndiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 2774. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The Spike Protein of SARS-CoV—A Target for Vaccine and Therapeutic Development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef]
- How Have Covid-19 Vaccines Been Made Quickly and Safely?|News|Wellcome. Available online: https://wellcome.org/news/quick-safe-covid-vaccine-development (accessed on 24 August 2022).
- Science Brief: SARS-CoV-2 Infection-Induced and Vaccine-Induced Immunity CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html (accessed on 24 July 2022).
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 Vaccines. Curr. Opin. Immunol. 2021, 71, 111. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Live Attenuated Vaccines: Historical Successes and Current Challenges. Virology 2015, 479–480, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Difference between Sinopharm and Sinovac—Healthsprings. Available online: https://www.healthspringsgroup.com.sg/difference-between-sinopharm-and-sinovac/ (accessed on 15 September 2022).
- COVAXIN—India’s First Indigenous COVID-19 Vaccine|Bharat Biotech. Available online: https://www.bharatbiotech.com/covaxin.html (accessed on 15 September 2022).
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- The Pfizer BioNTech (BNT162b2) COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine-what-you-need-to-know (accessed on 15 September 2022).
- The Moderna COVID-19 (MRNA-1273) Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know (accessed on 15 September 2022).
- GRADE: Moderna COVID-19 Vaccine|CDC. Available online: https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-moderna-vaccine.html (accessed on 15 September 2022).
- What You Need to Know about AstraZeneca vs. Pfizer Vaccine. Available online: https://www.healthline.com/health/astrazeneca-vs-pfizer-vaccine (accessed on 15 September 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Facts about the Johnson and Johnson Vaccine. Available online: https://www.healthline.com/health/vaccinations/johnson-and-johnson-vaccine (accessed on 15 September 2022).
- Dolgin, E. How Protein-Based COVID Vaccines Could Change the Pandemic. Nature 2021, 599, 359–360. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Calina, D.; Docea, A.O.; Petrakis, D.; Egorov, A.M.; Ishmukhametov, A.A.; Gabibov, A.G.; Shtilman, M.I.; Kostoff, R.; Carvalho, F.; Vinceti, M.; et al. Towards Effective COVID-19 Vaccines: Updates, Perspectives and Challenges (Review). Int. J. Mol. Med. 2020, 46, 3. [Google Scholar] [CrossRef]
- Liljeqvist, S.; Ståhl, S. Production of Recombinant Subunit Vaccines: Protein Immunogens, Live Delivery Systems and Nucleic Acid Vaccines. J. Biotechnol. 1999, 73, 1–33. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, S.; Ou, J.; Zhang, J.; Lan, W.; Guan, W.; Wu, X.; Yan, Y.; Zhao, W.; Wu, J.; et al. COVID-19: Coronavirus Vaccine Development Updates. Front. Immunol. 2020, 11, 3435. [Google Scholar] [CrossRef] [PubMed]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. AAPS PharmSciTech 2020, 21, 225. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological Considerations for COVID-19 Vaccine Strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Manjunath, K.; Ranjan, R.K.; Kaushik, S.; Kumar, S.; Verma, V. COVID-19 Pandemic: Insights into Structure, Function, and HACE2 Receptor Recognition by SARS-CoV-2. PLoS Pathog. 2020, 16, e1008762. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.K. Delivery Routes for COVID-19 Vaccines. Vaccines 2021, 9, 524. [Google Scholar] [CrossRef]
- Zhang, N.; Li, K.; Liu, Z.; Nandakumar, K.S.; Jiang, S. A Perspective on the Roles of Adjuvants in Developing Highly Potent COVID-19 Vaccines. Viruses 2022, 14, 387. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 2896. [Google Scholar] [CrossRef]
- Adjuvants and Vaccines|Vaccine Safety|CDC. Available online: https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html (accessed on 24 August 2022).
- Tomljenovic, L.; Shaw, C.A. Aluminum Vaccine Adjuvants: Are They Safe? Curr. Med. Chem. 2011, 18, 2630–2637. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in Aluminum Hydroxide-Based Adjuvant Research and Its Mechanism. Hum. Vaccin. Immunother. 2015, 11, 477. [Google Scholar] [CrossRef]
- Liao, H.C.; Wu, W.L.; Chiang, C.Y.; Huang, M.S.; Shen, K.Y.; Huang, Y.L.; Wu, S.C.; Liao, C.L.; Chen, H.W.; Liu, S.J. Low-Dose SARS-CoV-2 S-Trimer with an Emulsion Adjuvant Induced Th1-Biased Protective Immunity. Int. J. Mol. Sci. 2022, 23, 4902. [Google Scholar] [CrossRef]
- Petrovsky, N. Freeing Vaccine Adjuvants from Dangerous Immunological Dogma. Expert Rev. Vaccines 2008, 7, 7–10. [Google Scholar] [CrossRef] [PubMed]
- TLR AGONISTS: Are They Good Adjuvants? Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2922045/ (accessed on 24 August 2022).
