Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted N-[2-(Phenyliminomethyl)phenyl]-4-methylbenzenesulfamide and Their Zinc(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Azomethines and Their Zinc Complexes
2.2. Elemental Analysis and Spectroscopic Data
2.3. X-ray Absorption Spectroscopy
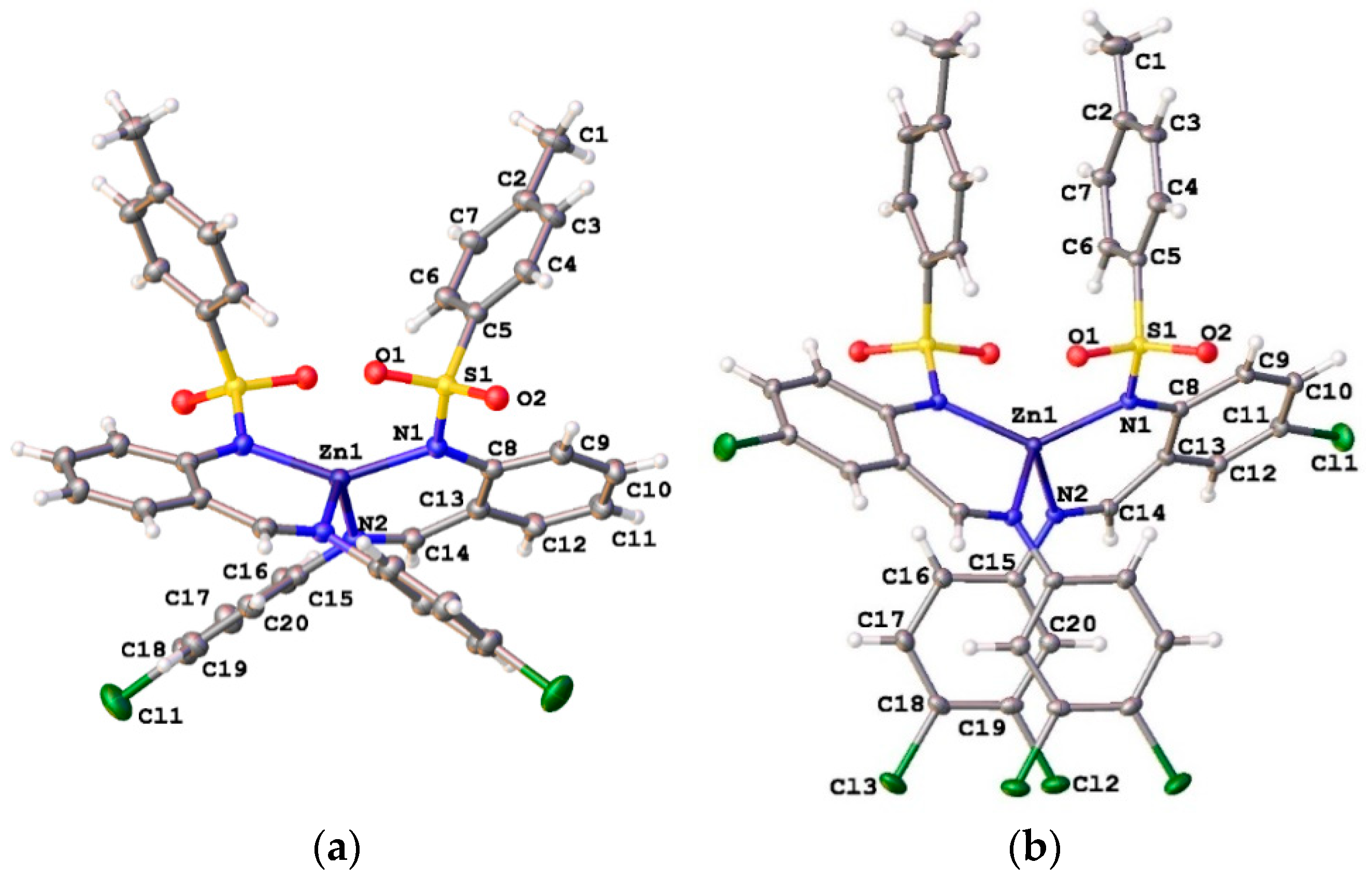
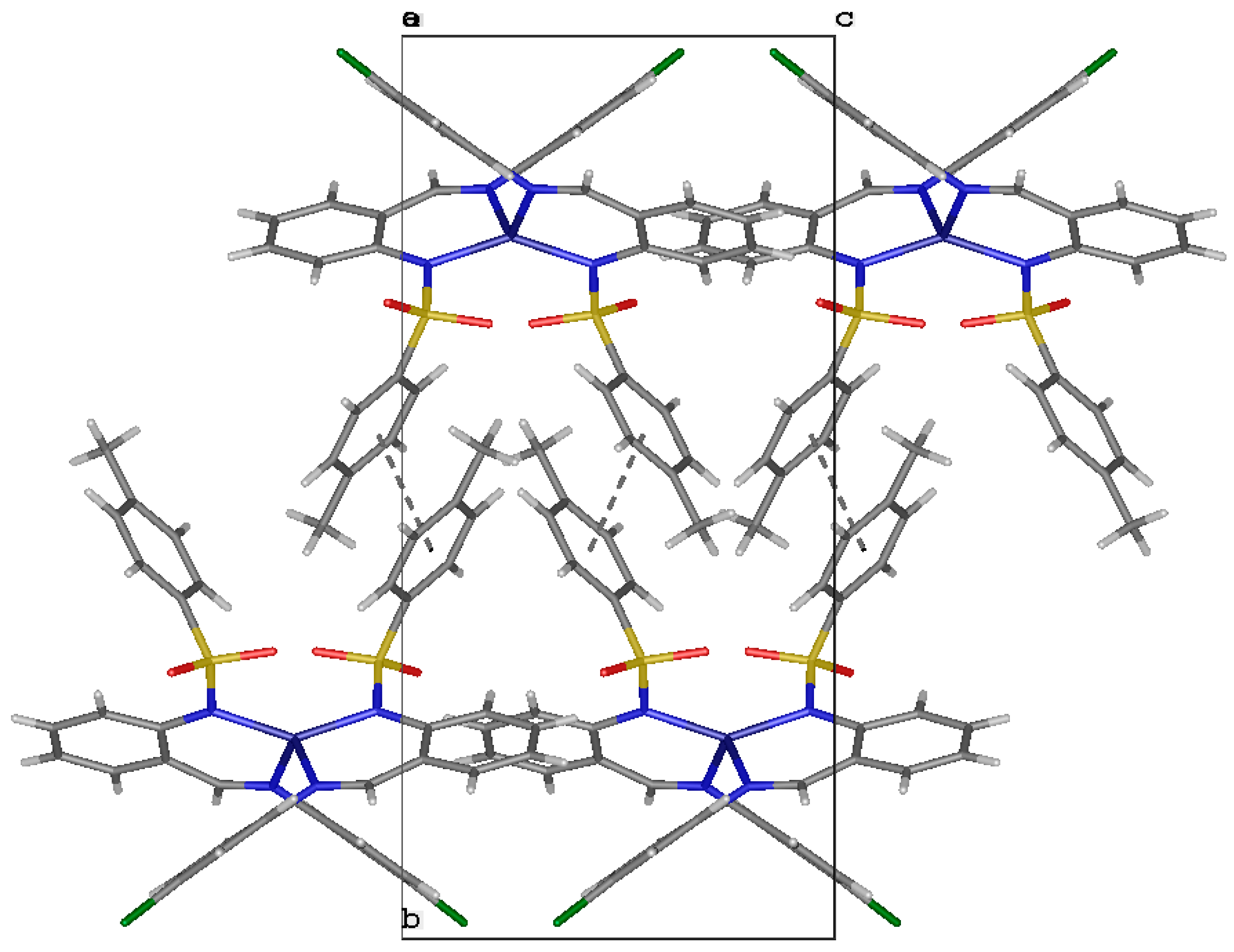
2.4. A Single-Crystal X-ray Diffraction
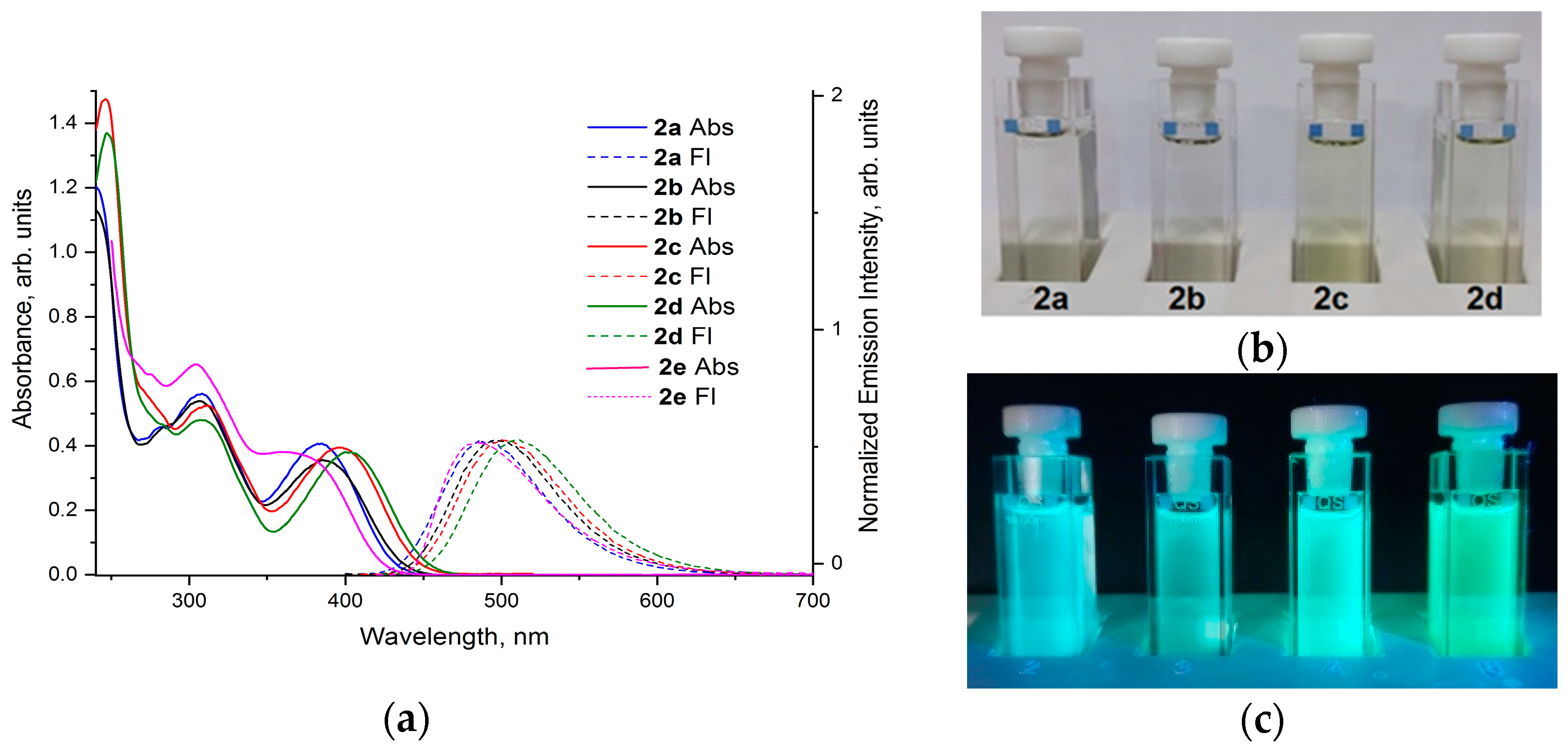
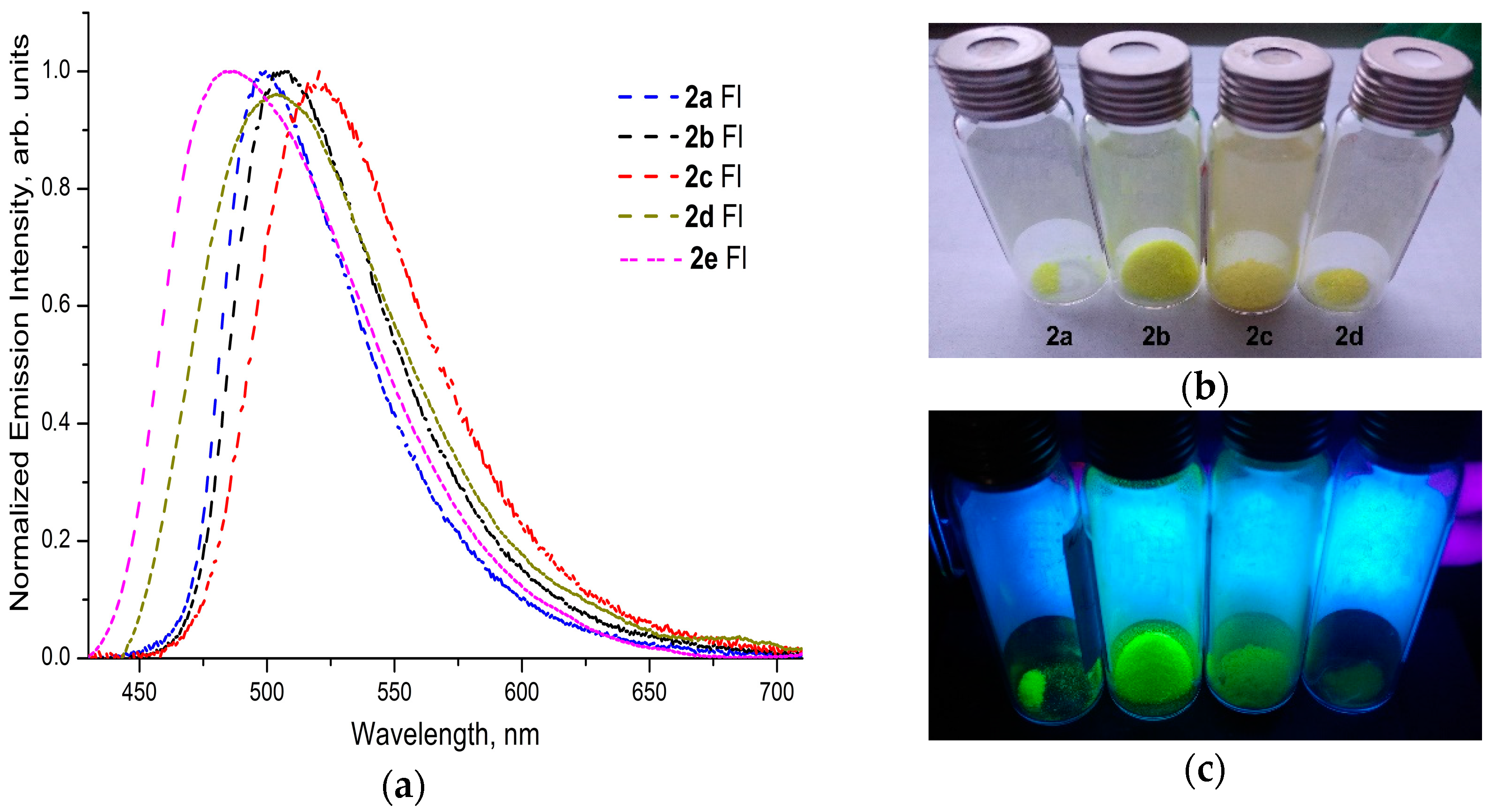
2.5. The Electron Absorption and Photoluminescence Spectra
2.6. Thermal and Electrochemical Properties
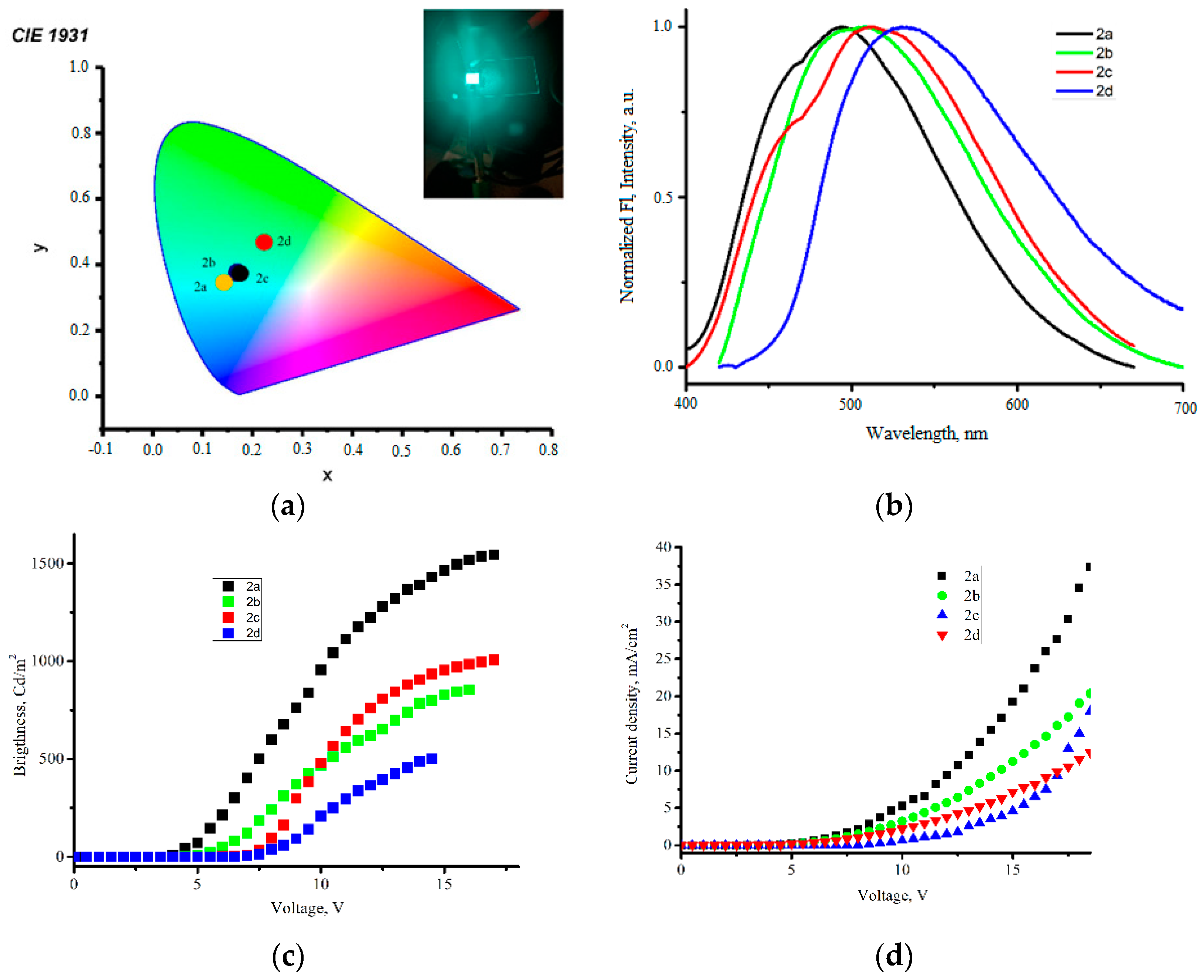
2.7. Electroluminescence Studies
2.8. The Biological Activity
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of Azomethines 1a–e
3.3. General Procedure for the Synthesis of Complexes 2a–e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S.; Bertolo, L. The development of compartmental macrocyclic Schiff bases and related polyamine derivatives. Coord. Chem. Rev. 2007, 251, 1311–1492. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. Advances in acyclic compartmental ligands and related complexes. Coord. Chem. Rev. 2008, 252, 1871–1995. [Google Scholar] [CrossRef]
