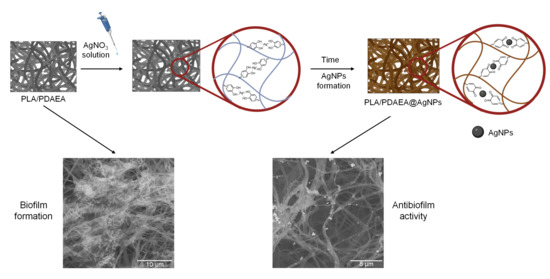
Developing Antibiofilm Fibrillar Scaffold with Intrinsic Capacity to Produce Silver Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
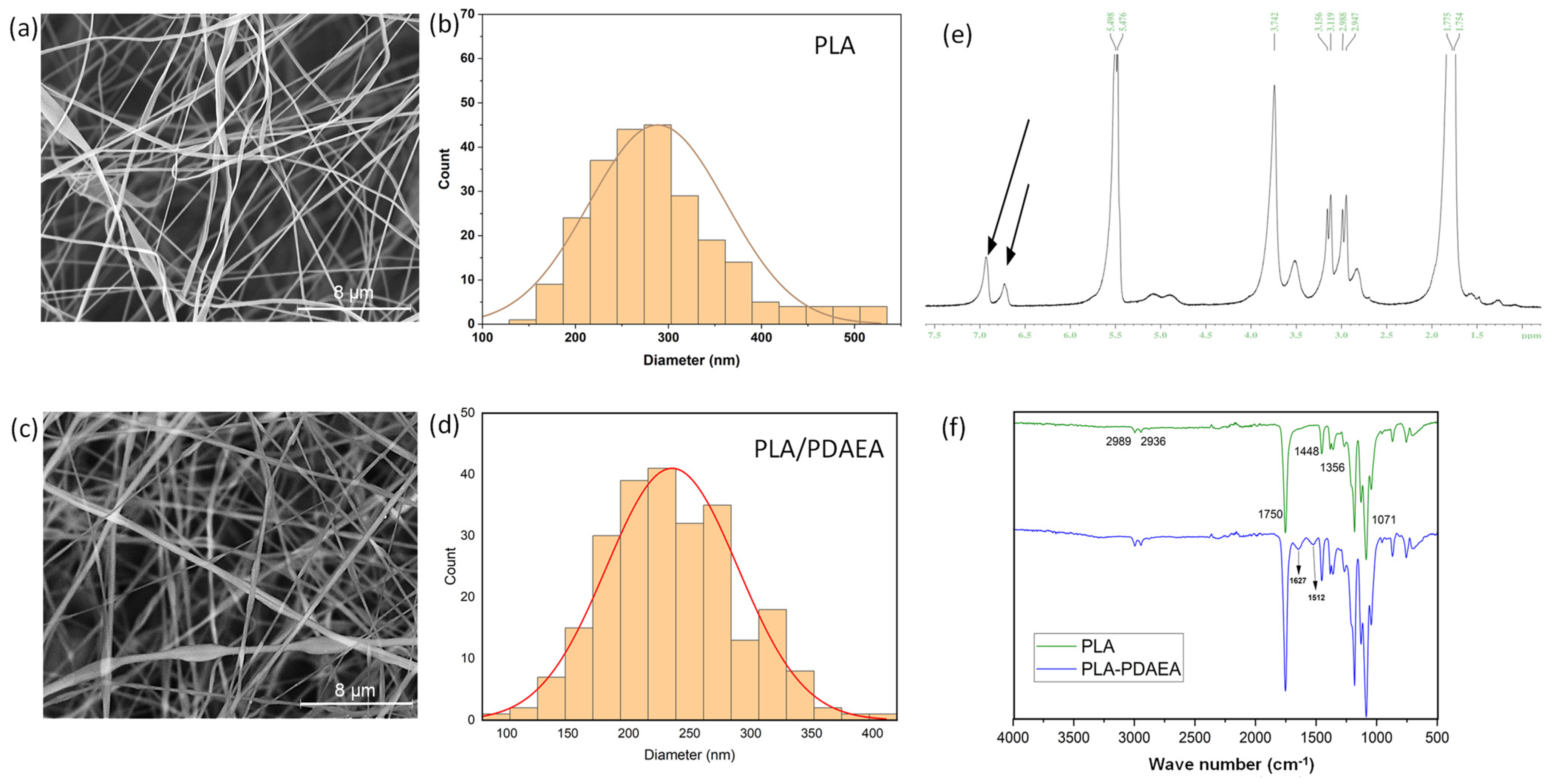
2.1. Synthesis and Characterization of PDAEA and PLA/PDAEA Electrospun Scaffolds
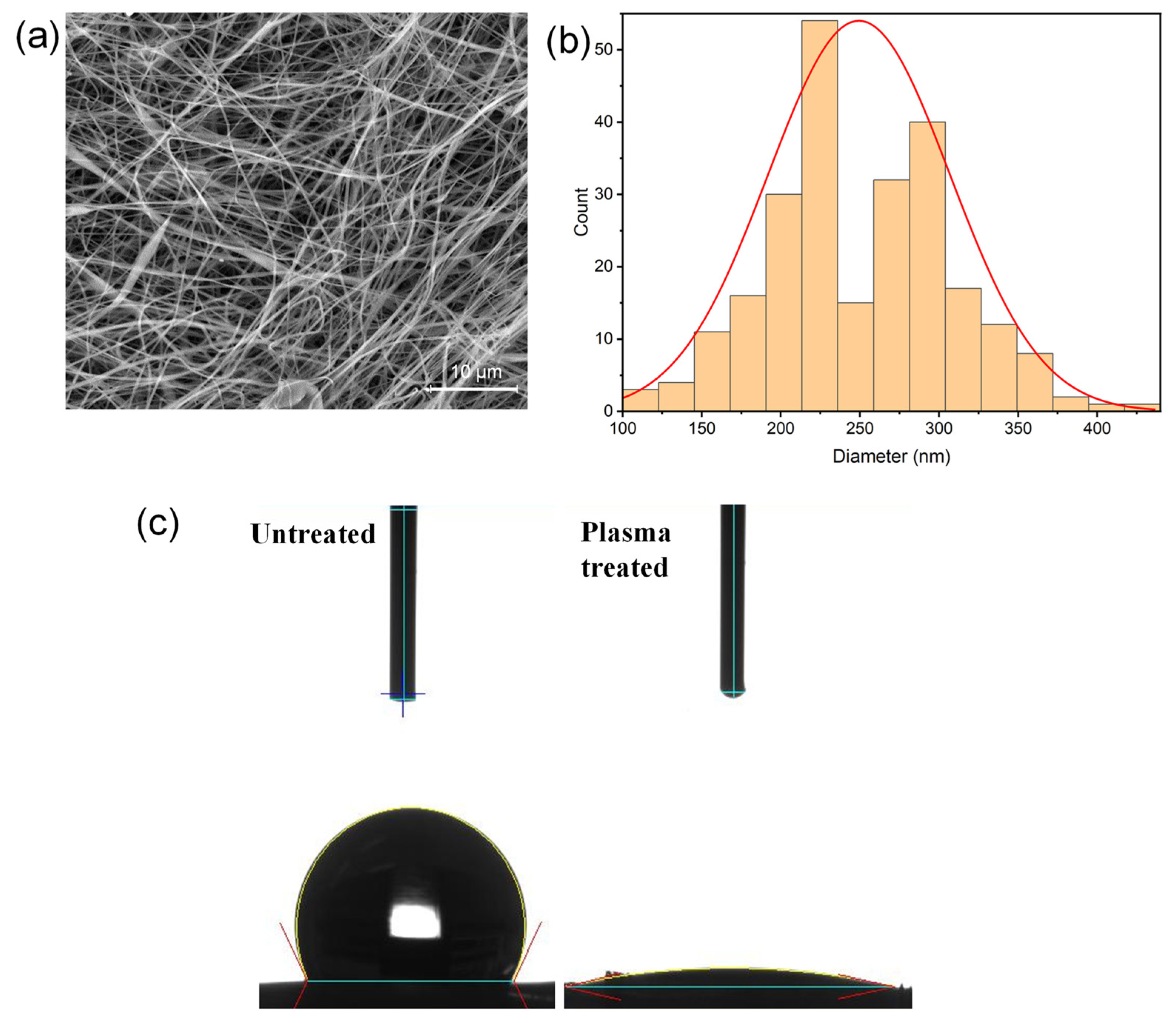
2.2. Plasma Treatment of the Scaffolds
2.3. SEM Analyses and Contact Angle Measurements on Plasma-Treated Scaffolds
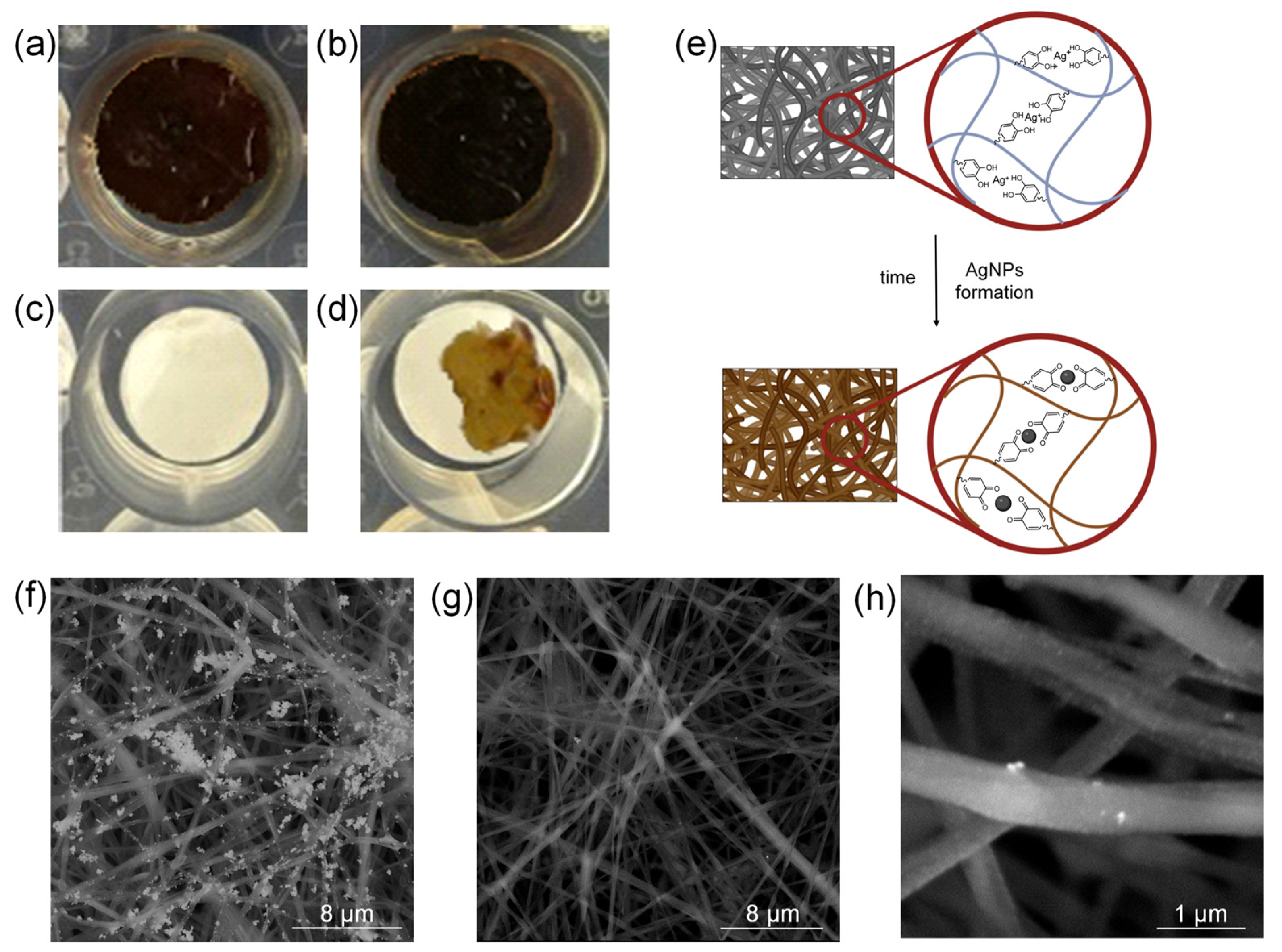
2.4. AgNPs In Situ Production
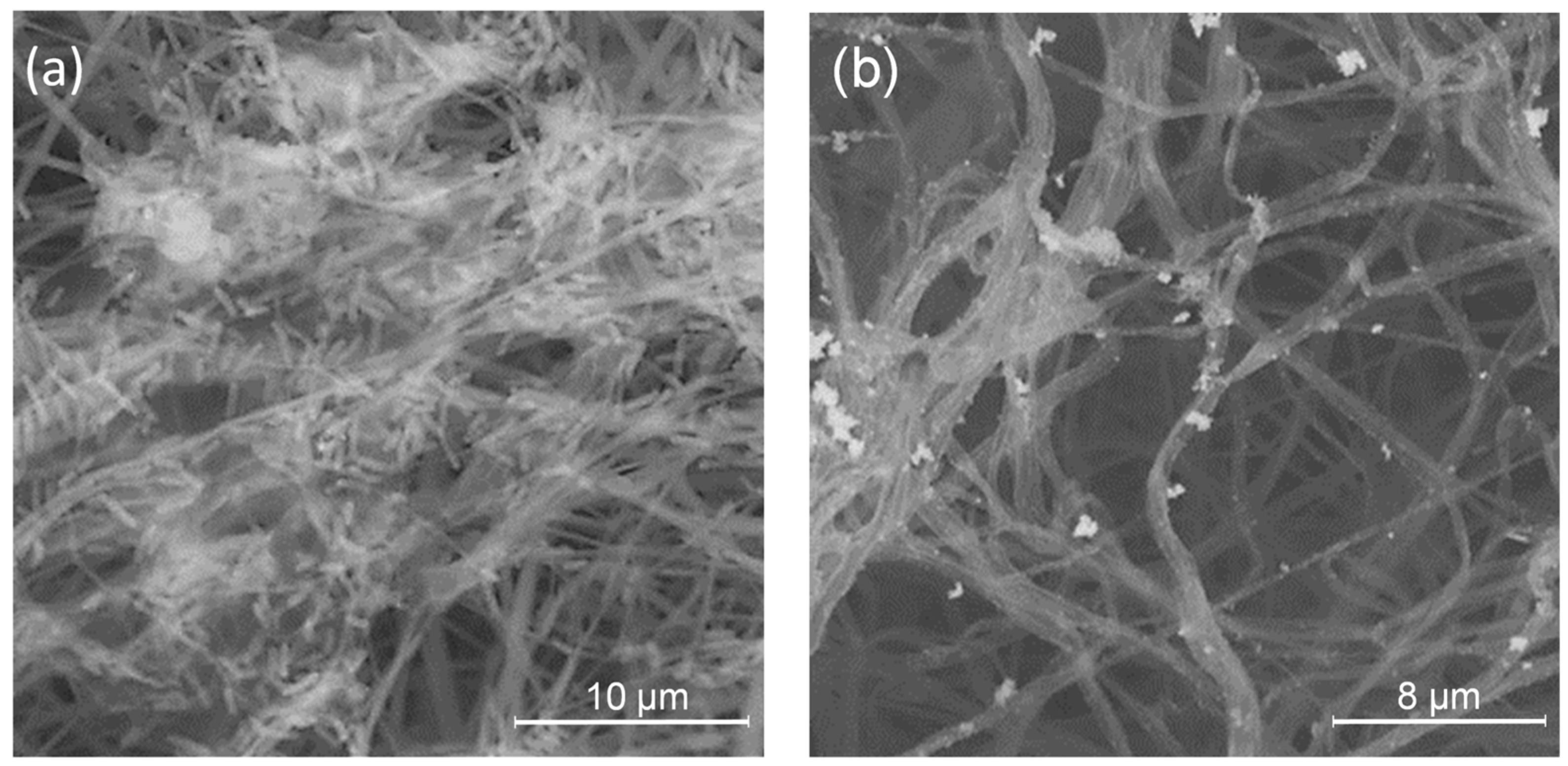
2.5. PLA/PDAEA@AgNPs Scaffolds Morphological Characterization
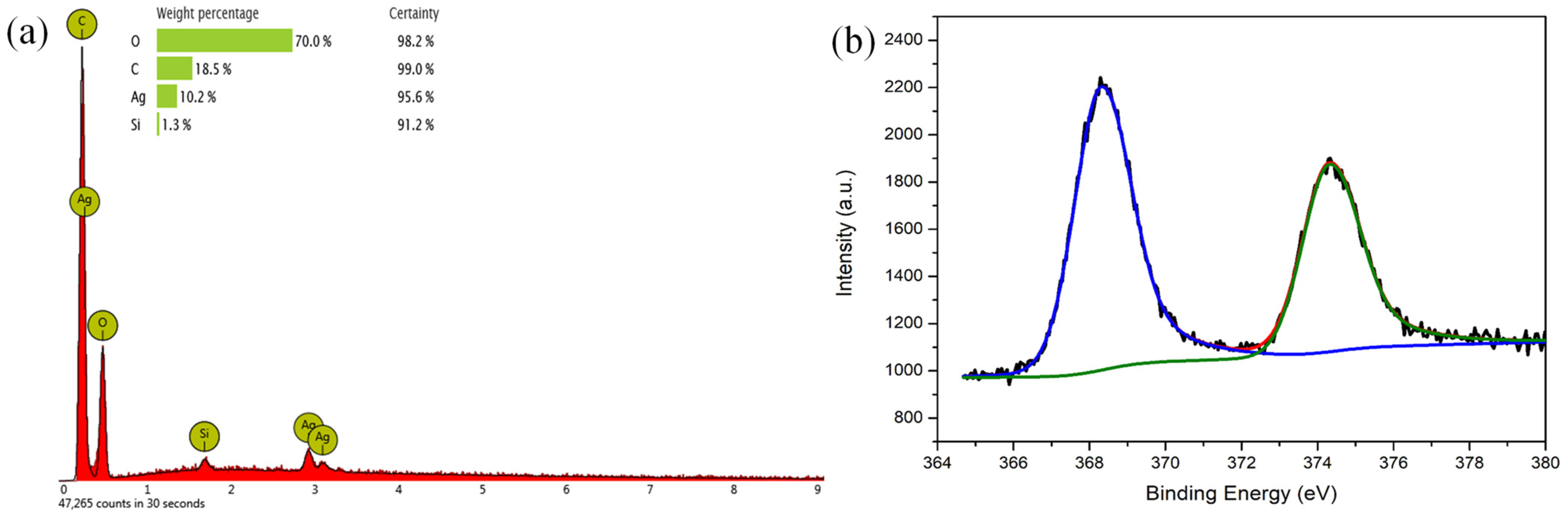
2.6. PLA/PDAEA@AgNPs Scaffolds Surface Characterization
2.7. Ag Quantification on PLA/PDAEA@AgNPs Scaffolds
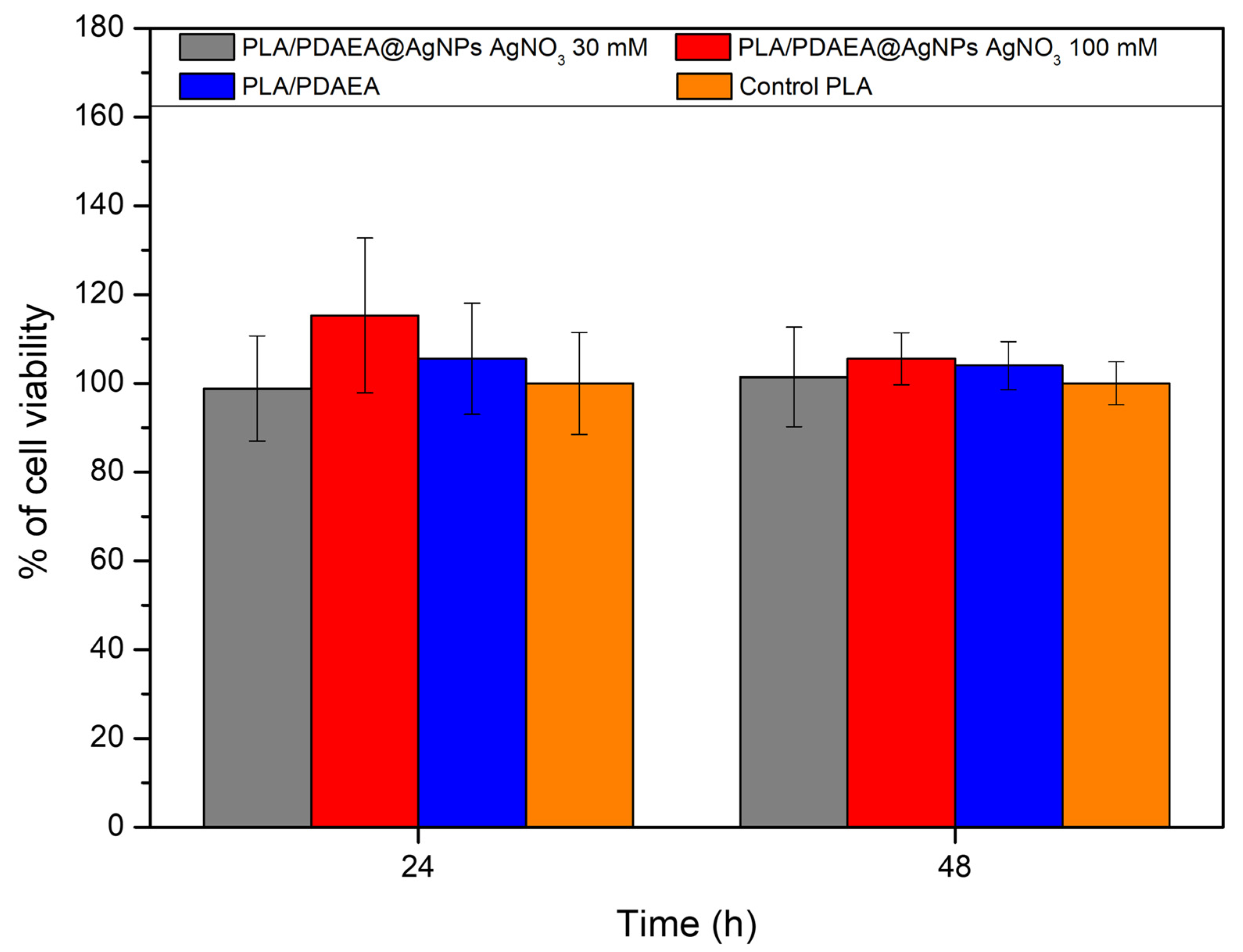
2.8. In Vitro Cytocompatibility Studies
2.9. Inhibition of Bacterial Biofilm Formation of PLA/PDAEA@AgNPs Scaffolds
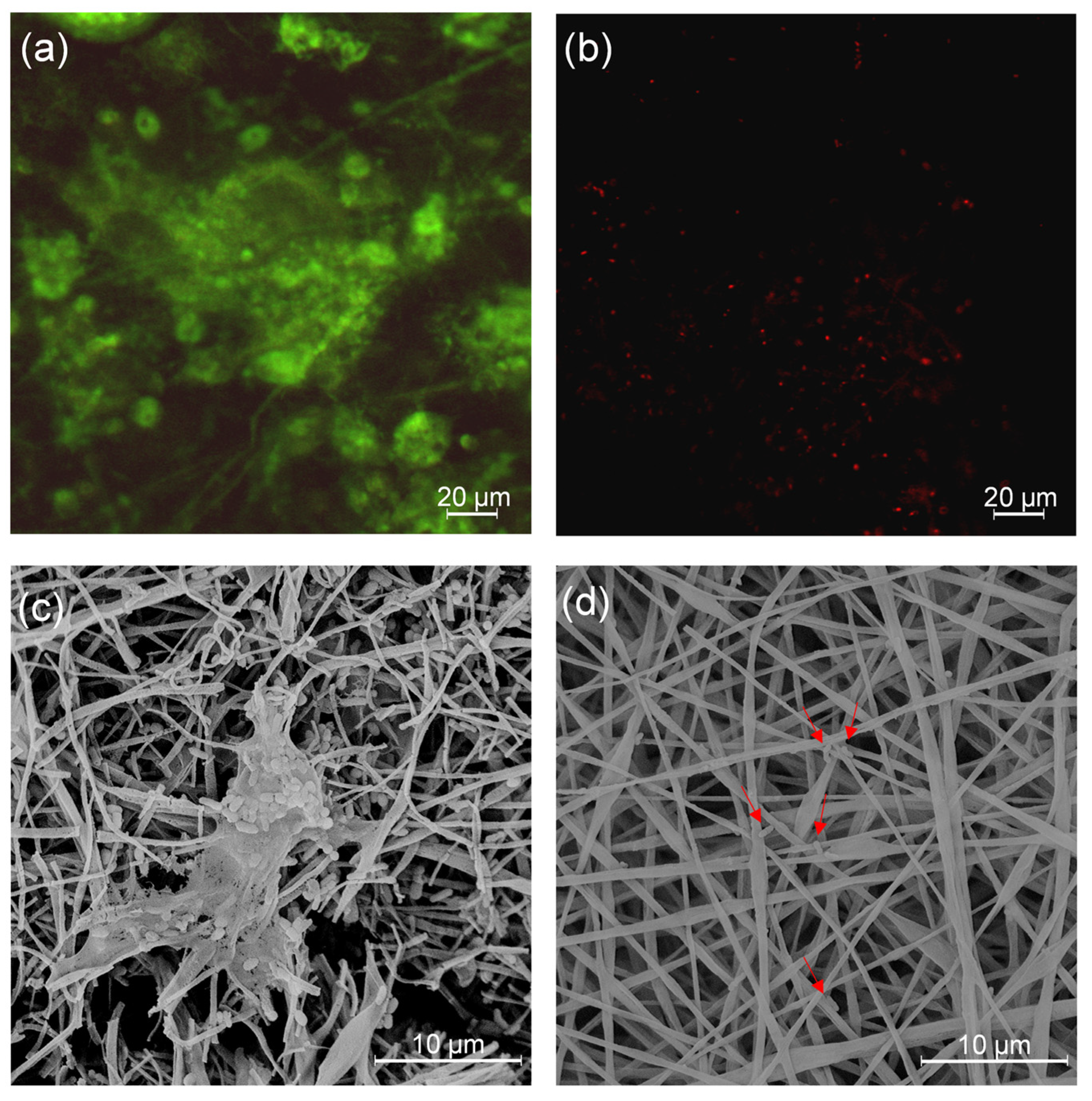
2.10. Antibiofilm Activity Evaluation
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Synthesis of PDAEA
3.4. Production of PLA/PDAEA Electrospun Scaffold
3.5. Plasma Treatment of Scaffolds
3.6. Production and Characterization of PLA/PDAEA@AgNPs Scaffolds
3.7. Ag Quantification on PLA/PDAEA@AgNPs Scaffolds
3.8. In Vitro Cytocompatibility Studies
3.9. Inhibition of Bacterial Biofilm Formation of PLA/PDAEA@AgNPs Scaffolds
3.10. Antibiofilm Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blaskovich, M.A.T. The Fight Against Antimicrobial Resistance Is Confounded by a Global Increase in Antibiotic Usage. ACS Infect. Dis. 2018, 4, 868–870. [Google Scholar] [CrossRef]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Pesset, C.M.; Fonseca, C.O.d.; Antunes, M.; dos Santos, A.L.L.; Teixeira, I.M.; Ribeiro, T.A.N.; Sachs, D.; Penna, B. Characterizing biofilm formation of Staphylococcus pseudintermedius in different suture materials. Microb. Pathog. 2022, 172, 105796. [Google Scholar] [CrossRef] [PubMed]
