Osteoarthritis as an Umbrella Term for Different Subsets of Humans Undergoing Joint Degeneration: The Need to Address the Differences to Develop Effective Conservative Treatments and Prevention Strategies
Abstract
1. Introduction
1.1. Background
1.2. Purpose of the Review
2. Subsets of Knee Osteoarthritis
2.1. Background
2.2. A Focus on Knee Osteoarthritis Associated with Knee Injury (Post-Traumatic Osteoarthritis; PTOA)
2.3. Metabolic OA
2.4. Post-Menopausal Onset Knee OA in Females
2.5. OA Risks Associated with Growth and Maturation Variations
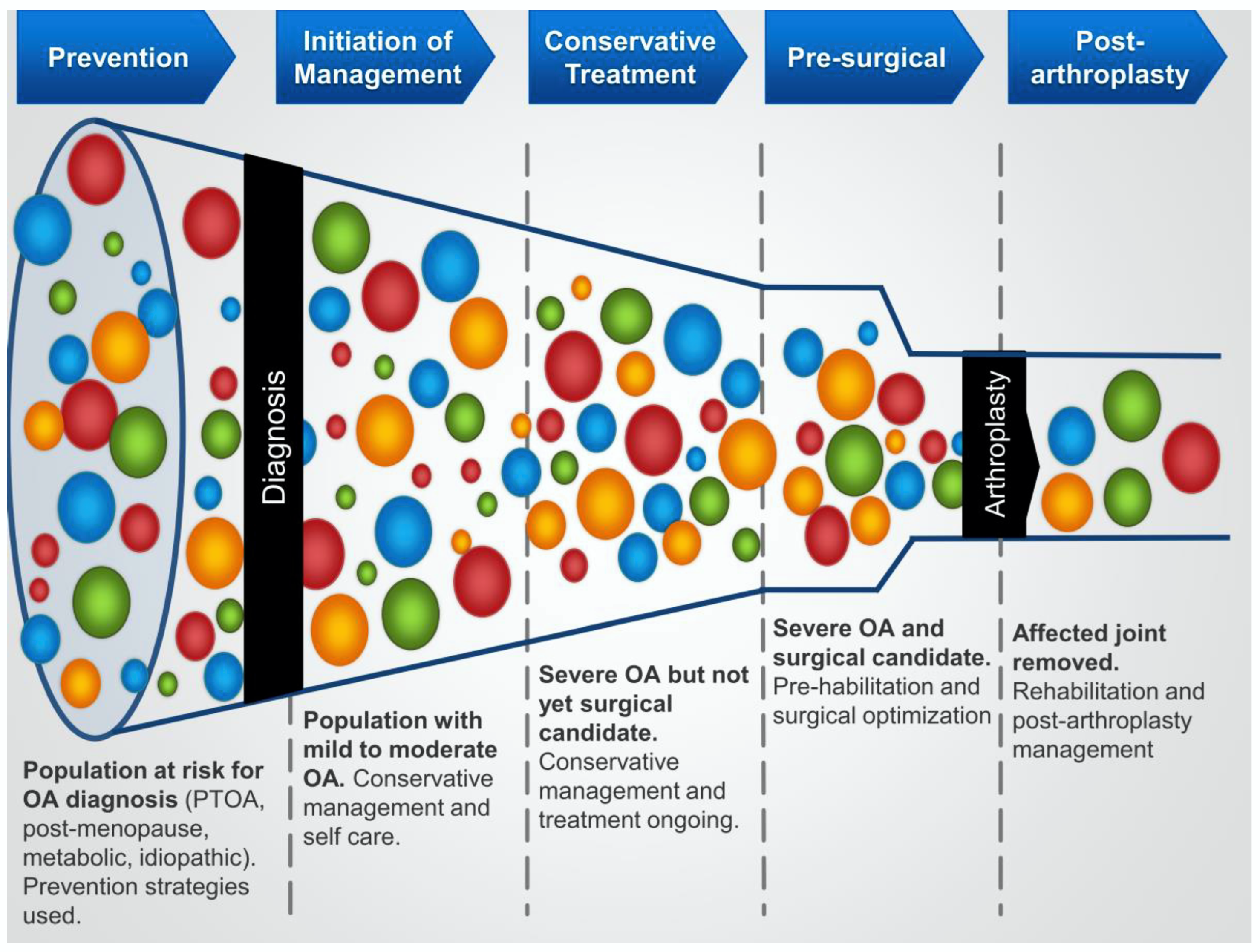
3. Implications from Subtyping for Conservative Treatment for Knee OA
3.1. Background
3.2. OA Phenotyping and Conservative Care
4. The Way Forward
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Y.; Wu, Z.; Zhao, P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. Front. Pharmacol. 2022, 13, 952560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, Y.; Yan, C.H.; Zhang, W. Adipokine signaling pathways in osteoarthritis. Front. Bioeng. Biotechnol. 2022, 10, 865370. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.; Slovek, A.; Miro, P.; Vij, N.; Traube, B.; Lee, C.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Sherman, W.F.; et al. A comprehensive review of viscosupplementation in osteoarthritis of the knee. Orthop. Rev. 2021, 13, 25549. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A.; Nakamura, N. Creating an optimal in vivo environment to enhance outcomes using cell therapy to repair/regenerate injured tissues of the musculoskeletal system. Biomedicines 2022, 10, 1570. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.B.; Shrive, N.G.; Boorman, R.S.; Lo, I.K.Y.; Hart, D.A. New perspectives on bioengineering of joint tissues: Joint adaptation creates a moving target for engineering replacement tissues. Ann. Biomed. Eng. 2004, 32, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Radin, E.L.; Burr, D.B.; Caterson, B.; Fyhrie, D.; Brown, T.; Boyd, R.D. Mechanical determinants of osteoarthrosis. Semin. Arthritis Rheum. 1991, 21 (Suppl. S2), 12–21. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and osteoarthritis. Clin. Rev. Allergy Immunol. 2022. [Google Scholar] [CrossRef]
- Heard, B.J.; Fritzler, M.J.; Wiley, P.; McAllister, J.; Martin, L.; El-Gabalawy, H.; Hart, D.A.; Frank, C.B.; Krawetz, R. Intraarticular and systemic inflammatory profiles may identify patients with osteoarthritis. J. Rheumatol. 2013, 40, 379–387. [Google Scholar] [CrossRef]
- Roskar, S.; Hafner-Bratkovic, I. The role of inflammasomes in osteoarthritis and secondary joint degeneration diseases. Life 2022, 12, 731. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, P.; Rodriguez-Nogales, C.; Jordan, O.; Allemann, E. Combination of mesenchymal cells and bioactive molecules in hydrogels for osteoarthritis treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef]
- Hart, D.A.; Werle, J.; Robert, J.; Kania-Richmond, A. Long wait times for knee and hip total joint replacement in Canada: An isolated health problem, or a symptom of a larger problem? Osteoarthr. Cartil. Open 2021, 3, 100141. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Lech, O.; Nakama, G.Y.; O’Gorman, D.M. Disease-modifying adjunctive therapy (DMAT) in osteoarthritis-the biological effects of a multi-mineral complex, LithoLexal Joint—A review. Clin. Pract. 2021, 11, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.; Platt, B.; Joiner, J.; Jacobs, C.; Conley, C.; Landy, D.; Stone, A.V. An investigation of Google searches for knee osteoarthritis and stem cell therapy: What are patients searching online. HSS J. 2022, 18, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Yu, H.; Huang, X.; Shen, J.; Xiao, G.; Chen, L.; Wang, H.; Xing, L.; Chen, D. Current understanding of osteoarthritis pathogenesis and relevant new approaches. Bone Res. 2022, 10, 60. [Google Scholar] [CrossRef]
- Allen, K.D.; Thomas, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Yuan, C.; Pan, Z.; Zhao, K.; Li, J.; Sheng, Z.; Yao, X.; Liu, H.; Zhang, X.; Yang, Y.; Yu, D. Classification of four distinct osteoarthritis subtypes with a knee joint tissue transcriptome atlas. Bone Res. 2020, 8, 38. [Google Scholar] [CrossRef]