- Arunadevi, A.; Raman, N. Biological response of Schiff base metal complexes incorporating amino acids–A short review. J. Coord. Chem. 2020, 73, 2095–2116. [Google Scholar] [CrossRef]
- Loginova, N.V.; Harbatsevich, H.I.; Osipovich, N.P.; Ksendzova, G.A.; Koval’chuk, T.V.; Polozov, G.I. Metal Complexes as Promising Agents for Biomedical Applications. Curr. Med. Chem. 2020, 27, 5213–5249. [Google Scholar] [CrossRef]
- Vlasenko, V.G.; Burlov, A.S.; Koshchienko, Y.V.; Kiskin, M.A.; Garnovskii, D.A.; Zubavichus, Y.V.; Kolodina, A.A.; Trigub, A.L.; Zubenko, A.A.; Drobin, Y.D. Synthesis, characterization, and biological activity of Co(II), Ni(II), and Cu(II) complexes derived from N,N′-bis(2-N-tozylaminobenzylidene)diaminodipropyliminate ligand. Inorg. Chim. Acta 2020, 510, 119776. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Makarova, N.I.; Zubenko, A.A.; Drobin, Y.D.; Fetisov, L.N.; Kolodina, A.A.; Zubavichus, Y.V.; Trigub, A.L.; et al. Synthesis, characterization, luminescent properties and biological activities of zinc complexes with bidentate azomethine Schiff-base ligands. Polyhedron 2018, 154, 65–76. [Google Scholar] [CrossRef]
