The Impact of Corticosteroids on Human Airway Smooth Muscle Contractility and Airway Hyperresponsiveness: A Systematic Review
Abstract
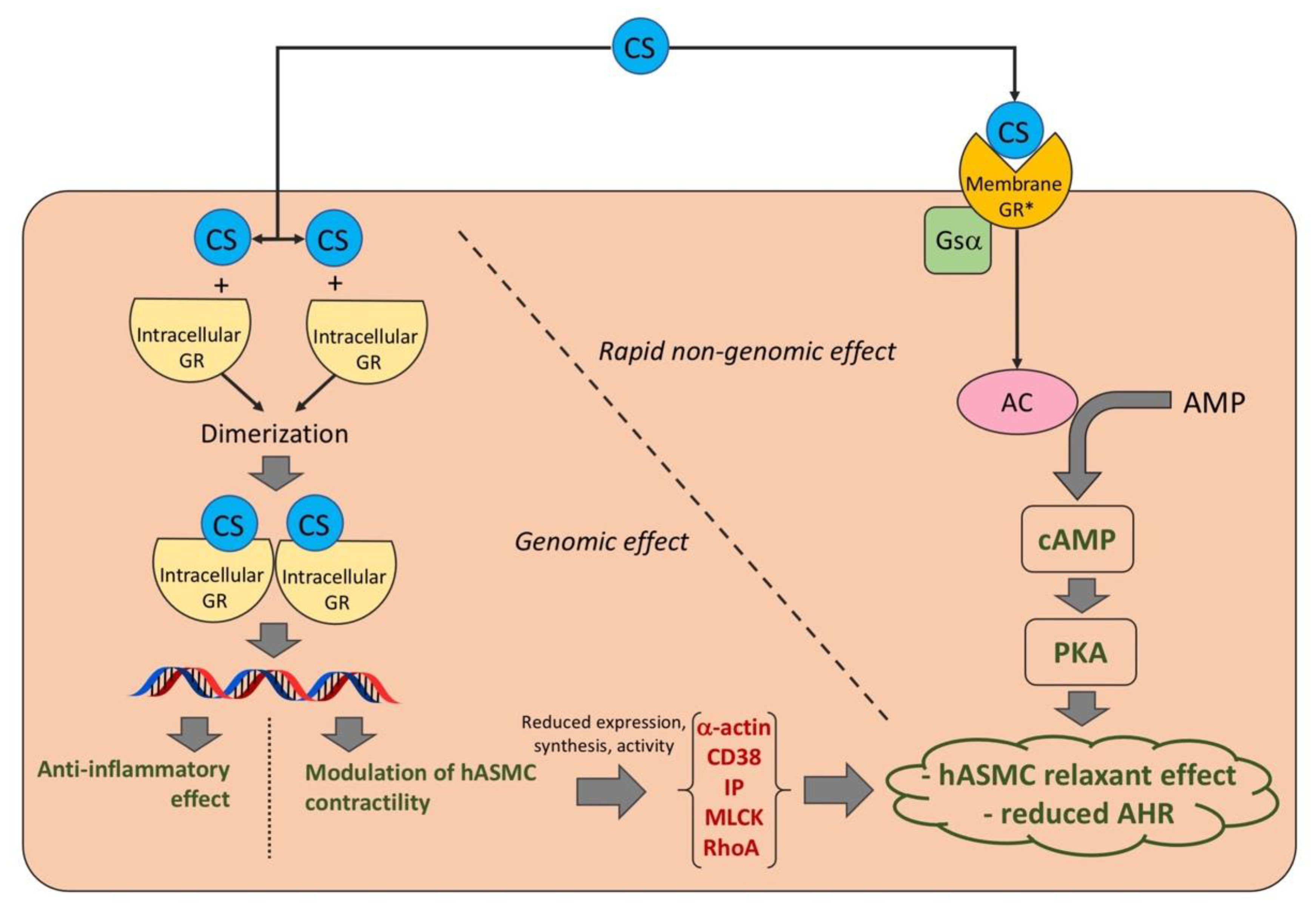
:1. Introduction
2. Methods
2.1. Review Question
2.2. Search Strategy and Study Eligibility
2.3. Data Extraction
2.4. Endpoints
2.5. Strategy for Data Analysis
2.6. Quality Score and RoB
3. Results
3.1. Study Characteristics
3.2. Impact of CS Administered Alone In Vitro
3.2.1. DEX
3.2.2. FP
3.2.3. BUD
3.2.4. PSL
3.3. Impact of CS Administered in Combination In Vitro
3.3.1. DEX Plus LABA
3.3.2. FP Plus LABA
3.4. Impact of CS Administered Alone Ex Vivo
3.4.1. DEX
3.4.2. BDP
3.5. Impact of CS Administered in Combination Ex Vivo
3.5.1. BDP Plus LABA
3.5.2. MF Plus LABA
3.5.3. BDP Plus LAMA
3.5.4. Triple Combinations Including BDP
3.5.5. Triple Combinations Including MF
3.6. Impact of CS Administered Alone in Clinical Studies
3.6.1. FP
3.6.2. BUD
3.6.3. PSL
3.7. RoB and Quality of Evidence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panettieri, R.A.; Schaafsma, D.; Amrani, Y.; Koziol-White, C.; Ostrom, R.; Tliba, O. Non-genomic Effects of Glucocorticoids: An Updated View. Trends Pharmacol. Sci. 2019, 40, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Lemanske, R.F.; Busse, W.W. 6. Asthma. J. Allergy Clin. Immunol. 2003, 111, S502–S519. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, E.; Tillmann, H.C.; Christ, M.; Feuring, M.; Wehling, M. Multiple actions of steroid hormones—A focus on rapid, nongenomic effects. Pharmacol. Rev. 2000, 52, 513–556. [Google Scholar] [PubMed]
- Cazzola, M.; Calzetta, L.; Rogliani, P.; Puxeddu, E.; Facciolo, F.; Matera, M.G. Interaction between corticosteroids and muscarinic antagonists in human airways. Pulm. Pharmacol. Ther. 2016, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.P. Glucocorticoids and Airway Smooth Muscle: A Few More Answers, Still More Questions. Am. J. Respir. Cell Mol. Biol. 2019, 61, 9–10. [Google Scholar] [CrossRef]
- Pedder, H.; Sarri, G.; Keeney, E.; Nunes, V.; Dias, S. Data extraction for complex meta-analysis (DECiMAL) guide. Syst. Rev. 2016, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019); Cochrane: London, UK, 2019; pp. 205–228. Available online: www.training.cochrane.org/handbook (accessed on 13 September 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuinness, L.A. Robvis: An R package and Web Application for Visualising Risk-of-Bias Assessments. 2019. Available online: https://github.com/mcguinlu/robvis (accessed on 13 September 2022).