- Vongsirinavarat, M.; Nilmart, P.; Somprasong, S.; Apinokul, B. Identification of knee osteoarthritis disability phenotypes regarding activity limitation: A cluster analysis. BMC Musculoskelet. Disord. 2020, 21, 237. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, Y.X.; Li, J.; Fei, Y.; Guo, H.; Sun, Z.; Lu, J.; Xu, X.; Jiang, Q.; Ikegawa, S.; et al. Molecular classification of knee osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 725568. [Google Scholar] [CrossRef]
- Li, M.; Lan, L.; Luo, J.; Peng, L.; Li, X.; Zhou, X. Identifying the phenotypic and temporal heterogeneity of knee osteoarthritis: Data from the Ostoearthritis Initiative. Front. Public Health 2021, 9, 726140. [Google Scholar] [CrossRef]
- Mulugeta, A.; Eshetie, T.C.; Kassie, G.M.; Erku, D.; Mekonnen, A.; Lumsden, A.; Hypponen, E. Association between metabolically different adiposity subtypes and osteoarthritis: A Mendelian randomization study. Arthritis Care Res. 2022. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Bihlet, A.; Byrjalsen, J.; Andersen, J.R.; Riis, B.J.; Christiansen, C.; Michaelis, M.; Guehring, H.; Ladel, C.; Karsdal, M.A. Serum C-reactive protein metabolite (CRPM) is associated with incidence of contralateral knee osteoarthritis. Sci. Rep. 2021, 11, 6583. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kim, H.A.; Felson, D.T.; Xu, L.; Kim, D.H.; Hewitt, M.C.; Yoshimura, N.; Kawaquichi, H.; Lin, J.; Kan, X.; et al. Radiographic knee osteoarthritis and knee pain: Cross-sectional study from five different racial/ethnic populations. Sci. Rep. 2018, 8, 1364. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Osteoarthritis: Where are we for pain and therapy in 2013? Aust. Fam. Physician 2013, 42, 766–769. [Google Scholar] [PubMed]
- Giordano, R.; Petersen, K.K.-S.; Arendt-Nielsen, L. The link between epigenetics, pain sensitivity and chronic pain. Scand. J. Pain 2022, 22, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Laigaard, J.; Karlsen, A.; Maagaard, M.; Rosenberg, L.K.; Creutzburg, A.; Lunn, T.H.; Mathiesen, O.; Overgaard, S. Perioperative prevention of persistent pain after total hip and knee arthroplasty-protocol for two systematic reviews. Acta Anaesthesiol. Scand. 2022, 66, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Kluger, M.T.; Rice, D.A.; Borotkanics, R.; Lewis, G.N.; Somogyi, A.A.; Barratt, D.T.; Walker, M.; McNair, P.J. Factors associated with persistent opioid use 6–12 months after primary total knee arthroplasty. Anaesthesia 2022, 77, 882–891. [Google Scholar] [CrossRef]
- Klasan, A.; Rice, D.A.; Kluger, M.T.; Borotkanics, R.; McNair, P.J.; Lewis, G.N.; Young, S.W. A combination of high preoperative pain and low radiological grade of arthritis is associated with greater intensity of persistent pain 12 months after total knee arthroplasty. Bone Jt. J. 2022, 104-B, 1202–1208. [Google Scholar] [CrossRef]
- Hart, D.A.; Nakamura, N.; Shrive, N.G. Perspective: Challenges presented for regeneration of heterogeneous musculoskeletal tissues that normally develop in unique biomechanical environments. Front. Bioeng. Biotechnol. 2021, 9, 760273. [Google Scholar] [CrossRef]
- Bapat, S.; Hubbard, D.; Munjal, A.; Hunter, M.; Fulzele, S. Pros and cons of mouse models for studying osteoarthritis. Clin. Transl. Med. 2018, 7, 36. [Google Scholar] [CrossRef]
- Serra, C.I.; Soler, C. Animal models of osteoarthritis in small mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 211–221. [Google Scholar] [CrossRef]
- Hart, D.A.; Martin, C.R.; Scott, M.; Shrive, N.G. The instrumented sheep knee to elucidate insights into osteoarthritis development and progression: A sensitive and reproducible platform to integrated research effects. Clin. Biomech. 2021, 87, 105404. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.M. Animal models of osteoarthritis: Comparisons and key considerations. Vet. Pathol. 2015, 52, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.R.; Viegas, C.A.; Carvallo, P.P. Large animal models for osteochondral regeneration. Adv. Exp. Med. Biol. 2018, 1059, 441–501. [Google Scholar] [CrossRef]