- Channappanavar, R.; Fett, C.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Virus-Specific Memory CD8 T Cells Provide Substantial Protection from Lethal Severe Acute Respiratory Syndrome Coronavirus Infection. J. Virol. 2014, 88, 11034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-kohara, K. An Overview of Recent Insights into the Response of TLR to SARS-CoV-2 Infection and the Potential of TLR Agonists as SARS-CoV-2 Vaccine Adjuvants. Viruses 2021, 13, 2302. [Google Scholar] [CrossRef]
- Cook, I.F. Evidence Based Route of Administration of Vaccines. Hum. Vaccines 2008, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wei, C.; Zhang, Z.; Liu, T.; Wang, T. Aluminum Nanoparticles Acting as a Pulmonary Vaccine Adjuvant-Delivery System (VADS) Able to Safely Elicit Robust Systemic and Mucosal Immunity. J. Inorg. Organomet. Polym. Mater 2020, 30, 4203–4217. [Google Scholar] [CrossRef] [PubMed]
- Severe Acute Respiratory Syndrome Coronavirus 2: Host-Pathogen Interactions…—Google Books. Available online: https://books.google.ae/books?hl=en&lr=&id=ohVdEAAAQBAJ&oi=fnd&pg=PA203&dq=1.%09Meseko+CA,+Song+R,+Zu%C3%B1iga+S,+Ansari+MA,+Khan+WH,+Hashmi+Z,+Goel+A,+Ahmad+R,+Gupta+K,+Khan+N,+Alam+I.+COVID-19+Pandemic+and+Vaccines+Update+on+Challenges+and+Resolutions.+Severe+Acute+Respiratory+Syndrome+Coronavirus+2:+Host-Pathogen+Interactions+and+Ce&ots=YRygmg53aO&sig=Z79LPgjzdmkojaVQjNLOklm3Wk4&redir_esc=y#v=onepage&q&f=false (accessed on 29 August 2022).
- Freeze-Dried COVID Vaccines? UB Is Working on It|WBFO. Available online: https://www.wbfo.org/health-wellness/2021-12-06/freeze-dried-covid-vaccines-ub-is-working-on-it (accessed on 24 August 2022).
- How to Freeze-Dry a Potential COVID-19 Vaccine—UBNow: News and Views for UB Faculty and Staff—University at Buffalo. Available online: https://www.buffalo.edu/ubnow/stories/2021/12/freeze-dried-vaccine.html (accessed on 24 August 2022).
- CDC. Ncird Pfizer-BioNTech COVID-19 Vaccine Pfizer-BioNTech COVID-19 Vaccine Storage and Handling Summary; CDC: Singapore, 2021. [Google Scholar]
- What’s Needed to Protect COVID-19 Vaccines during Transport—European Pharmaceutical Manufacturer. Available online: https://pharmaceuticalmanufacturer.media/pharmaceutical-industry-insights/pharmaceutical-logistics-distribution/what-s-needed-to-protect-covid-19-vaccines-during-transport/ (accessed on 24 August 2022).
- Oxford AstraZeneca Covid Vaccine Has up to 90% Efficacy, Data Reveals|Coronavirus|The Guardian. Available online: https://www.theguardian.com/society/2020/nov/23/astrazeneca-says-its-coronavirus-vaccine-has-70-per-cent-efficacy-covid-oxford-university (accessed on 24 August 2022).
- CONVIDECIA|WHO—Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). Available online: https://extranet.who.int/pqweb/vaccines/convidecia (accessed on 24 August 2022).
- CDC. Ncird Novavax COVID-19 Vaccine Storage and Handling Label—Intended for Print Only; CDC: Singapore, 2022. [Google Scholar]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Polack, F.P.; Zerbini, C.; et al. Six Month Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. medRxiv 2021. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Study Looks at Moderna COVID-19 Vaccine Effectiveness|Kaiser Permanente. Available online: https://about.kaiserpermanente.org/our-story/health-research/news/study-looks-at-moderna-covid-19-vaccine-effectiveness (accessed on 8 August 2022).
- How AstraZeneca Is Made and What It Contains|The Immunisation Advisory Centre. Available online: https://covid.immune.org.nz/covid-19-vaccines-nz/astrazeneca-vaccine/how-astrazeneca-made-and-what-it-contains (accessed on 24 August 2022).
