Cucurbitacin B-, E-, and I-Induced Browning of White Adipocytes Is Promoted by the Inhibition of Phospholipase D2
Abstract
:1. Introduction
2. Results
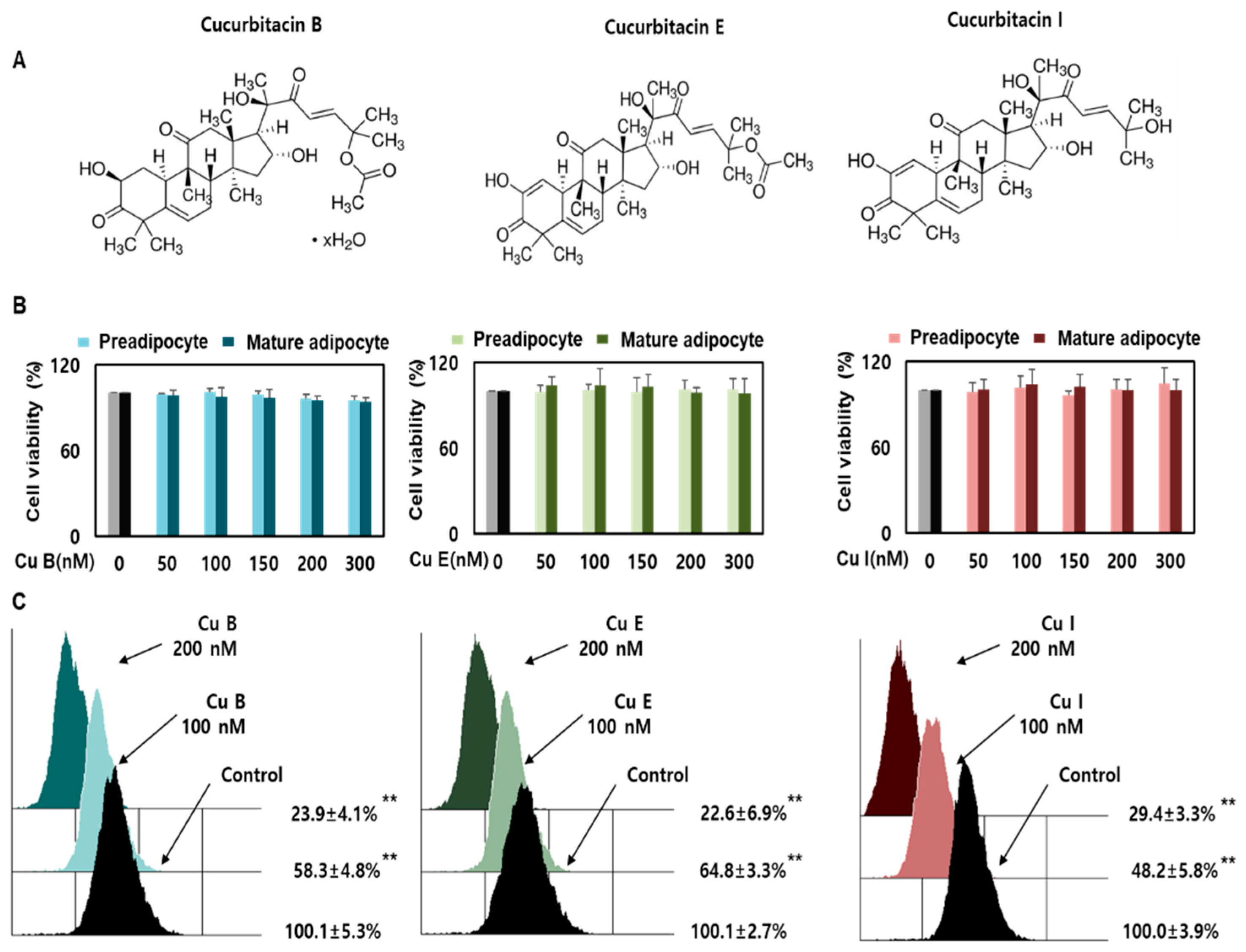
2.1. Effect of Cu B, Cu E, and Cu I on Nile Red-O Staining Activity and Cell Viability
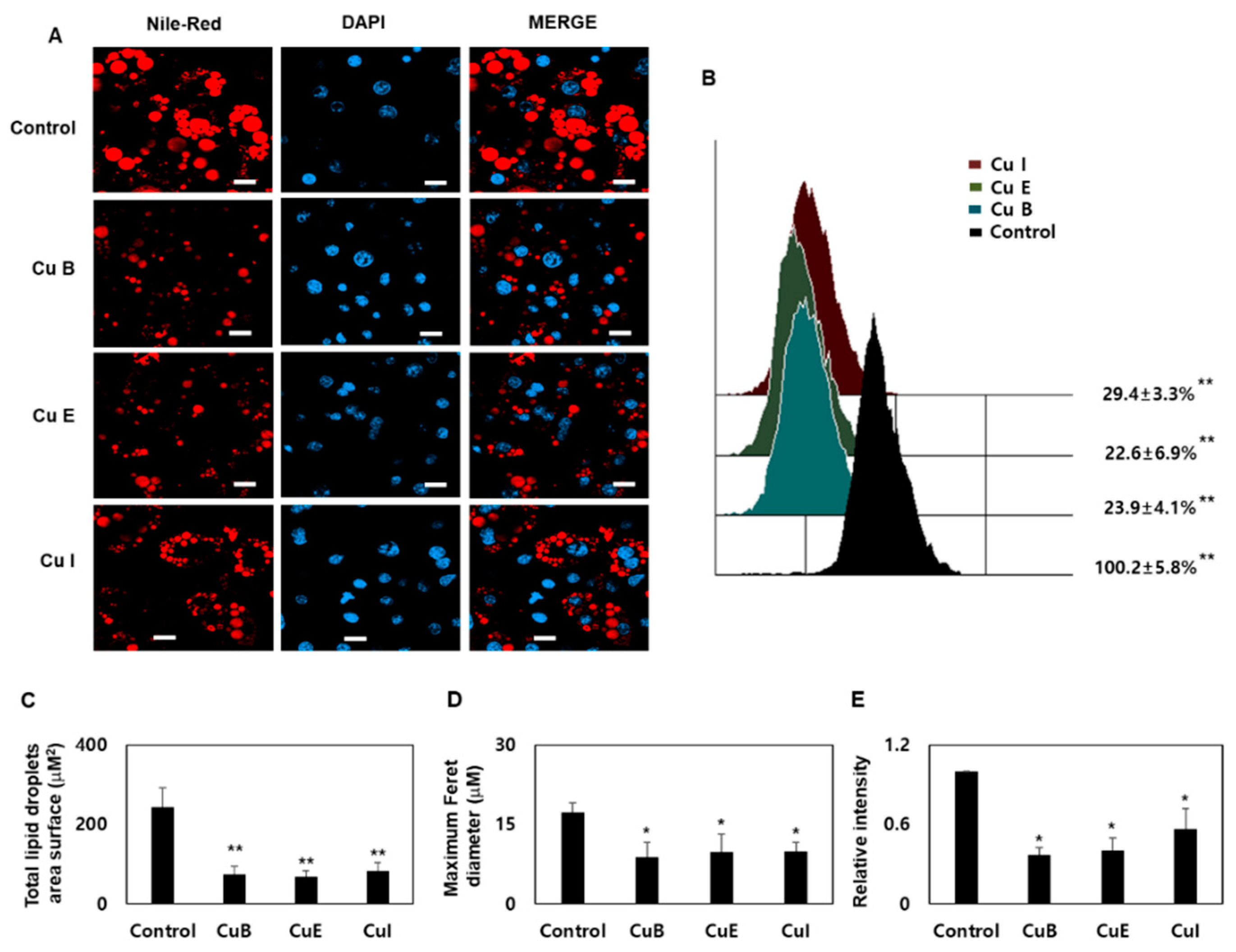
2.2. Effect of Cu B, Cu E, and Cu I on Formation of Smaller Lipid Droplets
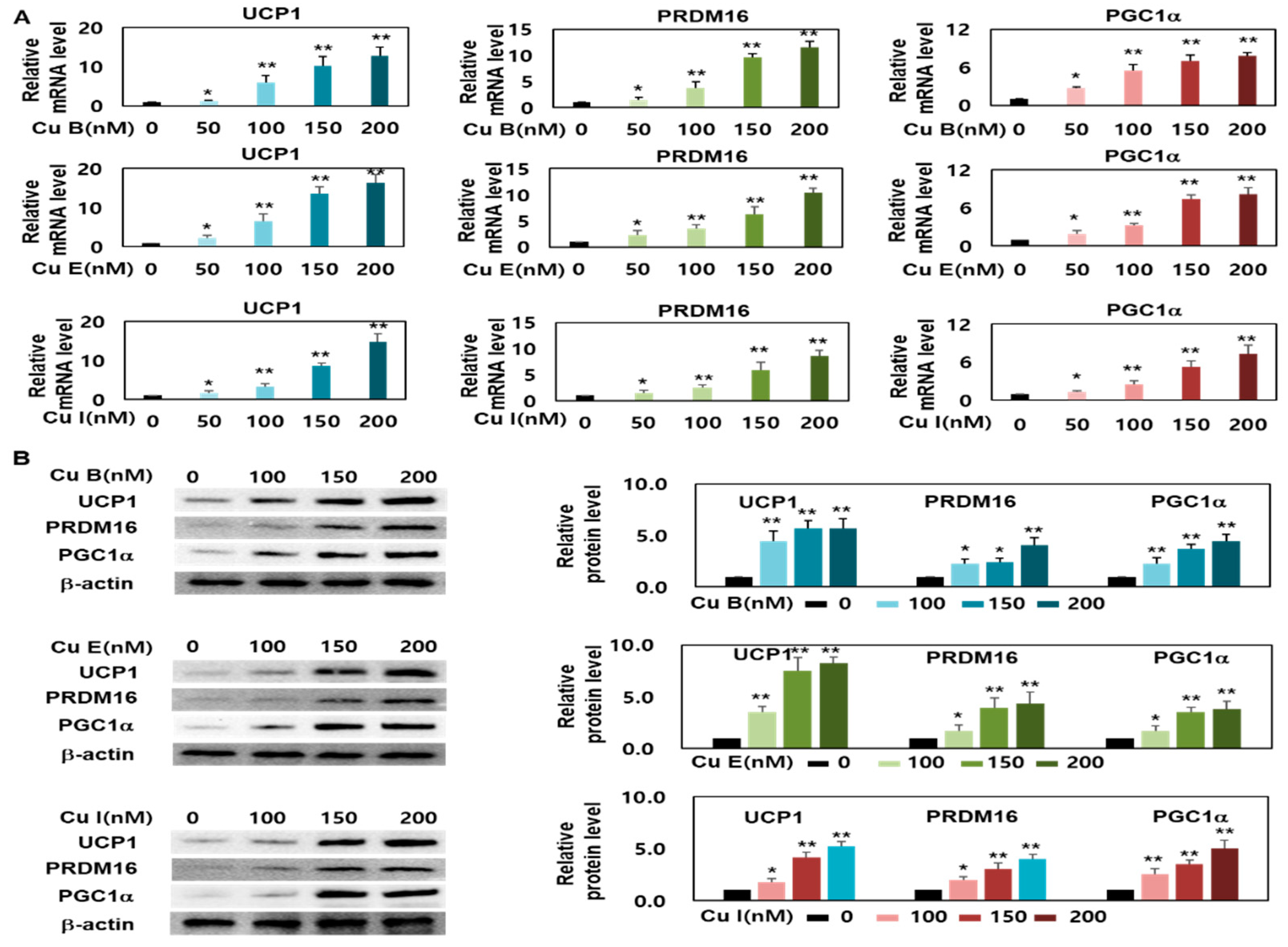
2.3. Effect of Cu B, Cu E, and Cu I on Expression Levels of Brown- and Beige-Fat Specific Marker Genes
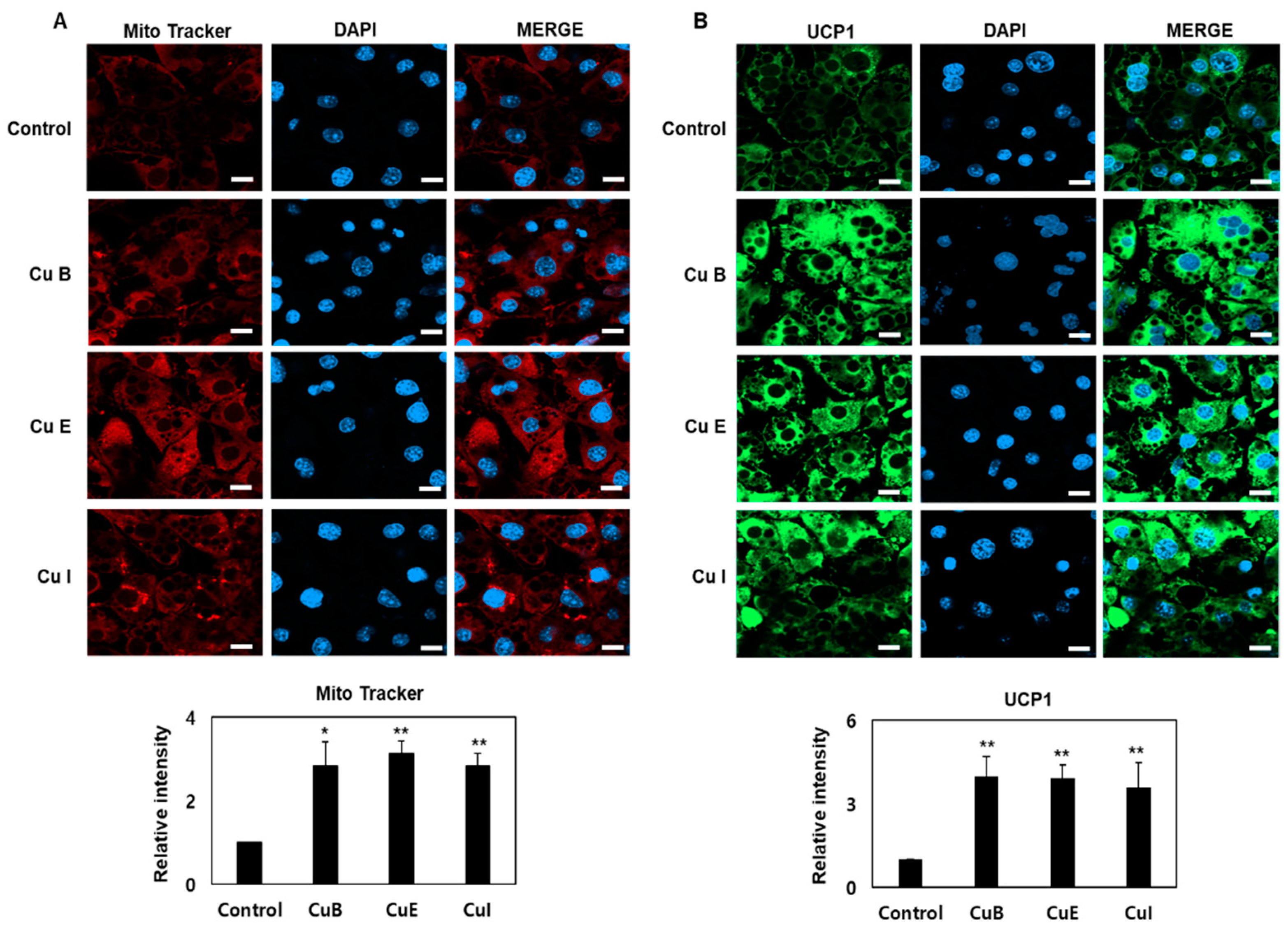
2.4. Effect of Cu B, Cu E, and Cu I on Mitochondrial Biogenesis
2.5. Effect of Adipocyte Browning of Cu B, Cu E, and Cu I through PLD2 Inhibition or Knockdown
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Pre- and Mature Adipocyte Culture and Treatment
4.3. Cell Viability of Pre- and Mature Adipocytes
4.4. qRT-PCR (Quantitative Real-Time PCR)
4.5. Protein Preparation and Western Blotting Analysis
4.6. Evaluation of Lipid Droplets Using Nile-Red Staining
4.7. Immunofluorescence
4.8. Mitochondrial Staining and Quantification
4.9. Short Interfering RNA (siRNA) and Transfection
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bäuerle, A.; Frewer, A.; Rentrop, V.; Schüren, L.C.; Niedergethmann, M.; Lortz, J.; Skoda, E.; Teufel, M. Determinants of Acceptance of Weight Management Applications in Overweight and Obese Individuals: Using an Extended Unified Theory of Acceptance and Use of Technology Model. Nutrients 2022, 14, 1968. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jin, Y.; Li, D.; Zhang, J.; Han, J.; Li, Y. Multidisciplinary Progress in Obesity Research. Genes 2022, 13, 1772. [Google Scholar] [CrossRef] [PubMed]
- Orzikulova, S.A. Obesity and the Cardiovascular System. Res. J. Trauma Disabil. Stud. 2022, 1, 9–16. [Google Scholar]
- Tsui, L. Adipocyte-based high throughput screening for anti-obesity drug discovery: Current status and future perspectives. SLAS Discov. Adv. Sci. Drug Discov. 2022, 27, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Estrada, J.; Chow, I.; Ruseva, A.; Ramasamy, A.; Burudpakdee, C.; Blanchette, C. EE72 The Relative Value of Anti-Obesity Medications Compared to Similar Therapies. Value Health 2022, 25, S348–S349. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Hu, F.; Zhou, Z.; Wang, L.; Liu, Y.; Hu, F.; Zhou, Z. The potential mechanisms of white adipose tissue browning: A novel target for the treatment of obesity. Nutr. Hosp. 2022, 39, 411–424. [Google Scholar] [PubMed]
- Manríquez-Núñez, J.; Ramos-Gómez, M. Bioactive Compounds and Adipocyte Browning Phenomenon. Curr. Issues Mol. Biol. 2022, 44, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Tu-Sekine, B.; Jin, S.; Anokye-Danso, F.; Ahima, R.S.; Brown, T.T.; Kim, S.F. Dolutegravir Suppresses Thermogenesis via Disrupting Uncoupling Protein 1 Expression and Mitochondrial Function in Brown/Beige Adipocytes in Preclinical Models. J. Infect. Dis. 2022, 226, 1626–1636. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 1–27. [Google Scholar] [CrossRef]
- Tanaka, E.; Mitani, T.; Nakashima, M.; Yonemoto, E.; Fujii, H.; Ashida, H. Theobromine enhances the conversion of white adipocytes into beige adipocytes in a PPARγ activation-dependent manner. J. Nutr. Biochem. 2022, 100, 108898. [Google Scholar] [CrossRef]
- Sathyanarayana, A.R.; Lu, C.; Liaw, C.; Chang, C.; Han, H.; Green, B.D.; Huang, W.; Huang, C.; He, W.; Lee, L. 1, 2, 3, 4, 6-Penta-O-galloyl-d-glucose Interrupts the Early Adipocyte Lifecycle and Attenuates Adiposity and Hepatic Steatosis in Mice with Diet-Induced Obesity. Int. J. Mol. Sci. 2022, 23, 4052. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Singh, S.P.; Raffaele, M.; Waldman, M.; Hochhauser, E.; Ospino, J.; Arad, M.; Peterson, S.J. Adipocyte-specific expression of PGC1α promotes adipocyte browning and alleviates obesity-induced metabolic dysfunction in an HO-1-Dependent fashion. Antioxidants 2022, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Kanani, S.H.; Pandya, D.J. Cucurbitacins: Nature’s Wonder Molecules. Curr. Tradit. Med. 2022, 8, 26–34. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Singha, S.; Kar, A.; Chanda, J.; Banerjee, S.; Dasgupta, B.; Haldar, P.K.; Sharma, N. Therapeutic importance of Cucurbitaceae: A medicinally important family. J. Ethnopharmacol. 2022, 282, 114599. [Google Scholar] [CrossRef] [PubMed]