- Goldsmith, A.M.; Hershenson, M.B.; Wolbert, M.P.; Bentley, J.K. Regulation of airway smooth muscle α-actin expression by glucocorticoids. Am. J. Physiol. Cell. Mol. Physiol. 2007, 292, L99–L106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, K.; Chiba, Y.; Sakai, H.; Misawa, M. Mechanism of Inhibitory Effect of Prednisolone on RhoA Upregulation in Human Bronchial Smooth Muscle Cells. Biol. Pharm. Bull. 2010, 33, 710–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, E.; Farahani, M.; Hall, I.P. Regulation of histamine H1 receptor coupling by dexamethasone in human cultured airway smooth muscle. J. Cereb. Blood Flow Metab. 1996, 118, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Schmidlin, F.; Scherrer, D.; Landry, Y.; Gies, J.-P. Glucocorticoids inhibit the bradykinin B2 receptor increase induced by interleukin-1β in human bronchial smooth muscle cells. Eur. J. Pharmacol. 1998, 354, R7–R8. [Google Scholar] [CrossRef]
- Tirumurugaan, K.G.; Kang, B.N.; Panettieri, R.A., Jr.; Foster, D.N.; Walseth, T.F.; Kannan, M.S. Regulation of the cd38 promoter in human airway smooth muscle cells by TNF-α and dexamethasone. Respir. Res. 2008, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Tliba, O.; Amrani, Y. CD38 Expression Is Insensitive to Steroid Action in Cells Treated with Tumor Necrosis Factor-α and Interferon-γ by a Mechanism Involving the Up-Regulation of the Glucocorticoid Receptor beta Isoform. FASEB J. 2006, 62, 588–596. [Google Scholar] [CrossRef]
- Lewis, R.J.; Chachi, L.; Newby, C.; Amrani, Y.; Bradding, P. Bidirectional Counterregulation of Human Lung Mast Cell and Airway Smooth Muscle β2 Adrenoceptors. J. Immunol. 2015, 196, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Baouz, S.; Giron-Michel, J.; Azzarone, B.; Giuliani, M.; Cagnoni, F.; Olsson, S.; Testi, R.; Gabbiani, G.; Canonica, G.W. Lung myofibroblasts as targets of salmeterol and fluticasone propionate: Inhibition of α-SMA and NF-κB. Int. Immunol. 2005, 17, 1473–1481. [Google Scholar] [CrossRef]
- Calzetta, L.; Matera, M.G.; Facciolo, F.; Cazzola, M.; Rogliani, P. Beclomethasone dipropionate and formoterol fumarate synergistically interact in hyperresponsive medium bronchi and small airways. Respir. Res. 2018, 19, 65. [Google Scholar] [CrossRef]
- Koziol-White, C.J.; Jia, Y.; Baltus, G.A.; Cooper, P.R.; Zaller, D.M.; Crackower, M.A.; Sirkowski, E.E.; Smock, S.; Northrup, A.B.; Himes, B.E.; et al. Inhibition of spleen tyrosine kinase attenuates IgE-mediated airway contraction and mediator release in human precision cut lung slices. J. Cereb. Blood Flow Metab. 