- Griffin, S. COVID-19: AstraZeneca Vaccine Prevents 79% of Symptomatic Disease and 100% of Severe Disease, US Study Finds. BMJ 2021, 372, n793. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine AstraZeneca Real-World Evidence Summary. Available online: https://www.astrazeneca.com/content/dam/az/covid-19/media/factsheets/COVID-19_Vaccine_AstraZeneca_Real-World_Evidence_Summary.pdf (accessed on 12 January 2022).
- Johnson & Johnson’s Janssen COVID-19 Vaccine Overview and Safety|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html (accessed on 24 August 2022).
- The Janssen Ad26.COV2.S COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the-j-j-covid-19-vaccine-what-you-need-to-know (accessed on 24 August 2022).
- Johnson & Johnson COVID-19 Vaccine Demonstrates 85 Percent Effectiveness against Hospitalization in South Africa When Omicron Was Dominant|Johnson & Johnson. Available online: https://www.jnj.com/johnson-johnson-covid-19-vaccine-demonstrates-85-percent-effectiveness-against-hospitalization-in-south-africa-when-omicron-was-dominant (accessed on 24 August 2022).
- You Got the J&J Vaccine: Should You Get the Booster? <News> Yale Medicine. Available online: https://www.yalemedicine.org/news/johnson-and-johnson-covid-booster (accessed on 24 August 2022).
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Muñoz Navarro, S.R.; et al. Final Efficacy Analysis, Interim Safety Analysis, and Immunogenicity of a Single Dose of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) in Adults 18 Years and Older: An International, Multicentre, Randomised, Double-Blinded, Placebo-Controlled Phase 3 Trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef]
- The CanSino Biologics Ad5-NCoV-S [Recombinant] COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the--cansino-biologics-ad5-ncov-s--recombinant---covid-19-vaccine--what-you-need-to-know (accessed on 24 August 2022).
- Convidecia Vaccine CanSino—Precision Vaccinations. Available online: https://www.precisionvaccinations.com/vaccines/convidecia-vaccine-cansino (accessed on 24 August 2022).
- Jin, P.; Li, J.; Guo, X.; Gou, J.; Hou, L.; Song, Z.; Zhu, T.; Pan, H.; Zhu, J.; Shi, F.; et al. Heterologous CoronaVac plus Ad5-NCOV versus Homologous CoronaVac Vaccination among Elderly: A Phase 4, Non-Inferiority, Randomized Study. medRxiv 2022. [Google Scholar] [CrossRef]
- Jin, P.; Guo, X.-L.; Chen, W.; Ma, S.; Pan, H.-X.; Dai, L.; Du, P.; Wang, L.; Jin, L.; Chen, Y.; et al. Safety and Immunogenicity of Heterologous Boosting with a Protein-Subunit-Based COVID-19 Vaccine (ZF2001) in Healthy Adults Previously Received One Dose of Convidecia: A Randomised, Observer-Blinded, Placebo-Controlled Trial. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Background Document on the Inactivated Vaccine Sinovac-CoronaVac against COVID-19; World Health Organization: Geneva, Switzerland, 2021.
- Wilder-Smith, A.; Mulholland, K. Effectiveness of an Inactivated SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 385, 946. [Google Scholar] [CrossRef] [PubMed]
- Medline ® Abstract for Reference 211 of “COVID-19: Vaccines”—UpToDate. Available online: https://www.uptodate.com/contents/covid-19-vaccines/abstract/211 (accessed on 24 August 2022).
- (PDF) COVID-19 in Older Adults Antibody Responses of Inactivated SARS-CoV-2 (Vero Cell-Sinovac) Vaccine for Elderly Comparing with Younger. Available online: https://www.researchgate.net/publication/351776336_COVID-19_in_older_adults_antibody_responses_of_inactivated_SARS-CoV-2_vero_cell-sinovac_vaccine_for_elderly_comparing_with_younger (accessed on 24 August 2022).
- Mallapaty, S. China’s COVID Vaccines Have Been Crucial—Now Immunity Is Waning. Nature 2021, 598, 398–399. [Google Scholar] [CrossRef] [PubMed]
- The Sinopharm COVID-19 Vaccine: What You Need to Know. Available online: https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know (accessed on 22 August 2022).