- Turtiainen, J.; Hakala, T.; Hakkarainen, T.; Karhukorpi, J. The Impact of Surgical Wound Bacterial Colonization on the Incidence of Surgical Site Infection After Lower Limb Vascular Surgery: A Prospective Observational Study. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.; Catania, V.; Palumbo, F.S.; Fiorica, C.; Schillaci, D.; Pitarresi, G.; Giammona, G. Photothermal nanofibrillar membrane based on hyaluronic acid and graphene oxide to treat Staphylococcus aureus and Pseudomonas aeruginosa infected wounds. Int. J. Biol. Macromol. 2022, 214, 470–479. [Google Scholar] [CrossRef]
- Biscari, G.; Pitarresi, G.; Fiorica, C.; Schillaci, D.; Catania, V.; Salvatore Palumbo, F.; Giammona, G. Near-infrared light-responsive and antibacterial injectable hydrogels with antioxidant activity based on a Dopamine-functionalized Gellan Gum for wound healing. Int. J. Pharm. 2022, 627, 122257. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Pitarresi, G.; Palumbo, F.S.; Catania, V.; Schillaci, D.; Mauro, N.; Fiorica, C.; Giammona, G. Fabrication of silver nanoparticles by a diethylene triamine-hyaluronic acid derivative and use as antibacterial coating. Carbohydr. Polym. 2022, 295, 119861. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Biscari, G.; Martorana, A.; Calà, C.; Maida, C.M.; Giammona, G. Ciprofloxacin releasing gellan gum/polydopamine based hydrogels with near infrared activated photothermal properties. Int. J. Pharm. 2021, 610, 121231. [Google Scholar] [CrossRef]
- Das, C.G.A.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Govindaraju, K.; Joselin, J.M.; Baalamurugan, J. Antibacterial activity of silver nanoparticles (biosynthesis): A short review on recent advances. Biocatal. Agric. Biotechnol. 2020, 27, 101593. [Google Scholar] [CrossRef]