- Adhikary, C.; Banerjee, S.; Chakraborty, J.; Ianelli, S. Copper(II) azide complexes with NNO donor ligands: Syntheses, structure, catalysis and biological studies. Polyhedron 2013, 65, 48–53. [Google Scholar] [CrossRef]
- Garoufis, A.; Hadjikakou, S.K.; Hadjiliadis, N. Palladium coordination compounds as anti-viral, anti-fungal, anti-microbial and anti-tumor agents. Coord. Chem. Rev. 2009, 253, 1384–1397. [Google Scholar] [CrossRef]
- Chen, H.Q.; Hall, S.; Zheng, B.; Rhodes, J. Potentiation of the immune system by Schiff-base forming drugs: Mechanism of action and therapeutic potential. Biodrugs 1997, 7, 217–231. [Google Scholar] [CrossRef]
- Parsekar, S.U.; Haldar, P.; Antharjanam, P.K.S.; Kumar, M.; Koley, A.P. Synthesis, characterization, crystal structure, DNA and human serum albumin interactions, as well as antiproliferative activity of a Cu(II) complex containing a Schiff base ligand formed in situ from the Cu(II)-induced cyclization of 1,5-bis(salicylidene)thiocarbohydrazide. Appl. Organomet. Chem. 2021, 35, e6152. [Google Scholar] [CrossRef]