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Nadeem, I.; Ul Munamm, S.A.; Ur Rasool, M.; Fatimah, M.; Abu Bakar, M.; Rana, Z.K.; Khatana, U.F.; Jordon, L.; Saqlain, M.; Mahdi, N.; et al. Safety and Efficacy of Sinopharm Vaccine (BBIBP-CorV) in Elderly Population of Faisalabad District of Pakistan. Postgrad. Med. J. 2022. [Google Scholar] [CrossRef]
- Behera, P.; Singh, A.K.; Subba, S.H.; Mc, A.; Sahu, D.P.; Chandanshive, P.D.; Pradhan, S.K.; Parida, S.P.; Mishra, A.; Patro, B.K.; et al. Effectiveness of COVID-19 Vaccine (Covaxin) against Breakthrough SARS-CoV-2 Infection in India. Hum. Vaccines Immunother. 2022, 18, 2034456. [Google Scholar] [CrossRef]
- Bhatnagar, T.; Chaudhuri, S.; Ponnaiah, M.; Yadav, P.D.; Sabarinathan, R.; Sahay, R.R.; Ahmed, F.; Aswathy, S.; Bhardwaj, P.; Bilimale, A.; et al. Effectiveness of BBV152/Covaxin and AZD1222/Covishield Vaccines against Severe COVID-19 and B.1.617.2/Delta Variant in India, 2021: A Multi-Centric Hospital-Based Case-Control Study. Int J. Infect. Dis. 2022, 122, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Phatak, S.R.; Singh, R.; Bhattacharjee, K.; Singh, N.K.; Gupta, A.; Sharma, A. Humoral Antibody Kinetics with ChAdOx1-NCOV (CovishieldTM) and BBV-152 (CovaxinTM) Vaccine among Indian Healthcare Workers: A 6-Month Longitudinal Cross-Sectional Coronavirus Vaccine-Induced Antibody Titre (COVAT) Study. Diabetes Metab. Syndr. 2022, 16, 102424. [Google Scholar] [CrossRef] [PubMed]
- Covovax Vaccine Now Available For 12–17 Age Group at Private Centres. Available online: https://www.ndtv.com/india-news/covovax-vaccine-now-available-for-12-17-age-group-at-private-centres-2940396 (accessed on 24 August 2022).
- Warude, B.J.; Dhariwal, R.M. The Systemic Study of Various Vaccines in Term of Current Status of Clinical Trial, Safety and Effectiveness of Covid Vaccine. J. Med. Pharm. Allied Sci. 2021, 10, 3973–3977. [Google Scholar] [CrossRef]
- Juno, J.A.; Wheatley, A.K. Boosting Immunity to COVID-19 Vaccines. Nat. Med. 2021, 27, 1874–1875. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 MRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Adverse Events Following Immunization (AEFIs) for COVID-19 in Ontario; Public Health Ontario: West Chatham, ON, Canada, 13 December 2020–14 August 2022.
- Kochhar, S.; Salmon, D.A. Planning for COVID-19 Vaccines Safety Surveillance. Vaccine 2020, 38, 6194–6198. [Google Scholar] [CrossRef]
- Remmel, A. COVID Vaccines and Safety: What the Research Says. Nature 2021, 590, 538–540. [Google Scholar] [CrossRef]
- MRNA Vaccines. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/vaccines/mrna-vaccines/ (accessed on 15 September 2022).
- McMurry, R.; Lenehan, P.; Awasthi, S.; Silvert, E.; Puranik, A.; Pawlowski, C.; Venkatakrishnan, A.J.; Anand, P.; Agarwal, V.; O’Horo, J.C.; et al. Real-Time Analysis of a Mass Vaccination Effort Confirms the Safety of FDA-Authorized MRNA COVID-19 Vaccines. Med 2021, 2, 965–978.e5. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca Pauses Vaccine Trial for Safety Review—The New York Times. Available online: https://www.nytimes.com/2020/09/08/health/coronavirus-astrazeneca-vaccine-safety.html (accessed on 23 August 2022).
- Mahase, E. COVID-19: WHO Says Rollout of AstraZeneca Vaccine Should Continue, as Europe Divides over Safety. BMJ 2021, 372, n728. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. COVID-19: European Countries Suspend Use of Oxford-AstraZeneca Vaccine after Reports of Blood Clots. BMJ 2021, 372, n699. [Google Scholar] [CrossRef]
- Roy, C.J.; Ault, A.; Sivasubramani, S.K.; Gorres, J.P.; Wei, C.J.; Andersen, H.; Gall, J.; Roederer, M.; Rao, S.S. Aerosolized Adenovirus-Vectored Vaccine as an Alternative Vaccine Delivery Method. Respir. Res. 2011, 12, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejczak-Grządko, S.; Czudy, Z.; Donderska, M. Side Effects after COVID-19 Vaccinations among Residents of Poland. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4418–4421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. [Google Scholar] [CrossRef]
- Vaccines|Free Full-Text|Vaccination with the Inactivated Vaccine (Sinopharm BBIBP-CorV) Ensures Protection against SARS-CoV-2 Related Disease. Available online: https://www.mdpi.com/2076-393X/10/6/920 (accessed on 24 August 2022).
