Psychedelic-Induced Serotonin 2A Receptor Downregulation Does Not Predict Swim Stress Coping in Mice
Abstract
:1. Introduction
2. Results
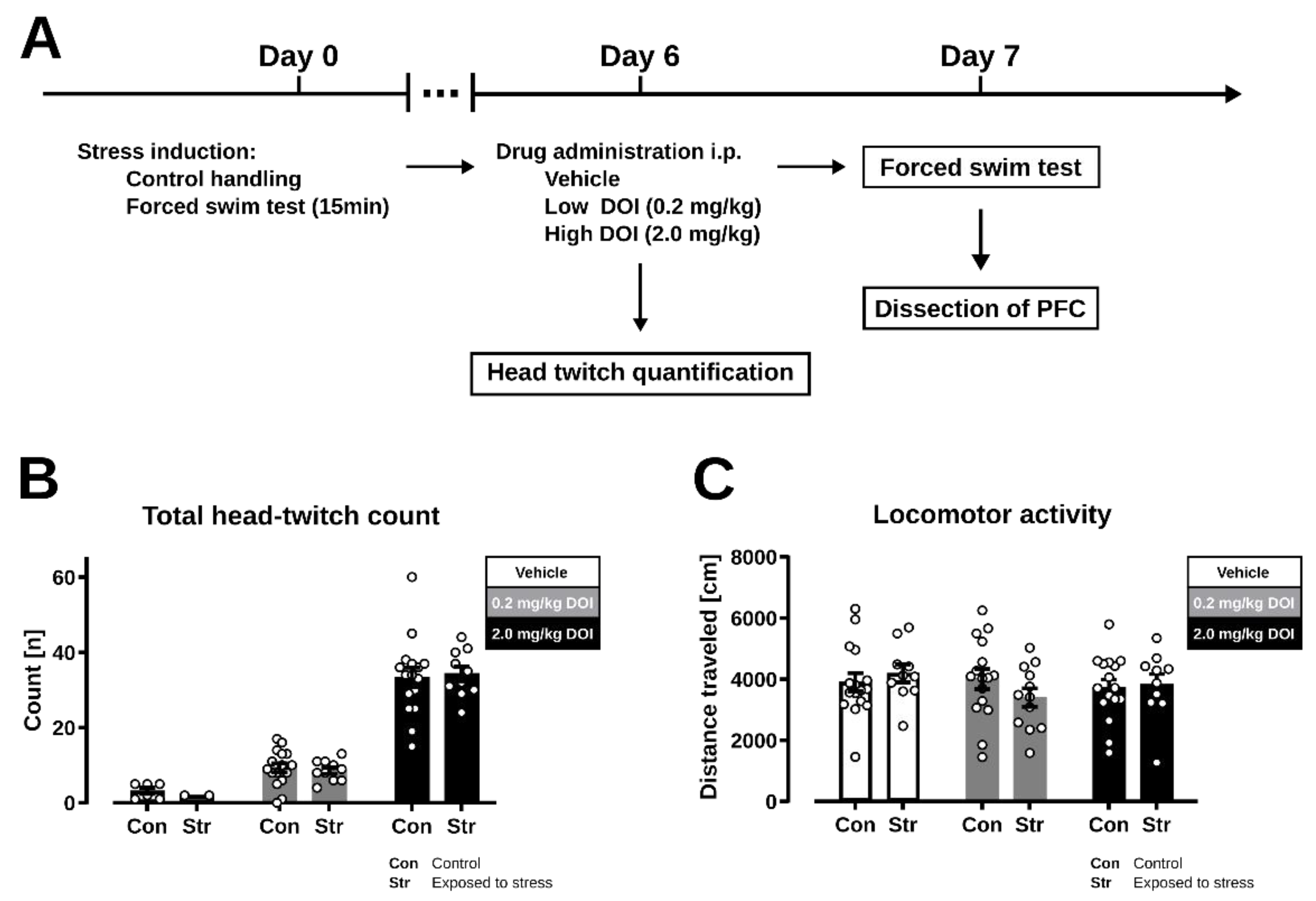
2.1. Head Twitch Response
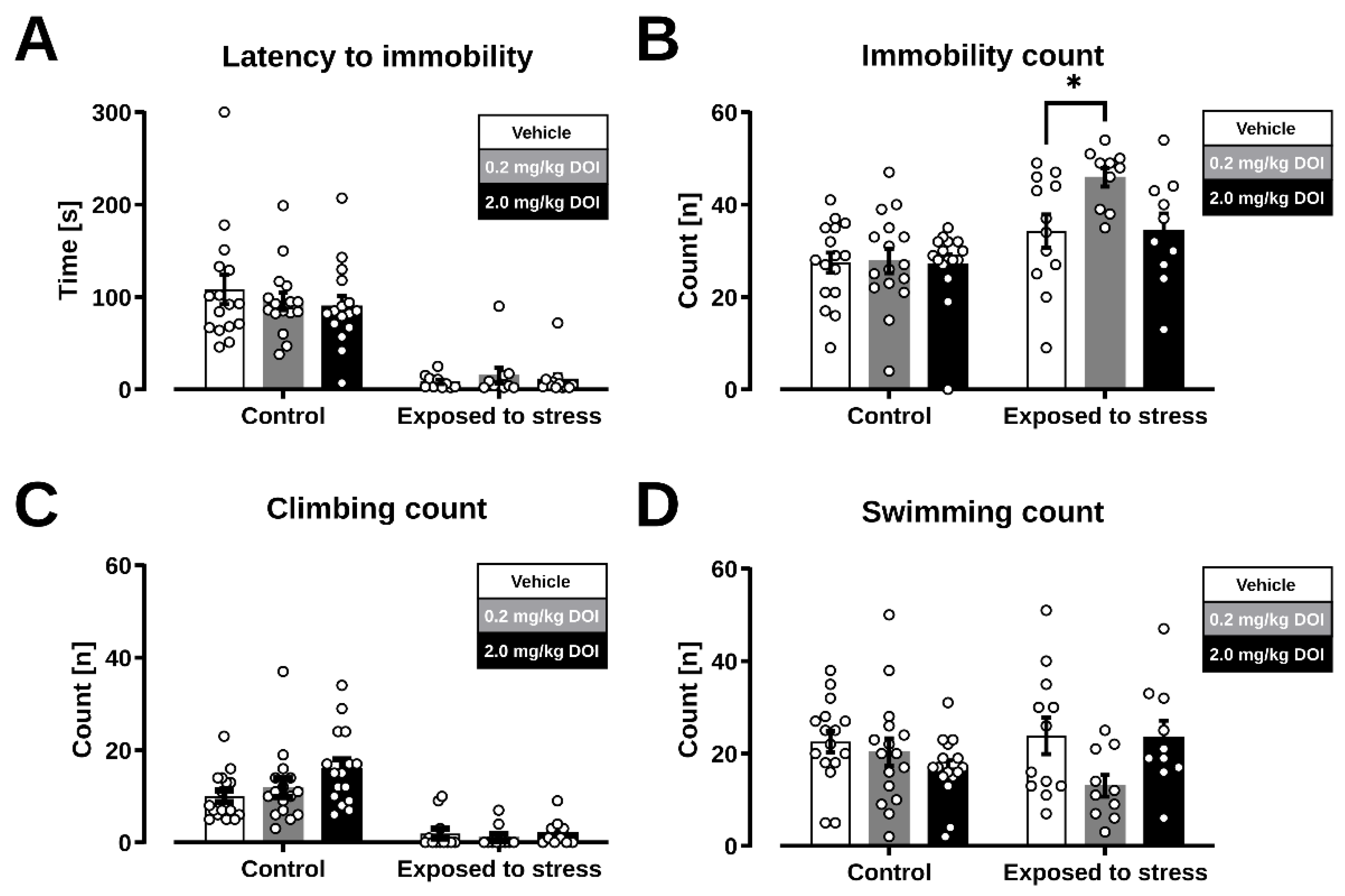
2.2. Forced Swim Test
2.3. 5-HT2A Receptor Protein Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Administration
4.3. Head Twitch Response
4.4. Locomotor Activity
4.5. Forced Swim Test
4.6. Western Blot
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naghavi, M. Global, Regional, and National Burden of Suicide Mortality 1990 to 2016: Systematic Analysis for the Global Burden of Disease Study 2016. BMJ 2019, 364, l94. [Google Scholar] [CrossRef] [Green Version]
- Jaffe, D.H.; Rive, B.; Denee, T.R. The Humanistic and Economic Burden of Treatment-Resistant Depression in Europe: A Cross-Sectional Study. BMC Psychiatry 2019, 19, 247. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, R.S.; Filteau, M.-J.; Martin, L.; Patry, S.; Carvalho, A.; Cha, D.S.; Barakat, M.; Miguelez, M. Treatment-Resistant Depression: Definitions, Review of the Evidence, and Algorithmic Approach. J. Affect. Disord. 2014, 156, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharm. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with Psychological Support for Treatment-Resistant Depression: An Open-Label Feasibility Study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Carhart-Harris, R.L.; Bolstridge, M.; Day, C.M.J.; Rucker, J.; Watts, R.; Erritzoe, D.E.; Kaelen, M.; Giribaldi, B.; Bloomfield, M.; Pilling, S.; et al. Psilocybin with Psychological Support for Treatment-Resistant Depression: Six-Month Follow-Up. Psychopharmacology 2018, 235, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.K.; Barrett, F.S.; Griffiths, R.R. Psychological Flexibility Mediates the Relations between Acute Psychedelic Effects and Subjective Decreases in Depression and Anxiety. J. Context. Behav. Sci. 2020, 15, 39–45. [Google Scholar] [CrossRef]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Coelho, N.L.; Marx, W.; Gonzalez, M.; Sinclair, J.; de Manincor, M.; Perkins, D.; Sarris, J. Classic Serotonergic Psychedelics for Mood and Depressive Symptoms: A Meta-Analysis of Mood Disorder Patients and Healthy Participants. Psychopharmacology 2021, 238, 341–354. [Google Scholar] [CrossRef]
- Cameron, L.P.; Nazarian, A.; Olson, D.E. Psychedelic Microdosing: Prevalence and Subjective Effects. J. Psychoact. Drugs 2020, 52, 113–122. [Google Scholar] [CrossRef]
- Hesselgrave, N.; Troppoli, T.A.; Wulff, A.B.; Cole, A.B.; Thompson, S.M. Harnessing Psilocybin: Antidepressant-like Behavioral and Synaptic Actions of Psilocybin Are Independent of 5-HT2R Activation in Mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2022489118. [Google Scholar] [CrossRef] [PubMed]
- Marek, G.J.; Aghajanian, G.K. LSD and the Phenethylamine Hallucinogen DOI Are Potent Partial Agonists at 5-HT2A Receptors on Interneurons in Rat Piriform Cortex. J. Pharm. Exp. Ther. 1996, 278, 1373–1382. [Google Scholar]
- González-Maeso, J.; Yuen, T.; Ebersole, B.J.; Wurmbach, E.; Lira, A.; Zhou, M.; Weisstaub, N.; Hen, R.; Gingrich, J.A.; Sealfon, S.C. Transcriptome Fingerprints Distinguish Hallucinogenic and Nonhallucinogenic 5-Hydroxytryptamine 2A Receptor Agonist Effects in Mouse Somatosensory Cortex. J. Neurosci. 2003, 23, 8836–8843. [Google Scholar] [CrossRef] [Green Version]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaki, S.; Becamel, C.; Murat, S.; la Cour, C.M.; Millan, M.J.; Prézeau, L.; Bockaert, J.; Marin, P.; Vandermoere, F. Quantitative Phosphoproteomics Unravels Biased Phosphorylation of Serotonin 2A Receptor at Ser280 by Hallucinogenic versus Nonhallucinogenic Agonists*. Mol. Cell. Proteom. 2014, 13, 1273–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. In Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A.L., Vollenweider, F.X., Nichols, D.E., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2017; Volume 36, pp. 45–73. ISBN 978-3-662-55878-2. [Google Scholar]
- Pottie, E.; Dedecker, P.; Stove, C.P. Identification of Psychedelic New Psychoactive Substances (NPS) Showing Biased Agonism at the 5-HT2AR through Simultaneous Use of β-Arrestin 2 and MiniGαq Bioassays. Biochem. Pharmacol. 2020, 182, 114251. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Hallak, J.E.; Baker, G.; Dursun, S. Hallucinogenic/Psychedelic 5HT2A Receptor Agonists as Rapid Antidepressant Therapeutics: Evidence and Mechanisms of Action. J. Psychopharmacol. 2021, 35, 453–458. [Google Scholar] [CrossRef]
