Heterozygote Dopamine Transporter Knockout Rats Display Enhanced Cocaine Locomotion in Adolescent Females
Abstract
1. Introduction
2. Results
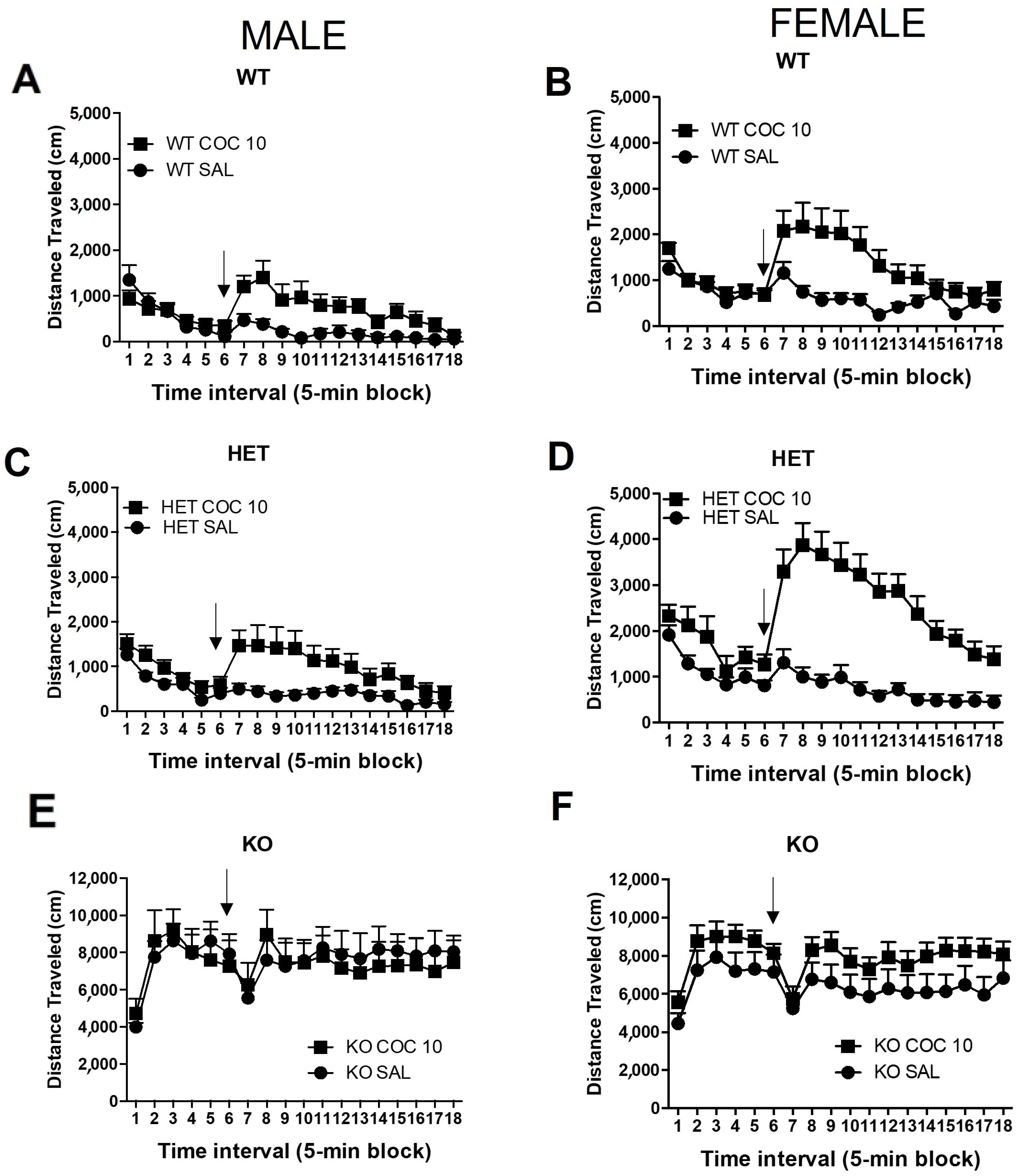
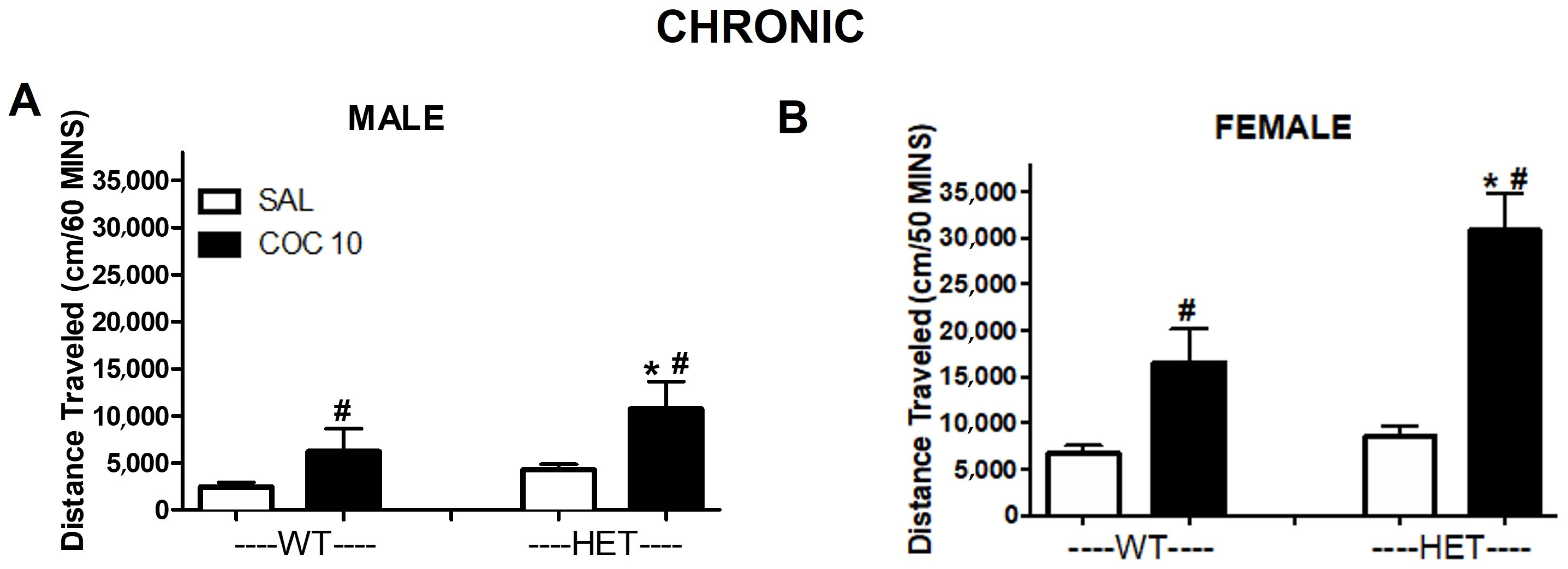
2.1. Experiment 1: Characterization of DAT HET and KO Rat’s Phenotype and Response to Cocaine Administration
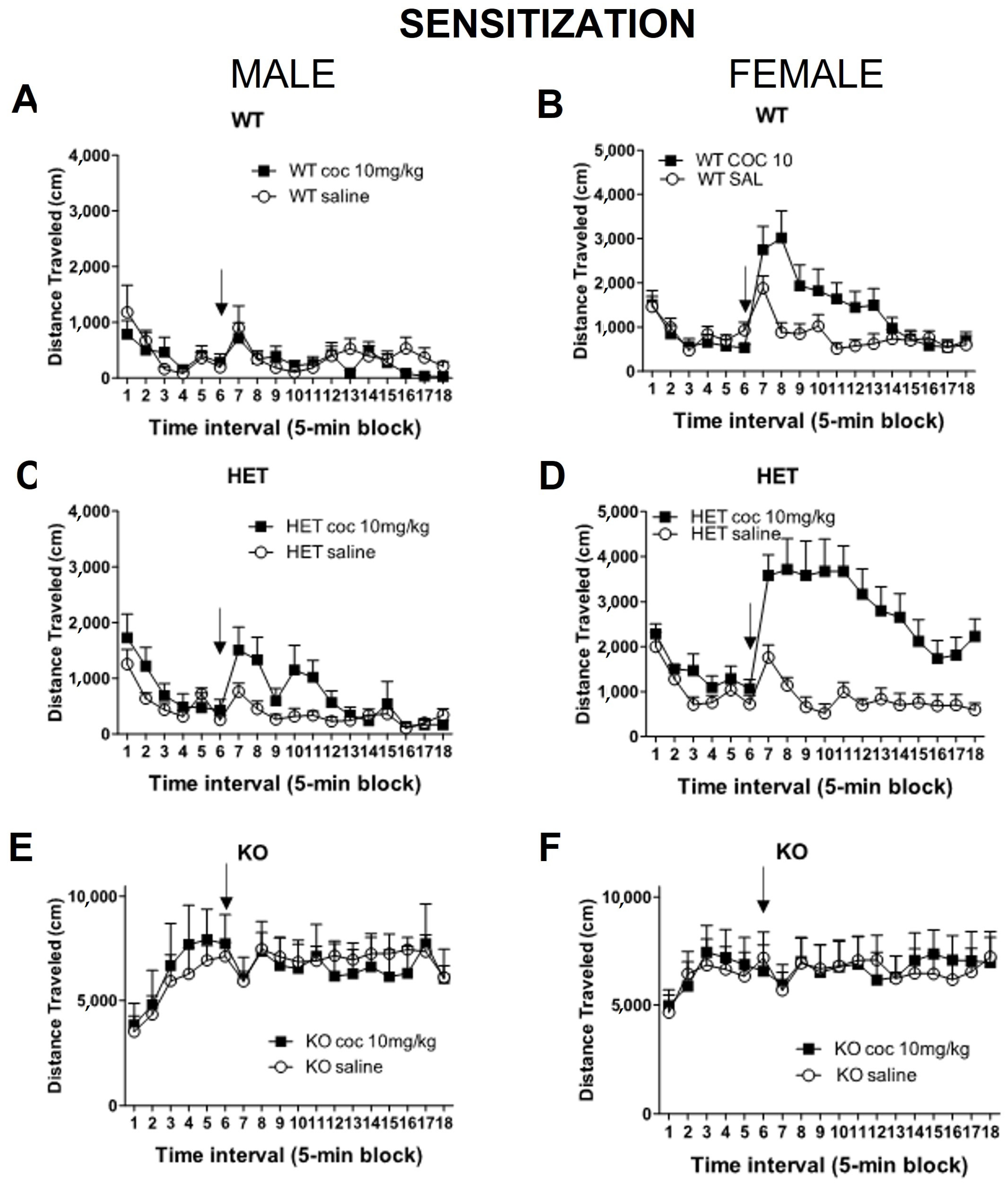
2.2. Experiment 2: Sensitization of Locomotor Activity to Cocaine in DAT Rats in the Open Field
2.3. Experiment 3: Dopamine Pathway Protein Alterations in DAT KO Rats
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animals
4.3. Immunoblot Analysis
4.4. Open Field (OF) Apparatus
4.5. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- McHugh, P.C.; Buckley, D.A. The structure and function of the dopamine transporter and its role in CNS diseases. Vitam. Horm. 2015, 98, 339–369. [Google Scholar] [PubMed]
- Chen, L.; Segal, D.M.; Moraes, C.T.; Mash, D.C. Dopamine transporter mRNA in autopsy studies of chronic cocaine users. Brain Res. Mol. Brain Res. 1999, 73, 181–185. [Google Scholar] [CrossRef]
- Qin, Y.; Ouyang, Q.; Pablo, J.; Mash, D.C. Cocaine abuse elevates alpha-synuclein and dopamine transporter levels in the human striatum. Neuroreport 2005, 16, 1489–1493. [Google Scholar] [CrossRef]
- Izenwasser, S. The role of the dopamine transporter in cocaine abuse. Neurotox. Res. 2004, 6, 379–383. [Google Scholar] [CrossRef]
- Cagniard, B.; Sotnikova, T.D.; Gainetdinov, R.R.; Zhuang, X. The dopamine transporter expression level differentially affects responses to cocaine and amphetamine. J. Neurogenet. 