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBIBP-CorV: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.X.; Zhang, T.T.; Shi, G.F.; Cheng, F.M.; Zheng, Y.M.; Tung, T.H.; Chen, H.X. Safety of an Inactivated SARS-CoV-2 Vaccine among Healthcare Workers in China. Expert Rev. Vaccines 2021, 20, 891–898. [Google Scholar] [CrossRef]
- Kezia, V.; Ramatillah, D.L. Intensive Monitroing of Sinovac Vaccine for Safety and Efficacy among Indonesian Population. Int. J. Appl. Pharm. 2022, 14, 44–48. [Google Scholar] [CrossRef]
- Dar, M.A.; Chauhan, P.; Kumar, P.; Chauhan, R.; Murti, K.; Charan, J.; Ravichandiran, V.; Dhingra, S. Safety, Efficacy, and Immunogenicity of COVAXIN: A Review. J. Appl. Pharm. Sci. 2021, 11, 18–25. [Google Scholar] [CrossRef]
- Parida, S.P.; Sahu, D.P.; Singh, A.K.; Alekhya, G.; Subba, S.H.; Mishra, A.; Padhy, B.M.; Patro, B.K. Adverse Events following Immunization of COVID-19 (Covaxin) Vaccine at a Tertiary Care Center of India. J. Med. Virol. 2022, 94, 2453–2459. [Google Scholar] [CrossRef]
- Rajpurohit, P.; Suva, M.; Rajpurohit, H.; Singh, Y.; Boda, P.; Scholar, M.D. A Retrospective Observational Survey of Adverse Events Following Immunization Comparing Tolerability of Covishield and Covaxin Vaccines in the Real World. J. Pharmacovigil. Drug Res. 2021, 2, 20–25. [Google Scholar] [CrossRef]
- Corbevax vs. Covovax COVID-19 Jabs for Those under 18 Years: Which One to Choose?|Deccan Herald. Available online: https://www.deccanherald.com/national/corbevax-vs-covovax-covid-19-jabs-for-those-under-18-years-which-one-to-choose-1105263.html (accessed on 24 August 2022).
- Kulkarni, P.S.; Kadam, A.; Godbole, S.; Bhatt, V.; Raut, A.; Kohli, S.; Tripathi, S.; Kulkarni, P.; Ludam, R.; Prabhu, M.; et al. Safety and Immunogenicity of SII-NVX-CoV2373 (COVID-19 Vaccine) in Adults in a Phase 2/3, Observer-Blind, Randomised, Controlled Study. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Immune Responses Support COVID-19 Vaccination Regardless of When People Were Infected: Scientists Study How Vaccinations Affect the Immune Response—ScienceDaily. Available online: https://www.sciencedaily.com/releases/2022/04/220405115230.htm (accessed on 15 September 2022).
- COVID-19 Immune Response Improves for Months after Vaccination|National Institutes of Health (NIH). Available online: https://www.nih.gov/news-events/nih-research-matters/covid-19-immune-response-improves-months-after-vaccination (accessed on 15 September 2022).
- Kim, W.; Zhou, J.Q.; Horvath, S.C.; Schmitz, A.J.; Sturtz, A.J.; Lei, T.; Liu, Z.; Kalaidina, E.; Thapa, M.; Alsoussi, W.B.; et al. Germinal Centre-Driven Maturation of B Cell Response to MRNA Vaccination. Nature 2022, 604, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Jeewandara, C.; Aberathna, I.S.; Pushpakumara, P.D.; Kamaladasa, A.; Guruge, D.; Wijesinghe, A.; Gunasekera, B.; Ramu, S.T. Persistence of Immune Responses to the Sinopharm/BBIBP-CorV Vaccine. Immun. Inflamm. Dis. 2022, 10, e621. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Walls, A.C.; Golden, N.; Atyeo, C.; Fischinger, S.; Li, C.; Aye, P.; Navarro, M.J.; Lai, L.; Edara, V.V.; et al. Adjuvanting a Subunit COVID-19 Vaccine to Induce Protective Immunity. Nature 2021, 594, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Andreano, E.; Paciello, I.; Pierleoni, G.; Piccini, G.; Abbiento, V.; Antonelli, G.; Pileri, P.; Manganaro, N.; Pantano, E.; Maccari, G.; et al. COVID-19 MRNA Third Dose Induces a Unique Hybrid Immunity-like Antibody Response. bioRxiv 2022. [Google Scholar] [CrossRef]
- Interim Statement on Hybrid Immunity and Increasing Population Seroprevalence Rates. Available online: https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates (accessed on 15 September 2022).