- Kannan, K.; Govindaraj, M.; Rajeswari, B.; Vijayakumar, K. Green synthesis silver nanoparticles using medicinal plant and antimicrobial activity against human pathogens. Mater. Today Proc. 2022, 69, 1346–1350. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.C.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Kim, K.D.; Han, D.N.; Kim, H.T. Optimization of experimental conditions based on the Taguchi robust design for the formation of nano-sized silver particles by chemical reduction method. Chem. Eng. J. 2004, 104, 55–61. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.W.; Hahm, D.H. Lignin-mediated green synthesis of AgNPs in carrageenan matrix for wound dressing applications. Int. J. Biol. Macromol. 2020, 159, 859–869. [Google Scholar] [CrossRef]
- Yang, X.; Jia, M.; Li, Z.; Ma, Z.; Lv, J.; Jia, D.; He, D.; Zeng, R.; Luo, G.; Yu, Y. In-situ synthesis silver nanoparticles in chitosan/Bletilla striata polysaccharide composited microneedles for infected and susceptible wound healing. Int. J. Biol. Macromol. 2022, 215, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, X.; Huang, T.; Ren, Y.; Gong, W.; Guo, Y.; Wang, J.; Tu, Q. Preparation of sodium hyaluronate/dopamine/AgNPs hydrogel based on the natural eutetic solvent as an antibaterial wound dressing. Int. J. Biol. Macromol. 2021, 191, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.; Pitarresi, G.; Palumbo, F.S.; Fiorica, C.; Catania, V.; Schillaci, D.; Giammona, G. An asymmetric electrospun membrane for the controlled release of ciprofloxacin and FGF-2: Evaluation of antimicrobial and chemoattractant properties. Mater. Sci. Eng. C 2021, 123, 112001. [Google Scholar] [CrossRef] [PubMed]
- Aragón, J.; Costa, C.; Coelhoso, I.; Mendoza, G.; Aguiar-Ricardo, A.; Irusta, S. Electrospun asymmetric membranes for wound dressing applications. Mater. Sci. Eng. C 2019, 103, 109822. [Google Scholar] [CrossRef] [PubMed]
- Miceli, G.C.; Palumbo, F.S.; Bonomo, F.P.; Zingales, M.; Licciardi, M. Polybutylene Succinate Processing and Evaluation as a Micro Fibrous Graft for Tissue Engineering Applications. Polymers 2022, 14, 4486. [Google Scholar] [CrossRef]