2014, 28, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, C.W. Dynamics of extracellular dopamine in the acute and chronic actions of cocaine. Neuroscientist 2002, 8, 315–322. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.R.; Hu, X.T.; Cooper, D.C.; Wightman, R.M.; White, F.J.; Caron, M.G. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat. Neurosci. 1999, 2, 649–655. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Kahlig, K.M.; Galli, A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur. J. Pharmacol. 2003, 479, 153–158. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Jones, S.R.; Fumagalli, F.; Wightman, R.M.; Caron, M.G. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res. Brain Res. Rev. 1998, 26, 148–153. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.R.; Wightman, R.M.; Caron, M.G. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J. Neurosci. 1998, 18, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Fumagalli, F.; Gainetdinov, R.R.; Jones, S.R.; Ator, R.; Giros, B.; Miller, G.W.; Caron, M.G. Cocaine self-administration in dopamine-transporter knockout mice. Nat. Neurosci. 1998, 1, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Wetsel, W.C.; Jones, S.R.; Levin, E.D.; Jaber, M.; Caron, M.G. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 1999, 283, 397–401. [Google Scholar] [CrossRef]
- Spielewoy, C.; Roubert, C.; Hamon, M.; Nosten-Bertrand, M.; Betancur, C.; Giros, B. Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav. Pharmacol. 2000, 11, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Sotnikova, T.D.; Yao, W.D.; Kockeritz, L.; Woodgett, J.R.; Gainetdinov, R.R.; Caron, M.G. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA 2004, 101, 5099–5104. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Tirotta, E.; Sotnikova, T.D.; Masri, B.; Salahpour, A.; Gainetdinov, R.R.; Borrelli, E.; Caron, M.G. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J. Neurosci. 2007, 27, 881–885. [Google Scholar] [CrossRef]
- Choudhary, A.; Ibdah, J.A. Animal models in today’s translational medicine world. Mo. Med. 2013, 110, 220–222. [Google Scholar]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef]
- Efimova, E.V.; Gainetdinov, R.R.; Budygin, E.A.; Sotnikova, T.D. Dopamine transporter mutant animals: A translational perspective. J. Neurogenet. 2016, 30, 5–15. [Google Scholar] [CrossRef]
- Martinez, L.A.; Gross, K.S.; Himmler, B.T.; Emmitt, N.L.; Peterson, B.M.; Zlebnik, N.E.; Olive, M.F.; Carroll, M.E.; Meisel, R.L.; Mermelstein, P.G. Estradiol Facilitation of Cocaine Self-Administration in Female Rats Requires Activation of mGluR5. eNeuro 2016, 3, ENEURO.0140-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.L.; Weiss, R.D.; Mirin, S.M.; Lange, U. A comparison of male and female cocaine abusers. Arch. Gen. Psychiatry 1989, 46, 122–126. [Google Scholar] [CrossRef]
- McCance-Katz, E.F.; Hart, C.L.; Boyarsky, B.; Kosten, T.; Jatlow, P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst. Use Misuse 2005, 40, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Izenwasser, S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology 2004, 46, 349–362. [Google Scholar] [CrossRef]
- Zakharova, E.; Wade, D.; Izenwasser, S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Behav. 2009, 92, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, M.; Starosciak, A.K.; Ledon, J.; Booth, C.; Zakharova, E.; Wade, D.; Vignoli, B.; Izenwasser, S. Sex differences in conditioned nicotine reward are age-specific. Pharmacol. Biochem. Behav. 2015, 132, 56–62. [Google Scholar] [CrossRef]
