Autophagy: A Double-Edged Sword in Male Reproduction
Abstract
:1. Introduction
2. What Is Autophagy?
3. Physiological Effects of Autophagy on Male Reproduction
3.1. Autophagy in Different Types of Testicular Cells
3.2. Autophagy in Spermatogenesis
3.3. Autophagy in the Endocrinology of Testis
3.4. The Role of mTORC1 in Autophagy in Male Reproduction
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancilla, H.; Maldonado, R.; Cereceda, K.; Villarroel-Espíndola, F.; Montes de Oca, M.; Angulo, C.; Castro, M.A.; Slebe, J.C.; Vera, J.C.; Lavandero, S.; et al. Glutathione Depletion Induces Spermatogonial Cell Autophagy. J. Cell. Biochem. 2015, 116, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wan, H.; Li, X.; Liu, W.; Chen, Q.; Wang, Y.; Yang, L.; Tang, H.; Zhang, X.; Duan, E.; et al. Atg7 is required for acrosome biogenesis during spermatogenesis in mice. Cell Res. 2014, 24, 852–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azhar, S.; Leers-Sucheta, S.; Reaven, E. Cholesterol uptake in adrenal and gonadal tissues: The SR-BI and ‘selective’ pathway connection. Front. Biosci. J. Virtual Libr. 2003, 8, s998–s1029. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Li, G.; Liu, C.; Gao, H.; Wang, H.; Liu, W.; Chen, M.; Shang, Y.; Wang, L.; Shi, J.; et al. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J. Cell Biol. 2018, 217, 2103–2119. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhou, Y.; Zhu, Y.C.; Wang, S.Q.; Ping, P.; Chen, X.F. Lipophagy Contributes to Testosterone Biosynthesis in Male Rat Leydig Cells. Endocrinology 2018, 159, 1119–1129. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, H.Z.; Xu, L.M.; Huang, Y.R.; Dai, H.L.; Kang, X.N. Testosterone regulates the autophagic clearance of androgen binding protein in rat Sertoli cells. Sci. Rep. 2015, 5, 8894. [Google Scholar] [CrossRef] [Green Version]
- Della-Maria, J.; Gerard, A.; Franck, P.; Gerard, H. Effects of androgen-binding protein (ABP) on spermatid Tnp1 gene expression in vitro. Mol. Cell. Endocrinol. 2002, 198, 131–141. [Google Scholar] [CrossRef]
- Ahmed, N.; Liu, Y.; Chen, H.; Yang, P.; Waqas, Y.; Liu, T.; Gandahi, J.A.; Huang, Y.; Wang, L.; Song, X.; et al. Novel cellular evidence of lipophagy within the Sertoli cells during spermatogenesis in the turtle. Aging 2016, 9, 41–51. [Google Scholar] [CrossRef]
- Horibe, A.; Eid, N.; Ito, Y.; Otsuki, Y.; Kondo, Y. Ethanol-Induced Autophagy in Sertoli Cells Is Specifically Marked at Androgen-Dependent Stages of the Spermatogenic Cycle: Potential Mechanisms and Implications. Int. J. Mol. Sci. 2019, 20, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Khawar, M.B.; Li, W. Autophagy in Reproduction. Adv. Exp. Med. Biol. 2019, 1206, 453–468. [Google Scholar] [CrossRef]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganschow, R.E.; Schimke, R.T. Independent genetic control of the catalytic activity and the rate of degradation of catalase in mice. J. Biol. Chem. 1969, 244, 4649–4658. [Google Scholar] [CrossRef] [PubMed]
- Kalish, F.; Chovick, N.; Dice, J.F. Rapid in vivo degradation of glycoproteins isolated from cytosol. J. Biol. Chem. 1979, 254, 4475–4481. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Klionsky, D.J. Autophagy in health and disease: A double-edged sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef] [Green Version]
- De Duve, C.; Pressman, B.C.; Gianetto, R.; Wattiaux, R.; Appelmans, F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem. J. 1955, 60, 604–617. [Google Scholar] [CrossRef] [Green Version]
- Novikoff, A.B.; Beaufay, H.; de Duve, C. Electron microscopy of lysosomerich fractions from rat liver. J. Biophys. Biochem. Cytol. 1956, 2, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Arstila, A.U.; Trump, B.F. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am. J. Pathol. 1968, 53, 687–733. [Google Scholar] [PubMed] [Green Version]
- Clark, S.L., Jr. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J. Biophys. Biochem. Cytol. 1957, 3, 349–362. [Google Scholar] [CrossRef]
- Cohlan S, Q. Lysosomes Ciba Foundation Symposium. Postgrad Med. J. 1964, 40, 557. [Google Scholar] [CrossRef]