2016, 173, 3080–3087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritondo, B.L.; Rogliani, P.; Facciolo, F.; Falco, S.; Vocale, A.; Calzetta, L. Beclomethasone dipropionate and sodium cromoglycate protect against airway hyperresponsiveness in a human ex vivo model of cow's milk aspiration. Curr. Res. Pharmacol. Drug Discov. 2020, 2, 100010. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Matera, M.G.; Facciolo, F.; Page, C.; Cazzola, M.; Calzetta, L. Beclomethasone dipropionate, formoterol fumarate and glycopyrronium bromide: Synergy of triple combination therapy on human airway smooth muscle ex vivo. J. Cereb. Blood Flow Metab. 2019, 177, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Ritondo, B.L.; Facciolo, F.; Matera, M.G.; Nikolaev, I.; Calzetta, L. Indacaterol, glycopyrronium, and mometasone: Pharmacological interaction and anti-inflammatory profile in hyperresponsive airways. Pharmacol. Res. 2021, 172, 105801. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Ora, J.; Girolami, A.; Rossi, I.; de Guido, I.; Facciolo, F.; Cazzola, M.; Calzetta, L. Ceiling effect of beclomethasone/formoterol/glycopyrronium triple fixed-dose combination in COPD: A translational bench-to-bedside study. Pulm. Pharmacol. Ther. 2021, 69, 102050. [Google Scholar] [CrossRef]
- Clearie, K.L.; McKinlay, L.; Williamson, P.A.; Lipworth, B.J. Fluticasone/Salmeterol Combination Confers Benefits in People With Asthma Who Smoke. Chest 2012, 141, 330–338. [Google Scholar] [CrossRef]
- Currie, G.P.; Stenback, S.; Lipworth, B.J. Effects of fluticasone vs. fluticasone/salmeterol on airway calibre and airway hyperresponsiveness in mild persistent asthma. Br. J. Clin. Pharmacol. 2003, 56, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.M.; O'Connor, T.M.; Leigh, R.; Otis, J.; Gwozd, C.; Gauvreau, G.M.; Gauldie, J.; O'Byrne, P.M. Effects of budesonide and formoterol on allergen-induced airway responses, inflammation, and airway remodeling in asthma. J. Allergy Clin. Immunol. 2010, 125, 349–356. [Google Scholar] [CrossRef]
- O'Connor, B.J.; Ridge, S.M.; Barnes, P.J.; Fuller, R.W. Greater Effect of Inhaled Budesonide on Adenosine 5′-Monophosphate-induced than on Sodium-Metabisulfite-induced Bronchoconstriction in Asthma. Am. Rev. Respir. Dis. 1992, 146, 560–564. [Google Scholar] [CrossRef]
- Williams, A.E.; Larner-Svensson, H.; Perry, M.; Campbell, G.A.; Herrick, S.; Adcock, I.; Erjefalt, J.S.; Chung, K.F.; Lindsay, M.A. MicroRNA Expression Profiling in Mild Asthmatic Human Airways and Effect of Corticosteroid Therapy. PLoS ONE 2009, 4, e5889. [Google Scholar] [CrossRef] [Green Version]
