Addressing the Clinical Feasibility of Adopting Circulating miRNA for Breast Cancer Detection, Monitoring and Management with Artificial Intelligence and Machine Learning Platforms
Abstract
1. Introduction
2. miRNA as Liquid Biopsy in Personalized Breast Cancer Management and Targeted Therapy
3. Current Trends and Research Outcomes of Circulating miRNA as Liquid Biopsy
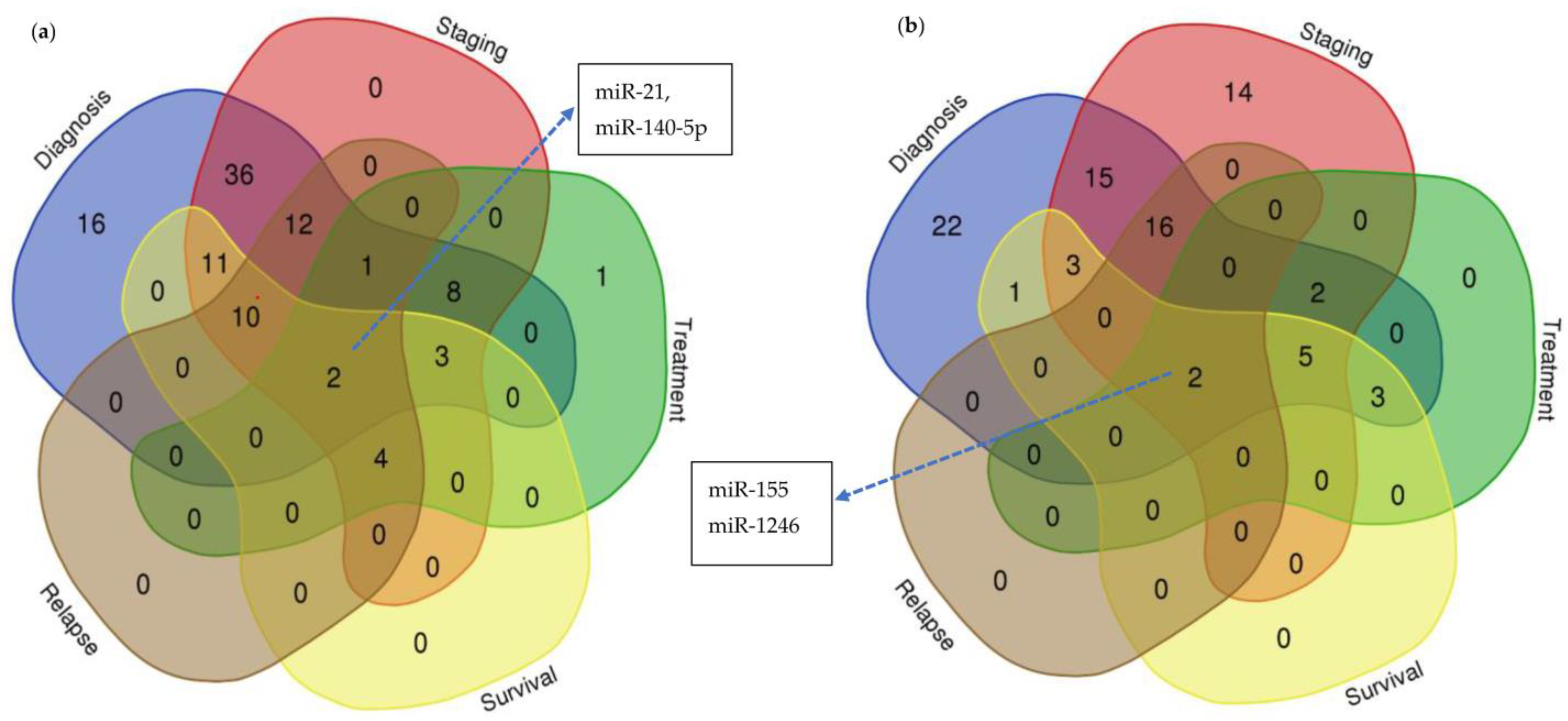
3.1. Diagnostic Significance of Circulating miRNAs in Human Breast Cancer
3.2. Prognostic Significance of Circulating miRNAs in Human Breast Cancer
| miRNAs | miRNAs Source | Diagnostic Significance | Significance in Grading/Classification | Prognostic Significance | Ref. | ||
|---|---|---|---|---|---|---|---|
| Response to Treatment | Overall Survival | Relapse/Recurrence | |||||
| miR-21 | Serum | ↑ miR-21 in BC | ↑ miR-21 in advanced BC | ↑ miR-21 linked to ↑ radioresistance | ↑ miR-21 in BC ↓ survival | NIA | [36] |
| miR-125b | Serum | NIA | ↑ miR-125b linked to ↑ disease staging | ↑ miR-125b linked to ↑ chemoresistance | NIA | NIA | [61] |
| miR-140-5p | Plasma | ↓ miR-140-5p in BC as compared to CT | ↓ miR-140-5p linked to worst disease prognosis | ↓ miR-140-5p linked to ↑ chemoresistance | ↓ miR-140-5p linked to ↓ EFS | ↓ miR-140-5p linked to ↑ relapse/recurrence | [39] |
| miR-335 | Serum | ↓ miR-335 in BC as compared to CT | ↓ miR-335 in TNBC | NIA | ↓ miR-335 linked to ↓ OS | ↓ miR-335 linked to ↑ relapse/recurrence | [54] |
| miR-34a/b/c | Plasma | ↓ In the 3 miRNAs levels in BC as compared to CT | ↓ miR-34a levels linked to advanced clinical staging & histopathological grading | NIA | ↓ miR-34a levels linked to ↓ survival | NIA | [37] |
| miR-21, miR-23b, miR-200c, miR-190 | Plasma | ↑ miR-21, miR-23b & miR-200c levels & ↓ miR-190 in BC | The 4 miRNAs distinguished relapsed & non-relapsed BC cases | NIA | ↑ miR-21 & miR-200c linked to short DFS | ↑ miR-21, miR-23b & miR-200c & ↓ miR-190 in relapsed as compared to non-relapsed case | [42] |
| miR-16-5p, miR-17-3p, miR-451a, miR-940 | Serum | No significant difference in the 4 miRNAs levels between BC & CT cases | The 4 miRNAs distinguished metastatic & non-metastatic BC cases | ↓ In the 4 miRNAs in trastuzumab-resistant BC | ↑ In the 4 miRNAs in improved BC survival | ↑ In the 4 miRNAs in reduced incidence of relapse/recurrence | [50] |
| miR-18b, miR-103, miR-107, miR-652 | Serum | ↑ In the 4 miRNAs levels in TNBC | ↑ In the 4 miRNAs levels linked to advanced clinical staging & histopathological grading | NIA | ↑ In the 4 miRNAs ↓ RFS & OS | ↑ In the 4 miRNAs in relapse group | [66] |
| Let-7a, miR-10b, miR-21, miR-145, miR-181a | Plasma | ↑ miR-10b, miR-21 & miR-181a & ↓ let-7a & miR-145 in BC | ↑ miR-10b, miR-21 & miR-181a & ↓ let-7a & miR-145 in locally advanced BC | NIA | ↑ miR-10b & ↓ miR-21 linked to survival | ↑ miR-10b & miR-21 linked to ↑ relapse | [57] |
| miR-26b-5p, miR-106b-5p, miR-142-3p, miR-142-5p, miR-185-5p, miR-362-5p | Whole blood | ↑ In the 6 miRNAs levels in BC | ↑ In the 6 miRNAs levels in early BC | NIA | ↑ In the 6 miRNAs levels linked to ↓ OS/DFS | NIA | [45] |
| miR-19a, miR-19b-3p, miR-22-3p, miR-25-3p, miR-93-5p, miR-199a-3p, miR-210-3p | Plasma | ↑ In the 7 miRNAs levels in BC | These miRNAs predicted BC patient survival & relapse | The 7 miRNAs regulate chemotherapy & targeted therapy resistance | ↑ miR-19a, miR-19b, miR-93 & miR-201 linked to poor OS in TNBC patients | NIA | [30] |
| miR-296-3p, miR-575, miR-3610-5p, miR-4483, miR-4710, miR-4755-3p, miR-5698, miR-8089 | Serum | ↑ In the 8 miRNAs levels in BC | The 8 miRNAs distinguished metastatic & non-metastatic BC cases | NIA | ↓ miR-5698 & miR-8089 linked to ↑ improved survival | The 8 miRNAs predicted distant metastases | [31] |
| miRNAs | miRNAs Source | Diagnostic Significance | Significance in Grading/Classification | Prognostic Significance | Ref. | ||
|---|---|---|---|---|---|---|---|
| Response to Treatment | Overall Survival | Relapse/Recurrence | |||||
| miR-24-3p | Plasma | ↑ miR-24-3p in BC | ↑ miR-24-3p linked to advanced clinical & histopathological grading | NIA | ↓ miR-24-3p linked to improved survival | NIA | [47] |
| miR-363-5p | Plasma | ↓ miR-363-5p in BC | ↓ miR-363-5p in LN+ve BC cases as compared to LN –ve BC cases | NIA | ↑ miR-363-5p linked to ↑ survival | NIA | [25] |
| miR-141, miR-200c | Plasma | ↑ miR-141 & miR-200c in BC | ↑ miR-141 in invasive BC; ↑ miR-141 & miR-200c in metastatic BC | NIA | ↑ miR-200c linked to short OS | NIA | [64] |