- Bardo, M.T.; Klebaur, J.E.; Valone, J.M.; Deaton, C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology 2001, 155, 278–284. [Google Scholar] [PubMed]
- Pena, Y.; Prunell, M.; Dimitsantos, V.; Nadal, R.; Escorihuela, R.M. Environmental enrichment effects in social investigation in rats are gender dependent. Behav. Brain Res. 2006, 174, 181–187. [Google Scholar] [CrossRef]
- Goldstein, R.B. Comorbidity of substance use with independent mood and anxiety disorders in women: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. In Women and Addiction: A Comprehensive Handbook; Brady, K.T., Back, S.E., Greenfield, S.F., Eds.; Guilford Press: New York, NY, USA, 2009; pp. 173–192. [Google Scholar]
- Spear, L.P.; Varlinskaya, E.I. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: Implications for prevention science? ” Dev. Psychobiol. 2010, 52, 236–243. [Google Scholar] [CrossRef]
- Giedd, J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef]
- Casey, B.J.; Getz, S.; Galvan, A. The adolescent brain. Dev. Rev. 2008, 28, 62–77. [Google Scholar] [CrossRef]
- Winters, K.C.; Arria, A. Adolescent Brain Development and Drugs. Prev. Res. 2011, 18, 21–24. [Google Scholar]
- Romer, D. Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Dev. Psychobiol. 2010, 52, 263–276. [Google Scholar] [CrossRef]
- Harmon, K. Dopamine Determines Impulsive Behavior—Scientific American. 2010. Available online: www.scientificamerican.com/article/dopamine-impulsive-addiction/ (accessed on 15 June 2022).