- Klionsky, D.J. The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 2005, 118, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Nie, T.; Zhu, L.; Yang, Q. The Classification and Basic Processes of Autophagy. Adv. Exp. Med. Biol. 2021, 1208, 3–16. [Google Scholar] [CrossRef]
- Tolkovsky, A.M. Mitophagy. Biochim. Biophys. Acta 2009, 1793, 1508–1515. [Google Scholar] [CrossRef] [Green Version]
- Lemasters, J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005, 8, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Marchetti, S.; Ricci, J.E. No Parkin Zone: Mitophagy without Parkin. Trends Cell Biol. 2018, 28, 882–895. [Google Scholar] [CrossRef]
- Liu, K.; Czaja, M.J. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 2013, 20, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- Weckman, A.; Di Ieva, A.; Rotondo, F.; Syro, L.V.; Ortiz, L.D.; Kovacs, K.; Cusimano, M.D. Autophagy in the endocrine glands. J. Mol. Endocrinol. 2014, 52, R151–R163. [Google Scholar] [CrossRef]
- Eberhart, T.; Kovacs, W.J. Pexophagy in yeast and mammals: An update on mysteries. Histochem. Cell Biol. 2018, 150, 473–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.; Verma, S.; Seranova, E.; Sarkar, S.; Kumar, D. Selective Autophagy and Xenophagy in Infection and Disease. Front. Cell Dev. Biol. 2018, 6, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidberg, H.; Shvets, E.; Elazar, Z. Biogenesis and cargo selectivity of autophagosomes. Annu. Rev. Biochem. 2011, 80, 125–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Eaten alive: A history of macroautophagy. Nat. Cell Biol. 2010, 12, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Mercer, C.A.; Kaliappan, A.; Dennis, P.B. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy 2009, 5, 649–662. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, N.; Sasaki, T.; Iemura, S.; Natsume, T.; Hara, T.; Mizushima, N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy 2009, 5, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Hara, T.; Takamura, A.; Kishi, C.; Iemura, S.; Natsume, T.; Guan, J.L.; Mizushima, N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell Biol. 2008, 181, 497–510. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.Y.; Longatti, A.; McKnight, N.C.; Tooze, S.A. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol. Cell. Biol. 2009, 29, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Itakura, E.; Mizushima, N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy 2010, 6, 764–776. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yin, Q.; Wei, D.; Yang, Z.; Du, Y.; Ma, Y. Autophagy in male reproduction. Syst. Biol. Reprod. Med. 2019, 65, 265–272. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deretic, V.; Levine, B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 2009, 5, 527–549. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, B.; Duden, R.; Rubinsztein, D.C. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 2002, 11, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Rubinsztein, D.C.; Gestwicki, J.E.; Murphy, L.O.; Klionsky, D.J. Potential therapeutic applications of autophagy. Nat. Rev.. Drug Discov. 2007, 6, 304–312. [Google Scholar] [CrossRef]
- Goodwin, J.M.; Dowdle, W.E.; DeJesus, R.; Wang, Z.; Bergman, P.; Kobylarz, M.; Lindeman, A.; Xavier, R.J.; McAllister, G.; Nyfeler, B.; et al. Autophagy-Independent Lysosomal Targeting Regulated by ULK1/2-FIP200 and ATG9. Cell Rep. 2017, 20, 2341–2356. [Google Scholar] [CrossRef] [Green Version]
- Hess, R.A.; Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef]
- Purvis, K.; Cusan, L.; Hansson, V. Regulation of steroidogenesis and steroid action in Leydig cells. J. Steroid Biochem. 1981, 15, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Chen, L.; Chang, Z.J.; Xin, H.; Liu, T.; Zhang, Y.Q.; Li, G.Y.; Zhou, F.; Gong, Y.Q.; Gao, Z.Z.; et al. Autophagic deficiency is related to steroidogenic decline in aged rat Leydig cells. Asian J. Androl. 2011, 13, 881–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Wang, H.; Shang, Y.; Liu, W.; Song, Z.; Zhao, H.; Wang, L.; Jia, P.; Gao, F.; Xu, Z.; et al. Autophagy is required for ectoplasmic specialization assembly in sertoli cells. Autophagy 2016, 12, 814–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, J.; Tang, X.M. Functional implication of autophagy in steroid-secreting cells of the rat. Anat. Rec. 1995, 242, 137–146. [Google Scholar] [CrossRef]