- Vakiel, P.; Shekarforoush, M.; Dennison, C.R.; Scott, M.; Frank, C.B.; Hart, D.A.; Shrive, N.G. Stress measurements on the articular cartilage surface using fiber optic technology and in-vivo gait kinematics. Ann. Biomed. Eng. 2020, 48, 2836–2845. [Google Scholar] [CrossRef]
- Vakiel, P.; Shekarforoush, M.; Dennison, C.R.; Scott, M.; Muench, G.; Hart, D.A.; Shrive, N.G. Mapping stresses on the tibial plateau cartilage in an ovine model using in-vivo gait kinematics. Ann. Biomed. Eng. 2021, 49, 1288–1297. [Google Scholar] [CrossRef]
- Shekarforoush, M.; Vakiel, P.; Scott, M.; Muench, G.; Hart, D.A.; Shrive, N.G. Relative surface velocity of the tibiofemoral joint and its relation to the development of osteoarthritis after joint injury. Ann. Biomed. Eng. 2020, 48, 695–708. [Google Scholar] [CrossRef]
- Richmond, S.A.; Fukuchi, R.K.; Ezzat, A.; Schneider, K.; Schneider, G.; Emery, C.A. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J. Orthop. Sport. Phys. Ther. 2013, 43, 515–524. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Toomey, C.M.; Woodhouse, L.J.; Laremko, J.L.; Nettel-Aquirre, A.; Emery, C.A. Association between MRI-defined osteoarthritis, pain, function and strength 3–10 years following knee joint injury in youth sport. Br. J. Sport. Med. 2018, 52, 934–939. [Google Scholar] [CrossRef]
- Ezzat, A.M.; Brussoni, M.; Masse, L.C.; Barton, C.J.; Emery, C.A. New or recurrent knee injury, physical activity, and osteoarthritis beliefs in a cohort of female athletes 2–3 years after ACL reconstruction and matched healthy peers. Sport. Health 2022, 14, 842–848. [Google Scholar] [CrossRef]
- Evers, B.J.; Van Den Bosch, M.H.J.; Blom, A.B.; van den Kraan, K.S.; Thurlings, R.M. Post-traumatic knee osteoarthritis; the role of inflammation and hemarthrosis on disease progression. Front. Med. 2022, 9, 973870. [Google Scholar] [CrossRef]
- Suarez, T.; Laudani, L.; Giombini, A.; Saraceni, V.M.; Mariani, P.P.; Pigozzi, F.; Macaluso, A. Comparison in joint-position sense and muscle coactivation between anterior cruciate ligament-deficient and healthy individuals. J. Sport Rehabil. 2016, 25, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Arhos, E.K.; Thoma, L.M.; Grindem, H.; Logerstedt, D.; Risberg, M.A.; Snyder-Mackler, L. Association of quadriceps strength symmetry and surgical status with clinical osteoarthrhritis five years after anterior cruciate ligament rupture. Arthritis Care Res. 2022, 74, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Zargi, T.G.; Drobnic, M.; Jkoder, J.; Strazar, K.; Kacin, A. The effects of preconditioning with ischemic exercise on quadriceps femoris muscle atrophy following anterior cruciate ligament reconstruction: A quasi-randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 310–320. [Google Scholar]
- Tourville, T.W.; Voight, T.B.; Choquette, R.H.; Failla, M.J.; Endres, N.K.; Slauterbeck, J.R.; Beynnon, B.D.; Toth, M.J. Skeletal muscle cellular contractile dysfunction after anterior cruciate ligament reconstruction contributes to quadriceps weakness at 6-month follow-up. J. Orthop. Res. 2022, 40, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Jorge, A.; de Sa, D.; Kay, J.; Fu, F.H.; Musahl, V. Anatomic ACL reconstruction reduces risk of post-traumatic osteoarthritis: A systematic review with minimum 10-year follow-up. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 1072–1084. [Google Scholar] [CrossRef]
- Solbak, N.M.; Heard, B.J.; Achari, Y.; Chung, M.; Shrive, N.G.; Frank, C.B.; Hart, D.A. Alterations in Hoffa’s fat pad induced by an inflammatory response following idealized anterior cruciate ligament surgery. Inflamm. Res. 2015, 64, 615–626. [Google Scholar] [CrossRef]
- Heard, B.J.; Barton, K.I.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J. Orthop. Res. 2015, 33, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Heard, B.J.; Solbak, N.M.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. The infrapatellar fat pad is affected by injury induced inflammation in the rabbit knee: Use of dexamethasone to mitigate damage. Inflamm. Res. 2016, 65, 459–470. [Google Scholar] [CrossRef]