- Yick, C.Y.; Zwinderman, A.H.; Kunst, P.W.; Grünberg, K.; Mauad, T.; Fluiter, K.; Bel, E.H.; Lutter, R.; Baas, F.; Sterk, P.J. Glucocorticoid-induced Changes in Gene Expression of Airway Smooth Muscle in Patients with Asthma. Am. J. Respir. Crit. Care Med. 2013, 187, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Sathish, V.; Thompson, M.A.; Bailey, J.P.; Pabelick, C.M.; Prakash, Y.S.; Sieck, G.C. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am. J. Physiol. Cell. Mol. Physiol. 2009, 297, L26–L34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, M.J.; Delmotte, P.; Bai, Y.; Perez-Zogbhi, J.F. Regulation of Airway Smooth Muscle Cell Contractility by Ca2+ Signaling and Sensitivity. Proc. Am. Thorac. Soc. 2008, 5, 23–31. [Google Scholar] [CrossRef]
- Jain, D.; Keslacy, S.; Tliba, O.; Cao, Y.; Kierstein, S.; Amin, K.; Panettieri, R.A.; Haczku, A.; Amrani, Y. Essential role of IFNβ and CD38 in TNFα-induced airway smooth muscle hyper-responsiveness. Immunobiology 2008, 213, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabe, K.F.; Watson, N.; Dent, G.; Morton, B.E.; Wagner, K.; Magnussen, H.; Heusser, C.H. Inhibition of Human Airway Sensitization by a Novel Monoclonal Anti-IgE Antibody, 17–19. Am. J. Respir. Crit. Care Med. 1998, 157, 1429–1435. [Google Scholar] [CrossRef]
- GINA. 2022 GINA Main Report|Global Initiative for Asthma. 2022. Available online: https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf (accessed on 13 September 2022).
- Lin, C.-H.; Hsu, J.-Y.; Hsiao, Y.-H.; Tseng, C.-M.; Su, V.Y.-F.; Chen, Y.-H.; Yang, S.-N.; Lee, Y.-C.; Su, K.-C.; Perng, D.-W. Budesonide/formoterol maintenance and reliever therapy in asthma control: Acute, dose-related effects and real-life effectiveness. Respirology 2014, 20, 264–272. [Google Scholar] [CrossRef]
- Bourdin, A.; Molinari, N.; Ferguson, G.T.; Singh, B.; Siddiqui, M.K.; Holmgren, U.; Ouwens, M.; Jenkins, M.; De Nigris, E. Efficacy and Safety of Budesonide/Glycopyrronium/Formoterol Fumarate versus Other Triple Combinations in COPD: A Systematic Literature Review and Network Meta-analysis. Adv. Ther. 2021, 38, 3089–3112. [Google Scholar] [CrossRef]
- Buhl, R.; Banerji, D. Profile of glycopyrronium for once-daily treatment of moderate-to-severe COPD. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Dhungana, S.; Criner, G.J. Spotlight on glycopyrronium/formoterol fumarate inhalation aerosol in the management of COPD: Design, development, and place in therapy. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2307–2312. [Google Scholar] [CrossRef]
- EMA. Trimbow, INN-Beclometasone Dipropionate, Formoterol Fumarate Dihydrate, Glycopyrronium. Available online: https://www.ema.europa.eu/en/documents/variation-report/trimbow-h-c-4257-x-0008-g-epar-assessment-report-extension_en.pdf (accessed on 13 September 2022).