- Attar, U.A.; Ghane, S.G.; Chavan, N.S.; Shiragave, P.D. Simultaneous detection of anticancer compounds (Cucurbitacin I, B and E) and some pharmacological properties of Indian Blastania species. S. Afr. J. Bot. 2022, 147, 871–881. [Google Scholar] [CrossRef]
- Mir, S.A.; Mukherjee, S.; Makar, S.; Pal, G. Cucurbitacins a vibrant triterpenoid: A review on its anticancer property. PharmaTutor 2019, 7, 43–54. [Google Scholar]
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms. Int. J. Oncol. 2018, 52, 19–37. [Google Scholar] [CrossRef]
- Kusagawa, E.; Okuda, C.; Yamaguchi, R.; Nakano, K.; Miyake, Y.; Kataoka, T. Cucurbitacin B Down-Regulates TNF Receptor 1 Expression and Inhibits the TNF-α-Dependent Nuclear Factor κB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells. Int. J. Mol. Sci. 2022, 23, 7130. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Park, G. Cucurbitacins attenuate microglial activation and protect from neuroinflammatory injury through Nrf2/ARE activation and STAT/NF-κB inhibition. Neurosci. Lett. 2015, 609, 129–136. [Google Scholar] [CrossRef]
- Debnath, P.; Das, B.; Singha, S.; Kar, A.; Haldar, P.K.; Sharma, N.; Mukherjee, P.K. Quantification of cucurbitacin E in different varieties of melon (Cucumis melo L.) fruit through validated RP-HPLC method. Nat. Prod. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Raineri, A.; Campagnari, R.; Dal Toso, R.; Copetti, S.; Gomez-Lira, M.; Menegazzi, M. 3, 5-Dicaffeoylquinic Acid Lowers 3T3-L1 Mitotic Clonal Expansion and Adipocyte Differentiation by Enhancing Heme Oxygenase-1 Expression. Molecules 2021, 26, 5027. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, H.M.; Song, W.C.; Oh, J.; Park, G.; Choi, Y. Characterization of Plocamium telfairiae Extract-Functionalized Au Nanostructures and Their Anti-Adipogenic Activity through PLD1. Mar. Drugs 2022, 20, 421. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, M.; Khan, G.; Aftab, M.F.; Afridi, S.K.; Ghaffar, S.; Ahmed, A.; Hafizur, R.M.; Waraich, R.S. Cucurbitacin E reduces obesity and related metabolic dysfunction in mice by targeting JAK-STAT5 signaling pathway. PLoS ONE 2017, 12, e0178910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, C.; Yang, D.K.; Song, N.; Yun, U.J.; Gwon, A.; Jo, D.; Cho, J.Y.; Yoon, K.; Ahn, J.; Nho, C.W.; et al. Cucurbitacin B and cucurbitacin I suppress adipocyte differentiation through inhibition of STAT3 signaling. Food Chem. Toxicol. 2014, 64, 217–224. [Google Scholar] [CrossRef]