- Heard, B.J.; Barton, K.I.; Abubacker, S.; Chung, M.; Martin, C.R.; Schmidt, T.A.; Shrive, N.G.; Hart, D.A. Synovial and cartilage responsiveness to peri-operative hyaluronic acid =/_ dexamethasone administration following a limited injury to the rabbit stifle joint. J. Orthop. Res. 2022, 40, 838–845. [Google Scholar] [CrossRef]
- Barton, K.I.; Heard, B.J.; Sevick, J.L.; Martin, C.R.; Shekarforoush, S.M.M.; Chung, M.; Achari, Y.; Frank, C.B.; Shrive, N.G.; Hart, D.A. Posttraumatic osteoarthritis development and progression in an ovine model of partial anterior cruciate ligament transection and effect of repeated intra-articular methylprednisolone acetate injections on early disease. Am. J. Sport. Med. 2018, 46, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Sieker, J.T.; Ayturk, U.M.; Proffen, B.L.; Weissenberger, M.H.; Kiapour, A.M.; Murray, M.M. Immediate administration of intraarticular triamcinolone acetnide after joint injury modulates molecular outcomes associated with early synovitis. Arthritis Rheumatol. 2016, 68, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Griffin, T.M.; Liu-Bryan, R. Review: Metabolic regulation of inflammation in osteoarthritis. Arthritis Rheumatol. 2017, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Courties, A.; Sellam, J.; Berenbaum, F. Metabolic syndrome-associated osteoarthritis. Curr. Opin. Rheumatol. 2017, 29, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Linares, N.; Eymard, F.; Berenbaum, F.; Houard, X. Role of adipose tissues in osteoarthritis. Curr. Opin. Rheumatol. 2021, 33, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Batushansky, A.; Zhu, S.; Komaravolu, R.K.; South, S.; Mehta-D’souza, P.; Griffin, T.M. Fundamentals of OA: An initiative of osteoarthritis and cartilage. Obesity and metabolic factors in OA. Osteoarthr. Cartil. 2022, 30, 501–515. [Google Scholar] [CrossRef]
- Nemet, M.; Blazin, T.; Milutinovic, S.; Cebovic, T.; Stanojevic, D.; Svorcan, J.Z. Association between metabolic syndrome, its components, and knee osteoarthritis in premenopausal and menopausal women: A pilot study. Cureus 2022, 14, e26726. [Google Scholar] [CrossRef]
- Collins, K.H.; Herzog, W.; MacDonald, G.Z.; Reimer, R.A.; Rios, J.L.; Smith, I.C.; Zernicke, R.F.; Hart, D.A. Obesity, metabolic syndrome, and musculoskeletal disease: Common inflammatory pathways suggest a central role for loss of muscle integrity. Front. Physiol. 2018, 9, 112. [Google Scholar] [CrossRef]
- Collins, K.H.; Hart, D.A.; Seerattan, R.A.; Reimer, R.A.; Herzog, W. High-fat/high-sucrose diet-induced obesity results in joint-specific development of osteoarthritis-like degeneration in a rat model. Bone Jt. Res. 2018, 7, 274–281. [Google Scholar] [CrossRef]
- Rios, J.L.; Bomhof, M.R.; Reimer, R.A.; Hart, D.A.; Collins, K.H.; Herzog, W. Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci. Rep. 2019, 9, 3893. [Google Scholar] [CrossRef]
- Fortuna, R.; Hart, D.A.; Sharkey, K.A.; Schachar, R.A.; Johnston, K.; Reimer, R.A. Effect of a prebiotic supplement on knee joint function, gut microbiota, and inflammation in adults with co-morbid obesity and knee osteoarthritis: Study protocol for a randomized controlled trial. Trials 2021, 22, 255. [Google Scholar] [CrossRef]
- Berenbaum, F.; Walker, C. Osteoarthritis and inflammation: A serious disease with overlapping phenotypic patterns. Postgrad. Med. 2020, 132, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.A. Sex differences in biological systems and the conundrum of menopause: Potential commonalities in post-menopausal disease mechanisms. Int. J. Mol. Sci. 2022, 23, 4119. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hart, D.A.; Enoka, R.M.; Nicolella, D.P.; Resnick, E.; Berkley, K.L.; Sluka, K.A.; Kwoh, C.K.; Tosi, L.L.; O’Conner, M.I.; et al. Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol. Sex Diff. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Stefanyshyn, D.J.; Loitz-Ramage, B.; Hart, D.A.; Ronsky, J.L. Changing hormone levels during the menstrual cycle affect knee laxity and stiffness in healthy female subjects. Am. J. Sport. Med. 2009, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.R.; Boyer, E.H.; Beynnon, B.; Segal, N.A. Pregnancy results in lasting changes in knee joint laxity. PM&R 2019, 11, 117–124. [Google Scholar] [CrossRef]