- Papi, A.; Fabbri, L.M.; Kerstjens, H.A.; Rogliani, P.; Watz, H.; Singh, D. Inhaled long-acting muscarinic antagonists in asthma—A narrative review. Eur. J. Intern. Med. 2021, 85, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Calzetta, L.; Gritti, G.; Gallo, L.; Perfetto, B.; Donnarumma, G.; Cazzola, M.; Rogliani, P.; Donniacuo, M.; Rinaldi, B. Role of statins and mevalonate pathway on impaired HDAC2 activity induced by oxidative stress in human airway epithelial cells. Eur. J. Pharmacol. 2018, 832, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Spina, D.; Page, C.P. Xanthines and Phosphodiesterase Inhibitors. In Pharmacology and Therapeutics of Asthma and COPD; Springer: Cham, Switzerland, 2016; Volume 237, pp. 63–91. [Google Scholar] [CrossRef]
- Calzetta, L.; Matera, M.G.; Goldstein, M.F.; Fairweather, W.R.; Howard, W.W.; Cazzola, M.; Rogliani, P. A long-term clinical trial on the efficacy and safety profile of doxofylline in Asthma: The LESDA study. Pulm. Pharmacol. Ther. 2019, 60, 101883. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Ora, J.; Cazzola, M.; Matera, M.G. Efficacy and safety profile of doxofylline compared to theophylline in asthma: A meta-analysis. Multidiscip. Respir. Med. 2019, 14, 1–8. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Barnes, P.J.; Criner, G.J.; Martinez, F.J.; Papi, A.; Matera, M.G. Efficacy and safety profile of xanthines in COPD: A network meta-analysis. Eur. Respir. Rev. 2018, 27, 180010. [Google Scholar] [CrossRef] [Green Version]
- Calzetta, L.; Hanania, N.A.; Dini, F.L.; Goldstein, M.F.; Fairweather, W.R.; Howard, W.W.; Cazzola, M. Impact of doxofylline compared to theophylline in asthma: A pooled analysis of functional and clinical outcomes from two multicentre, double-blind, randomized studies (DOROTHEO 1 and DOROTHEO 2). Pulm. Pharmacol. Ther. 2018, 53, 20–26. [Google Scholar] [CrossRef]
- Tasker, J.G.; Di, S.; Malcher-Lopes, R. Rapid Glucocorticoid Signaling via Membrane-Associated Receptors. Endocrinology 2006, 147, 5549–5556. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-Z.; Qiu, J. Pleiotropic Signaling Pathways in Rapid, Nongenomic Action of Glucocorticoid. Mol. Cell Biol. Res. Commun. 1999, 2, 145–149. [Google Scholar] [CrossRef]


| Study and Year | Type of Study | Type of Human Cells, Tissue Donors, or Characteristics of Analyzed Patients | Contractile Stimulus (Dose) | Number of Patients or Tissue Donors | Age | Male (%) | CS with Dose (Exposure Time or Treatment Duration) | Route of Administration | Jadad Score | Investigated Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Ritondo et al., 2021 [24] | Ex vivo study | Medium bronchi from patients undergoing lobectomy surgery for lung cancer | Cow’s milk (1:10 v/v) | 16 | 50.0 | 50.0 | BDP 0.1–10 μM (1 hr) | Incubation | / | Bronchorelaxant effect |
| Rogliani et al., 2021 [26] | Ex vivo study | Medium bronchi and PCLS from patients undergoing surgery for lung cancer with normal lung function and without history of chronic airway disease (tissues were passively sensitized with serum from atopic asthma patients) | His in passively sensitized tissues (EC70); CCh in COPD tissues (EC70) | 13 | 50.0 | 50.0 | MF/IND at 100:45 or 100:90 concentration-ratio (cumulative concentrations); MF/IND/GLY at 100:37:45 or 100:37:90 concentration-ratio | Incubation | / | Bronchorelaxant effect and pharmacological interaction |
| Rogliani et al., 2021 [27] | Ex vivo study | Medium bronchi and PCLS from COPD donors | CCh in COPD tissues (EC70) | 16 | 69.3 | 93.8 | BDP/FOR at 100:6 concentration-ratio (cumulative concentration); BDP/FOR/GLY at 100:6:12.5 concentration-ratio (cumulative concentrations) | Incubation | / | Bronchorelaxant effect and pharmacological interaction |