- Kargar, H.; Behjatmanesh-Ardakani, R.; Torabi, V.; Sarvian, A.; Kazemi, Z.; Chavoshpour-Natanzi, Z.; Mirkhani, V.; Sahraei, A.; Tahir, M.N.; Ashfaq, M. Novel copper(II) and zinc(II) complexes of halogenated bidentate N,O-donor Schiff base ligands: Synthesis, characterization, crystal structures, DNA binding, molecular docking, DFT and TD-DFT computational studies. Inorg. Chim. Acta 2021, 514, 120004. [Google Scholar] [CrossRef]
- Ribeiro, N.; Bulut, I.; Cevatemre, B.; Teixeira, C.; Yildizhan, Y.; Andre, V.; Adao, P.; Pessoa, J.C.; Acilan, C.; Correia, I. Cu(ii) and V(iv)O complexes with tri- or tetradentate ligands based on (2-hydroxybenzyl)-l-alanines reveal promising anticancer therapeutic potential. Dalton Trans. 2021, 50, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Shah, D.; Khan, I.; Ahmad, S.; Ali, U.; Rahman, A.U. Synthesis and Antioxidant Activities of Schiff Bases and Their Complexes: An Updated Review. Res. Appl. Chem. 2020, 10, 6936–6963. [Google Scholar] [CrossRef]
- Deda, M.D.; Ghedini, M.; Aiello, I.; Grisolia, A. A new blue photoluminescent Salen-like zinc complex with excellent emission quantum yield. Chem. Lett. 2004, 33, 1060–1061. [Google Scholar] [CrossRef]