- Wahlstrom, D.; Collins, P.; White, T.; Luciana, M. Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain Cogn. 2010, 72, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.Y.; O’Donnell, P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb. Cortex 2007, 17, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Kandel, D.B.; Yamaguchi, K.; Chen, K. Stages of progression in drug involvement from adolescence to adulthood: Further evidence for the gateway theory. J. Stud. Alcohol. 1992, 53, 447–457. [Google Scholar] [CrossRef]
- Chambers, R.A.; Taylor, J.R.; Potenza, M.N. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am. J. Psychiatry 2003, 160, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.D.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2010; Institute for Social Research, the University of Michigan: Ann Arbor, MI, USA, 2011. [Google Scholar]
- Collins, S.L.; Izenwasser, S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res. Dev. Brain Res. 2002, 138, 27–34. [Google Scholar] [CrossRef]
- Faraday, M.M.; Elliott, B.M.; Phillips, J.M.; Grunberg, N.E. Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacol. Biochem. Behav. 2003, 74, 917–931. [Google Scholar] [CrossRef]
- Trauth, J.A.; Seidler, F.J.; Slotkin, T.A. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res. 2000, 880, 167–172. [Google Scholar] [CrossRef]
- Trinh, J.V.; Nehrenberg, D.L.; Jacobsen, J.P.; Caron, M.G.; Wetsel, W.C. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neuroscience 2003, 118, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.W.; Hagino, Y.; Kobayashi, H.; Shinohara-Tanaka, K.; Ikeda, K.; Yamamoto, H.; Yamamoto, T.; Lesch, K.P.; Murphy, D.L.; Hall, F.S.; et al. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacology 2004, 29, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, I.O.; Gainetdinov, R.R.; Sotnikova, T.D.; Bohn, L.M.; Caron, M.G.; Dykstra, L.A. Characterization of conditioned place preference to cocaine in congenic dopamine transporter knockout female mice. Psychopharmacology 2005, 180, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.S.; Li, X.F.; Randall-Thompson, J.; Sora, I.; Murphy, D.L.; Lesch, K.P.; Caron, M.; Uhl, G.R. Cocaine-conditioned locomotion in dopamine transporter, norepinephrine transporter and 5-HT transporter knockout mice. Neuroscience 2009, 162, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Morice, E.; Denis, C.; Giros, B.; Nosten-Bertrand, M. Evidence of long-term expression of behavioral sensitization to both cocaine and ethanol in dopamine transporter knockout mice. Psychopharmacology 2010, 208, 57–66. [Google Scholar] [CrossRef]
- Adinolfi, A.; Carbone, C.; Leo, D.; Gainetdinov, R.R.; Laviola, G.; Adriani, W. Novelty-related behavior of young and adult dopamine transporter knockout rats: Implication for cognitive and emotional phenotypic patterns. Genes Brain Behav. 2018, 17, e12463. [Google Scholar] [CrossRef]
- Mariano, S.; Pardo, M.; Buccheri, C.; Illiano, P.; Adinolfi, A.; Russo, S.L.M.L.; Alleva, E.; Carbone, C.; Adriani, W. Own or dam’s genotype? Classical colony breeding may bias spontaneous and stress-challenged activity in DAT-mutant rats. Dev. Psychobiol. 2020, 62, 505–518. [Google Scholar] [CrossRef]
- Illiano, P.; Bigford, G.E.; Gainetdinov, R.R.; Pardo, M. Rats Lacking Dopamine Transporter Display Increased Vulnerability and Aberrant Autonomic Response to Acute Stress. Biomolecules 2020, 10, 842. [Google Scholar] [CrossRef]
- Illiano, P.; Leo, D.; Gainetdinov, R.R.; Pardo, M. Early Adolescence Prefrontal Cortex Alterations in Female Rats Lacking Dopamine Transporter. Biomedicines 2021, 9, 157. [Google Scholar] [CrossRef]
- Zakharova, E.; Starosciak, A.; Wade, D.; Izenwasser, S. Sex differences in the effects of social and physical environment on novelty-induced exploratory behavior and cocaine-stimulated locomotor activity in adolescent rats. Behav. Brain Res. 2012, 230, 92–99. [Google Scholar] [CrossRef]