| Rogliani et al., 2020 [25] | Ex vivo study | Medium bronchi and PCLS from patients undergoing surgery for lung cancer with normal lung function and without history of chronic airway disease (asthma model, tissues were passively sensitized with serum from atopic asthma patients); medium bronchi and PCLS from COPD donors with a lung function in agreement with spirometric diagnosis of COPD FEV1/FVC < 0.7 (COPD model) | His in passively sensitized tissues (EC70); CCh in COPD tissues (EC70) | 32 | 50.4 | 53.1 | BDP 0.3–300 nM (overnight); BDP/FOR/GLY at 100:6:12.5 concentration-ratio (cumulative concentrations) | Incubation | / | Bronchorelaxant effect and pharmacological interaction |
| Calzetta et al., 2018 [22] | Ex vivo study | Medium bronchi and PCLS from patients undergoing lobectomy surgery for lung cancer, without a history of chronic airway disease; tissues were non-sensitized (incubated with serum from non-atopic donors) or passively sensitized (with serum from atopic asthma patients) | His (EC70) | 16 | 50.0 | 50.0 | BDP 1–100 nM (overnight); BDP/FOR at 100:5 concentration-ratio (cumulative concentrations) | Incubation | / | Bronchorelaxant effect and pharmacological interaction |
| Cazzola et al., 2016 [4] | Ex vivo study | Medium bronchi and PCLS from patients undergoing lobectomy surgery for lung cancer, without a history of chronic airway disease; tissues were non-sensitized (incubated with serum from non-atopic donors) or passively sensitized (with serum from atopic asthma patients) | His (EC70) | 14 | 63.3 | 57.1 | BDP (0.1 nM–10 μM); BDP/GLY at concentrations inducing EC30 | Incubation | / | Bronchorelaxant effect and pharmacological interaction |
| Koziol-White et al., 2016 [23] | Ex vivo study | PCLS from non-asthmatic donors | CCh (100 μM) | 11 | 43.2 | 72.7 | DEX 1 μM (overnight) | Incubation | / | FcεRI-cross-linking-induced ASM contraction |
| Lewis et al., 2015 [20] | In vitro study | hASMC from asthmatic and healthy donors, cultured alone or co-cultured with hLMC | Not present | ≃6 | NA | NA | FP 10 μM; FP 10 μM + FOR 1 nM or OLO 1 nM | Incubation | / | Spontaneous contraction in collagen gel assay |
| Yick et al., 2013 [33] | Double-blind, randomized, PCB-controlled, parallel study | Atopic asthma patients (non-smoking or stopped for >12 months with smoking history of <5 pack-years; no exacerbations within 6 weeks before participation; steroid-naive or stopped using CS by any dosing route for ≥8 wks before participation; MCh PC20 ≤ 8 mg/mL; post-bronchodilator FEV1 >70% predicted) | MCh (NA) | 12 | 24.5 | NA | PSL 0.5 mg/kg/day | Oral | 3 | Methacholine PC20 |
| Clearie et al., 2012 [28] | Single-center, double-blind, crossover, randomized, PCB-controlled study | Mild to moderate persistent asthma patients (FEV1 ≥ 60% [<30% PEF variability], prescribed ≤1000 μg BDP or equivalent) | MCh (NA) | 31 | 38.4 | 48.4 | FP 500 μg BID (2 wks); FP/SAL 250/50 μg BID (2 wks) | Oral inhalation (pMDI) | 3 | MCh PC20 |
| Goto et al., 2010 [15] | In vitro study | Cultured hASMC | IL-13 (100 ng/mL), TNF-α (10 ng/mL) | NA | NA | NA | Prednisolone 10 μM (24 hrs) | Incubation | / | RhoA protein expression and RhoA promoter activity |
| Kelly et al., 2010 [30] | Prospective, double-blind, crossover, randomized, PCB-controlled study | Mild atopic asthma patients (MCh PC20 < 16 mg/mL and allergen-induced early and late bronchoconstrictor responses of ≥15% reduction in FEV1 during screening challenge) | MCh (NA) | 14 | 26.0 | 42.9 | BUD 400 μg BID (11 days); BUD/FOR 400/12 μg BID (11 days); PCB BID | Oral inhalation (DPI) | 3 | MCh PC20 |