- Sciore, P.; Frank, C.B.; Hart, D.A. Identification of sex hormone receptors in human and rabbit ligament of the knee by reverse transcription-polymerase chain reaction: Evidence that receptors are present in tissue from both male and female subjects. J. Orthop. Res. 1998, 16, 604–610. [Google Scholar] [CrossRef]
- Liu, S.H.; al-Shaikh, R.; Ponossian, V.; Yang, R.S.; Nelson, S.D.; Soleiman, N.; Fineman, G.A.; Lane, J.M. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. J. Orthop. Res. 1996, 14, 526–533. [Google Scholar] [CrossRef]
- Achari, Y.; Lu, T.; Hart, D.A. Polymorphisms in the promoter regions for human MMP-1 and MMP-13 lead to differential responses to the alpha and beta isoforms of estrogen receptor and their ligand in vitro. Biochim. Biophys. Acta 2008, 1782, 391–400. [Google Scholar] [CrossRef]
- Achari, Y.; Lu, T.; Katzenellenbogen, B.S.; Hart, D.A. Distinct roles for AF-1 and -2 of ER-alpha in regulation of MMP-13 promoter activity. Biochim. Biophys. Acta 2009, 1792, 211–220. [Google Scholar] [CrossRef]
- Lu, T.; Achari, Y.; Sciore, P.; Hart, D.A. Estrogen receptor alpha regulates metalloproteinase-13 promoter activity primarily through the AP-1 transcriptional regulatory site. Biochim. Biophys. Acta 2006, 1762, 719–731. [Google Scholar] [CrossRef]
- Lu, T.; Achari, Y.; Rattner, J.B.; Hart, D.A. Evidence that estrogen receptor beta enhances MMP-13 promoter activity in HIG-cells and that this enhancement can be influenced by ligands and involves specific promoter sites. Biochem. Cell Biol. 2007, 85, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Linkage between muscle and bone: Common catabolic signals resulting in osteoporosis and sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 272–277. [Google Scholar] [CrossRef]
- Agostini, D.; Donati, S.Z.; Lucertini, F.; Annibalini, G.; Gervasi, M.; Marini, C.F.; Piccoli, G.; Stocchi, V.; Barbieri, E.; Sestili, P. Muscle and bone health in postmenopausal women: Role of protein and vitamin D supplementation combined with exercise training. Nutrients 2018, 10, 1103. [Google Scholar] [CrossRef] [PubMed]
- Helmuth, H. Body height, body mass and surface area of the Neanderthals. Z. Für Morphol. Anthropol. 1998, 82, 1–12. [Google Scholar] [CrossRef]
- Quatman, C.E.; Ford, K.R.; Myer, G.D.; Hewett, T.E. Maturation leads to gender differences in landing force and vertical jump performance: A longitudinal study. Am. J. Sport. Med. 2006, 34, 806–813. [Google Scholar] [CrossRef]
- Ford, K.R.; Shapiro, R.; Myere, G.D.; Van Den Bogert, A.J.; Hewett, T.E. Longitudinal sex differences during landing in knee abduction in young athletes. Med. Sci. Sport. Exerc. 2010, 42, 1923–1931. [Google Scholar] [CrossRef]
- Carson, D.W.; Ford, K.R. Sex differences in knee abduction during landing: A systematic review. Sport. Health 2011, 3, 373–382. [Google Scholar] [CrossRef]
- Hewett, T.E. Longitudinal increases in knee abduction moments in females during adolescent growth. Med. Sci. Sport. Exerc. 2015, 47, 2579–2585. [Google Scholar] [CrossRef]
- Hewett, T.E.; Roewer, B.; Ford, K.; Myer, G. Multicenter trial of motion analysis for injury risk prediction: Lessons learned from prospective longitudinal large cohort combined biomechanical -epidemiological studies. Braz. J. Phys. Ther. 2015, 19, 398–409. [Google Scholar] [CrossRef][Green Version]
- De Souza, L.F.; Danielewicz, A.L.; Rech, C.R.; d’Orsi, E.; Mendonca, V.A.; Lacerda, A.C.R.; de Avelar, N.C.P. How much time in sedentary behavior is associated with probable sarcopenia in older adults? Geriatr. Nurs. 2022, 48, 123–127. [Google Scholar] [CrossRef]