- Unique Antibody Responses after Third COVID-19 MRNA Vaccination. Available online: https://www.news-medical.net/news/20220516/Unique-antibody-responses-after-third-COVID-19-mRNA-vaccination.aspx (accessed on 25 July 2022).
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; PaulChourdhury, R.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid Decline in Vaccine-Boosted Neutralizing Antibodies Against SARS-CoV-2 Omicron Variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef]
- Salajegheh Tazerji, S.; Shahabinejad, F.; Tokasi, M.; Ali Rad, M.; Sajjad Khan, M.; Safdar, M.; Filipiak, K.J.; Szarpak, L.; Dzieciatkowski, T.; Jurgiel, J.; et al. Global Data Analysis and Risk Factors Associated with Morbidity and Mortality of COVID-19. Gene Rep. 2022, 26, 101505. [Google Scholar] [CrossRef]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations among Adults without Immunocompromising Conditions—United States, March–August 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1337. [Google Scholar] [CrossRef]
- Al-Sadeq, D.W.; Shurrab, F.M.; Ismail, A.; Amanullah, F.H.; Thomas, S.; Aldewik, N.; Yassine, H.M.; Abdul Rahim, H.F.; Abu-Raddad, L.; Nasrallah, G.K. Comparison of Antibody Immune Responses between BNT162b2 and MRNA-1273 SARS-CoV-2 Vaccines in Naïve and Previously Infected Individuals. J. Travel Med. 2021, 28, taab190. [Google Scholar] [CrossRef]
- Kanokudom, S.; Assawakosri, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Yorsaeng, R.; Srimuan, D.; Thatsanatorn, T.; et al. Safety and Immunogenicity of the Third Booster Dose with Inactivated, Viral Vector, and MRNA COVID-19 Vaccines in Fully Immunized Healthy Adults with Inactivated Vaccine. Vaccines 2022, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Alqassieh, R.; Suleiman, A.; Abu-Halaweh, S.; Santarisi, A.; Shatnawi, O.; Shdaifat, L.; Tarifi, A.; Al-Tamimi, M.; Al-Shudifat, A.E.; Alsmadi, H.; et al. Pfizer-BioNTech and Sinopharm: A Comparative Study on Post-Vaccination Antibody Titers. Vaccines 2021, 9, 1223. [Google Scholar] [CrossRef]
- Fernandez-Davila, N.; Taylor, M.G.; Anvari, S. Hypersensitivity Reactions to COVID-19 Vaccines—Identify High-Risk Children and Vaccinate the Rest. JAMA Pediatr. 2022, 176, 443–444. [Google Scholar] [CrossRef]
- Hung, S.I.; Preclaro, I.A.C.; Chung, W.H.; Wang, C.W. Immediate Hypersensitivity Reactions Induced by COVID-19 Vaccines: Current Trends, Potential Mechanisms and Prevention Strategies. Biomedicines 2022, 10, 1260. [Google Scholar] [CrossRef]
- Desai, H.D.; Sharma, K.; Shah, A.; Patoliya, J.; Patil, A.; Hooshanginezhad, Z.; Grabbe, S.; Goldust, M. Can SARS-CoV-2 Vaccine Increase the Risk of Reactivation of Varicella Zoster? A Systematic Review. J. Cosmet. Dermatol. 2021, 20, 3350–3361. [Google Scholar] [CrossRef]
- Psichogiou, M.; Samarkos, M.; Mikos, N.; Hatzakis, A. Reactivation of Varicella Zoster Virus after Vaccination for SARS-CoV-2. Vaccines 2021, 9, 572. [Google Scholar] [CrossRef]
- COVID-19 MRNA Vaccination Side Effects: Varicellar Zoster Reactivation. Available online: https://www.emjreviews.com/dermatology/article/varicella-zoster-reactivation-following-mrna-vaccination-two-case-reports-and-a-review-of-cutaneous-adverse-events-of-covid-19-vaccines/ (accessed on 28 September 2022).