- Kometer, M.; Schmidt, A.; Bachmann, R.; Studerus, E.; Seifritz, E.; Vollenweider, F.X. Psilocybin Biases Facial Recognition, Goal-Directed Behavior, and Mood State Toward Positive Relative to Negative Emotions Through Different Serotonergic Subreceptors. Biol. Psychiatry 2012, 72, 898–906. [Google Scholar] [CrossRef]
- Kometer, M.; Schmidt, A.; Jancke, L.; Vollenweider, F.X. Activation of Serotonin 2A Receptors Underlies the Psilocybin-Induced Effects on Oscillations, N170 Visual-Evoked Potentials, and Visual Hallucinations. J. Neurosci. 2013, 33, 10544–10551. [Google Scholar] [CrossRef] [Green Version]
- Valle, M.; Maqueda, A.E.; Rabella, M.; Rodríguez-Pujadas, A.; Antonijoan, R.M.; Romero, S.; Alonso, J.F.; Mañanas, M.À.; Barker, S.; Friedlander, P.; et al. Inhibition of Alpha Oscillations through Serotonin-2A Receptor Activation Underlies the Visual Effects of Ayahuasca in Humans. Eur. Neuropsychopharmacol. 2016, 26, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Kraehenmann, R.; Schmidt, A.; Friston, K.; Preller, K.H.; Seifritz, E.; Vollenweider, F.X. The Mixed Serotonin Receptor Agonist Psilocybin Reduces Threat-Induced Modulation of Amygdala Connectivity. NeuroImage Clin. 2016, 11, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, F.S.; Carbonaro, T.M.; Hurwitz, E.; Johnson, M.W.; Griffiths, R.R. Double-Blind Comparison of the Two Hallucinogens Psilocybin and Dextromethorphan: Effects on Cognition. Psychopharmacology 2018, 235, 2915–2927. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Burt, J.B.; Ji, J.L.; Schleifer, C.H.; Adkinson, B.D.; Stämpfli, P.; Seifritz, E.; Repovs, G.; Krystal, J.H.; Murray, J.D.; et al. Changes in Global and Thalamic Brain Connectivity in LSD-Induced Altered States of Consciousness Are Attributable to the 5-HT2A Receptor. eLife 2018, 7, e35082. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L. How Do Psychedelics Work? Curr. Opin. Psychiatry 2019, 32, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.J.; Sorensen, S.M.; Kehne, J.H.; Carr, A.A.; Palfreyman, M.G. The Role of 5-HT2A Receptors in Antipsychotic Activity. Life Sci. 1995, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.L.; Jocic, Z.; Hall, J.M.; Rasmussen, S.A.; Price, L.H. Mirtazapine Augmentation in the Treatment of Refractory Depression. J. Clin. Psychiatry 1999, 60, 45–49. [Google Scholar] [CrossRef]
- Macs, M.; Vandoolaeghe, E.; Desnyder, R. Efficacy of Treatment with Trazodone in Combination with Pindolol or Fluoxetine in Major Depression. J. Affect. Disord. 1996, 41, 201–210. [Google Scholar] [CrossRef]
- Roth, B.L.; Willins, D.L.; Kristiansen, K.; Kroeze, W.K. Activation Is Hallucinogenic and Antagonism Is Therapeutic: Role of 5-HT2A Receptors in Atypical Antipsychotic Drug Actions. Neuroscientist 1999, 5, 254–262. [Google Scholar] [CrossRef]
- Shi, J.; Landry, M.; Carrasco, G.A.; Battaglia, G.; Muma, N.A. Sustained Treatment with a 5-HT2A Receptor Agonist Causes Functional Desensitization and Reductions in Agonist-Labeled 5-HT2A Receptors despite Increases in Receptor Protein Levels in Rats. Neuropharmacology 2008, 55, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente Revenga, M.; Jaster, A.M.; McGinn, J.; Silva, G.; Saha, S.; González-Maeso, J. Tolerance and Cross-Tolerance among Psychedelic and Nonpsychedelic 5-HT 2A Receptor Agonists in Mice. ACS Chem. Neurosci. 2022, 13, 2436–2448. [Google Scholar] [CrossRef]
- Willins, D.L.; Berry, S.A.; Alsayegh, L.; Backstrom, J.R.; Sanders-Bush, E.; Friedman, L.; Roth, B.L. Clozapine and Other 5-Hydroxytryptamine-2A Receptor Antagonists Alter the Subcellular Distribution of 5-Hydroxytryptamine-2A Receptors in Vitro and in Vivo. Neuroscience 1999, 91, 599–606. [Google Scholar] [CrossRef]
- Gray, J.A.; Roth, B.L. Paradoxical Trafficking and Regulation of 5-HT2A Receptors by Agonists and Antagonists. Brain Res. Bull. 2001, 56, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.I.; Yadav, P.N.; Yao, W.-D.; Arbuckle, M.I.; Grant, S.G.N.; Caron, M.G.; Roth, B.L. PSD-95 Is Essential for Hallucinogen and Atypical Antipsychotic Drug Actions at Serotonin Receptors. J. Neurosci. 2009, 29, 7124–7136. [Google Scholar] [CrossRef] [Green Version]
- Sanders-Bush, E.; Breeding, M.; Knoth, K.; Tsutsumi, M. Sertraline-Induced Desensitization of the Serotonin 5HT-2 Receptor Transmembrane Signaling System. Psychopharmacology 1989, 99, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Puig, M.V.; Amargós-Bosch, M.; Adell, A.; Artigas, F. The Therapeutic Role of 5-HT1A and 5-HT2A Receptors in Depression. J. Psychiatry Neurosci. 2004, 29, 252–265. [Google Scholar]