- Kuhar, M.J.; Ritz, M.C.; Boja, J.W. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991, 14, 299–302. [Google Scholar] [CrossRef]
- Ji, J.; Bourque, M.; di Paolo, T.; Dluzen, D.E. Genetic alteration in the dopamine transporter differentially affects male and female nigrostriatal transporter systems. Biochem. Pharmacol. 2009, 78, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Sanna, F.; Bratzu, J.; Serra, M.P.; Leo, D.; Quartu, M.; Boi, M.; Espinoza, S.; Gainetdinov, R.R.; Melis, M.R.; Argiolas, A. Altered Sexual Behavior in Dopamine Transporter (DAT) Knockout Male Rats: A Behavioral, Neurochemical and Intracerebral Microdialysis Study. Front. Behav. Neurosci. 2020, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- Ritz, M.C.; Lamb, R.J.; Goldberg, S.R.; Kuhar, M.J. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 1987, 237, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Mateo, Y.; Budygin, E.A.; John, C.E.; Jones, S.R. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc. Natl. Acad. Sci. USA 2004, 101, 372–377. [Google Scholar] [CrossRef]
- Cartier, E.A.; Parra, L.A.; Baust, T.B.; Quiroz, M.; Salazar, G.; Faundez, V.; Egaña, L.; Torres, G.E. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J. Biol. Chem. 2010, 285, 1957–1966. [Google Scholar] [CrossRef]
- Carboni, E.; Spielewoy, C.; Vacca, C.; Nosten-Bertrand, M.; Giros, B.; Di Chiara, G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J. Neurosci. 2001, 21, 141–144. [Google Scholar] [CrossRef]
- Jaber, M.; Dumartin, B.; Sagné, C.; Haycock, J.W.; Roubert, C.; Giros, B.; Bloch, B.; Caron, M.G. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur. J. Neurosci. 1999, 11, 3499–3511. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Calipari, E.S.; Jones, S.R. Regulation of Tyrosine Hydroxylase Expression and Phosphorylation in Dopamine Transporter-Deficient Mice. ACS Chem. Neurosci. 2016, 7, 941–951. [Google Scholar] [CrossRef]
- Cinque, S.; Zoratto, F.; Poleggi, A.; Leo, D.; Cerniglia, L.; Cimino, S.; Tambelli, R.; Alleva, E.; Gainetdinov, R.R.; Laviola, G.; et al. Behavioral Phenotyping of Dopamine Transporter Knockout Rats: Compulsive Traits, Motor Stereotypies, and Anhedonia. Front. Psychiatry 2018, 22, 43. [Google Scholar] [CrossRef]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the vesicular monoamine transporter-2: A novel mechanism for cocaine and other psychostimulants. J. Pharmacol. Exp. Ther. 2001, 296, 762–767. [Google Scholar] [PubMed]
- Miller, G.W.; Wang, Y.M.; Gainetdinov, R.R.; Caron, M.G. Dopamine transporter and vesicular monoamine transporter knockout mice: Implications for Parkinson’s disease. Methods Mol. Med. 2001, 62, 179–190. [Google Scholar] [PubMed]
- Wang, Y.M.; Gainetdinov, R.R.; Fumagalli, F.; Xu, F.; Jones, S.R.; Bock, C.B.; Miller, G.W.; Wightman, R.M.; Caron, M.G. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron 1997, 19, 1285–1296. [Google Scholar] [CrossRef]
- Isingrini, E.; Perret, L.; Rainer, Q.; Sagueby, S.; Moquin, L.; Gratton, A.; Giros, B. Selective genetic disruption of dopaminergic, serotonergic and noradrenergic neurotransmission: Insights into motor, emotional and addictive behaviour. J. Psychiatry Neurosci. 2016, 41, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Trubetckaia, O.; Lane, A.E.; Qian, L.; Zhou, P.; Lane, D.A. Alpha-synuclein is strategically positioned for afferent modulation of midbrain dopamine neurons and is essential for cocaine preference. Commun. Biol. 2019, 15, 418. [Google Scholar] [CrossRef]
- Wawer, A.; Joniec-Maciejak, I.; Sznejder-Pachołek, A.; Schwenkgrub, J.; Ciesielska, A.; Mirowska-Guzel, D. Exogenous α-Synuclein Monomers Alter Dopamine Metabolism in Murine Brain. Neurochem. Res. 2016, 41, 2102–2109. [Google Scholar] [CrossRef]