- Kaplunov, M.G.; Krasnikova, S.S.; Yakushchenko, I.K.; Shamaev, S.N.; Pivovarov, A.P.; Efimov, O.N. New organic electroluminescent materials. Mol. Cryst. Liq. Cryst. 2005, 426, 287–293. [Google Scholar] [CrossRef]
- Yu, T.; Su, W.; Li, W.; Hong, Z.; Hua, R.; Li, M.; Chu, B.; Li, B.; Zhang, Z.; Hu, Z.Z. Synthesis, crystal structure and electroluminescent properties of a Schiff base zinc complex. Inorg. Chim. Acta 2006, 359, 2246–2251. [Google Scholar] [CrossRef]
- Xie, J.; Qiao, J.; Wang, L.; Xie, J.; Qiu, Y. An azomethin-zinc complex for organic electroluminescence: Crystal structure, thermal stability and optoelectronic properties. Inorg. Chim. Acta 2005, 358, 4451–4458. [Google Scholar] [CrossRef]
- Chen, L.; Qiao, J.; Xie, J.; Duan, L.; Zhang, D. Substituted azomethine-zinc complexes: Thermal stability, electrochemical and electron transport properties. Inorg. Chim. Acta 2009, 362, 2327–2333. [Google Scholar] [CrossRef]