- Saewu, A.; Kongmanas, K.; Raghupathy, R.; Netherton, J.; Kadunganattil, S.; Linton, J.J.; Chaisuriyong, W.; Faull, K.F.; Baker, M.A.; Tanphaichitr, N. Primary Sertoli Cell Cultures from Adult Mice Have Different Properties Compared With Those Derived From 20-Day-Old Animals. Endocrinology 2020, 161, bqz020. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, H.; Jia, P.; Zhao, H.; Liu, C.; Liu, W.; Song, Z.; Xu, Z.; Yang, L.; Wang, Y.; et al. Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy 2016, 12, 1575–1592. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Chen, H.; Zirkin, B.R. Temporal relationships among testosterone production, steroidogenic acute regulatory protein (StAR), and P450 side-chain cleavage enzyme (P450scc) during Leydig cell aging. J. Androl. 2005, 26, 25–31. [Google Scholar]
- Qian, X.; Mruk, D.D.; Cheng, Y.H.; Tang, E.I.; Han, D.; Lee, W.M.; Wong, E.W.; Cheng, C.Y. Actin binding proteins, spermatid transport and spermiation. Semin. Cell Dev. Biol. 2014, 30, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Schlatt, S.; Ehmcke, J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Semin. Cell Dev. Biol. 2014, 29, 2–16. [Google Scholar] [CrossRef]
- Tamargo-Gómez, I.; Mariño, G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 2018, 19, 3812. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.Y.; Yang, B.; Shi, Y.X.; Zhang, W.L.; Liu, F.; Zhao, W.; Yang, M.W. High glucose downregulates the effects of autophagy on osteoclastogenesis via the AMPK/mTOR/ULK1 pathway. Biochem. Biophys. Res. Commun. 2018, 503, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Li, F.; Feng, X.; Yang, H.; Han, L.; Fan, Y.; Nie, H.; Wang, Z.; Wang, F.; Zhang, Y. Influences of different dietary energy level on sheep testicular development associated with AMPK/ULK1/autophagy pathway. Theriogenology 2018, 108, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Liu, Y.; Su, Z.; Dawar, F.U.; Dan, H.; He, Y.; Gui, J.F.; Mei, J. Leucine mediates autophagosome-lysosome fusion and improves sperm motility by activating the PI3K/Akt pathway. Oncotarget 2017, 8, 111807–111818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zheng, Y.; Lv, Y.; Li, F.; Su, L.; Qin, Y.; Zeng, W. Melatonin protects the mouse testis against heat-induced damage. Mol. Hum. Reprod. 2020, 26, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Orth, J.M.; Gunsalus, G.L.; Lamperti, A.A. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 1988, 122, 787–794. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells--immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.H.; Cheng, C.Y. The blood-testis barrier: Its biology, regulation, and physiological role in spermatogenesis. Curr. Top. Dev. Biol. 2005, 71, 263–296. [Google Scholar] [CrossRef]
- Yan, H.H.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Ectoplasmic specialization: A friend or a foe of spermatogenesis? BioEssays News Rev. Mol. Cell. Dev. Biol. 2007, 29, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Toyama, Y.; Maekawa, M.; Yuasa, S. Ectoplasmic specializations in the Sertoli cell: New vistas based on genetic defects and testicular toxicology. Anat. Sci. Int. 2003, 78, 1–16. [Google Scholar] [CrossRef]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, C.; Ji, Y.; Chen, Z.; Kitazato, K.; Xiang, Y.; Zhong, M.; Wang, Q.; Pei, Y.; Ju, H.; Wang, Y. Proteomics analysis of autophagy-deficient Atg7-/- MEFs reveals a close relationship between F-actin and autophagy. Biochem. Biophys. Res. Commun. 2013, 437, 482–488. [Google Scholar] [CrossRef]
- Da Ros, M.; Lehtiniemi, T.; Olotu, O.; Fischer, D.; Zhang, F.P.; Vihinen, H.; Jokitalo, E.; Sironen, A.; Toppari, J.; Kotaja, N. FYCO1 and autophagy control the integrity of the haploid male germ cell-specific RNP granules. Autophagy 2017, 13, 302–321. [Google Scholar] [CrossRef] [PubMed]