- Denard, J.; Rouillon, J.; Leger, T.; Garcia, C.; Lambert, M.P.; Griffith, G.; Jenny, C.; Camadro, J.M.; Garcia, L.; Svinartchouk, F. AAV-8 and AAV-9 Vectors Cooperate with Serum Proteins Differently Than AAV-1 and AAV-6. Mol. Ther. Methods Clin. Dev. 2018, 10, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Franchini, M.; Liumbruno, G.M.; Pezzo, M. COVID-19 Vaccine-Associated Immune Thrombosis and Thrombocytopenia (VITT): Diagnostic and Therapeutic Recommendations for a New Syndrome. Eur. J. Haematol. 2021, 107, 173–180. [Google Scholar] [CrossRef]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Østerlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T Cell Immune Responses Following MRNA COVID-19 Vaccination in Patients with Cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Segal, B.H. Immune Responses to COVID-19 Vaccines in Patients with Cancer: Promising Results and a Note of Caution. Cancer Cell 2021, 39, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.J.; Shah, G.L.; Devlin, S.M.; Ramanathan, L.V.; Doddi, S.; Pessin, M.S.; Hoover, E.; Marcello, L.T.; Young, J.C.; Boutemine, S.R.; et al. Disease- and Therapy-Specific Impact on Humoral Immune Responses to COVID-19 Vaccination in Hematologic Malignancies. Blood Cancer Discov. 2021, 2, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Nault, L.; Marchitto, L.; Goyette, G.; Tremblay-Sher, D.; Fortin, C.; Martel-Laferrière, V.; Trottier, B.; Richard, J.; Durand, M.; Kaufmann, D.; et al. COVID-19 Vaccine Immunogenicity in People Living with HIV-1. Vaccine 2022, 40, 3633–3637. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral Immune Response to COVID-19 MRNA Vaccine in Patients with Multiple Sclerosis Treated with High-Efficacy Disease-Modifying Therapies. Ther. Adv. Neurol. Disord. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Dolev, M.; Menascu, S.; Magalashvili, D.; Flechter, S.; Givon, U.; Guber, D.; et al. Humoral Immune Response in Multiple Sclerosis Patients Following PfizerBNT162b2 COVID19 Vaccination: Up to 6 Months Cross-Sectional Study. J. Neuroimmunol. 2021, 361, 577746. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 MRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 MRNA COVID-19 Vaccine and SARS-CoV-2 Infection in Lung Transplant Recipients. J. Heart Lung Transplant. 2021, 40, 754–758. [Google Scholar] [CrossRef]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Kragh, A.; Frederiksen, H.; Ditzel, H.J. Increased Antibody Titers and Reduced Seronegativity Following Fourth MRNA COVID-19 Vaccination in Patients with Cancer. Cancer Cell 2022, 40, 800–801. [Google Scholar] [CrossRef]
- Mencoboni, M.; Fontana, V.; Damiani, A.; Spitaleri, A.; Raso, A.; Bottaro, L.C.; Rossi, G.; Canobbio, L.; La Camera, A.; Filiberti, R.A.; et al. Antibody Response to COVID-19 MRNA Vaccines in Oncologic and Hematologic Patients Undergoing Chemotherapy. Curr. Oncol. 2022, 29, 3364–3374. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; Li, X.; Chen, D.; Zhao, X.; Qiu, Y.; Zhang, L.; Xiao, J.; Li, B.; Zhao, H. Covid-19 Vaccination in People Living with Hiv (Plwh) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines 2021, 9, 1458. [Google Scholar] [CrossRef]
- Netto, L.C.; Ibrahim, K.Y.; Picone, C.M.; Alves, A.P.P.S.; Aniceto, E.V.; Santiago, M.R.; Parmejani, P.S.S.; Aikawa, N.E.; Medeiros-Ribeiro, A.C.; Pasoto, S.G.; et al. Safety and Immunogenicity of CoronaVac in People Living with HIV: A Prospective Cohort Study. Lancet HIV 2022, 9, e323–e331. [Google Scholar] [CrossRef]
- Cherian, S.; Paul, A.; Ahmed, S.; Alias, B.; Manoj, M.; Santhosh, A.K.; Varghese, D.R.; Krishnan, N.; Shenoy, P. Safety of the ChAdOx1 NCoV-19 and the BBV152 Vaccines in 724 Patients with Rheumatic Diseases: A Post-Vaccination Cross-Sectional Survey. Rheumatol. Int. 2021, 41, 1441–1445. [Google Scholar] [CrossRef]
- Safary, A.; Esalatmanesh, K.; Eftekharsadat, A.T.; Jafari Nakjavani, M.R.; Khabbazi, A. Autoimmune Inflammatory Rheumatic Diseases Post-COVID-19 Vaccination. Int. Immunopharmacol. 2022, 110, 109061. [Google Scholar] [CrossRef] [PubMed]