| Williams et al., 2008 [32] | Clinical study | Mild asthma patients (history of intermittent wheeze, treatment with albutamol inhaler on an intermittent basis, CS-naive, with positive skin-prick test to common aeroallergens) | MCh (NA) | 5 | 29.0 | 12.5 | BUD 200 μg BID (4 wks) | Oral inhalation (DPI) | / | MCh PC20 |
| Tirumurugaan et al., 2008 [18] | In vitro | Cultured hASMC from donors | TNF-α (50 ng/mL) | 3 | NA | NA | DEX 1 μM (24 hrs) | Incubation | / | CD38 gene expression |
| Goldsmith et al., 2007 [14] | In vitro | Primary hASMC | TGFβ (1 ng/mL) | NA | NA | NA | DEX 0.1,1 μM (6 days); DEX 0.1,1 μM + SAL 1 nM (6 days); FP 10, 100 nM (48 hrs, 6 days); FP 10 nM + SAL 1 nM (6 days) | Incubation | / | Gene and protein expression of α-actin, protein expression of MLCK, rate of α-actin mRNA degradation, synthesis of α-actin in presence of actinomycin D, α-actin turnover, and contractile response to aCh and KCl-induced stimulation |
| Tliba et al., 2006 [19] | In vitro | hASMC from lung transplant donors | TNF-α (10 mg/mL), IFNγ (500 IU/mL), IFNβ (500 IU/mL) | NA | NA | NA | DEX 1 μM (2 hrs); FP 1, 10, 50, 100 nM (2 hrs); BUD 100 nM (2 hrs) | Incubation | / | CD38 gene overexpression |
| Baouz et al., 2005 [21] | In vitro | Myofibroblasts | TGFβ (5 ng/mL) | NA | NA | NA | FP 1 pM (24 hrs); FP 1 pM + SAL 10 nM (24 hrs) | Incubation | / | α-actin protein expression and contractile activity of single myofibroblasts evaluated within 30 min from treatment administration |
| Currie et al., 2003 [29] | Single-center, double-blind, crossover, randomized study | Mild asthma patients (FEV1 > 80% predicted and MCh PD20 < 500 μg) | MCh (NA) | 14 | 21.4 | 36.0 | FP 250 μg BID (3 wks); FP/SAL 125/25 μg BID (3 wks) | Oral inhalation (pMDI) | 3 | MCh PD20 |
| Schmidilin et al., 1998 [17] | In vitro | Primary hASMC | IL-1β (10 U/mL) | NA | NA | NA | DEX 1, 100 nM (1 hr); BUD 1, 100 nM (3 hrs, 6 hrs) | Incubation | / | Gene overexpression of bradykinin B2 receptor, and IP synthesis |
| Hardy et al., 1996 [16] | In vitro | Primary hASMC | His (100 μM or range of concentrations 1 μM–1 mM) | NA | NA | NA | DEX 1 nM–1 μM (1–22 hrs) | Incubation | / | IP response and synthesis |
| O’Connor et al., 1992 [31] | Randomized, double-blind, PCB-controlled, crossover study | Mild atopic asthma patients | AMP, MBS, MCh (NA) | NA | NA | NA | BUD 0.8 mg BID (2 wks) | Oral inhalation | 3 | PC20 to AMP, MBS, MCh |
| Corticosteroids Administered as Monocomponents | |||||
|---|---|---|---|---|---|
| Experimental Setting | BDP | BUD | DEX | FP | PSL |
| hASMC (in vitro) | NA | ↓ CD38 overexpression to TNF-α ↓ IP synthesis to bradykinin | ↓ CD38 overexpression to TNF-α ↓ IP synthesis to His ↓ α-actin overexpression to TGFβ ↓ short isoform of MLCK overexpression to TGFβ | ↓ CD38 overexpression to TNF-α ↓ α-actin overexpression to TGFβ ↑ α-actin protein turnover to TGFβ ↓ α-actin incorporation into filaments, reduced cell length and contractility to ACh and KCL Reversed the shift in MLCK expression from the long to the short isoform | ↓ RhoA overexpression to IL-13 ↓ RhoA overexpression to TNF-α |
| Human myofibroblasts (in vitro) | NA | NA | NA | ↓ α-actin overexpression to TGFβ | NA |
| Human medium bronchi (ex vivo) | Weak relaxant effect to His in non-sensitized tissue Strong relaxant effect to His in passively sensitized tissue ↑ Gsα–cAMP–PKA cascade in passively sensitized tissue ↓ contractility to EFS in cow’s milk challenged tissue | NA | NA | NA | NA |