- Berruti, G.; Paiardi, C. Acrosome biogenesis: Revisiting old questions to yield new insights. Spermatogenesis 2011, 1, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khawar, M.B.; Gao, H.; Li, W. Mechanism of Acrosome Biogenesis in Mammals. Front. Cell Dev. Biol. 2019, 7, 195. [Google Scholar] [CrossRef]
- Haas, A.K.; Yoshimura, S.; Stephens, D.J.; Preisinger, C.; Fuchs, E.; Barr, F.A. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J. Cell Sci. 2007, 120, 2997–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A.K.; Liegel, R.P.; Ronchetti, A.; Ebert, A.D.; Geurts, A.; Sidjanin, D.J. Targeted disruption of Tbc1d20 with zinc-finger nucleases causes cataracts and testicular abnormalities in mice. BMC Genet. 2014, 15, 135. [Google Scholar] [CrossRef]
- Liegel, R.P.; Handley, M.T.; Ronchetti, A.; Brown, S.; Langemeyer, L.; Linford, A.; Chang, B.; Morris-Rosendahl, D.J.; Carpanini, S.; Posmyk, R.; et al. Loss-of-function mutations in TBC1D20 cause cataracts and male infertility in blind sterile mice and Warburg micro syndrome in humans. Am. J. Hum. Genet. 2013, 93, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Sidjanin, D.J.; Park, A.K.; Ronchetti, A.; Martins, J.; Jackson, W.T. TBC1D20 mediates autophagy as a key regulator of autophagosome maturation. Autophagy 2016, 12, 1759–1775. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Song, Z.; Wang, L.; Yu, H.; Liu, W.; Shang, Y.; Xu, Z.; Zhao, H.; Gao, F.; Wen, J.; et al. Sirt1 regulates acrosome biogenesis by modulating autophagic flux during spermiogenesis in mice. Dev. Camb. Engl. 2017, 144, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Lehti, M.S.; Zhang, F.P.; Kotaja, N.; Sironen, A. SPEF2 functions in microtubule-mediated transport in elongating spermatids to ensure proper male germ cell differentiation. Dev. Camb. Engl. 2017, 144, 2683–2693. [Google Scholar] [CrossRef]
- Finetti, F.; Cassioli, C.; Cianfanelli, V.; Onnis, A.; Paccagnini, E.; Kabanova, A.; Baldari, C.T. The intraflagellar transport protein IFT20 controls lysosome biogenesis by regulating the post-Golgi transport of acid hydrolases. Cell Death Differ. 2020, 27, 310–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zeng, L.; Su, P.; Ma, L.; Zhang, M.; Zhang, Y.Z. Autophagy: A multifaceted player in the fate of sperm. Hum. Reprod. Update 2022, 28, 200–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Z.; Khawar, M.B.; Liu, C.; Li, W. The histone codes for meiosis. Reproduction 2017, 154, R65–R79. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.S.; Falciatori, I.; Tam, O.H.; Burgess, R.; Meikar, O.; Kotaja, N.; Hammell, M.; Hannon, G.J. piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes Dev. 2015, 29, 1032–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meikar, O.; Da Ros, M.; Korhonen, H.; Kotaja, N. Chromatoid body and small RNAs in male germ cells. Reproduction 2011, 142, 195–209. [Google Scholar] [CrossRef]
- Xiong, M.; Zhu, Z.; Tian, S.; Zhu, R.; Bai, S.; Fu, K.; Davis, J.G.; Sun, Z.; Baur, J.A.; Zheng, K.; et al. Conditional ablation of Raptor in the male germline causes infertility due to meiotic arrest and impaired inactivation of sex chromosomes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 3934–3949. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, I.M.; Espino, J.; Bejarano, I.; Gallardo-Soler, A.; Campo, M.L.; Salido, G.M.; Pariente, J.A.; Peña, F.J.; Tapia, J.A. Autophagy-related proteins are functionally active in human spermatozoa and may be involved in the regulation of cell survival and motility. Sci. Rep. 2016, 6, 33647. [Google Scholar] [CrossRef]
- Yang, P.; Ahmed, N.; Wang, L.; Chen, H.; Waqas, Y.; Liu, T.; Haseeb, A.; Bangulzai, N.; Huang, Y.; Chen, Q. In vivo autophagy and biogenesis of autophagosomes within male haploid cells during spermiogenesis. Oncotarget 2017, 8, 56791–56801. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Yang, P.; Huang, Y.; Chen, H.; Liu, T.; Wang, L.; Nabi, F.; Liu, Y.; Chen, Q. Entosis Acts as a Novel Way within Sertoli Cells to Eliminate Spermatozoa in Seminiferous Tubule. Front. Physiol. 2017, 8, 361. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Huang, W.; Liu, S.; Cai, S.; Hong, L.; Xiao, W.; Thiele, K.; Zeng, Y.; Song, M.; Diao, L. Impacts of Immunometabolism on Male Reproduction. Front. Immunol. 