- Yamauchi, M.; Miyara, T.; Matsushima, T.; Imanishi, T. Desensitization of 5-HT2A Receptor Function by Chronic Administration of Selective Serotonin Reuptake Inhibitors. Brain Res. 2006, 1067, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, E.K.; Mun, J.; Nye, J.A.; Kimmel, H.L.; Voll, R.J.; Stehouwer, J.S.; Rice, K.C.; Goodman, M.M.; Howell, L.L. Neurobiological Changes Mediating the Effects of Chronic Fluoxetine on Cocaine Use. Neuropsychopharmacology 2012, 37, 1816–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesseveur, G.; Repérant, C.; David, D.J.; Gardier, A.M.; Sanchez, C.; Guiard, B.P. 5-HT2A Receptor Inactivation Potentiates the Acute Antidepressant-like Activity of Escitalopram: Involvement of the Noradrenergic System. Exp. Brain Res. 2013, 226, 285–295. [Google Scholar] [CrossRef]
- Pandey, D.K.; Mahesh, R.; kumar, A.A.; Rao, V.S.; Arjun, M.; Rajkumar, R. A Novel 5-HT2A Receptor Antagonist Exhibits Antidepressant-like Effects in a Battery of Rodent Behavioural Assays: Approaching Early-Onset Antidepressants. Pharmacol. Biochem. Behav. 2010, 94, 363–373. [Google Scholar] [CrossRef]
- Redrobe, J.P.; Bourin, M. Partial Role of 5-HT2 and 5-HT3 Receptors in the Activity of Antidepressants in the Mouse Forced Swimming Test. Eur. J. Pharmacol. 1997, 325, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.G.; Bartoszyk, G.D.; Edwards, E.; Ashby, C.R., Jr. The Highly Selective 5-Hydroxytryptamine (5-HT)2A Receptor Antagonist, EMD 281014, Significantly Increases Swimming and Decreases Immobility in Male Congenital Learned Helpless Rats in the Forced Swim Test. Synapse 2004, 52, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Zaniewska, M.; McCreary, A.C.; Wydra, K.; Filip, M. Effects of Serotonin (5-HT)2 Receptor Ligands on Depression-like Behavior during Nicotine Withdrawal. Neuropharmacology 2010, 58, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Pilar-Cuéllar, F.; Vidal, R.; Pazos, A. Subchronic Treatment with Fluoxetine and Ketanserin Increases Hippocampal Brain-Derived Neurotrophic Factor, β-Catenin and Antidepressant-like Effects. Br. J. Pharm. 2012, 165, 1046–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, Y.; Yamada, S.; Yamada, J. The 5-HT2 Receptor Antagonist Reduces Immobility of Mice Treated with the Atypical Antidepressant Mianserin in the Forced Swimming Test. Biol. Pharm. Bull. 2002, 25, 1479–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, J.; Sugimoto, Y. Differential Effects of the 5-HT2 Receptor Antagonist on the Anti-Immobility Effects of Noradrenaline and Serotonin Reuptake Inhibitors in the Forced Swimming Test. Brain Res. 2002, 958, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Keitner, G.I.; Qin, B.; Ravindran, A.V.; Bauer, M.; Del Giovane, C.; Zhao, J.; Liu, Y.; Fang, Y.; Zhang, Y.; et al. Atypical Antipsychotic Augmentation for Treatment-Resistant Depression: A Systematic Review and Network Meta-Analysis. Int. J. Neuropsychopharmacol. 2015, 18, pyv060. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.R.; Woo, Y.S.; Ahn, H.S.; Ahn, I.M.; Kim, H.J.; Bahk, W.-M. Can Atypical Antipsychotic Augmentation Reduce Subsequent Treatment Failure More Effectively Among Depressed Patients with a Higher Degree of Treatment Resistance? A Meta-Analysis of Randomized Controlled Trials. Int. J. Neuropsychopharmacol. 2015, 18, pyv023. [Google Scholar] [CrossRef] [Green Version]
- Stanley, M.; Mann, J.J. Increased Serotonin-2 Binding Sites in Frontal Cortex of Suicide Victims. Lancet 1983, 321, 214–216. [Google Scholar] [CrossRef]
- Mann, J.J.; Stanley, M.; McBride, P.A.; McEwen, B.S. Increased Serotonin2 and β-Adrenergic Receptor Binding in the Frontal Cortices of Suicide Victims. Arch. Gen. Psychiatry 1986, 43, 954–959. [Google Scholar] [CrossRef]
- Arango, V.; Ernsberger, P.; Marzuk, P.M.; Chen, J.-S.; Tierney, H.; Stanley, M.; Reis, D.J.; Mann, J.J. Autoradiographic Demonstration of Increased Serotonin 5-HT2 and β-Adrenergic Receptor Binding Sites in the Brain of Suicide Victims. Arch. Gen. Psychiatry 1990, 47, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G.; Brière, R.; Dewar, K.; Antonetti, T.; Lesage, A.D.; Séguin, M.; Chawky, N.; Vanier, C.; Alda, M.; Joober, R.; et al. Prediction of Level of Serotonin 2A Receptor Binding by Serotonin Receptor 2A Genetic Variation in Postmortem Brain Samples from Subjects Who Did or Did Not Commit Suicide. Am. J. Psychiatry 1999, 156, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Oquendo, M.A.; Russo, S.A.; Underwood, M.D.; Kassir, S.A.; Ellis, S.P.; Mann, J.J.; Arango, V. Higher Postmortem Prefrontal 5-HT2A Receptor Binding Correlates with Lifetime Aggression in Suicide. Biol. Psychiatry 2006, 59, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Galfalvy, H.; Dwork, A.J.; Mann, J.J.; Arango, V. Serotonin Receptors and Suicide, Major Depression, Alcohol Use Disorder and Reported Early Life Adversity. Transl. Psychiatry 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Stockmeier, C.A.; Dilley, G.E.; Shapiro, L.A.; Overholser, J.C.; Thompson, P.A.; Meltzer, H.Y. Serotonin Receptors in Suicide Victims with Major Depression. Neuropsychopharmacology 1997, 16, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Underwood, M.D.; Kassir, S.A.; Bakalian, M.J.; Galfalvy, H.; Mann, J.J.; Arango, V. Neuron Density and Serotonin Receptor Binding in Prefrontal Cortex in Suicide. Int. J. Neuropsychopharmacol. 2012, 15, 435–447. [Google Scholar] [CrossRef] [Green Version]