- Venda, L.L.; Cragg, S.J.; Buchman, V.L.; Wade-Martins, R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010, 33, 559–568. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Impaired dopamine storage resulting from alpha-synuclein mutations may contribute to the pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 2002, 11, 2395–2407. [Google Scholar] [CrossRef]
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002, 22, 3090–3099. [Google Scholar] [CrossRef]
- Mash, D.C.; Adi, N.; Duque, L.; Pablo, J.; Kumar, M.; Ervin, F.R. Alpha synuclein protein levels are increased in serum from recently abstinent cocaine abusers. Drug Alcohol Depend. 2008, 94, 246–250. [Google Scholar] [CrossRef]
- Wersinger, C.; Sidhu, A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci. Lett. 2003, 340, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Barreto, B.R.; D’Acunzo, P.; Ungania, J.M.; Das, S.; Hashim, A.; Goulbourne, C.N.; Canals-Baker, S.; Saito, M.; Saito, M.; Sershen, H.; et al. Cocaine Modulates the Neuronal Endosomal System and Extracellular Vesicles in a Sex-Dependent Manner. Neurochem. Res. 2022, 47, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.E.; Yao, W.D.; Mohn, A.R.; Quan, H.; Kim, K.M.; Levey, A.I.; Staudinger, J.; Caron, M.G. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 2001, 30, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Haglerød, C.; Hussain, S.; Nakamura, Y.; Xia, J.; Haug, F.S.; Ottersen, O.P.; Henley, J.M.; Davanger, S. Presynaptic PICK1 facilitates trafficking of AMPA-receptors between active zone and synaptic vesicle pool. Neuroscience 2017, 344, 102–112. [Google Scholar] [CrossRef]
- Haglerød, C.; Kapic, A.; Boulland, J.L.; Hussain, S.; Holen, T.; Skare, O.; Laake, P.; Ottersen, O.P.; Haug, F.M.; Davanger, S. Protein interacting with C kinase 1 (PICK1) and GluR2 are associated with presynaptic plasma membrane and vesicles in hippocampal excitatory synapses. Neuroscience 2009, 158, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, J.; Zhou, J.; Burgess, R.; Elledge, S.J.; Olson, E.N. PICK1: A perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J. Cell Biol. 1995, 128, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Terashima, A.; Pelkey, K.A.; Rah, J.C.; Suh, Y.H.; Roche, K.W.; Collingridge, G.L.; McBain, C.J.; Isaac, J.T. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 2008, 57, 872–882. [Google Scholar] [CrossRef]
- Jensen, K.L.; Sørensen, G.; Dencker, D.; Owens, W.A.; Rahbek-Clemmensen, T.; Lever, M.B.; Runegaard, A.H.; Christensen, N.R.; Weikop, P.; Wörtwein, G.; et al. PICK1-Deficient Mice Exhibit Impaired Response to Cocaine and Dysregulated Dopamine Homeostasis. eNeuro 2018, 5, ENEURO.0422-17.2018. [Google Scholar] [CrossRef]
- Wickens, M.M.; Kirkland, J.M.; Knouse, M.C.; McGrath, A.G.; Briand, L.A. Sex-specific role for prefrontal cortical protein interacting with C kinase 1 in cue-induced cocaine seeking. Addict. Biol. 2021, 26, e13051. [Google Scholar] [CrossRef]
- Fonseca, F.; Robles-Martínez, M.; Tirado-Muñoz, J.; Alías-Ferri, M.; Mestre-Pintó, J.I.; Coratu, A.M.; Torrens, M. A Gender Perspective of Addictive Disorders. Curr. Addict. Rep. 2021, 8, 89–99. [Google Scholar] [CrossRef]
- Mandt, B.H.; Zahniser, N.R. Low and high cocaine locomotor responding male Sprague-Dawley rats differ in rapid cocaine-induced regulation of striatal dopamine transporter function. Neuropharmacology 2010, 58, 605–612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandt, B.H.; Allen, R.M.; Zahniser, N.R. Individual differences in initial low-dose cocaine-induced locomotor activity and locomotor sensitization in adult outbred female Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2009, 91, 511–516. [Google Scholar] [CrossRef]
- Gulley, J.M.; Hoover, B.R.; Larson, G.A.; Zahniser, N.R. Individual differences in cocaine-induced locomotor activity in rats: Behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology 2003, 28, 2089–2101. [Google Scholar] [CrossRef] [PubMed]