2021, 12, 658432. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharma, M. Basics of Human Andrology: A Textbook; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Plant, T.M. 60 years of neuroendocrinology: The hypothalamo-pituitary-gonadal axis. J. Endocrinol. 2015, 226, T41–T54. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.C. Endocrinology of testicular neoplasms. Urology 1981, 17, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Grossmann, M.; Gow, P.J.; Angus, P.W. Testosterone in men with advanced liver disease: Abnormalities and implications. J. Gastroenterol. Hepatol. 2015, 30, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.M.; Zhang, H.X.; Yi, J. Leydig cells—A normal cell model of cellular autophagy. Shi Yan Sheng Wu Xue Bao 1992, 25, 39–47. [Google Scholar] [PubMed]
- Gao, H.; Liu, C.; Li, W. Assessing Autophagy in the Leydig Cells. Methods Mol. Biol. 2019, 1854, 71–85. [Google Scholar] [CrossRef]
- Jones, T.H. Late onset hypogonadism. BMJ Clin. Res. Ed. 2009, 338, b352. [Google Scholar] [CrossRef]
- Gao, H.; Khawar, M.B.; Li, W. Essential role of autophagy in resource allocation during sexual reproduction. Autophagy 2020, 16, 18–27. [Google Scholar] [CrossRef]
- Chen, H.; Hardy, M.P.; Zirkin, B.R. Age-related decreases in Leydig cell testosterone production are not restored by exposure to LH in vitro. Endocrinology 2002, 143, 1637–1642. [Google Scholar] [CrossRef]
- Luo, L.; Chen, H.; Zirkin, B.R. Leydig cell aging: Steroidogenic acute regulatory protein (StAR) and cholesterol side-chain cleavage enzyme. J. Androl. 2001, 22, 149–156. [Google Scholar] [PubMed]
- Manna, P.R.; Dyson, M.T.; Stocco, D.M. Regulation of the steroidogenic acute regulatory protein gene expression: Present and future perspectives. Mol. Hum. Reprod. 2009, 15, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Joseph, D.R. Structure, function, and regulation of androgen-binding protein/sex hormone-binding globulin. Vitam. Horm. 1994, 49, 197–280. [Google Scholar] [CrossRef] [PubMed]
- Attramadal, A.; Bardin, C.W.; Gunsalus, G.L.; Musto, N.A.; Hansson, V. Immunocytochemical localization of androgen binding protein in rat Sertoli and epididymal cells. Biol. Reprod. 1981, 25, 983–988. [Google Scholar] [CrossRef] [Green Version]
- Selva, D.M.; Bassas, L.; Munell, F.; Mata, A.; Tekpetey, F.; Lewis, J.G.; Hammond, G.L. Human sperm sex hormone-binding globulin isoform: Characterization and measurement by time-resolved fluorescence immunoassay. J. Clin. Endocrinol. Metab. 2005, 90, 6275–6282. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dubocq, F.; Jiang, Y.; Tiguert, R.; Gheiler, E.L.; Dhabuwala, C.B. Effect of surgically induced varicocele on testicular blood flow and Sertoli cell function. Urology 1999, 53, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Danzo, B.J.; Pavlou, S.N.; Anthony, H.L. Hormonal regulation of androgen-binding protein in the rat. Endocrinology 1990, 127, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Aleem, M.; Padwal, V.; Choudhari, J.; Balasinor, N.; Parte, P.; Gill-Sharma, M.K. Estradiol affects androgen-binding protein expression and fertilizing ability of spermatozoa in adult male rats. Mol. Cell. Endocrinol. 2006, 253, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, N.; Osawa, Y. Purification of human placental aromatase cytochrome P-450 with monoclonal antibody and its characterization. Biochemistry 1991, 30, 3003–3010. [Google Scholar] [CrossRef]
- Robertson, K.M.; O’Donnell, L.; Simpson, E.R.; Jones, M.E. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology 2002, 143, 2913–2921. [Google Scholar] [CrossRef]
- Robertson, K.M.; Simpson, E.R.; Lacham-Kaplan, O.; Jones, M.E. Characterization of the fertility of male aromatase knockout mice. J. Androl. 2001, 22, 825–830. [Google Scholar] [PubMed]