- Frokjaer, V.G.; Mortensen, E.L.; Nielsen, F.A.; Haugbol, S.; Pinborg, L.H.; Adams, K.H.; Svarer, C.; Hasselbalch, S.G.; Holm, S.; Paulson, O.B.; et al. Frontolimbic Serotonin 2A Receptor Binding in Healthy Subjects Is Associated with Personality Risk Factors for Affective Disorder. Biol. Psychiatry 2008, 63, 569–576. [Google Scholar] [CrossRef]
- Baeken, C.; Bossuyt, A.; De Raedt, R. Dorsal Prefrontal Cortical Serotonin 2A Receptor Binding Indices Are Differentially Related to Individual Scores on Harm Avoidance. Psychiatry Res. 2014, 221, 162–168. [Google Scholar] [CrossRef]
- Bhagwagar, Z.; Hinz, R.; Taylor, M.; Fancy, S.; Cowen, P.; Grasby, P. Increased 5-HT(2A) Receptor Binding in Euthymic, Medication-Free Patients Recovered from Depression: A Positron Emission Study with [(11)C]MDL 100,907. Am. J. Psychiatry 2006, 163, 1580–1587. [Google Scholar] [CrossRef]
- Meyer, J.H.; Houle, S.; Sagrati, S.; Carella, A.; Hussey, D.F.; Ginovart, N.; Goulding, V.; Kennedy, J.; Wilson, A.A. Brain Serotonin Transporter Binding Potential Measured with Carbon 11-Labeled DASB Positron Emission Tomography: Effects of Major Depressive Episodes and Severity of Dysfunctional Attitudes. Arch. Gen. Psychiatry 2004, 61, 1271–1279. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.H.; Kapur, S.; Houle, S.; DaSilva, J.; Owczarek, B.; Brown, G.M.; Wilson, A.A.; Kennedy, S.H. Prefrontal Cortex 5-HT2 Receptors in Depression: An [18F] Setoperone PET Imaging Study. Am. J. Psychiatry 1999, 156, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Steiner, M.; Liddle, P.F.; Shiah, I.S.; Lam, R.W.; Zis, A.P.; Coote, M. A PET Study of Brain 5-HT2 Receptors and Their Correlation with Platelet 5-HT2 Receptors in Healthy Humans. Psychopharmacology 2000, 151, 424–427. [Google Scholar] [CrossRef] [PubMed]
- van Heeringen, C.; Audenaert, K.; Van Laere, K.; Dumont, F.; Slegers, G.; Mertens, J.; Dierckx, R.A. Prefrontal 5-HT2a Receptor Binding Index, Hopelessness and Personality Characteristics in Attempted Suicide. J. Affect. Disord. 2003, 74, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Sheline, Y.I.; Moerlein, S.M.; Vlassenko, A.G.; Huang, Y.; Snyder, A.Z. Decreased Hippocampal 5-HT2A Receptor Binding in Major Depressive Disorder: In Vivo Measurement with [18F]Altanserin Positron Emission Tomography. Biol. Psychiatry 2004, 55, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; De Raedt, R.; Bossuyt, A. Is Treatment-Resistance in Unipolar Melancholic Depression Characterized by Decreased Serotonin2A Receptors in the Dorsal Prefrontal—Anterior Cingulate Cortex? Neuropharmacology 2012, 62, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Dean, B.; Tawadros, N.; Seo, M.S.; Jeon, W.J.; Everall, I.; Scarr, E.; Gibbons, A. Lower Cortical Serotonin 2A Receptors in Major Depressive Disorder, Suicide and in Rats after Administration of Imipramine. Int. J. Neuropsychopharmacol. 2014, 17, 895–906. [Google Scholar] [CrossRef] [Green Version]
- Muguruza, C.; Miranda-Azpiazu, P.; Díez-Alarcia, R.; Morentin, B.; González-Maeso, J.; Callado, L.F.; Meana, J.J. Evaluation of 5-HT2A and MGlu2/3 Receptors in Postmortem Prefrontal Cortex of Subjects with Major Depressive Disorder: Effect of Antidepressant Treatment. Neuropharmacology 2014, 86, 311–318. [Google Scholar] [CrossRef]
- Meyer, J.H.; Kapur, S.; Eisfeld, B.; Brown, G.M.; Houle, S.; DaSilva, J.; Wilson, A.A.; Rafi-Tari, S.; Mayberg, H.S.; Kennedy, S.H. The Effect of Paroxetine on 5-HT(2A) Receptors in Depression: An [(18)F]Setoperone PET Imaging Study. Am. J. Psychiatry 2001, 158, 78–85. [Google Scholar] [CrossRef]
- Torda, T.; Culman, J.; Cechová, E.; Murgas, K. 3-H-Ketanserin (Serotonin Type 2) Binding in the Rat Frontal Cortex: Effect of Immobilization Stress. Endocrinol. Exp. 1988, 22, 99–105. [Google Scholar]
- Davis, S.; Heal, D.J.; Stanford, S.C. Long-Lasting Effects of an Acute Stress on the Neurochemistry and Function of 5-Hydroxytryptaminergic Neurones in the Mouse Brain. Psychopharmacology 1995, 118, 267–272. [Google Scholar] [CrossRef]