- Ollivier, B.; Berger, P.; Depuydt, C.; Vandenneucjer, H. Good long-term survival and patient-reported outcomes after high tibial osteotomy for medial compartment osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 3569–3584. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.C.; Lavoie-Gagne, O.Z.; Knapik, D.M.; Korrapati, A.; Chala, J.; Forsythe, B. Outcomes of distal femoral osteotomy for valgus malalignment: A systematic review and meta-analysis of closing wedge versus opening wedge techniques. Am. J. Sport. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.M.; Pelletier, J.-P.; Abram, F.; Boily, M.; Antoniou, J.; Martineau, P.A.; Morelli, M.; Martel-Pelletier, J. Gait risk factors for disease progression differ between non-traumatic and post-traumatic knee osteoarthritis. Osteoarthr. Cartil. 2021, 29, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Kydd, A.S.R.; Hart, D.A. Efficacy and safety of platelet-rich plasma injections for osteoarthritis. Curr. Treat. Options Rheumatol. 2020, 6, 87–98. [Google Scholar] [CrossRef]
- Vina, E.R.; Tsoukas, P.H.; Abdollahi, S.; Mody, N.; Roth, S.C.; Redford, A.H.; Kwoh, C.K. Racial and ethnic differences in the pharmacologic management of osteoarthritis: Rapid systematic review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221105011. [Google Scholar] [CrossRef]
- Roemer, F.W.; Jarraya, M.; Collins, J.E.; Kwoh, C.K.; Hayashi, D.; Hunter, D.J.; Guermazi, A. Structural phenotypes of knee osteoarthritis: Potential clinical and research relevance. Skeletal. Radiol. 2022. [Google Scholar] [CrossRef]
- Caneiro, J.P.; O’Sullivan, P.B.; Roos, E.M.; Smith, A.J.; Choong, P.; Dowsey, M.; Hunter, D.J.; Kemp, J.; Rodriguez, J.; Lohmander, S.; et al. Three steps to changing the narrative about knee osteoarthritis care: A call to action. Br. J. Sport. Med. 2020, 54, 256–258. [Google Scholar] [CrossRef]
- Overton, C.; Nelson, A.E.; Neogi, T. Osteoarthritis treatment guidelines from six professional societies: Similarities and differences. Rheum. Dis. Clin. N. Am. 2022, 48, 637–657. [Google Scholar] [CrossRef]
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-rich PRP for knee osteoarthritis: Current concepts. J. Clin. Orthop. Trauma 2019, 10 (Suppl. S1), 5179–5182. [Google Scholar] [CrossRef]
- Zhang, W. The powerful placebo effect in osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 118–123. [Google Scholar]
- Fazeli, M.S.; McIntyre, L.; Huang, Y.; Chevalier, X. Intra-articular placebo effect in the treatment of knee osteoarthritis: A survey of the current clinical evidence. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X211066689. [Google Scholar] [CrossRef] [PubMed]
- Vannabouathong, C.; Bhandari, M.; Bedi, A.; Khanna, V.; Yung, P.; Shetty, V.; Khan, M. Nonoperative treatments of knee osteoarthritis: An evaluation of treatment characteristics and the intra-articular placebo effect: A systematic review. JBJS Rev. 2018, 6, e5. [Google Scholar] [CrossRef] [PubMed]
- Thorlund, J.B.; Simic, M.; Pihl, K.; Berthelsen, D.B.; Day, R.; Koes, B.; Juhl, C.B. Similar effects of exercise therapy, nonsteroidal anti-inflammatory drugs, and opioids for knee osteoarthritis pain: A systematic review with network meta-analysis. J. Orthop. Sport. Phys. Ther. 2022, 52, 207–2016. [Google Scholar] [CrossRef] [PubMed]
- Brumat, P.; Kunsic, O.; Novak, S.; Slokar, U.; Psenica, J.; Topolovec, M.; Mihalic, R.; Trebse, R. The surgical treatment of osteoarthritis. Life 2022, 12, 982. [Google Scholar] [CrossRef]
- Hoburg, A.; Niemyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuzma, T.; Widuchowski, W.; et al. Sustained superiority in KOOS subscores after matrix-associated chondrocyte implantation using spheroids compared to microfracture. Knee Surg. Sport. Traumatol. Arthrosc. 2022. [CrossRef]
- Tirtosuharto, H.; Wiratnaya, G.E.; Astawa, P. Adjunctive platelet-rich plasma and hyaluronic acid injection after arthroscopic debridement in Kellgren-Lawrence grade 3 and 4 knee osteoarthritis. World J. Orthop. 2022, 13, 911–920. [Google Scholar] [CrossRef]
- Lu, M.; Jin, Y. Efficacy evaluation of the combined platelet-rich plasma and hyaluronic acid after arthroscopic joint debridement in treating knee osteoarthritis. Scanning 2022, 2022, 6994017. [Google Scholar] [CrossRef]
- Lin, T.; Liu, Z.; Ji, W.; Zhang, P. Effects of knee debridement with flurbiprofen on knee function, inflammatory levels, and bone metabolism activity in patients with knee osteoarthritis. Comput. Math. Methods Med. 2022, 2022, 8031360. [Google Scholar] [CrossRef]