- Wang, F.; Hu, S.; Jia, Q.; Zhang, L. Advances in Electrospinning of Natural Biomaterials for Wound Dressing. J. Nanomater. 2020, 2020, 8719859. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and ε-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the Field of Electrospinning for Tissue Engineering Applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications —A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Xu, H.; Shen, M.; Shang, H.; Xu, W.; Zhang, S.; Yang, H.R.; Zhou, D.; Hakkarainen, M. Osteoconductive and Antibacterial Poly(lactic acid) Fibrous Membranes Impregnated with Biobased Nanocarbons for Biodegradable Bone Regenerative Scaffolds. Ind. Eng. Chem. Res. 2021, 60, 12021–12031. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, M.; Escobar-Barrios, V.A.; Pozos-Guillén, A.; Escobar-García, D.M. RGD-functionalization of PLA/starch scaffolds obtained by electrospinning and evaluated in vitro for potential bone regeneration. Mater. Sci. Eng. C 2019, 96, 798–806. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef]
- Dong, H.; Li, D.; Mao, D.; Bai, N.; Chen, Y.; Li, Q. Enhanced performance of magnesium alloy for drug-eluting vascular scaffold application. Appl. Surf. Sci. 2018, 435, 320–328. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Drago, S.E.; Nicosia, A.; Costa, S.; Cavallaro, G.; Giammona, G. Hyaluronic acid dressing of hydrophobic carbon nanodots: A self-assembling strategy of hybrid nanocomposites with theranostic potential. Carbohydr. Polym. 2021, 267, 118213. [Google Scholar] [CrossRef] [PubMed]
- Drago, S.E.; Craparo, E.F.; Luxenhofer, R.; Cavallaro, G. Development of polymer-based nanoparticles for zileuton delivery to the lung: PMeOx and PMeOzi surface chemistry reduces interactions with mucins. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102451. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Visai, L.; Renò, F.; Cangini, I.; Rizzi, M.; Poggi, A.; Montanaro, L.; Rimondini, L.; Arciola, C.R. Bacterial adhesion to poly-(d,l)lactic acid blended with vitamin E: Toward gentle anti-infective biomaterials. J. Biomed. Mater. Res. Part A 2015, 103, 1447–1458. [Google Scholar] [CrossRef]