| Human PCLS (ex vivo) | Weak relaxant effect to His in non-sensitized tissue Strong relaxant effect to His in passively sensitized tissue | NA | NA | NA | NA |
| Mild asthmatic patients (clinical trials) | NA | ↓ AHR to MBS ↓ AHR to MCh ↓ AHR to AMP Improvement in PD20 to MCh, also post allergen challenge Prevention of allergen-induced AHR | NA | Improvement in PD20 to MCh (greater effect in non-smokers than in current smokers) | Correlation of FAM129A and SYNPO2 genes with AHR |
| Corticosteroids Administered in Combination with Bronchodilators | ||||||||
|---|---|---|---|---|---|---|---|---|
| Experimental Setting | BDP + LABA | BDP + LAMA | BDP + LABA + LAMA | DEX + LABA | FP + LABA | FP + LAMA | MF + LABA | MF + LABA + LAMA |
| hASMC (in vitro) | NA | NA | NA | ↓ α-actin overexpression to TGFβ ↓ short isoform of MLCK to TGFβ | ↓ α-actin overexpression to TGFβ ↓ short isoform of MLCK to TGFβ ↓ spontaneous contractility | ↓ spontaneous contractility | NA | NA |
| Human myofibroblasts (in vitro) | NA | NA | NA | NA | ↓ α-actin overexpression to TGFβ | NA | NA | NA |
| Human medium bronchi (ex vivo) | Strong synergistic relaxant effect to His in passively sensitized tissue ↓ CCh-induced contractile tone in tissue from COPD donors | Synergistic relaxant effect to His in passively sensitized tissue | Very strong synergistic relaxant effect to His in passively sensitized tissue Very strong synergistic relaxant effect to CCh in tissue from COPD donors | NA | NA | NA | Strong to very strong synergistic relaxant effect to His in passively sensitized tissue | Very strong synergistic relaxant effect to His in passively sensitized tissue |
| Human PCLS (ex vivo) | Very strong synergistic relaxant effect to His in passively sensitized tissue ↓ CCh-induced contractile tone in tissue from COPD donors | Synergistic relaxant effect to His in passively sensitized tissue | Very strong synergistic relaxant effect to His in passively sensitized tissue Strong to very strong synergistic relaxant effect to CCh in tissue from COPD donors | NA | NA | NA | Mild to very strong synergistic relaxant effect to His in passively sensitized tissue | Very strong synergistic relaxant effect to His in passively sensitized tissue |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calzetta, L.; Chetta, A.; Aiello, M.; Pistocchini, E.; Rogliani, P. The Impact of Corticosteroids on Human Airway Smooth Muscle Contractility and Airway Hyperresponsiveness: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 15285. https://doi.org/10.3390/ijms232315285
Calzetta L, Chetta A, Aiello M, Pistocchini E, Rogliani P. The Impact of Corticosteroids on Human Airway Smooth Muscle Contractility and Airway Hyperresponsiveness: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(23):15285. https://doi.org/10.3390/ijms232315285
Chicago/Turabian StyleCalzetta, Luigino, Alfredo Chetta, Marina Aiello, Elena Pistocchini, and Paola Rogliani. 2022. "The Impact of Corticosteroids on Human Airway Smooth Muscle Contractility and Airway Hyperresponsiveness: A Systematic Review" International Journal of Molecular Sciences 23, no. 23: 15285. https://doi.org/10.3390/ijms232315285
APA StyleCalzetta, L., Chetta, A., Aiello, M., Pistocchini, E., & Rogliani, P. (2022). The Impact of Corticosteroids on Human Airway Smooth Muscle Contractility and Airway Hyperresponsiveness: A Systematic Review. International Journal of Molecular Sciences, 23(23), 15285. https://doi.org/10.3390/ijms232315285