- Jin, L.; Deng, L.; Bartlett, M.; Ren, Y.; Lu, J.; Chen, Q.; Pan, Y.; Wang, H.; Guo, X.; Liu, C. A Novel Herbal Extract Blend Product Prevents Particulate Matters-Induced Inflammation by Improving Gut Microbiota and Maintaining the Integrity of the Intestinal Barrier. Nutrients 2022, 14, 2010. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Won, Y.J.; Kim, D.Y. Time-lapse in situ fluorescence lifetime imaging of lipid droplets in differentiating 3T3-L1 preadipocytes with Nile Red. Curr. Appl. Phys. 2015, 15, 1634–1640. [Google Scholar] [CrossRef]
- Vliora, M.; Grillo, E.; Corsini, M.; Ravelli, C.; Nintou, E.; Karligiotou, E.; Flouris, A.D.; Mitola, S. Irisin regulates thermogenesis and lipolysis in 3T3-L1 adipocytes. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2022, 1866, 130085. [Google Scholar] [CrossRef]
- Nintou, E.; Karligiotou, E.; Vliora, M.; Fatouros, I.G.; Jamurtas, A.Z.; Sakellaridis, N.; Dimas, K.; Flouris, A.D. Effects of In Vitro Muscle Contraction on Thermogenic Protein Levels in Co-Cultured Adipocytes. Life 2021, 11, 1227. [Google Scholar] [CrossRef]
- Wu, J.; Du, J.; Li, Z.; He, W.; Wang, M.; Jin, M.; Yang, L.; Liu, H. Pentamethylquercetin Regulates Lipid Metabolism by Modulating Skeletal Muscle-Adipose Tissue Crosstalk in Obese Mice. Pharmaceutics 2022, 14, 1159. [Google Scholar] [CrossRef]
- Millar, C.A.; Jess, T.J.; Saqib, K.M.; Wakelam, M.J.; Gould, G.W. 3T3-L1 adipocytes express two isoforms of phospholipase D in distinct subcellular compartments. Biochem. Biophys. Res. Commun. 1999, 254, 734–738. [Google Scholar] [CrossRef]