- McKittrick, C.R.; Blanchard, D.C.; Blanchard, R.J.; McEwen, B.S.; Sakai, R.R. Serotonin Receptor Binding in a Colony Model of Chronic Social Stress. Biol. Psychiatry 1995, 37, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Klimek, V.; Willner, P. Effects of Imipramine on Serotonergic and Beta-Adrenergic Receptor Binding in a Realistic Animal Model of Depression. Psychopharmacology 1994, 114, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Nagatani, T.; Kitamura, Y.; Kawasaki, K.; Hayakawa, H.; Yamawaki, S. Chronic Forced Swim Stress of Rats Increases Frontal Cortical 5-HT2 Receptors and the Wet-Dog Shakes They Mediate, but Not Frontal Cortical Beta-Adrenoceptors. Eur. J. Pharm. 1995, 294, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Ossowska, G.; Nowa, G.; Kata, R.; Klenk-Majewska, B.; Danilczuk, Z.; Zebrowska-Lupina, I. Brain Monoamine Receptors in a Chronic Unpredictable Stress Model in Rats. J. Neural. Transm. 2001, 108, 311–319. [Google Scholar] [CrossRef]
- Metz, A.; Heal, D.J. In Mice Repeated Administration of Electroconvulsive Shock or Desmethylimipramine Produces Rapid Alterations in 5-HT2-Mediated Head-Twitch Responses and Cortical 5-HT2 Receptor Number. Eur. J. Pharmacol. 1986, 126, 159–162. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Hanson, L.A.; Brotto, L.A. Chronic Stress Effects on Sexual Behavior in Male and Female Rats: Mediation by 5-HT2A Receptors. Pharm. Biochem. Behav. 1998, 61, 405–412. [Google Scholar] [CrossRef]
- Gorzalka, B.B.; Hanson, L.A. Sexual Behavior and Wet Dog Shakes in the Male Rat: Regulation by Corticosterone. Behav. Brain Res. 1998, 97, 143–151. [Google Scholar] [CrossRef]
- Sood, A.; Pati, S.; Bhattacharya, A.; Chaudhari, K.; Vaidya, V.A. Early Emergence of Altered 5-HT2A Receptor-Evoked Behavior, Neural Activation and Gene Expression Following Maternal Separation. Int. J. Dev. Neurosci. 2018, 65, 21–28. [Google Scholar] [CrossRef]
- Ueki, T.; Mizoguchi, K.; Yamaguchi, T.; Nishi, A.; Sekiguchi, K.; Ikarashi, Y.; Kase, Y. Yokukansan, a Traditional Japanese Medicine, Decreases Head-Twitch Behaviors and Serotonin 2A Receptors in the Prefrontal Cortex of Isolation-Stressed Mice. J. Ethnopharmacol. 2015, 166, 23–30. [Google Scholar] [CrossRef]
- Kitamura, Y.; Shibata, K.; Akiyama, K.; Kimoto, S.; Fujitani, Y.; Kitagawa, K.; Kanzaki, H.; Ouchida, M.; Shimizu, K.; Kawasaki, H.; et al. Increased DOI-Induced Wet-Dog Shakes in Adrenocorticotropic Hormone-Treated Rats Are Not Affected by Chronic Imipramine Treatment: Possible Involvement of Enhanced 5-HT(2A)-Receptor Expression in the Frontal Cortex. J. Pharm. Sci. 2008, 106, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Trajkovska, V.; Kirkegaard, L.; Krey, G.; Marcussen, A.B.; Thomsen, M.S.; Chourbaji, S.; Brandwein, C.; Ridder, S.; Halldin, C.; Gass, P.; et al. Activation of Glucocorticoid Receptors Increases 5-HT2A Receptor Levels. Exp. Neurol. 2009, 218, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; McKittrick, C.R.; File, S.E.; McEwen, B.S. Decreased 5-HT1A and Increased 5-HT2A Receptor Binding after Chronic Corticosterone Associated with a Behavioural Indication of Depression but Not Anxiety. Psychoneuroendocrinology 1997, 22, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Thompson, K.S.J.; Akhtar, S.; Handley, S.L. Increased 5-HT2A Receptor Expression and Function Following Central Glucocorticoid Receptor Knockdown in Vivo. Eur. J. Pharmacol. 2004, 502, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Suemaru, K.; Cui, R.; Umeda, Y.; Li, B.; Gomita, Y.; Kawasaki, H.; Araki, H. Effects of Physical and Psychological Stress on 5-HT2A Receptor-Mediated Wet-Dog Shake Responses in Streptozotocin-Induced Diabetic Rats. Acta Med. Okayama 2007, 61, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.K.D.; Meerlo, P.; Ettrup, A.; Knudsen, G.M.; Bosker, F.J.; den Boer, J.A.; Dierckx, R.A.J.O.; van Waarde, A. Acute Social Defeat Does Not Alter Cerebral 5-HT2A Receptor Binding in Male Wistar Rats. Synapse 2014, 68, 379–386. [Google Scholar] [CrossRef]
- Carneiro-Nascimento, S.; Powell, W.; Uebel, M.; Buerge, M.; Sigrist, H.; Patterson, M.; Pryce, C.R.; Opacka-Juffry, J. Region- and Receptor-Specific Effects of Chronic Social Stress on the Central Serotonergic System in Mice. IBRO Neurosci. Rep. 2021, 10, 8–16. [Google Scholar] [CrossRef]
- Yamada, S.; Watanabe, A.; Nankai, M.; Toru, M. Acute Immobilization Stress Reduces (+/-)DOI-Induced 5-HT2A Receptor-Mediated Head Shakes in Rats. Psychopharmacology 1995, 119, 9–14. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benson, C.J.; Dunlap, L.E.; Olson, D.E. Effects of N,N-Dimethyltryptamine on Rat Behaviors Relevant to Anxiety and Depression. ACS Chem. Neurosci. 2018, 9, 1582–1590. [Google Scholar] [CrossRef]