- Mainil-Varlet, P.; Hauke, C.; Maquet, V.; Printzen, G.; Arens, S.; Schaffner, T.; Jérô, R.; Perren, S.; Schlegel, U. Polylactide implants and bacterial contamination: An animal study. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2000, 54, 335–343. [Google Scholar] [CrossRef]
- Popelka, A.; Abdulkareem, A.; Mahmoud, A.A.; Nassr, M.G.; Al-Ruweidi, M.K.A.A.; Mohamoud, K.J.; Hussein, M.K.; Lehocky, M.; Vesela, D.; Humpolíček, P.; et al. Antimicrobial modification of PLA scaffolds with ascorbic and fumaric acids via plasma treatment. Surf. Coat. Technol. 2020, 400, 126216. [Google Scholar] [CrossRef]
- Xiang, J.; Zhu, R.; Lang, S.; Yan, H.; Liu, G.; Peng, B. Mussel-inspired immobilization of zwitterionic silver nanoparticles toward antibacterial cotton gauze for promoting wound healing. Chem. Eng. J. 2021, 409, 128291. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Zhang, W.; Hickey, M.E.; Lin, Z.; Tu, Q.; Wang, J. In situ assembly of well-dispersed Ag nanoparticles on the surface of polylactic acid-Au@polydopamine nanofibers for antimicrobial applications. Colloids Surf. B Biointerfaces 2019, 184, 110506. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Lee, S.W.; Ryu, J.H.; Do, M.J.; Namkoong, E.; Lee, H.; Park, K. NiCHE Platform: Nature-Inspired Catechol-Conjugated Hyaluronic Acid Environment Platform for Salivary Gland Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Gowda, A.H.J.; Bu, Y.; Kudina, O.; Krishna, K.V.; Bohara, R.A.; Eglin, D.; Pandit, A. Design of tunable gelatin-dopamine based bioadhesives. Int. J. Biol. Macromol. 2020, 164, 1384–1391. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Ghobeira, R.; Esbah Tabaei, P.S.; Morent, R.; De Geyter, N. Chemical characterization of plasma-activated polymeric surfaces via XPS analyses: A review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Meyer-Plath, A.A.; Schröder, K.; Finke, B.; Ohl, A. Current trends in biomaterial surface functionalization—Nitrogen-containing plasma assisted processes with enhanced selectivity. Vacuum 2003, 71, 391–406. [Google Scholar] [CrossRef]