- Yokota, N.; Lyman, S.; Hanai, H.; Shimomura, K.; Ando, W.; Nakamura, N. Clinical safety and effectiveness of adipose-derived stromal cell vs stromal vascular fraction injection for treatment of knee osteoarthritis: 2-year results of parallel single-arm trials. Am. J. Sport. Med. 2022, 50, 2659–2668. [Google Scholar] [CrossRef]
- Kim, K.; Lee, W.S.; Kim, J.H.; Bae, J.K.; Jin, W. Safety and efficacy of the intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritic knee: A 5-year follow-up study. Stem Cells Transl. Med. 2022, 11, 586–596. [Google Scholar] [CrossRef]
- Ferracini, R.; Alessio-Mazzola, M.; Sonzogni, B.; Stambazzi, C.; Ursino, C.; Roato, I.; Mussano, F.; Bistolfi, A.; Furlan, A.; Godio, L.; et al. Age and synovitis affect the results of the treatment of knee osteoarthritis with microfragmented autologous fat tissue. Knee Surg. Sport. Traumatol. Arthrosc. 2022. [Google Scholar] [CrossRef]
- Kwon, D.G.; Kim, M.K.; Jeon, Y.S.; Nam, Y.C.; Park, J.S.; Ryu, D.J. State of the art: The immunomodulatory role of MSCs for osteoarthritis. Int. J. Mol. Sci. 2022, 23, 1618. [Google Scholar] [CrossRef]
- Wang, S.; Lei, B.; Zhang, E.; Gong, P.; Gu, J.; He, L.; Han, L.; Yuan, Z. Targeted therapy for inflammatory diseases with mesenchymal stem cells and their derived oxosome: From basics to clinics. Int. J. Nanomed. 2022, 17, 1757–1781. [Google Scholar] [CrossRef]
- Shimomura, K.; Yasui, Y.; Koizumi, K.; Chijimatsu, R.; Hart, D.A.; Yonetani, Y.; Ando, W.; Nishii, T.; Kanomoto, T.; Horibe, S.; et al. First-in-human pilot study of implantation of a scaffold-free tissue-engineered construct generated from autologous synovial mesenchymal stem cells for repair of knee chondral lesions. Am. J. Sport. Med. 2018, 46, 2384–2393. [Google Scholar] [CrossRef]
- Shimomura, K.; Hamada, H.; Hart, D.A.; Ando, W.; Nishii, T.; Tratting, S.; Neher, S.; Nakamura, N. Histological analysis of cartilage defects repaired with an autologous human stem cell construct 48 weeks postimplantation reveals structural details not detected by T2-mapping MRI. Cartilage 2021, 13 (Suppl. S1), 684S–706S. [Google Scholar] [CrossRef]
- D’Arrigo, D.; Roffi, A.; Cucchiarini, M.; Moretti, M.; Candrian, C.; Filardo, G. Secretome and extracellular vesicles as new biological therapies for knee osteoarthritis: A systematic review. J. Clin. Med. 2019, 8, 1867. [Google Scholar] [CrossRef]
- Tang, S.; Chen, P.; Zhang, H.; Weng, H.; Fang, Z.; Chen, C.; Peng, G.; Gao, H.; Hu, K.; Chen, J.; et al. Comparison of curative effect of human umbilical cord-derived mesenchymal stem cells and their small extracellular vesicles in treatment osteoarthritis. Int. J. Nanomed. 2021, 16, 8185–8202. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Shehabaz, S.; Jeyaraman, N.; Rajendran, R.L.; Hong, C.M.; Nallakumarasamy, A.; Packkyarathinam, R.P.; Sharma, S.; Ranjan, R.; et al. Current understanding of MSC-derived exosomes in the management of knee osteoarthritis. Exp. Cell Res. 2022, 418, 113274. [Google Scholar] [CrossRef]
- Pandey, V.; Madi, S.; Gupta, P. The promising role of autologous and allogenic mesenchymal stromal cells in managing knee osteoarthritis. What is beyond mesenchymal stromal cells? J. Clin. Orthop. Trauma 2022, 26, 101804. [Google Scholar] [CrossRef]
- Phelps, J.; Leonanrd, C.; Shah, S.; Krawetz, R.; Hart, D.A.; Duncan, N.A.; Sen, A. Production of mesenchymal progenitor cell-derived extracellular vesicles in suspension bioreactors for use in articular cartilage repair. Stem Cells Trans. Med. 2022, 11, 73–87. [Google Scholar] [CrossRef]
- Baumbach, L.; Gronne, D.T.; Moller, N.C.; Skou, S.T.; Roos, E.M. Changes in physical activity and the association between pain and physical activity-a longitudinal analysis of 17,454 patients with knee or hip osteoarthritis from the GLA:D registry. Osteoarthr. Cartil. 2022. [Google Scholar] [CrossRef]
- Panoutsopoulou, K.; Zeggini, E. Advances in osteoarthritis genetics. J. Med. Genet. 2013, 50, 715–724. [Google Scholar] [CrossRef]