- Rodríguez, P.; Urbanavicius, J.; Prieto, J.P.; Fabius, S.; Reyes, A.L.; Havel, V.; Sames, D.; Scorza, C.; Carrera, I. A Single Administration of the Atypical Psychedelic Ibogaine or Its Metabolite Noribogaine Induces an Antidepressant-Like Effect in Rats. ACS Chem. Neurosci. 2020, 11, 1661–1672. [Google Scholar] [CrossRef]
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci. 2020, 11, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Knight, A.R.; Misra, A.; Quirk, K.; Benwell, K.; Revell, D.; Kennett, G.; Bickerdike, M. Pharmacological Characterisation of the Agonist Radioligand Binding Site of 5-HT2A, 5-HT2B and 5-HT2C Receptors. Naunyn-Schmiedeberg’s Arch. Pharm. 2004, 370, 114–123. [Google Scholar] [CrossRef]
- May, J.A.; Chen, H.-H.; Rusinko, A.; Lynch, V.M.; Sharif, N.A.; McLaughlin, M.A. A Novel and Selective 5-HT 2 Receptor Agonist with Ocular Hypotensive Activity: (S)-(+)-1-(2-Aminopropyl)-8,9-Dihydropyrano[3,2-e]Indole. J. Med. Chem. 2003, 46, 4188–4195. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; van der Heijden, I.; Ruderman, M.A.; Risbrough, V.B.; Gingrich, J.A.; Geyer, M.A.; Powell, S.B. 5-HT2A and 5-HT2C Receptors Exert Opposing Effects on Locomotor Activity in Mice. Neuropsychopharmacology 2009, 34, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Pędzich, B.D.; Rubens, S.; Sekssaoui, M.; Pierre, A.; Van Schuerbeek, A.; Marin, P.; Bockaert, J.; Valjent, E.; Bécamel, C.; De Bundel, D. Effects of a Psychedelic 5-HT2A Receptor Agonist on Anxiety-Related Behavior and Fear Processing in Mice. Neuropsychopharmacology 2022, 47, 1304–1314. [Google Scholar] [CrossRef]
- Peričić, D. Swim Stress Inhibits 5-HT2A Receptor-Mediated Head Twitch Behaviour in Mice. Psychopharmacology 2003, 167, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; Durand, M.; Aguerre, S.; Mormède, P.; Chaouloff, F. Behavioral, Neuroendocrine and Serotonergic Consequences of Single Social Defeat and Repeated Fluoxetine Pretreatment in the Lewis Rat Strain. Neuroscience 1999, 92, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Jaggar, M.; Weisstaub, N.; Gingrich, J.A.; Vaidya, V.A. 5-HT2A Receptor Deficiency Alters the Metabolic and Transcriptional, but Not the Behavioral, Consequences of Chronic Unpredictable Stress. Neurobiol. Stress 2017, 7, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Weisstaub, N.V.; Zhou, M.; Lira, A.; Lambe, E.; González-Maeso, J.; Hornung, J.-P.; Sibille, E.; Underwood, M.; Itohara, S.; Dauer, W.T.; et al. Cortical 5-HT 2A Receptor Signaling Modulates Anxiety-Like Behaviors in Mice. Science 2006, 313, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Qesseveur, G.; Petit, A.C.; Nguyen, H.T.; Dahan, L.; Colle, R.; Rotenberg, S.; Seif, I.; Robert, P.; David, D.; Guilloux, J.-P.; et al. Genetic Dysfunction of Serotonin 2A Receptor Hampers Response to Antidepressant Drugs: A Translational Approach. Neuropharmacology 2016, 105, 142–153. [Google Scholar] [CrossRef]
- Raote, I.; Bhattacharyya, S.; Panicker, M.M. Functional Selectivity in Serotonin Receptor 2A (5-HT2A) Endocytosis, Recycling, and Phosphorylation. Mol. Pharm. 2013, 83, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Günther, L.; Liebscher, S.; Jähkel, M.; Oehler, J. Effects of Chronic Citalopram Treatment on 5-HT1A and 5-HT2A Receptors in Group- and Isolation-Housed Mice. Eur. J. Pharmacol. 2008, 593, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Jefsen, O.; Højgaard, K.; Christiansen, S.L.; Elfving, B.; Nutt, D.J.; Wegener, G.; Müller, H.K. Psilocybin Lacks Antidepressant-like Effect in the Flinders Sensitive Line Rat. Acta Neuropsychiatr. 2019, 31, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Gregorio, D.; Inserra, A.; Enns, J.P.; Markopoulos, A.; Pileggi, M.; El Rahimy, Y.; Lopez-Canul, M.; Comai, S.; Gobbi, G. Repeated Lysergic Acid Diethylamide (LSD) Reverses Stress-Induced Anxiety-like Behavior, Cortical Synaptogenesis Deficits and Serotonergic Neurotransmission Decline. Neuropsychopharmacology 2022, 47, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.P.; Benson, C.J.; DeFelice, B.C.; Fiehn, O.; Olson, D.E. Chronic, Intermittent Microdoses of the Psychedelic N,N -Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem. Neurosci. 2019, 10, 3261–3270. [Google Scholar] [CrossRef] [Green Version]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D.; et al. A Non-Hallucinogenic Psychedelic Analogue with Therapeutic Potential. Nature 2021, 589, 474–479. [Google Scholar] [CrossRef]
- Wojtas, A.; Bysiek, A.; Wawrzczak-Bargiela, A.; Szych, Z.; Majcher-Maślanka, I.; Herian, M.; Maćkowiak, M.; Gołembiowska, K. Effect of Psilocybin and Ketamine on Brain Neurotransmitters, Glutamate Receptors, DNA and Rat Behavior. Int. J. Mol. Sci. 2022, 23, 6713. [Google Scholar] [CrossRef]