| miR-155, miR-1246 | Plasma | ↑ Both miRNAs in trastuzumab-resistant BC | ↑ Both miRNAs advanced BC as compared to non-advanced BC | ↑ Both miRNAs in trastuzumab-resistant BC | ↑ Both miRNAs linked to poor survival | ↑ Both miRNAs linked to relapse & poor EFS | [38] |
| miR-21, miR-105, miR-222 | Serum | ↑ In the 3 miRNAs levels linked to presence of circulating BC cells | ↑ miR-222 linked to advanced clinical staging & histopathological grading; ↑ miR-21 & miR-105 in metastatic than non-metastatic BC | ↑ miR-21 reduced NACT response | NIA | NIA | [58] |
| miR-150-5p, miR-576-3p, miR-4665-5p | Plasma | ↑ In the 3 miRNAs levels in BC | The 3 miRNAs distinguished recurrence & non-recurrence in BC cases | NIA | NIA | ↑ In the 3 miRNAs levels in recurrent BC as compared to non-recurrent BC | [34] |
| miR-16, miR-30b, miR-93 | Plasma | ↑ miR-16 in BC & ↑ miR-93 in DCIS | ↑ miR-93 in ER & PR +ve BC | NIA | NIA | ↓ miR-30b linked to recurrence | [63] |
| miR-195-5p, miR-548ab, miR-2392, miR-2467-3p, miR-4448, miR-4800-3p | Serum | ↑ miR-2392, miR-2467-3p, miR-4448 & miR-4800-3p levels in BC | The 6 miRNAs distinguished recurrence & non-recurrence in BC cases | ↑ miR-2392, miR-2467-3p, miR-4448 & miR-4800-3p levels in BC with complete NACT response | ↑ miR-2392, miR-2467-3p, miR-4448 & miR-4800-3p levels linked to ↑ OS in BC | ↑ In miR-195-5p & ↓ miR-548ab in recurrent BC cases | [60] |
| miR-30b, miR-34a, miR-127, miR-141, miR-182, miR-183, miR-328, miR-423 | Plasma | Dysregulation in the 8 miRNA levels in BC | ↓ miR-30b, miR-127 & miR-328 in invasive BC | ↑ miR-127 & miR-141 linked to complete NACT response; ↑ miR-34a, miR-182 & miR-183 linked to poor NACT response | ↓ miR-141, miR-34a, miR-423, miR-182 & miR-183 linked to ↑ OS | NIA | [59] |
3.3. Multifunctional Roles of Circulating miRNAs as Potential Biomarker for Human Breast Cancer
3.4. Sensitivity and Specificity Levels of miRNA Detection in BC Patients
4. Current Challenges and Issues in Circulating miRNAs as a Common Candidate for Liquid Biopsy in BC Management
4.1. Biological Parameters
4.2. Statistical Models
5. Machine Learning and Deep Learning Approaches in BC Research
5.1. Machine Learning and Deep Learning for Detection and Diagnosis
5.2. Studies with miRNAs as Breast Cancer Biomarkers with ML/DL Approaches
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global Breast Cancer Incidence and Mortality Trends by Region, Age-Groups, and Fertility Patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef] [PubMed]
- Mubarik, S.; Malik, S.S.; Wang, Z.; Li, C.; Fawad, M.; Yu, C. Recent Insights into Breast Cancer Incidence Trends among Four Asian Countries Using Age-Period-Cohort Model. Cancer Manag. Res. 2019, 11, 8145–8155. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, D.; Hoe, W.M.K.; Mohan, D.; Allotey, P.; Reidpath, D.D.; Tan, M.M.; Taib, N.A.M.; Donnelly, M.; Su, T.T. Challenges and Opportunities for Breast Cancer Early Detection among Rural Dwelling Women in Segamat District, Malaysia: A Qualitative Study. PLoS ONE 2022, 17, 267308. [Google Scholar] [CrossRef] [PubMed]
- Tuasha, N.; Petros, B. Heterogeneity of Tumors in Breast Cancer: Implications and Prospects for Prognosis and Therapeutics. Scientifica (Cairo) 2020, 2020, 4736091. [Google Scholar] [CrossRef]
- Zambelli, A.; Tondini, C.; Munkácsy, G.; Santarpia, L.; Gyorffy, B. Gene Expression Profiling in Early Breast Cancer-Patient Stratification Based on Molecular and Tumor Microenvironment Features. Biomedicines 2022, 10, 248. [Google Scholar] [CrossRef]
- Pan, J.W.; Zabidi, M.M.A.; Ng, P.S.; Meng, M.Y.; Hasan, S.N.; Sandey, B.; Sammut, S.J.; Yip, C.H.; Rajadurai, P.; Rueda, O.M.; et al. The Molecular Landscape of Asian Breast Cancers Reveals Clinically Relevant Population-Specific Differences. Nat. Commun. 2020, 11, 6433. [Google Scholar] [CrossRef]
- Eliyatkin, N.; Yalcin, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2021, 11, 632079. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Lukong, K.E. Breast Cancer Stem-Like Cells in Drug Resistance: A Review of Mechanisms and Novel Therapeutic Strategies to Overcome Drug Resistance. Front. Oncol. 2022, 12, 961. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, R.G.; Otoni, K.M. Histological and Molecular Classification of Breast Cancer: What Do We Know? Mastology 2020, 30, e20200024. [Google Scholar] [CrossRef]