- Lin, L.; Baehrecke, E.H. Autophagy, cell death, and cancer. Mol. Cell. Oncol. 2015, 2, e985913. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, H.; Liao, J.; Chen, K.; Javed, M.T.; Qiao, N.; Zeng, Q.; Liu, B.; Yi, J.; Tang, Z.; et al. Long-term copper exposure promotes apoptosis and autophagy by inducing oxidative stress in pig testis. Environ. Sci. Pollut. Res. Int. 2021, 28, 55140–55153. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh, M.; Karimfar, B.; Amiri, A.A.; Akbari, A. Protective Effect of Nano-Vitamin C on Infertility due to Oxidative Stress Induced by Lead and Arsenic in Male Rats. J. Chem. 2021, 2021, 9589345. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Naghani, E.S.; Fallah, M.M. The comparison of effect of origanum vulgar aqueous extract and vitamin C on the control of cadmium chlorie damage in testicular tissue in male rats. J. Babol Univ. Med. Sci. 2020, 20, 44–50. [Google Scholar]
- Silva, J.V.; Cabral, M.; Correia, B.R.; Carvalho, P.; Sousa, M.; Oliveira, P.F.; Fardilha, M. mTOR Signaling Pathway Regulates Sperm Quality in Older Men. Cells 2019, 8, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yao, Y.; Pan, J.; Guo, X.; Han, X.; Zhou, J.; Meng, X. Maternal exposure to Di-(2-ethylhexyl) phthalate (DEHP) activates the PI3K/Akt/mTOR signaling pathway in F1 and F2 generation adult mouse testis. Exp. Cell Res. 2020, 394, 112151. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, L.; Geng, Y.; He, J.; Chen, X.; Xu, H.; Li, R.; Wang, Y.; Ding, Y.; Liu, X. Rapamycin inhibits spermatogenesis by changing the autophagy status through suppressing mechanistic target of rapamycin-p70S6 kinase in male rats. Mol. Med. Rep. 2017, 16, 4029–4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Shen, L.; Chen, X.; Ding, Y.; He, J.; Zhu, J.; Wang, Y.; Liu, X. mTOR/P70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats. Reprod. Biomed. Online 2016, 32, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. mTORC1 as the main gateway to autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Domke, L.M.; Rickelt, S.; Dörflinger, Y.; Kuhn, C.; Winter-Simanowski, S.; Zimbelmann, R.; Rosin-Arbesfeld, R.; Heid, H.; Franke, W.W. The cell-cell junctions of mammalian testes: I. The adhering junctions of the seminiferous epithelium represent special differentiation structures. Cell Tissue Res. 2014, 357, 645–665. [Google Scholar] [CrossRef] [Green Version]
- Bowles, J.; Knight, D.; Smith, C.; Wilhelm, D.; Richman, J.; Mamiya, S.; Yashiro, K.; Chawengsaksophak, K.; Wilson, M.J.; Rossant, J.; et al. Retinoid signaling determines germ cell fate in mice. Science 2006, 312, 596–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, B.; DePinho, R.A. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle 2009, 8, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Zheng, W.; Shen, Y.; Gorre, N.; Hämäläinen, T.; Cooney, A.J.; Huhtaniemi, I.; Lan, Z.J.; Liu, K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum. Mol. Genet. 2010, 19, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Busada, J.T.; Niedenberger, B.A.; Velte, E.K.; Keiper, B.D.; Geyer, C.B. Mammalian target of rapamycin complex 1 (mTORC1) Is required for mouse spermatogonial differentiation in vivo. Dev. Biol. 2015, 407, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Serra, N.; Velte, E.K.; Niedenberger, B.A.; Kirsanov, O.; Geyer, C.B. The mTORC1 component RPTOR is required for maintenance of the foundational spermatogonial stem cell pool in mice. Biol. Reprod. 2019, 100, 429–439. [Google Scholar] [CrossRef]
- Riera, M.F.; Regueira, M.; Galardo, M.N.; Pellizzari, E.H.; Meroni, S.B.; Cigorraga, S.B. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E914–E923. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Wang, C.; Wang, Z.; Dai, H.; Song, Q.; Zou, Z.; Xiao, B.; Zhao, A.Z.; Bai, X.; Chen, Z. Raptor directs Sertoli cell cytoskeletal organization and polarity in the mouse testis. Biol. Reprod. 2018, 99, 1289–1302. [Google Scholar] [CrossRef]