- de la Fuente Revenga, M.; Zhu, B.; Guevara, C.A.; Naler, L.B.; Saunders, J.M.; Zhou, Z.; Toneatti, R.; Sierra, S.; Wolstenholme, J.T.; Beardsley, P.M.; et al. Prolonged Epigenomic and Synaptic Plasticity Alterations Following Single Exposure to a Psychedelic in Mice. Cell Rep. 2021, 37, 109836. [Google Scholar] [CrossRef]
- Barre, A.; Berthoux, C.; De Bundel, D.; Valjent, E.; Bockaert, J.; Marin, P.; Bécamel, C. Presynaptic Serotonin 2A Receptors Modulate Thalamocortical Plasticity and Associative Learning. Proc. Natl. Acad. Sci. USA 2016, 113, E1382–E1391. [Google Scholar] [CrossRef] [Green Version]
- Morici, J.F.; Miranda, M.; Gallo, F.T.; Zanoni, B.; Bekinschtein, P.; Weisstaub, N.V. 5-HT2a Receptor in MPFC Influences Context-Guided Reconsolidation of Object Memory in Perirhinal Cortex. eLife 2018, 7, e33746. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Renner, M.C.; Gonzalez, M.C.; Weisstaub, N. Role of Medial Prefrontal Cortex Serotonin 2A Receptors in the Control of Retrieval of Recognition Memory in Rats. J. Neurosci. 2013, 33, 15716–15725. [Google Scholar] [CrossRef] [Green Version]
- Healy, C.J. The Acute Effects of Classic Psychedelics on Memory in Humans. Psychopharmacology 2021, 238, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Wießner, I.; Olivieri, R.; Falchi, M.; Palhano-Fontes, F.; Oliveira Maia, L.; Feilding, A.; B Araujo, D.; Ribeiro, S.; Tófoli, L.F. LSD, Afterglow and Hangover: Increased Episodic Memory and Verbal Fluency, Decreased Cognitive Flexibility. Eur. Neuropsychopharmacol. 2022, 58, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Simoneau, J.; Cohen, M.S.; Zimmerman, S.M.; Henson, C.M.; Rice, K.C.; Woods, J.H. Interaction of 5-HT 2A and 5-HT 2C Receptors in R (−)-2,5-Dimethoxy-4-Iodoamphetamine-Elicited Head Twitch Behavior in Mice. J. Pharm. Exp. Ther. 2010, 335, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Siuciak, J.A.; Chapin, D.S.; McCarthy, S.A.; Guanowsky, V.; Brown, J.; Chiang, P.; Marala, R.; Patterson, T.; Seymour, P.A.; Swick, A.; et al. CP-809,101, a Selective 5-HT2C Agonist, Shows Activity in Animal Models of Antipsychotic Activity. Neuropharmacology 2007, 52, 279–290. [Google Scholar] [CrossRef]
- Gibson, E.L.; Barnfield, A.M.C.; Curzon, G. Evidence That MCPP-Induced Anxiety in the plus-Maze Is Mediated by Postsynaptic 5-HT2C Receptors but Not by Sympathomimetic Effects. Neuropharmacology 1994, 33, 457–465. [Google Scholar] [CrossRef]
- Mora, P.O.; Netto, C.F.; Graeff, F.G. Role of 5-HT2A and 5-HT2C Receptor Subtypes in the Two Types of Fear Generated by the Elevated T-Maze. Pharmacol. Biochem. Behav. 1997, 58, 1051–1057. [Google Scholar] [CrossRef]
- Kimura, A.; Stevenson, P.L.; Carter, R.N.; MacColl, G.; French, K.L.; Paul Simons, J.; Al-Shawi, R.; Kelly, V.; Chapman, K.E.; Holmes, M.C. Overexpression of 5-HT 2C Receptors in Forebrain Leads to Elevated Anxiety and Hypoactivity. Eur. J. Neurosci. 2009, 30, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Heisler, L.K.; Zhou, L.; Bajwa, P.; Hsu, J.; Tecott, L.H. Serotonin 5-HT 2C Receptors Regulate Anxiety-like Behavior. Genes Brain Behav. 2007, 6, 491–496. [Google Scholar] [CrossRef]
- Ferré, S.; Baler, R.; Bouvier, M.; Caron, M.G.; Devi, L.A.; Durroux, T.; Fuxe, K.; George, S.R.; Javitch, J.A.; Lohse, M.J.; et al. Building a New Conceptual Framework for Receptor Heteromers. Nat. Chem. Biol. 2009, 5, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Moutkine, I.; Quentin, E.; Guiard, B.P.; Maroteaux, L.; Doly, S. Heterodimers of Serotonin Receptor Subtypes 2 Are Driven by 5-HT2C Protomers. J. Biol. Chem. 2017, 292, 6352–6368. [Google Scholar] [CrossRef] [Green Version]
- Pokorny, T.; Preller, K.H.; Kraehenmann, R.; Vollenweider, F.X. Modulatory Effect of the 5-HT1A Agonist Buspirone and the Mixed Non-Hallucinogenic 5-HT1A/2A Agonist Ergotamine on Psilocybin-Induced Psychedelic Experience. Eur. Neuropsychopharmacol. 2016, 26, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Fantegrossi, W.E.; Reissig, C.J.; Katz, E.B.; Yarosh, H.L.; Rice, K.C.; Winter, J.C. Hallucinogen-like Effects of N,N-Dipropyltryptamine (DPT): Possible Mediation by Serotonin 5-HT1A and 5-HT2A Receptors in Rodents. Pharmacol. Biochem. Behav. 2008, 88, 358–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchborn, T.; Schröder, H.; Höllt, V.; Grecksch, G. Repeated Lysergic Acid Diethylamide in an Animal Model of Depression: Normalisation of Learning Behaviour and Hippocampal Serotonin 5-HT 2 Signalling. J. Psychopharmacol. 2014, 28, 545–552. [Google Scholar] [CrossRef]