- Zou, R.; Loke, S.Y.; Tan, V.K.M.; Quek, S.T.; Jagmohan, P.; Tang, Y.C.; Madhukumar, P.; Tan, B.K.T.; Yong, W.S.; Sim, Y.; et al. Development of a Microrna Panel for Classification of Abnormal Mammograms for Breast Cancer. Cancers (Basel) 2021, 13, 2130. [Google Scholar] [CrossRef] [PubMed]
- Oluwaseyi Temilola, D.; Wium, M.; Herve Coulidiati, T.; Ademola Adeola, H.; Maria Carbone, G.; Vittorio Catapano, C.; Fernando Zerbini, L. The Prospect and Challenges to the Flow of Liquid Biopsy in Africa. Cells 2019, 8, 862. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nishimura, S.; Ito, T.; Oka, N.; Akagi, M. Limitations and Usefulness of Biopsy Techniques for the Diagnosis of Metastatic Bone and Soft Tissue Tumors. Ann. Med. Surg. 2021, 68, 102581. [Google Scholar] [CrossRef]
- Rodríguez, J.; Avila, J.; Rolfo, C.; Ruíz-Patiño, A.; Russo, A.; Ricaurte, L.; Ordóñez-Reyes, C.; Arrieta, O.; Zatarain-Barrón, Z.L.; Recondo, G.; et al. When Tissue Is an Issue the Liquid Biopsy Is Nonissue: A Review. Oncol. Ther. 2021, 9, 89–110. [Google Scholar] [CrossRef]
- Hirahata, T.; ul Quraish, R.; ul Quraish, A.; ul Quraish, S.; Naz, M.; Razzaq, M.A. Liquid Biopsy: A Distinctive Approach to the Diagnosis and Prognosis of Cancer. Cancer Inform. 2022, 21, 11769351221076062. [Google Scholar] [CrossRef]
- Liu, X.; Papukashvili, D.; Wang, Z.; Liu, Y.; Chen, X.; Li, J.; Li, Z.; Hu, L.; Li, Z.; Rcheulishvili, N.; et al. Potential Utility of MiRNAs for Liquid Biopsy in Breast Cancer. Front. Oncol. 2022, 12, 940314. [Google Scholar] [CrossRef]
- Zeng, X.; Shi, G.; He, Q.; Zhu, P. Screening and Predicted Value of Potential Biomarkers for Breast Cancer Using Bioinformatics Analysis. Sci. Rep. 2021, 11, 20799. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qu, H.; Wang, S.; Chater, J.M.; Wang, X.; Cui, Y.; Yu, L.; Zhou, R.; Jia, Q.; Traband, R.; et al. CancerMIRNome: An Interactive Analysis and Visualization Database for MiRNome Profiles of Human Cancer. Nucleic Acids Res. 2022, 50, D1139–D1146. [Google Scholar] [CrossRef] [PubMed]
- Lánczky, A.; Nagy, Á.; Bottai, G.; Munkácsy, G.; Szabó, A.; Santarpia, L.; Győrffy, B. MiRpower: A Web-Tool to Validate Survival-Associated MiRNAs Utilizing Expression Data from 2178 Breast Cancer Patients. Breast Cancer Res. Treat. 2016, 160, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Eichelser, C.; Flesch-Janys, D.; Chang-Claude, J.; Pantel, K.; Schwarzenbach, H. Deregulated Serum Concentrations of Circulating Cell-Free MicroRNAs MiR-17, MiR-34a, MiR-155, and MiR-373 in Human Breast Cancer Development and Progression. Clin. Chem. 2013, 59, 1489–1496. [Google Scholar] [CrossRef]
- Wang, X.; Qian, T.; Bao, S.; Zhao, H.; Chen, H.; Xing, Z.; Li, Y.; Zhang, M.; Meng, X.; Wang, C.; et al. Circulating Exosomal MiR-363-5p Inhibits Lymph Node Metastasis by Downregulating PDGFB and Serves as a Potential Noninvasive Biomarker for Breast Cancer. Mol. Oncol. 2021, 15, 2466–2479. [Google Scholar] [CrossRef]
- Moloney, B.M.; Gilligan, K.E.; Joyce, D.P.; O’Neill, C.P.; O’Brien, K.P.; Khan, S.; Glynn, C.L.; Waldron, R.M.; Maguire, C.M.; Holian, E.; et al. Investigating the Potential and Pitfalls of EV-Encapsulated MicroRNAs as Circulating Biomarkers of Breast Cancer. Cells 2020, 9, 141. [Google Scholar] [CrossRef]
- Li, M.; Zou, X.; Xia, T.; Wang, T.; Liu, P.; Zhou, X.; Wang, S.; Zhu, W. A Five-MiRNA Panel in Plasma Was Identified for Breast Cancer Diagnosis. Cancer Med. 2019, 8, 7006–7017. [Google Scholar] [CrossRef]
- Chen, W.; Cao, R.; Su, W.; Zhang, X.; Xu, Y.; Wang, P.; Gan, Z.; Xie, Y.; Li, H.; Qin, J. Simple and Fast Isolation of Circulating Exosomes with a Chitosan Modified Shuttle Flow Microchip for Breast Cancer Diagnosis. Lab Chip 2021, 21, 1759–1770. [Google Scholar] [CrossRef]
- Sueta, A.; Yamamoto, Y.; Tomiguchi, M.; Takeshita, T.; Yamamoto-Ibusuki, M.; Iwase, H. Differential Expression of Exosomal MiRNAs between Breast Cancer Patients with and without Recurrence. Oncotarget 2017, 8, 69934–69944. [Google Scholar] [CrossRef]
- Qattan, A.; Al-Tweigeri, T.; Alkhayal, W.; Suleman, K.; Tulbah, A.; Amer, S. Clinical Identification of Dysregulated Circulating MicroRNAs and Their Implication in Drug Response in Triple Negative Breast Cancer (TNBC) by Target Gene Network and Meta-Analysis. Genes 2021, 12, 549. [Google Scholar] [CrossRef]
- Satomi-Tsushita, N.; Shimomura, A.; Matsuzaki, J.; Yamamoto, Y.; Kawauchi, J.; Takizawa, S.; Aoki, Y.; Sakamoto, H.; Kato, K.; Shimizu, C.; et al. Serum MicroRNA-Based Prediction of Responsiveness to Eribulin in Metastatic Breast Cancer. PLoS ONE 2019, 14, e0222024. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, Y.S.; Kang, K.N.; Kim, K.H.; Park, Y.J.; Kim, C.W. Multiple MicroRNAs as Biomarkers for Early Breast Cancer Diagnosis. Mol. Clin. Oncol. 2021, 14, 31. [Google Scholar] [CrossRef]