- Campos-Cabrera, G.; Torres-Salgado, F.-G.; Campos-Cabrera, S.; Campos-Villagomez, J.-L.; Campos-Cabrera, V. Autoimmune Cytopenias and COVID-19 Vaccination: Relapse and Suggested Treatment. Blood 2021, 138, 4141. [Google Scholar] [CrossRef]
- Andresciani, F.; Ricci, M.; Grasso, R.F.; Zobel, B.B.; Quattrocchi, C.C. COVID-19 Vaccination Simulating Lymph Node Progression in a Patient with Prostate Cancer. Radiol. Case Rep. 2022, 17, 2996–2999. [Google Scholar] [CrossRef] [PubMed]
- Klomjit, N.; Alexander, M.P.; Fervenza, F.C.; Zoghby, Z.; Garg, A.; Hogan, M.C.; Nasr, S.H.; Minshar, M.A.; Zand, L. COVID-19 Vaccination and Glomerulonephritis. Kidney Int. Rep. 2021, 6, 2969–2978. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Z.; Tan, R.Y.; Choo, J.C.J.; Lim, C.C.; Tan, C.S.; Loh, A.H.L.; Tien, C.S.Y.; Tan, P.H.; Woo, K.T. Is COVID-19 Vaccination Unmasking Glomerulonephritis? Kidney Int. 2021, 100, 469–471. [Google Scholar] [CrossRef]
- Tang, W.; Gartshteyn, Y.; Ricker, E.; Inzerillo, S.; Murray, S.; Khalili, L.; Askanase, A. The Use of COVID-19 Vaccines in Patients with SLE. Curr. Rheumatol. Rep. 2021, 23, 79. [Google Scholar] [CrossRef]
- Tang, Z.; Kong, N.; Zhang, X.; Liu, Y.; Hu, P.; Mou, S.; Liljeström, P.; Shi, J.; Tan, W.; Kim, J.S.; et al. A Materials-Science Perspective on Tackling COVID-19. Nat. Rev. Mater. 2020, 5, 847–860. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, K.; Costabel, U.; Zhang, X. Nanotechnology-Facilitated Vaccine Development during the Coronavirus Disease 2019 (COVID-19) Pandemic. Exploration 2022, 2, 20210082. [Google Scholar] [CrossRef]
- Mascellino, M.T.; di Timoteo, F.; de Angelis, M.; Oliva, A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021, 14, 3459. [Google Scholar] [CrossRef]
- Gelman, R.; Bayatra, A.; Kessler, A.; Schwartz, A.; Ilan, Y. Targeting SARS-CoV-2 Receptors as a Means for Reducing Infectivity and improving Antiviral and Immune Response: An Algorithm-Based Method for Overcoming to Antiviral Agents. Emerg. Microbes Infect. 2020, 9, 1397. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Preliminary Identification of Potential Vaccine Targets for the COVID-19 Coronavirus (SARS-CoV-2) Based on SARS-CoV Immunological Studies. Viruses 2020, 12, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.W.; Sahi, V.; Figueroa, A.; et al. Potent Neutralizing Antibodies against Multiple Epitopes on SARS-CoV-2 Spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, B.D.; Mou, H.; Zhang, L.; Guo, Y.; He, W.; Ojha, A.; Parcells, M.S.; Luo, G.; Li, W.; Zhong, G.; et al. The SARS-CoV-2 Receptor-Binding Domain Elicits a Potent Neutralizing Response without Antibody-Dependent Enhancement. bioRxiv 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abufares, H.I.; Oyoun Alsoud, L.; Alqudah, M.A.Y.; Shara, M.; Soares, N.C.; Alzoubi, K.H.; El-Huneidi, W.; Bustanji, Y.; Soliman, S.S.M.; Semreen, M.H. COVID-19 Vaccines, Effectiveness, and Immune Responses. Int. J. Mol. Sci. 2022, 23, 15415. https://doi.org/10.3390/ijms232315415
Abufares HI, Oyoun Alsoud L, Alqudah MAY, Shara M, Soares NC, Alzoubi KH, El-Huneidi W, Bustanji Y, Soliman SSM, Semreen MH. COVID-19 Vaccines, Effectiveness, and Immune Responses. International Journal of Molecular Sciences. 2022; 23(23):15415. https://doi.org/10.3390/ijms232315415
Chicago/Turabian StyleAbufares, Haneen Imad, Leen Oyoun Alsoud, Mohammad A. Y. Alqudah, Mohd Shara, Nelson C. Soares, Karem H. Alzoubi, Waseem El-Huneidi, Yasser Bustanji, Sameh S. M. Soliman, and Mohammad H. Semreen. 2022. "COVID-19 Vaccines, Effectiveness, and Immune Responses" International Journal of Molecular Sciences 23, no. 23: 15415. https://doi.org/10.3390/ijms232315415
APA StyleAbufares, H. I., Oyoun Alsoud, L., Alqudah, M. A. Y., Shara, M., Soares, N. C., Alzoubi, K. H., El-Huneidi, W., Bustanji, Y., Soliman, S. S. M., & Semreen, M. H. (2022). COVID-19 Vaccines, Effectiveness, and Immune Responses. International Journal of Molecular Sciences, 23(23), 15415. https://doi.org/10.3390/ijms232315415