- Zengini, E.; Finan, C.; Wilkinson, J.M. The genetic epidemiological landscape of hip and knee osteoarthritis: Where are we now and where are we going? J. Rheumatol. 2016, 43, 260–266. [Google Scholar] [CrossRef]
- Cai, Z.; Long, T.; Zhao, Y.; Lin, R.; Wang, Y. Epigenetic regulation in knee osteoarthritis. Front. Genet. 2022, 13, 942982. [Google Scholar] [CrossRef]
- Wang, T.; Liang, Y.; Li, H.; Li, H.; He, Q.; Xue, Y.; Shen, C.; Zhang, C.; Xiang, J.; Ding, J.; et al. Single nucleotide polymorphisms and osteoarthritis: An overview and a meta-analysis. Medicine 2016, 95, e2811. [Google Scholar] [CrossRef]
- Lee, Y.R.; Briggs, M.T.; Condina, M.R.; Puddy, H.; Anderson, P.H.; Hoffmann, P.; Kuliwaba, J.S. Mass spectrometry imaging as a potential tool to investigate human osteoarthritis at the tissue level. Int. J. Mol. Med. 2020, 21, 6414. [Google Scholar] [CrossRef]
- Timur, U.T.; Jahr, H.; Anderson, J.; Green, D.C.; Means, P.J.; Smagul, A.; van Rhijn, L.W.; Peffers, M.J.; Welting, T.J.M. Identification of tissue-dependent proteins in knee OA synovial fluid. Osteoarthr. Cartil. 2021, 29, 124–133. [Google Scholar] [CrossRef]
- Brophy, R.H.; Cai, L.; Zhang, Q.; Townsend, R.T.; Rai, M.F. Proteomic profile of synovial fluid in patients with anterior cruciate ligament tears. Am. J. Sport. Med. 2022, 50, 2935–2943. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Runhaar, J.; Bierma-Zeinstra, S.; Roos, E.M. A lifespan approach to osteoarthritis prevention. Osteoarthr. Cartil. 2021, 29, 1638–1653. [Google Scholar] [CrossRef]
- Roos, E.M.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 92–101. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Roos, E.M. A pragmatic approach to prevent post-traumatic osteoarthritis after sport or exercise-related joint injury. Best Pract. Res. Clin. Rheumatol. 2019, 33, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Peat, G.; Yu, D.; Gronne, D.T.; Marshall, M.; Skou, S.T.; Roos, E.M. Do patients with intersectional disadvantage have poorer outcomes form osteoarthritis management programs? A tapered balancing study of patient outcomes from the good life with osteoarthritis in Denmark program. Arthritis Care Res. 2022. [Google Scholar] [CrossRef] [PubMed]
| Subtype | Initiation | Mechanical Instability a | Inflammation Detected b |
|---|---|---|---|
| PTOA | Joint Injury | YES | YES (due to injury/repair) |
| Metabolic | Obesity | Not Initially | YES (metabolic syndrome) |
| Post-Menopausal | Menopause | Not Initially | YES (with tissue degeneration) |
| Growth/Maturation c | Age Transitions | Not Initially | YES (with tissue degeneration) |
| (malalignment, muscle) | |||
| Idiopathic d (cause?) | Aging?/Genetics? | Not Initially | YES (with tissue degeneration) |
| All likely have some genetic risk associated with subtype and all present with pain and some disability | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hart, D.A. Osteoarthritis as an Umbrella Term for Different Subsets of Humans Undergoing Joint Degeneration: The Need to Address the Differences to Develop Effective Conservative Treatments and Prevention Strategies. Int. J. Mol. Sci. 2022, 23, 15365. https://doi.org/10.3390/ijms232315365
Hart DA. Osteoarthritis as an Umbrella Term for Different Subsets of Humans Undergoing Joint Degeneration: The Need to Address the Differences to Develop Effective Conservative Treatments and Prevention Strategies. International Journal of Molecular Sciences. 2022; 23(23):15365. https://doi.org/10.3390/ijms232315365
Chicago/Turabian StyleHart, David A. 2022. "Osteoarthritis as an Umbrella Term for Different Subsets of Humans Undergoing Joint Degeneration: The Need to Address the Differences to Develop Effective Conservative Treatments and Prevention Strategies" International Journal of Molecular Sciences 23, no. 23: 15365. https://doi.org/10.3390/ijms232315365
APA StyleHart, D. A. (2022). Osteoarthritis as an Umbrella Term for Different Subsets of Humans Undergoing Joint Degeneration: The Need to Address the Differences to Develop Effective Conservative Treatments and Prevention Strategies. International Journal of Molecular Sciences, 23(23), 15365. https://doi.org/10.3390/ijms232315365