- Koi, Y.; Tsutani, Y.; Nishiyama, Y.; Ueda, D.; Ibuki, Y.; Sasada, S.; Akita, T.; Masumoto, N.; Kadoya, T.; Yamamoto, Y.; et al. Predicting the Presence of Breast Cancer Using Circulating Small RNAs, Including Those in the Extracellular Vesicles. Cancer Sci. 2020, 111, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Q.; Zhong, H.; Li, L.; Zhang, Q.; Huang, Q.; Yu, Z. Differentially Expressed MicroRNAs in Exosomes of Patients with Breast Cancer Revealed by Next-Generation Sequencing. Oncol. Rep. 2020, 43, 240–250. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Espinoza-Sánchez, N.A.; El-Damen, A.; Fahim, S.A.; Badawy, M.A.; Greve, B.; El-Shinawi, M.; Götte, M.; Ibrahim, S.A. Small Extracellular Vesicle-Encapsulated MiR-181b-5p, MiR-222-3p and Let-7a-5p: Next Generation Plasma Biopsy-Based Diagnostic Biomarkers for Inflammatory Breast Cancer. PLoS ONE 2021, 16, e0250642. [Google Scholar] [CrossRef] [PubMed]
- Dan, T.; Shastri, A.A.; Palagani, A.; Buraschi, S.; Neill, T.; Savage, J.E.; Kapoor, A.; Deangelis, T.; Addya, S.; Camphausen, K.; et al. MiR-21 Plays a Dual Role in Tumor Formation and Cytotoxic Response in Breast Tumors. Cancers 2021, 13, 888. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, X.; Zhu, D.; Luo, Z.; Yang, M. Low Expression of Circulating MicroRNA-34c Is Associated with Poor Prognosis in Triple-Negative Breast Cancer. Yonsei Med. J. 2017, 58, 697–702. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yu, G.; Sun, Z.; Wang, T.; Tian, X.; Duan, X.; Zhang, C. Exosomal MiR-1246 and MiR-155 as Predictive and Prognostic Biomarkers for Trastuzumab-Based Therapy Resistance in HER2-Positive Breast Cancer. Cancer Chemother. Pharmacol. 2020, 86, 761–772. [Google Scholar] [CrossRef]
- Di Cosimo, S.; Appierto, V.; Pizzamiglio, S.; Tiberio, P.; Iorio, M.V.; Hilbers, F.; De Azambuja, E.; De La Peña, L.; Izquierdo, M.; Huober, J.; et al. Plasma MiRNA Levels for Predicting Therapeutic Response to Neoadjuvant Treatment in HER2-Positive Breast Cancer: Results from the NeoALTTO Trial. Clin. Cancer Res. 2019, 25, 3887–3895. [Google Scholar] [CrossRef]
- Stevic, I.; Müller, V.; Weber, K.; Fasching, P.A.; Karn, T.; Marmé, F.; Schem, C.; Stickeler, E.; Denkert, C.; Van Mackelenbergh, M.; et al. Specific MicroRNA Signatures in Exosomes of Triple-Negative and HER2-Positive Breast Cancer Patients Undergoing Neoadjuvant Therapy within the GeparSixto Trial. BMC Med. 2018, 16, 179. [Google Scholar] [CrossRef]
- Zheng, X.; Lu, S.; He, Z.; Huang, H.; Yao, Z.; Miao, Y.; Cai, C.; Zou, F. MCU-Dependent Negative Sorting of MiR-4488 to Extracellular Vesicles Enhances Angiogenesis and Promotes Breast Cancer Metastatic Colonization. Oncogene 2020, 39, 6975–6989. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, C.; Stratigos, M.; Markakis, G.; Spiliotaki, M.; Mastrostamatis, G.; Nikolaou, C.; Mavroudis, D.; Agelaki, S. Circulating MicroRNAs in the Early Prediction of Disease Recurrence in Primary Breast Cancer. Breast Cancer Res. 2018, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Q.; Schrauder, M.; Yan, L.; Wang, D.; Medico, L.; Guo, Y.; Yao, S.; Zhu, Q.; Liu, B.; et al. Circulating MiR-148b and MiR-133a as Biomarkers for Breast Cancer Detection. Oncotarget 2014, 5, 5284–5294. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, C.; Stoupis, G.; Tsalikis, L.; Monastirioti, A.; Papadaki, M.; Maliotis, N.; Stratigos, M.; Mastrostamatis, G.; Mavroudis, D.; Agelaki, S. Circulating MiRNAs as a Marker of Metastatic Disease and Prognostic Factor in Metastatic Breast Cancer. Oncotarget 2019, 10, 966–981. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.Y.; Xu, Y.; Zhang, L.; Zhu, J.; Si, P.C.; Wang, Y.W.; Ma, R. A Two-MiRNA Signature of Upregulated MiR-185-5p and MiR-362-5p as a Blood Biomarker for Breast Cancer. Pathol. Res. Pract. 2021, 222, 153458. [Google Scholar] [CrossRef]
- Cuk, K.; Zucknick, M.; Madhavan, D.; Schott, S.; Golatta, M.; Heil, J.; Marmé, F.; Turchinovich, A.; Sinn, P.; Sohn, C.; et al. Plasma MicroRNA Panel for Minimally Invasive Detection of Breast Cancer. PLoS ONE 2013, 8, e76729. [Google Scholar] [CrossRef]