- Miller, S.; Oleksy, A.; Perisic, O.; Williams, R.L. Finding a fitting shoe for Cinderella: Searching for an autophagy inhibitor. Autophagy 2010, 6, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.F.; Li, Y.M.; Chen, N.; Fan, Y.; Huang, W.K.; Hu, S.F.; Rao, M.; Zhang, Y.Z.; Su, P. Cross-talk between autophagy and apoptosis regulates testicular injury/recovery induced by cadmium via PI3K with mTOR-independent pathway. Cell Death Dis. 2020, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Abraham, M.C.; Shaham, S. Death without caspases, caspases without death. Trends Cell Biol. 2004, 14, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Proud, C.G. mTORC1 signaling: What we still don’t know. J. Mol. Cell Biol. 2011, 3, 206–220. [Google Scholar] [CrossRef] [Green Version]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Anim. Int. J. Anim. Biosci. 2018, 12, s27–s35. [Google Scholar] [CrossRef] [Green Version]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ma, Y.; Zhang, J.; Jiang, S.; Yuan, G.; Cheng, J.; Lan, T.; Hao, J. Alteration in autophagy gene expression profile correlates with low sperm quality. Reprod. Biol. 2021, 21, 100546. [Google Scholar] [CrossRef] [PubMed]
- Vallenius, T.; Luukko, K.; Mäkelä, T.P. CLP-36 PDZ-LIM protein associates with nonmuscle alpha-actinin-1 and alpha-actinin-4. J. Biol. Chem. 2000, 275, 11100–11105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yefimova, M.G.; Messaddeq, N.; Harnois, T.; Meunier, A.C.; Clarhaut, J.; Noblanc, A.; Weickert, J.L.; Cantereau, A.; Philippe, M.; Bourmeyster, N.; et al. A chimerical phagocytosis model reveals the recruitment by Sertoli cells of autophagy for the degradation of ingested illegitimate substrates. Autophagy 2013, 9, 653–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, W.; Tarique, I.; Deng, B.; Zhang, Y.; Haseeb, A.; Chen, Q.; Yang, P. Cellular evidence of autophagy in Sertoli cells during spermatogenesis in goats. Theriogenology 2020, 154, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.X.; Zhu, H.L.; Shi, X.T.; Nan, Y.; Liu, W.B.; Dai, L.M.; Xiong, Y.W.; Yi, S.J.; Cao, X.L.; Xu, D.X.; et al. Autophagy in Sertoli cell protects against environmental cadmium-induced germ cell apoptosis in mouse testes. Environ. Pollut. 2021, 270, 116241. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yan, W.J.; Yin, T.L.; Zhang, Y.; Li, J.; Yang, J. Diet-induced obesity impairs spermatogenesis: A potential role for autophagy. Sci. Rep. 2017, 7, 43475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Sato, K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science 2011, 334, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.C.; Adekola, L.; Papadopoulos, V.; Chen, H.; Zirkin, B.R. Leydig cell aging and hypogonadism. Exp. Gerontol. 2015, 68, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassil, N. Late-onset hypogonadism. Med. Clin. North Am. 2011, 95, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Bassil, N.; Morley, J.E. Late-life onset hypogonadism: A review. Clin. Geriatr. Med. 2010, 26, 197–222. [Google Scholar] [CrossRef]
- Morales, A.; Bella, A.J.; Chun, S.; Lee, J.; Assimakopoulos, P.; Bebb, R.; Gottesman, I.; Alarie, P.; Dugré, H.; Elliott, S. A practical guide to diagnosis, management and treatment of testosterone deficiency for Canadian physicians. Can. Urol. Assoc. J. 2010, 4, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Pan, L.; Zou, Z.; Wang, D.; Lu, Y.; Dong, Z.; Zhu, L. Hypoxia reduces testosterone synthesis in mouse Leydig cells by inhibiting NRF1-activated StAR expression. Oncotarget 2017, 8, 16401–16413. [Google Scholar] [CrossRef] [Green Version]
- Azhar, S.; Reaven, E. Scavenger receptor class BI and selective cholesteryl ester uptake: Partners in the regulation of steroidogenesis. Mol. Cell. Endocrinol. 2002, 195, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pan, T. N6-methyladenosine–encoded epitranscriptomics. Nat. Struct. Mol. Biol. 2016, 23, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Xu, D.; Xiang, Z.; Ding, J.; Yang, X.; Li, D.; Han, X. m(6)A mRNA methylation regulates testosterone synthesis through modulating autophagy in Leydig cells. Autophagy 2021, 17, 457–475. [Google Scholar] [CrossRef]
- Valvezan, A.J.; Manning, B.D. Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat. Metab. 2019, 1, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Deleyto-Seldas, N.; Efeyan, A. The mTOR-Autophagy Axis and the Control of Metabolism. Front. Cell Dev. Biol. 2021, 9, 655731. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, M.; Tursi, N.J.; Yan, B.; Cao, X.; Che, Q.; Yang, N.; Dong, X. Uropathogenic Escherichia coli Infection Compromises the Blood-Testis Barrier by Disturbing mTORC1-mTORC2 Balance. Front. Immunol. 2021, 12, 582858. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Mruk, D.D. A local autocrine axis in the testes that regulates spermatogenesis. Nat. Rev. Endocrinol. 2010, 6, 380–395. [Google Scholar] [CrossRef] [Green Version]