- Mauro, N.; Scialabba, C.; Pitarresi, G.; Giammona, G. Enhanced adhesion and in situ photothermal ablation of cancer cells in surface-functionalized electrospun microfiber scaffold with graphene oxide. Int. J. Pharm. 2017, 526, 167–177. [Google Scholar] [CrossRef]
- Sumesh, E.; Bootharaju, M.S.; Anshup; Pradeep, T. A practical silver nanoparticle-based adsorbent for the removal of Hg2+ from water. J. Hazard. Mater. 2011, 189, 450–457. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Hall, R.E.; Bender, G.; Marquis, R.E. In vitro effects of low intensity direct current generated silver on eukaryotic cells. J. Oral Maxillofac. Surg. 1988, 46, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Osborne, O.J.; Lin, S.; Chang, C.H.; Ji, Z.; Yu, X.; Wang, X.; Lin, S.; Xia, T.; Nel, A.E. Organ-Specific and Size-Dependent Ag Nanoparticle Toxicity in Gills and Intestines of Adult Zebrafish. ACS Nano 2015, 9, 9573–9584. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 7, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Sekkarapatti Ramasamy, M.; Bhaskar, R.; Narayanan, K.B.; Purohit, S.D.; Park, S.S.; Manikkavel, A.; Kim, B.; Han, S.S. Combination of polydopamine and carbon nanomaterials coating enhances the piezoelectric responses and cytocompatibility of biodegradable PLLA nanofiber scaffolds for tissue engineering applications. Mater. Today Commun. 2022, 33, 104659. [Google Scholar] [CrossRef]
- Barroca, N.; Marote, A.; Vieira, S.I.; Almeida, A.; Fernandes, M.H.V.; Vilarinho, P.M.; da Cruz e Silva, O.A.B. Electrically polarized PLLA nanofibers as neural tissue engineering scaffolds with improved neuritogenesis. Colloids Surf. B Biointerfaces 2018, 167, 93–103. [Google Scholar] [CrossRef]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Jers, C.; Joshi, A.S.; Garnaes, J.; Mijakovic, I. Silver nanoparticles produced from Cedecea sp. exhibit antibiofilm activity and remarkable stability. Sci. Rep. 2021, 11, 12619. [Google Scholar] [CrossRef]