- Khodadadi-Jamayran, A.; Akgol-Oksuz, B.; Afanasyeva, Y.; Heguy, A.; Thompson, M.; Ray, K.; Giro-Perafita, A.; Sánchez, I.; Wu, X.; Tripathy, D.; et al. Prognostic Role of Elevated Mir-24-3p in Breast Cancer and Its Association with the Metastatic Process. Oncotarget 2018, 9, 12868–12878. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Iinuma, H.; Umemoto, Y.; Yanagisawa, T.; Matsumoto, A.; Jinno, H. Exosome-Encapsulated MicroRNA-223-3p as a Minimally Invasive Biomarker for the Early Detection of Invasive Breast Cancer. Oncol. Lett. 2018, 15, 9584–9592. [Google Scholar] [CrossRef]
- Mohmmed, E.A.; Shousha, W.G.; EL-Saiid, A.S.; Ramadan, S.S. A Clinical Evaluation of Circulating MiR-106a and Raf-1 as Breast Cancer Diagnostic and Prognostic Markers. Asian Pac. J. Cancer Prev. 2021, 22, 3513–3520. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Chen, J.; Wang, H.; Yang, L.; Chen, F.; Fan, S.; Wang, J.; Shao, B.; Yin, D.; et al. A Serum MicroRNA Signature Predicts Trastuzumab Benefit in HER2-Positive Metastatic Breast Cancer Patients. Nat. Commun. 2018, 9, 1614. [Google Scholar] [CrossRef]
- McAnena, P.; Tanriverdi, K.; Curran, C.; Gilligan, K.; Freedman, J.E.; Brown, J.A.L.; Kerin, M.J. Circulating MicroRNAs MiR-331 and MiR-195 Differentiate Local Luminal a from Metastatic Breast Cancer. BMC Cancer 2019, 19, 436. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating MicroRNAs as Novel Minimally Invasive Biomarkers for Breast Cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, Y.; Xia, T.; Zhou, X.; Huang, Z.; Zhang, H.; Zhu, W.; Ding, Q.; Wang, S. Circulating MicroRNAs from the MiR-106a–363 Cluster on Chromosome X as Novel Diagnostic Biomarkers for Breast Cancer. Breast Cancer Res. Treat. 2018, 170, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Swellam, M.; Mahmoud, M.S.; Hashim, M.; Hassan, N.M.; Sobeih, M.E.; Nageeb, A.M. Clinical Aspects of Circulating MiRNA-335 in Breast Cancer Patients: A Prospective Study. J. Cell. Biochem. 2018, 120, 8975–8982. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiecki, S.; Piekarski, J.; Szymańska, B.; Pawłowska, Z.; Jeziorski, A. Serum Levels of Circulating MiRNA-21, MiRNA-10b and MiRNA-200c in Triple-Negative Breast Cancer Patients. Ginekol. Pol. 2018, 89, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Hamam, R.; Ali, A.M.; Alsaleh, K.A.; Kassem, M.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. MicroRNA Expression Profiling on Individual Breast Cancer Patients Identifies Novel Panel of Circulating MicroRNA for Early Detection. Sci. Rep. 2016, 6, 25997. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Said, M.M.; Hilal, A.M.; Medhat, A.M.; Abd Elsalam, I.M. Candidate Circulating MicroRNAs as Potential Diagnostic and Predictive Biomarkers for the Monitoring of Locally Advanced Breast Cancer Patients. Tumor Biol. 2020, 42, 1010428320963811. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, A.; De Miguel-Pérez, D.; Ortega, F.G.; García-Puche, J.L.; Robles-Fernández, I.; Exposito, J.; Martorell-Marugan, J.; Carmona-Sáez, P.; Garrido-Navas, M.D.C.; Rolfo, C.; et al. Exosomal MiRNA Profile as Complementary Tool in the Diagnostic and Prediction of Treatment Response in Localized Breast Cancer under Neoadjuvant Chemotherapy. Breast Cancer Res. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.K.; Byrum, S.D.; Gies, A.J.; Haynie, C.; Smith, H.; Reyna, N.S.; Makhoul, I. Circulating Exosomal MicroRNAs as Predictive Biomarkers of Neoadjuvant Chemotherapy Response in Breast Cancer. Current Oncology 2022, 29, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Sueta, A.; Fujiki, Y.; Goto Yamaguchi, L.; Tomiguchi, M.; Yamamoto Ibusuki, M.; Iwase, H.; Yamamoto, Y. Exosomal MiRNA Profiles of Triple negative Breast Cancer in Neoadjuvant Treatment. Oncol. Lett. 2021, 22, 819. [Google Scholar] [CrossRef]
- Wang, H.; Tan, G.; Dong, L.; Cheng, L.; Li, K.; Wang, Z.; Luo, H. Circulating Mir-125b as a Marker Predicting Chemoresistance in Breast Cancer. PLoS ONE 2012, 7, e34210. [Google Scholar] [CrossRef] [PubMed]
- Bakr, N.M.; Mahmoud, M.S.; Nabil, R.; Boushnak, H.; Swellam, M. Impact of Circulating MiRNA-373 on Breast Cancer Diagnosis through Targeting VEGF and Cyclin D1 Genes. J. Genet. Eng. Biotechnol. 2021, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Stevic, I.; Pan, C.; Müller, V.; Oliviera-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Different Signatures of MiR-16, MiR-30b and MiR-93 in Exosomes from Breast Cancer and DCIS Patients. Sci. Rep. 2018, 8, 12974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, W.; Li, B.; Stringer-Reasor, E.; Chu, C.; Sun, L.; Bae, S.; Chen, D.; Wei, S.; Jiao, K.; et al. MicroRNA-200c and MicroRNA-141 Are Regulated by a FOXP3-KAT2B Axis and Associated with Tumor Metastasis in Breast Cancer. Breast Cancer Res. 2017, 19, 73. [Google Scholar] [CrossRef]