- Berthoux, C.; Barre, A.; Bockaert, J.; Marin, P.; Bécamel, C. Sustained Activation of Postsynaptic 5-HT2A Receptors Gates Plasticity at Prefrontal Cortex Synapses. Cereb. Cortex 2019, 29, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Bullich, S.; Delcourte, S.; Haddjeri, N.; Guiard, B.P. Learned Immobility Produces Enduring Impairment of the HPA Axis Reactivity in Mice without Replicating the Broad Spectrum of Depressive-Like Phenotype. Int. J. Mol. Sci. 2021, 22, 937. [Google Scholar] [CrossRef]
- Trunnell, E.R.; Carvalho, C. The Forced Swim Test Has Poor Accuracy for Identifying Novel Antidepressants. Drug Discov. Today 2021, 26, 2898–2904. [Google Scholar] [CrossRef]
- Kara, N.Z.; Stukalin, Y.; Einat, H. Revisiting the Validity of the Mouse Forced Swim Test: Systematic Review and Meta-Analysis of the Effects of Prototypic Antidepressants. Neurosci. Biobehav. Rev. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Yin, C.-Y.; Zhu, L.-J.; Zhu, X.-H.; Xu, C.; Luo, C.-X.; Chen, H.; Zhu, D.-Y.; Zhou, Q.-G. Sucrose Preference Test for Measurement of Stress-Induced Anhedonia in Mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef]
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased Global Integration in the Brain after Psilocybin Therapy for Depression. Nat. Med. 2022, 28, 844–851. [Google Scholar] [CrossRef]
- Darmani, N.A.; Gerdes, C.F. Temporal Differential Adaptation of Head-Twitch and Ear-Scratch Responses Following Administration of Challenge Doses of DOI. Pharmacol. Biochem. Behav. 1995, 50, 545–550. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Characterization of the Head-Twitch Response Induced by Hallucinogens in Mice. Psychopharmacology 2013, 227, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A.; Martin, B.R.; Glennon, R.A. Behavioral Evidence for Differential Adaptation of the Serotonergic System after Acute and Chronic Treatment with (+/-)-1-(2,5-Dimethoxy-4-Iodophenyl)-2-Aminopropane (DOI) or Ketanserin. J. Pharm. Exp. Ther. 1992, 262, 692–698. [Google Scholar]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazavchinsky, L.; Dafna, A.; Einat, H. Individual Variability in Female and Male Mice in a Test-Retest Protocol of the Forced Swim Test. J. Pharmacol. Toxicol. Methods 2019, 95, 12–15. [Google Scholar] [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Coping with the Forced Swim Stressor: Current State-of-the-Art. Behav. Brain Res. 2019, 364, 1–10. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji—An Open Source Platform for Biological Image Analysis. Nat Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Guiard, B.P.; Di Giovanni, G. (Eds.) 5-HT2A Receptors in the Central Nervous System; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-70472-2. [Google Scholar]
- Morici, J.F.; Ciccia, L.; Malleret, G.; Gingrich, J.A.; Bekinschtein, P.; Weisstaub, N.V. Serotonin 2a Receptor and Serotonin 1a Receptor Interact Within the Medial Prefrontal Cortex During Recognition Memory in Mice. Front. Pharmacol. 2015, 6, 298. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Ásgeirsdóttir, H.N.; Cohen, S.J.; Munchow, A.H.; Barrera, M.P.; Stackman, R.W. Stimulation of Serotonin 2A Receptors Facilitates Consolidation and Extinction of Fear Memory in C57BL/6J Mice. Neuropharmacology 2013, 64, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Martin, D.A.; Nichols, C.D. Psychedelics Recruit Multiple Cellular Types and Produce Complex Transcriptional Responses Within the Brain. EBioMedicine 2016, 11, 262–277. [Google Scholar] [CrossRef]
- Martin, D.A.; Nichols, C.D. The Effects of Hallucinogens on Gene Expression. In Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A.L., Vollenweider, F.X., Nichols, D.E., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2018; pp. 137–158. ISBN 978-3-662-55880-5. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pędzich, B.D.; Medrano, M.; Buckinx, A.; Smolders, I.; De Bundel, D. Psychedelic-Induced Serotonin 2A Receptor Downregulation Does Not Predict Swim Stress Coping in Mice. Int. J. Mol. Sci. 2022, 23, 15284. https://doi.org/10.3390/ijms232315284
Pędzich BD, Medrano M, Buckinx A, Smolders I, De Bundel D. Psychedelic-Induced Serotonin 2A Receptor Downregulation Does Not Predict Swim Stress Coping in Mice. International Journal of Molecular Sciences. 2022; 23(23):15284. https://doi.org/10.3390/ijms232315284
Chicago/Turabian StylePędzich, Błażej D., Mireia Medrano, An Buckinx, Ilse Smolders, and Dimitri De Bundel. 2022. "Psychedelic-Induced Serotonin 2A Receptor Downregulation Does Not Predict Swim Stress Coping in Mice" International Journal of Molecular Sciences 23, no. 23: 15284. https://doi.org/10.3390/ijms232315284