- Mruk, D.D.; Silvestrini, B.; Cheng, C.Y. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol. Rev. 2008, 60, 146–180. [Google Scholar] [CrossRef] [Green Version]
- Mok, K.W.; Mruk, D.D.; Cheng, C.Y. Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the “Yin” and “Yang” effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. Int. Rev. Cell Mol. Biol. 2013, 301, 291–358. [Google Scholar] [CrossRef] [Green Version]
- Mok, K.W.; Chen, H.; Lee, W.M.; Cheng, C.Y. rpS6 regulates blood-testis barrier dynamics through Arp3-mediated actin microfilament organization in rat sertoli cells. An in vitro study. Endocrinology 2015, 156, 1900–1913. [Google Scholar] [CrossRef] [Green Version]
- Den Hond, E.; Tournaye, H.; de Sutter, P.; Ombelet, W.; Baeyens, W.; Covaci, A.; Cox, B.; Nawrot, T.S.; van Larebeke, N.; D’Hooghe, T. Human exposure to endocrine disrupting chemicals and fertility: A case-control study in male subfertility patients. Environ. Int. 2015, 84, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, W.; Bian, X.; Yuan, Y.; Gu, J.; Liu, X.; Liu, Z.; Bian, J. Zearalenone induces apoptosis and cytoprotective autophagy in primary Leydig cells. Toxicol. Lett. 2014, 226, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, K.; Ling, X.; Wang, Z.; Zou, P.; Wang, X.; Gao, J.; Yin, L.; Zhang, X.; Liu, J.; et al. DBP-induced endoplasmic reticulum stress in male germ cells causes autophagy, which has a cytoprotective role against apoptosis in vitro and in vivo. Toxicol. Lett. 2016, 245, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, J.; Zeng, L.; Yang, D.; Shao, S.; Wang, J.; Wei, J.; Xiong, J.; Chen, J. Role of autophagy in di-2-ethylhexyl phthalate (DEHP)-induced apoptosis in mouse Leydig cells. Environ. Pollut. 2018, 243, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Y.; Wang, X.; Qian, W.; Han, X. Microcystin-LR induces autophagy and apoptosis in rat Sertoli cells in vitro. Toxicon Off. J. Int. Soc. Toxinology 2013, 76, 84–93. [Google Scholar] [CrossRef]
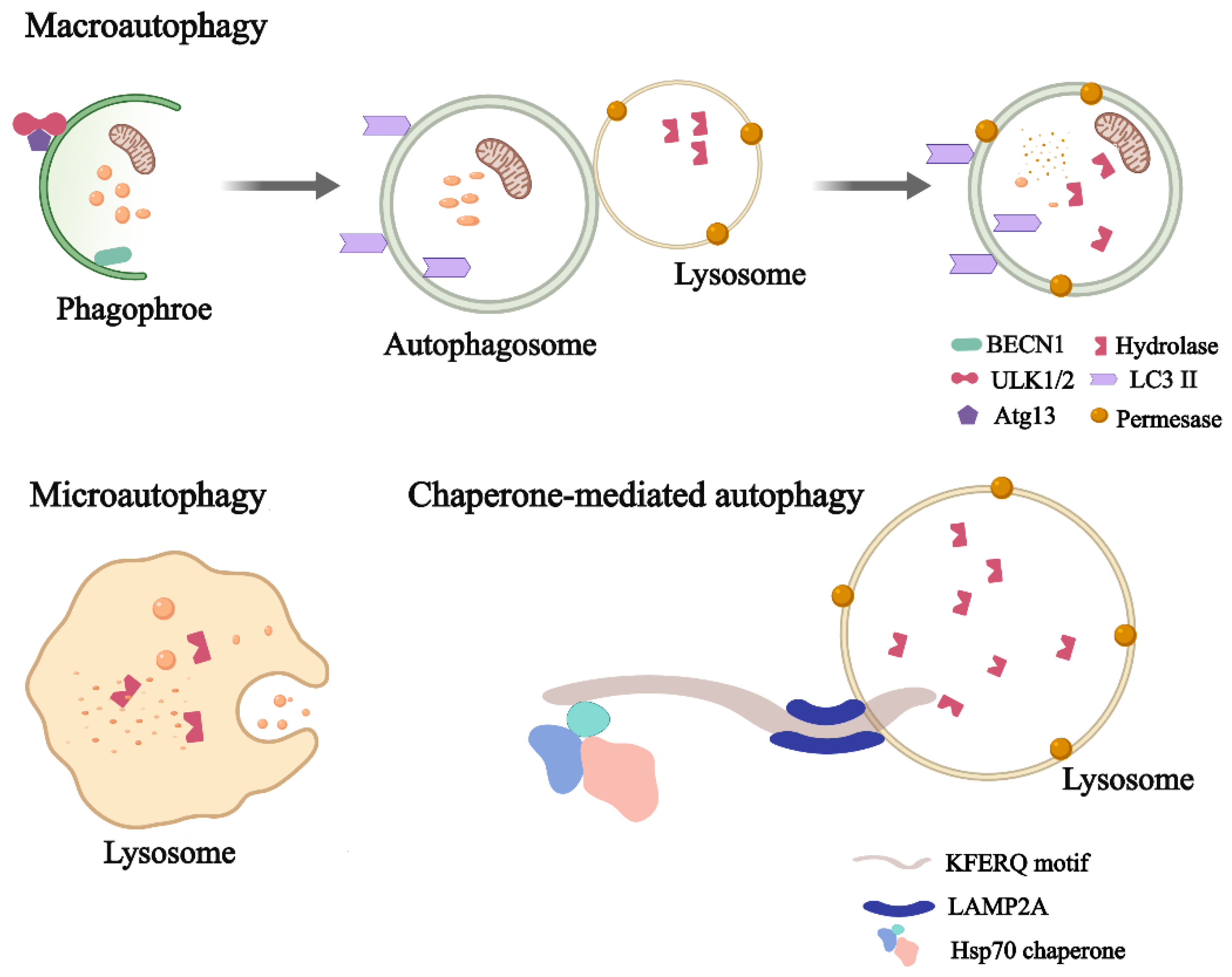
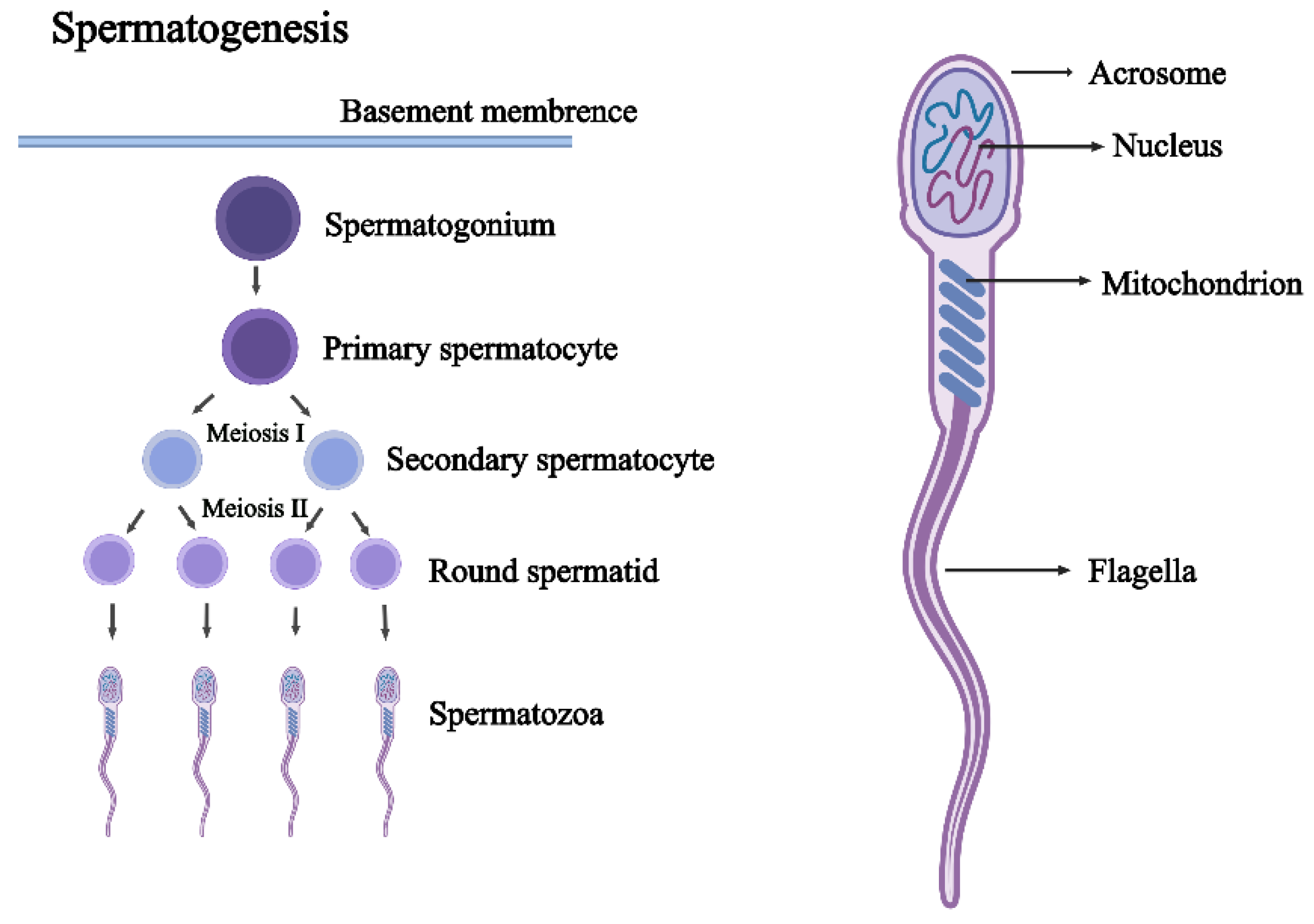
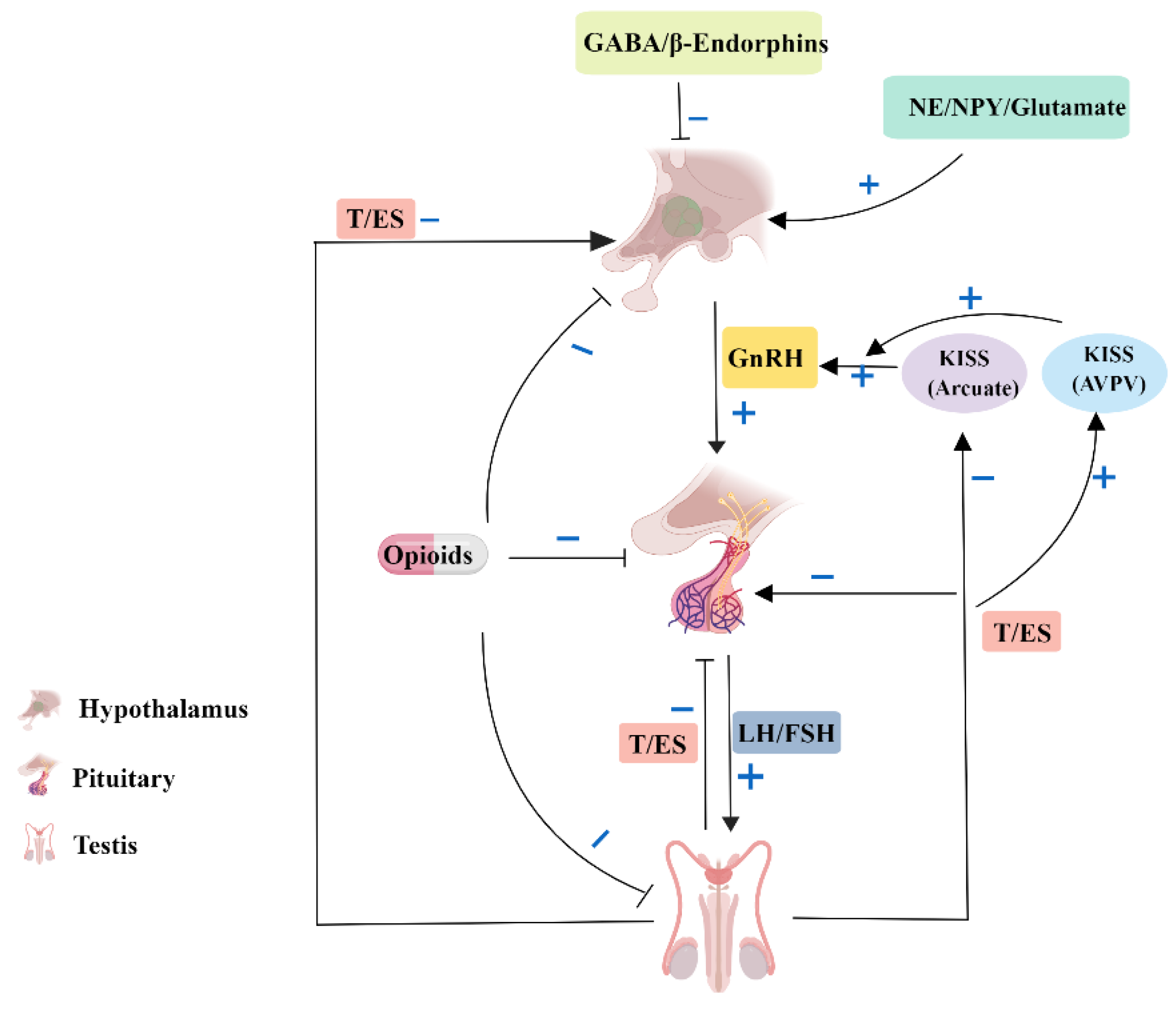
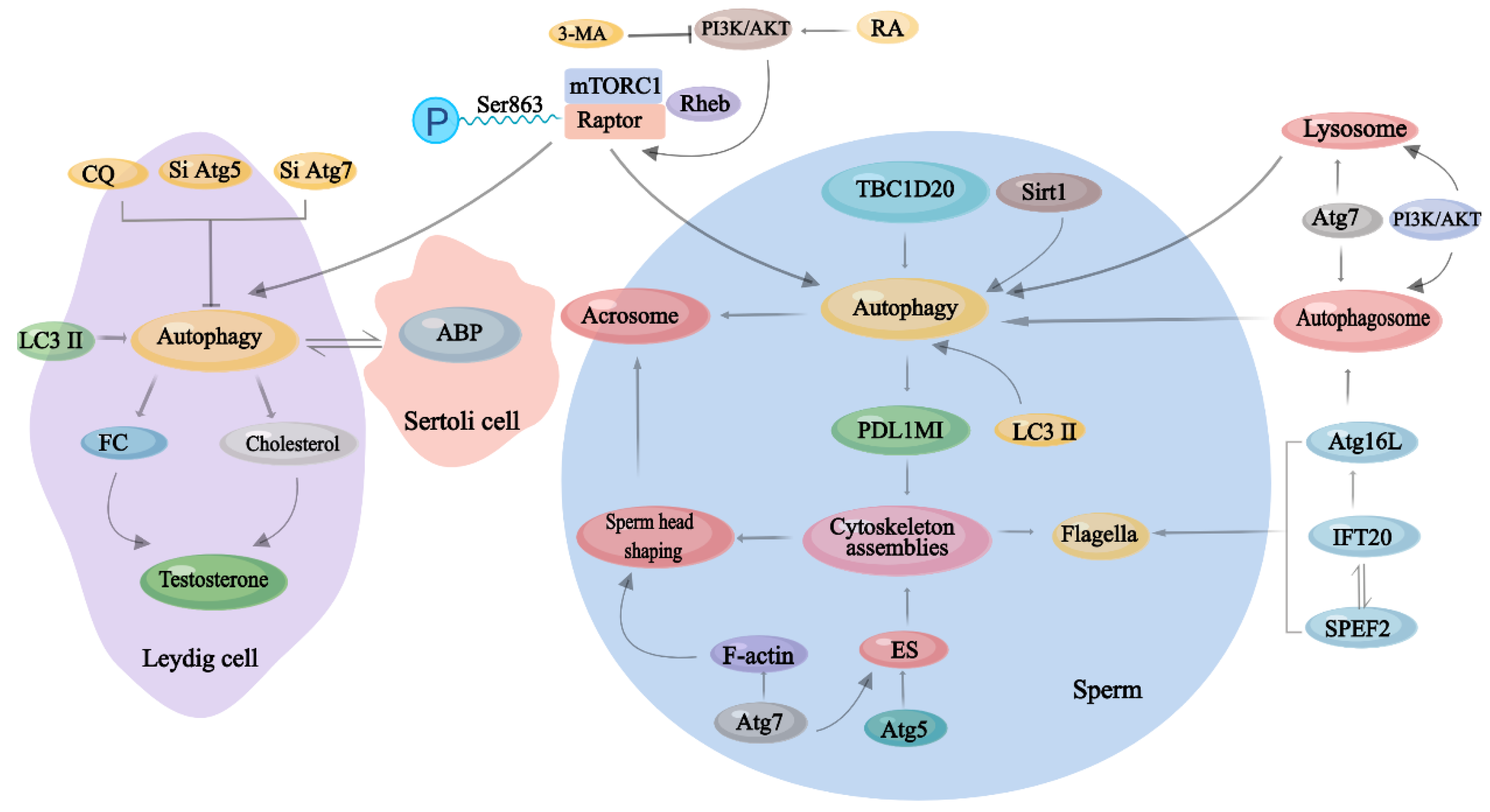
| Cell Types | Role of Autophagy | Dysfunction | References |
|---|---|---|---|
| Sertoli cells | clear ABP; regulate secretory activity; degrade useless components in seminiferous tubules; modulate ES formation; maintain the normal cytoskeletal organization | Prolongation of the half-life of ABP; disorders of the cytoskeleton of Sertoli cells; destruction of the ES structure | [8,53,54,55] |
| Spermatozoa | participate in acrosome biogenesis; regulate the formation of flagellum | malformation of spermatozoa head; decreasing of spermatozoa motility | [4,56] |
| Spermatogonial cells and Spermatogonial stem cells | adaptive protection | decreased ATP content; cell death | [3] |
| Leydig cells | maintain the production of testosterone; facilitate the uptake of cholesterol | steroidogenic decline | [6,52,57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Zhang, Y.; Wang, Q.; Yuan, L. Autophagy: A Double-Edged Sword in Male Reproduction. Int. J. Mol. Sci. 2022, 23, 15273. https://doi.org/10.3390/ijms232315273
Yan Q, Zhang Y, Wang Q, Yuan L. Autophagy: A Double-Edged Sword in Male Reproduction. International Journal of Molecular Sciences. 2022; 23(23):15273. https://doi.org/10.3390/ijms232315273
Chicago/Turabian StyleYan, Qiu, Yong Zhang, Qi Wang, and Ligang Yuan. 2022. "Autophagy: A Double-Edged Sword in Male Reproduction" International Journal of Molecular Sciences 23, no. 23: 15273. https://doi.org/10.3390/ijms232315273
APA StyleYan, Q., Zhang, Y., Wang, Q., & Yuan, L. (2022). Autophagy: A Double-Edged Sword in Male Reproduction. International Journal of Molecular Sciences, 23(23), 15273. https://doi.org/10.3390/ijms232315273