- Hesari, A.R.; Azizian, M.; Darabi, H.; Nesaei, A.; Hosseini, S.A.; Salarinia, R.; Motaghi, A.A.; Ghasemi, F. Expression of Circulating MiR-17, MiR-25, and MiR-133 in Breast Cancer Patients. J. Cell. Biochem. 2019, 120, 7109–7114. [Google Scholar] [CrossRef]
- Sahlberg, K.K.; Bottai, G.; Naume, B.; Burwinkel, B.; Calin, G.A.; Børresen-Dale, A.-L.; Santarpia, L. A Serum MicroRNA Signature Predicts Tumor Relapse and Survival in Triple-Negative Breast Cancer Patients. Clin. Cancer Res. 2015, 21, 1207–1214. [Google Scholar] [CrossRef]
- Diansyah, M.N.; Prayogo, A.A.; Sedana, M.P.; Savitri, M.; Romadhon, P.Z.; Amrita, P.N.A.; Wijaya, A.Y.; Hendrata, W.M.; Bintoro, U.Y. Early Detection Breast Cancer: Role of Circulating Plasma MiRNA-21 Expression as a Potential Screening Biomarker. Turk. J. Med. Sci. 2021, 51, 562–569. [Google Scholar] [CrossRef]
- Lopes, B.C.; Braga, C.Z.; Ventura, F.V.; de Oliveira, J.G.; Kato-Junior, E.M.; Bordin-Junior, N.A.; Zuccari, D.A.P.C. MiR-210 and MiR-152 as Biomarkers by Liquid Biopsy in Invasive Ductal Carcinoma. J. Pers. Med. 2021, 11, 31. [Google Scholar] [CrossRef]
- Motawi, T.M.K.; Sadik, N.A.H.; Shaker, O.G.; el Masry, M.R.; Mohareb, F. Study of MicroRNAs-21/221 as Potential Breast Cancer Biomarkers in Egyptian Women. Gene 2016, 590, 210–219. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Yang, J.; Zhen, J.; Zhang, D. Serum MicroRNA-21 as a Potential Diagnostic Biomarker for Breast Cancer: A Systematic Review and Meta-Analysis. Clin. Exp. Med. 2016, 16, 29–35. [Google Scholar] [CrossRef]
- Sehovic, E.; Urru, S.; Chiorino, G.; Doebler, P. Meta-Analysis of Diagnostic Cell-Free Circulating MicroRNAs for Breast Cancer Detection. BMC Cancer 2022, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Hamam, R.; Hamam, D.; Alsaleh, K.A.; Kassem, M.; Zaher, W.; Alfayez, M.; Aldahmash, A.; Alajez, N.M. Circulating MicroRNAs in Breast Cancer: Novel Diagnostic and Prognostic Biomarkers. Cell Death Dis. 2017, 8, e3045. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, Z.; Wang, J.; Sun, J.; Wang, P.; Cheng, X.; Fu, L.; Zhang, L.; Wang, Z.; Li, Z. Different MiRNA Expression Profiles between Human Breast Cancer Tumors and Serum. Front. Genet. 2014, 5, 149. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Incoronato, M. Clinical Translatability of “Identified” Circulating Mirnas for Diagnosing Breast Cancer: Overview and Update. Cancers 2019, 11, 901. [Google Scholar] [CrossRef]
- Chan, M.; Liaw, C.S.; Ji, S.M.; Tan, H.H.; Wong, C.Y.; Thike, A.A.; Tan, P.H.; Ho, G.H.; Lee, A.S.G. Identification of Circulating MicroRNA Signatures for Breast Cancer Detection. Clin. Cancer Res. 2013, 19, 4477–4487. [Google Scholar] [CrossRef]
- Uyisenga, J.P.; Debit, A.; Poulet, C.; Frères, P.; Poncin, A.; Thiry, J.; Mutesa, L.; Jerusalem, G.; Bours, V.; Josse, C. Differences in Plasma MicroRNA Content Impair MicroRNA-Based Signature for Breast Cancer Diagnosis in Cohorts Recruited from Heterogeneous Environmental Sites. Sci. Rep. 2021, 11, 11698. [Google Scholar] [CrossRef] [PubMed]
- Jusoh, A.R.; Mohan, S.V.; Ping, T.L.; Din, T.A.D.A.A.B.T.; Haron, J.; Romli, R.C.; Jaafar, H.; Nafi, S.N.; Salwani, T.I.T.; Yahya, M.M. Plasma Circulating Mirnas Profiling for Identification of Potential Breast Cancer Early Detection Biomarkers. Asian Pac. J. Cancer Prev. 2021, 22, 1375–1381. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Roles of Circulating MicroRNA(s) in Human Breast Cancer. Arch. Biochem. Biophys. 2020, 695, 108583. [Google Scholar] [CrossRef]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial Intelligence in Healthcare: Past, Present and Future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The Potential for Artificial Intelligence in Healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Dlamini, Z.; Francies, F.Z.; Hull, R.; Marima, R. Artificial Intelligence (AI) and Big Data in Cancer and Precision Oncology. Comput. Struct. Biotechnol. J. 2020, 18, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Chen, C.H.; Shi, S.; Chung, C.R.; Wen, Y.H.; Wu, M.H.; Lebowitz, M.S.; Zhou, J.; Lu, J.J. Improving Multi-Tumor Biomarker Health Check-up Tests with Machine Learning Algorithms. Cancers 2020, 12, 1442. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Chandorkar, P.; Dsouza, A.; Kazi, N. Breast Cancer Diagnosis And Recurrence Prediction Using Machine Learning Techniques. Int. J. Res. Eng. Technol. 2015, 4, 372–376. [Google Scholar]