- Laviola, G.; Macrì, S.; Morley-Fletcher, S.; Adriani, W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003, 27, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar]
- Siciliano, C.A.; Jones, S.R. Cocaine Potency at the Dopamine Transporter Tracks Discrete Motivational States During Cocaine Self-Administration. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 1893–1904. [Google Scholar] [CrossRef]
- Gibbs, R.A.; Weinstock, G.M.; Metzker, M.L.; Muzny, D.M.; Sodergren, E.J.; Scherer, S.; Scott, G.; Steffen, D.; Worley, K.C.; Burch, P.E.; et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 2004, 428, 493–521. [Google Scholar]
- Lin, J.H. Species similarities and differences in pharmacokinetics. Drug Metab. Dispos. 1995, 23, 1008–1021. [Google Scholar]
- Caspell-Garcia, C.; Simuni, T.; Tosun-Turgut, D.; Wu, I.W.; Zhang, Y.; Nalls, M.; Singleton, A.; Shaw, L.A.; Kang, J.H.; Trojanowski, J.Q.; et al. Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS ONE 2017, 12, e0175674. [Google Scholar] [CrossRef]
- Wang, R.; Xu, B.; Guo, Z.; Chen, T.; Zhang, J.; Chen, Y.; Zhu, H. Suite PET/CT neuroimaging for the diagnosis of Parkinson’s disease: Statistical parametric mapping analysis. Nucl. Med. Commun. 2017, 38, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Hansen, F.H.; Skjørringe, T.; Yasmeen, S.; Arends, N.V.; Sahai, M.A.; Erreger, K.; Andreassen, T.F.; Holy, M.; Hamilton, P.J.; Neergheen, V.; et al. Missense dopamine transporter mutations associate with adult parkinsonism and ADHD. J. Clin. Investig. 2014, 124, 3107–3120. [Google Scholar] [CrossRef] [PubMed]
- Drury, S.S.; Brett, Z.H.; Henry, C.; Scheeringa, M. The association of a novel haplotype in the dopamine transporter with preschool age posttraumatic stress disorder. J. Child Adolesc. Psychopharmacol. 2013, 23, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Segman, R.H.; Cooper-Kazaz, R.; Macciardi, F.; Goltser, T.; Halfon, Y.; Dobroborski, T.; Shalev, A.Y. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol. Psychiatry 2002, 7, 903–907. [Google Scholar] [CrossRef][Green Version]
- Vaughan, R.A.; Foster, J.D. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef]
- Mash, D.C.; Duque, L.; Pablo, J.; Qin, Y.; Adi, N.; Hearn, W.L.; Hyma, B.A.; Karch, S.B.; Druid, H.; Wetli, C.V. Brain biomarkers for identifying excited delirium as a cause of sudden death. Forensic Sci. Int. 2009, 190, e13–e19. [Google Scholar] [CrossRef]
- Wu, C.; Garamszegi, S.P.; Xie, X.; Mash, D.C. Altered Dopamine Synaptic Markers in Postmortem Brain of Obese Subjects. Front. Hum. Neurosci. 2017, 11, 386. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, M.; Martin, M.; Gainetdinov, R.R.; Mash, D.C.; Izenwasser, S. Heterozygote Dopamine Transporter Knockout Rats Display Enhanced Cocaine Locomotion in Adolescent Females. Int. J. Mol. Sci. 2022, 23, 15414. https://doi.org/10.3390/ijms232315414
Pardo M, Martin M, Gainetdinov RR, Mash DC, Izenwasser S. Heterozygote Dopamine Transporter Knockout Rats Display Enhanced Cocaine Locomotion in Adolescent Females. International Journal of Molecular Sciences. 2022; 23(23):15414. https://doi.org/10.3390/ijms232315414
Chicago/Turabian StylePardo, Marta, Michele Martin, Raul R. Gainetdinov, Deborah C Mash, and Sari Izenwasser. 2022. "Heterozygote Dopamine Transporter Knockout Rats Display Enhanced Cocaine Locomotion in Adolescent Females" International Journal of Molecular Sciences 23, no. 23: 15414. https://doi.org/10.3390/ijms232315414
APA StylePardo, M., Martin, M., Gainetdinov, R. R., Mash, D. C., & Izenwasser, S. (2022). Heterozygote Dopamine Transporter Knockout Rats Display Enhanced Cocaine Locomotion in Adolescent Females. International Journal of Molecular Sciences, 23(23), 15414. https://doi.org/10.3390/ijms232315414