- Barber, C.N.; Goldschmidt, H.L.; Lilley, B.; Bygrave, A.M.; Johnson, R.C.; Huganir, R.L.; Zack, D.J.; Raben, D.M. Differential expression patterns of phospholipase D isoforms 1 and 2 in the mammalian brain and retina. J. Lipid Res. 2022, 63, 100247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, M.Y.; Yun, N.J.; Go, H.S.; Kim, M.Y.; Seong, J.K.; Lee, M.; Kang, E.S.; Ghim, J.; Ryu, S.H. Targeting PLD2 in adipocytes augments adaptive thermogenesis by improving mitochondrial quality and quantity in mice. J. Exp. Med. 2021, 219, e20211523. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Kang, H.M.; Oh, J.-W.; Choi, Y.-W.; Park, G. Cucurbitacin B-, E-, and I-Induced Browning of White Adipocytes Is Promoted by the Inhibition of Phospholipase D2. Int. J. Mol. Sci. 2022, 23, 15362. https://doi.org/10.3390/ijms232315362
Park SY, Kang HM, Oh J-W, Choi Y-W, Park G. Cucurbitacin B-, E-, and I-Induced Browning of White Adipocytes Is Promoted by the Inhibition of Phospholipase D2. International Journal of Molecular Sciences. 2022; 23(23):15362. https://doi.org/10.3390/ijms232315362
Chicago/Turabian StylePark, Sun Young, Hye Mi Kang, Jin-Woo Oh, Young-Whan Choi, and Geuntae Park. 2022. "Cucurbitacin B-, E-, and I-Induced Browning of White Adipocytes Is Promoted by the Inhibition of Phospholipase D2" International Journal of Molecular Sciences 23, no. 23: 15362. https://doi.org/10.3390/ijms232315362