- Shravya, C.; Pravalika, K.; Subhani, S. Prediction of Breast Cancer Using Supervised Machine Learning Techniques. Int. J. Innov. Technol. Explor. Eng. (IJITEE) 2019, 8, 1106–1110. [Google Scholar]
- Naji, M.A.; el Filali, S.; Aarika, K.; Benlahmar, E.H.; Abdelouhahid, R.A.; Debauche, O. Machine Learning Algorithms for Breast Cancer Prediction and Diagnosis. Procedia Comput. Sci. 2021, 191, 487–492. [Google Scholar] [CrossRef]
- Chaudhury, S.; Krishna, A.N.; Gupta, S.; Sankaran, K.S.; Khan, S.; Sau, K.; Raghuvanshi, A.; Sammy, F. Effective Image Processing and Segmentation-Based Machine Learning Techniques for Diagnosis of Breast Cancer. Comput. Math. Methods Med. 2022, 2022, 6841334. [Google Scholar] [CrossRef]
- Wu, J.; Hicks, C. Breast Cancer Type Classification Using Machine Learning. J. Pers. Med. 2021, 11, 61. [Google Scholar] [CrossRef]
- Haque, M.N.; Tazin, T.; Khan, M.M.; Faisal, S.; Ibraheem, S.M.; Algethami, H.; Almalki, F.A. Predicting Characteristics Associated with Breast Cancer Survival Using Multiple Machine Learning Approaches. Comput. Math. Methods Med. 2022, 2022, 1249692. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.; Yu, Y.; Lin, Y.; Song, C. The Impact of Chemotherapy and Survival Prediction by Machine Learning in Early Elderly Triple Negative Breast Cancer (ETNBC): A Population Based Study from the SEER Database. BMC Geriatr. 2022, 22, 268. [Google Scholar] [CrossRef]
- Boughorbel, S.; Al-Ali, R.; Elkum, N. Model Comparison for Breast Cancer Prognosis Based on Clinical Data. PLoS ONE 2016, 11, 146413. [Google Scholar] [CrossRef]
- Hameed, Z.; Zahia, S.; Garcia-Zapirain, B.; Aguirre, J.J.; Vanegas, A.M. Breast Cancer Histopathology Image Classification Using an Ensemble of Deep Learning Models. Sensors 2020, 20, 4373. [Google Scholar] [CrossRef] [PubMed]
- Nomani, A.; Ansari, Y.; Nasirpour, M.H.; Masoumian, A.; Pour, E.S.; Valizadeh, A. PSOWNNs-CNN: A Computational Radiology for Breast Cancer Diagnosis Improvement Based on Image Processing Using Machine Learning Methods. Comput. Intell. Neurosci. 2022, 2022, 5667264. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, D.; Gao, Z.; Wang, S.; He, M.; Fan, J. Deep Learning Assisted Efficient AdaBoost Algorithm for Breast Cancer Detection and Early Diagnosis. IEEE Access 2020, 8, 96946–96954. [Google Scholar] [CrossRef]
- Song, T.; Liang, Y.; Cao, Z.; Du, W.; Li, Y. Computational Analysis of Specific MicroRNA Biomarkers for Noninvasive Early Cancer Detection. Biomed. Res. Int. 2017, 2017, 4680650. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rincon, A.; Mendoza-Maldonado, L.; Martinez-Archundia, M.; Schönhuth, A.; Kraneveld, A.D.; Garssen, J.; Tonda, A. Machine Learning-Based Ensemble Recursive Feature Selection of Circulating Mirnas for Cancer Tumor Classification. Cancers 2020, 12, 1785. [Google Scholar] [CrossRef]
- Robertus Fujii, Y. The Quantum Language of the MicroRNA Gene and Anti-Cancer: With a Dynamic Computer Simulation of Human Breast Cancer Drug Resistance. Integr. Mol. Med. 2018, 5. [Google Scholar] [CrossRef]
- Lopez-Rincon, A.; Martinez-Archundia, M.; Martinez-Ruiz, G.U.; Schoenhuth, A.; Tonda, A. Automatic Discovery of 100-MiRNA Signature for Cancer Classification Using Ensemble Feature Selection. BMC Bioinform. 2019, 20, 480. [Google Scholar] [CrossRef] [PubMed]
- Kotlarchyk, A.; Khoshgoftaar, T.; Pavlovic, M.; Zhuang, H.; Pandya, A.S. Identification of MicroRNA Biomarkers for Cancer by Combining Multiple Feature Selection Techniques. J. Comput. Methods Sci. Eng. 2011, 11, 283–298. [Google Scholar] [CrossRef]
- Rehman, O.; Zhuang, H.; Ali, A.M.; Ibrahim, A.; Li, Z. Validation of MiRNAs as Breast Cancer Biomarkers with a Machine Learning Approach. Cancers 2019, 11, 431. [Google Scholar] [CrossRef]
- Sherafatian, M. Tree-Based Machine Learning Algorithms Identified Minimal Set of MiRNA Biomarkers for Breast Cancer Diagnosis and Molecular Subtyping. Gene 2018, 677, 111–118. [Google Scholar] [CrossRef]
- Waspada, I.; Wibowo, A.; Meraz, N.S. Supervised Machine Learning Model For Microrna Expression Data In Cancer. J. Ilmu Komput. Dan Inf. 2017, 10, 108. [Google Scholar] [CrossRef]
| Cancer Type | Benign | Pre-Malignant/ In-Situ (20–25%) [13] | Malignant/Invasive [IDC (80%), ILC (20%)] [13] | |||||
|---|---|---|---|---|---|---|---|---|