- Giammona, G.; Carlisi, B.; Cavallaro, G.; Pitarresi, G.; Spampinato, S. A new water-soluble synthetic polymer, α,β-polyasparthydrazide, as potential plasma expander and drug carrier. J. Control. Release 1994, 29, 63–72. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cabibbo, M.; Scialabba, C.; Giammona, G.; Cavallaro, G. Inhalable Formulation Based on Lipid-Polymer Hybrid Nanoparticles for the Macrophage Targeted Delivery of Roflumilast. Biomacromolecules 2022, 23, 3439–3451. [Google Scholar] [CrossRef]
- Pitarresi, G.; Fiorica, C.; Palumbo, F.S.; Calascibetta, F.; Giammona, G. Polyaspartamide-polylactide electrospun scaffolds for potential topical release of Ibuprofen. J. Biomed. Mater. Res. Part A 2012, 100, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Ventura, C.A.; Pitarresi, G.; Giammona, G. Polyaspartamide based hydrogel with cell recruitment properties for the local administration of hydrophobic anticancer drugs. React. Funct. Polym. 2019, 138, 9–17. [Google Scholar] [CrossRef]
- Gong, C.; Lu, C.; Li, B.; Shan, M.; Wu, G. Injectable dopamine-modified poly(α,β-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J. Biomed. Mater. Res. Part A 2017, 105, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Plescia, F.; Venturella, F.; D’Anneo, A.; Catania, V.; Gargano, M.L.; Polito, G.; Schillaci, D.; Palumbo Piccionello, A.; Lauricella, M.; Venturella, G.; et al. Phytochemical-rich extracts of Helianthemum lippii possess antimicrobial, anticancer, and anti-biofilm activities. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 1–11. [Google Scholar] [CrossRef]