| Categories | Fibroadenoma Intraductal papilloma Lipoma | Early Breast Cancer Detection | Molecular Subtypes (St Gallen) | Recurrence/ Metastatic | ||||
| Lubular Carcinoma In-situ (LCIS) | Ductal Carcinoma In-situ (DCIS) | Luminal A | Luminal B (HER2-) | Luminal B (HER2+) | HER2+ Enriched | TNBC | ||
| Cancer/Bio markers [11,12] | ER, PR, HER2 & Ki67 (low < 10%); Germline test BRCA1 & 2 (High risk group) | ER+; PR+; HER2−; Ki67 low (<10–14%); Germline test BRCA1 & 2 (High Risk Group) | ER+; PR−; HER2−; Ki67 high (>14–30%); Germline test BRCA1 & 2 (High Risk Group) | ER+; PR+/−; HER2+; Ki67 high/low; Germline test BRCA1 & 2 (High Risk Group) | ER−; PR−; HER2+; Ki67 high; Germline test BRCA1 & 2 (High Risk Group) | ER−; PR−; HER2−; Ki67 high; (CK 5/6+; EGFR+); Germline test BRCA1 & BRCA2 (High Risk Group) | Metastatic Site: Bone, liver, lungs, brain ESCAT score: I = Good prognosis II = Poor Prognosis | |
| Frequency of cases [14] | 20–25% | 40–50% | 20–30% | 20–30% | 15–20% | 10–20% | 4% | |
| Histological grade (Majority) | Well differentiated (G1) | Moderately differentiated (G2) | Moderately differentiated (G2) | Poorly differentiated (G3) | Poorly differentiated (G3) | Poorly differentiated (G4) | ||
| TNM Stage | NR | I-III | I-III | I-III | I-III | I-III | IV | |
| Prognosis | NR | Good | Intermediate | Intermediate | Poor | Poor | Poor | |
| Response to therapies [11,12,14] | Surgery Breast-conserving surgery (BCS) Radiotherapy Lumpectomy Mastectomy | Endocrine | Endocrine Chemotherapy | Endocrine Chemotherapy Targeted Therapy | Chemotherapy Targeted Therapy | Chemotherapy PARP inhibitors | Chemotherapy CKD4/6 Inhibitor Fulvestrant | |
| Study Name | Year Launched | Study ID | Location | Status |
|---|---|---|---|---|
| Onco-liq: Kit for Breast Cancer Diagnosis. | 2021 | NCT04906330 | Argentina | On-going |
| Prospective Breast Cancer Biobanking (PBCB) | 2020 | NCT04488614 | Norway | On-going |
| Reference | Function/Purpose | Methods | Accuracy of Model |
|---|---|---|---|
| [97] | Cancer Classification | Gradient Boosting | Accuracy 93.59% |
| RF | Accuracy 93.24% | ||
| LR | Accuracy 92.37% | ||
| Passive Aggressive | Accuracy 88.31% | ||
| SGD | Accuracy 90.35% | ||
| SVM | Accuracy 91.54% | ||
| Ridge | Accuracy 83.05% | ||
| Bagging | Accuracy 91.1% | ||
| [98] | Cancer Classification | NB | Accuracy 94.9% |
| [99] | Cancer detection | RF | AUC 99.5–99.9% |
| SVM | AUC 93.8–99.6% | ||
| ANN | Accuracy 97.3% | ||
| KNN | Accuracy 99.2% | ||
| SVM | Accuracy 96.3% | ||
| LR | Accuracy 95.8% | ||
| [100] | Cancer Classification | Tree-based model | NIA |
| [101] | Cancer Classification | DT | Accuracy 99.12% |
| NB | Accuracy 93.86% | ||
| ANN | Accuracy 100% | ||
| DL | Accuracy 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, L.; Aldoghachi, A.F.; Chong, Z.X.; Ho, W.Y.; Yeap, S.K.; Chin, R.J.; Soo, E.Z.X.; Khor, J.F.; Yong, Y.L.; Ling, J.L.; et al. Addressing the Clinical Feasibility of Adopting Circulating miRNA for Breast Cancer Detection, Monitoring and Management with Artificial Intelligence and Machine Learning Platforms. Int. J. Mol. Sci. 2022, 23, 15382. https://doi.org/10.3390/ijms232315382
Ling L, Aldoghachi AF, Chong ZX, Ho WY, Yeap SK, Chin RJ, Soo EZX, Khor JF, Yong YL, Ling JL, et al. Addressing the Clinical Feasibility of Adopting Circulating miRNA for Breast Cancer Detection, Monitoring and Management with Artificial Intelligence and Machine Learning Platforms. International Journal of Molecular Sciences. 2022; 23(23):15382. https://doi.org/10.3390/ijms232315382
Chicago/Turabian StyleLing, Lloyd, Ahmed Faris Aldoghachi, Zhi Xiong Chong, Wan Yong Ho, Swee Keong Yeap, Ren Jie Chin, Eugene Zhen Xiang Soo, Jen Feng Khor, Yoke Leng Yong, Joan Lucille Ling, and et al. 2022. "Addressing the Clinical Feasibility of Adopting Circulating miRNA for Breast Cancer Detection, Monitoring and Management with Artificial Intelligence and Machine Learning Platforms" International Journal of Molecular Sciences 23, no. 23: 15382. https://doi.org/10.3390/ijms232315382
APA StyleLing, L., Aldoghachi, A. F., Chong, Z. X., Ho, W. Y., Yeap, S. K., Chin, R. J., Soo, E. Z. X., Khor, J. F., Yong, Y. L., Ling, J. L., Yan, N. S., & Ong, A. H. K. (2022). Addressing the Clinical Feasibility of Adopting Circulating miRNA for Breast Cancer Detection, Monitoring and Management with Artificial Intelligence and Machine Learning Platforms. International Journal of Molecular Sciences, 23(23), 15382. https://doi.org/10.3390/ijms232315382