- Plescia, F.; Venturella, F.; Lauricella, M.; Catania, V.; Polito, G.; Schillaci, D.; Piccionello, A.P.; Giuseppe, D.; D’Anneo, A.; Raffa, D. Chemical composition, cytotoxic effects, antimicrobial and antibiofilm activity of Artemisia arborescens (Vaill.) L. growing wild in the province of Agrigento, Sicily, Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 1–10. [Google Scholar] [CrossRef]
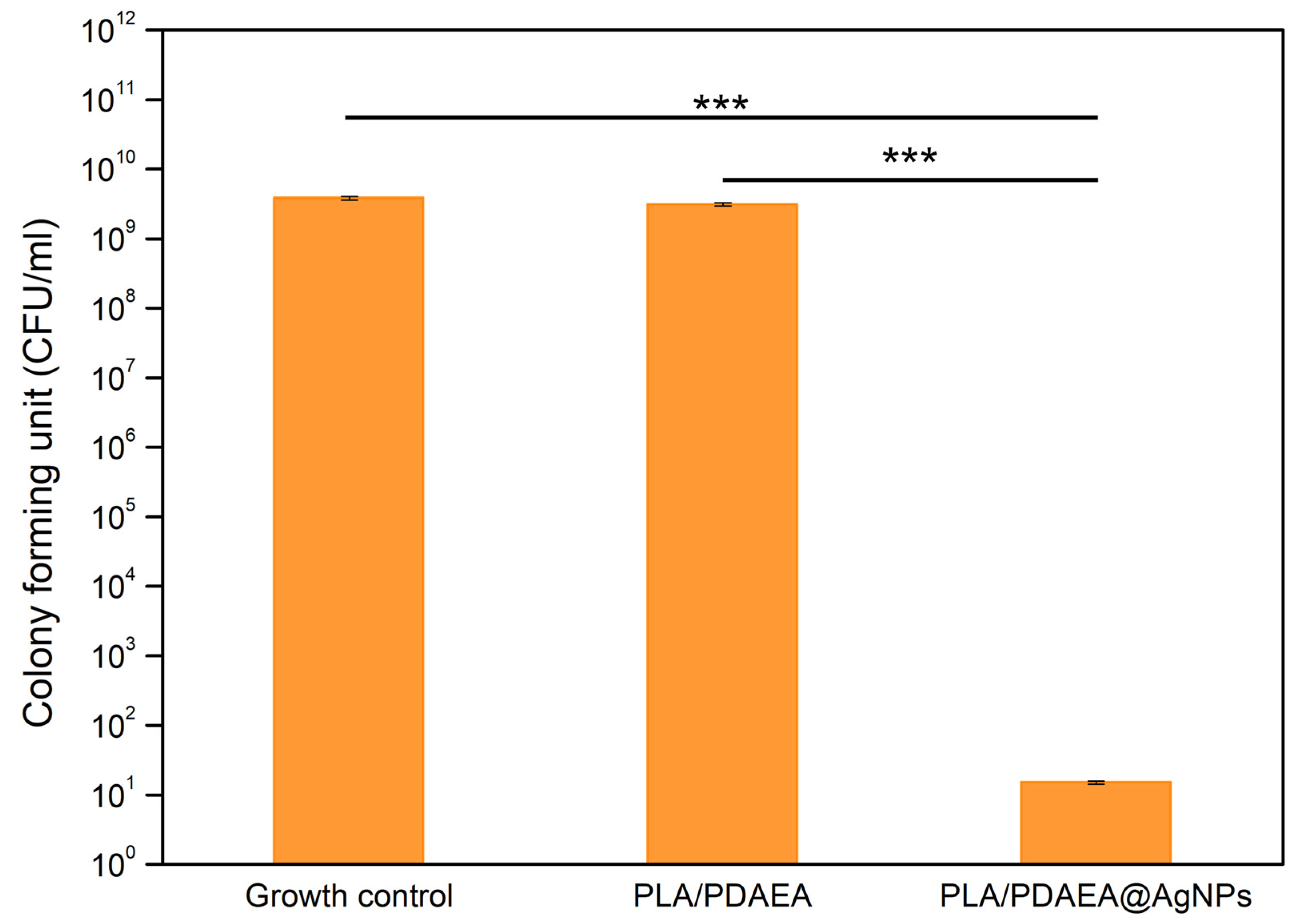

| Planktonic Growth | Optical Density Mean Values ± SD |
|---|---|
| Growth control | 0.391 (±0.049) |
| PLA/PDAEA | 0.403 (±0.013) |
| PLA/PDAEA@AgNPs | 0.079 (±0.007) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitarresi, G.; Barberi, G.; Palumbo, F.S.; Schillaci, D.; Fiorica, C.; Catania, V.; Indelicato, S.; Bongiorno, D.; Biscari, G.; Giammona, G. Developing Antibiofilm Fibrillar Scaffold with Intrinsic Capacity to Produce Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 15378. https://doi.org/10.3390/ijms232315378
Pitarresi G, Barberi G, Palumbo FS, Schillaci D, Fiorica C, Catania V, Indelicato S, Bongiorno D, Biscari G, Giammona G. Developing Antibiofilm Fibrillar Scaffold with Intrinsic Capacity to Produce Silver Nanoparticles. International Journal of Molecular Sciences. 2022; 23(23):15378. https://doi.org/10.3390/ijms232315378
Chicago/Turabian StylePitarresi, Giovanna, Giuseppe Barberi, Fabio Salvatore Palumbo, Domenico Schillaci, Calogero Fiorica, Valentina Catania, Serena Indelicato, David Bongiorno, Giuseppina Biscari, and Gaetano Giammona. 2022. "Developing Antibiofilm Fibrillar Scaffold with Intrinsic Capacity to Produce Silver Nanoparticles" International Journal of Molecular Sciences 23, no. 23: 15378. https://doi.org/10.3390/ijms232315378
APA StylePitarresi, G., Barberi, G., Palumbo, F. S., Schillaci, D., Fiorica, C., Catania, V., Indelicato, S., Bongiorno, D., Biscari, G., & Giammona, G. (2022). Developing Antibiofilm Fibrillar Scaffold with Intrinsic Capacity to Produce Silver Nanoparticles. International Journal of Molecular Sciences, 23(23), 15378. https://doi.org/10.3390/ijms232315378