- Kotova, O.V.; Eliseeva, S.V.; Averjushkin, A.S.; Lepnev, L.S.; Vaschenko, A.A.; Rogachev, A.Y.; Vitukhnovsky, A.G.; Kuzmina, N.P. Zinc(II) complexes with Schiff bases derived from ethylenediamine and salicylaldehyde: The synthesis and photoluminescent properties. Russ. Chem. Bull. Intern. Ed. 2008, 57, 1880–1889. [Google Scholar] [CrossRef]
- Chen, C.H.; Shi, J. Metal chelates as emitting materials for organic electroluminescence. Coord. Chem. Rev. 1998, 171, 161–174. [Google Scholar] [CrossRef]
- Gusev, A.N.; Braga, E.; Shul’gin, V.; Lyssenko, K.; Eremenko, I.; Samsonova, L.; Degtyarenko, K.; Kopylova, T.; Linert, W. Luminescent Properties of Zn and Mg Complexes on N-(2-Carboxyphenyl)salicylidenimine Basis. Materials 2017, 10, 897. [Google Scholar] [CrossRef] [Green Version]
- Kotova, O.; Lyssenko, K.; Rogachev, A.; Eliseeva, S.; Fedyanin, I.; Lepnev, L.; Pandey, L.; Burlov, A.; Garnovskii, A.; Vitukhnovsky, A.; et al. Low temperature X-ray diffraction analysis, electronic density distribution and photophysical properties of bidentate N,O-donor salicylaldehyde Schiff bases and zinc complexes in solid state. J. Photochem. Photobiol. A Chem. 2011, 218, 117–129. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Makarova, N.I.; Zubenko, A.A.; Drobin, Y.D.; Borodkin, G.S.; Metelitsa, A.V.; Zubavichus, Y.V.; Garnovskii, D.A. Complexes of zinc(II) with N-2-(hydroxyalkyliminomethyl)phenyl-4-methylbenzenesulfonamides: Synthesis, structure, photoluminescence properties and biological activity. Polyhedron 2018, 144, 249–258. [Google Scholar] [CrossRef]
- Burlov, A.S.; Mal’tsev, E.I.; Vlasenko, V.G.; Garnovskii, D.A.; Dmitriev, A.V.; Lypenko, D.A.; Vannikov, A.V.; Dorovatovskii, P.V.; Lazarensko, V.A.; Zubavichus, Y.V.; et al. Synthesis, structure, photo- and electroluminescent properties of bis{(4-methyl-N-2-(E)-2-pyridyliminomethylphenyl)benzenesulfonamide}zinc(II). Polyhedron 2017, 133, 231–237. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Dmitriev, A.V.; Chesnokov, V.V.; Uraev, A.I.; Garnovskii, D.A.; Zubavichus, Y.V.; Trigub, A.L.; Vasilchenko, I.S.; Lypenko, D.A.; et al. Synthesis, structure, photo- and electroluminescent properties of zinc(II) complexes with aminomethylene derivatives of 1-phenyl-3-methyl-4-formylpyrazol-5-one and 3- and 6-aminoquinolines. Synth. Metals 2015, 203, 156–163. [Google Scholar] [CrossRef]
- Burlov, A.S.; Koshchienko, Y.V.; Makarova, N.I.; Kuz’menko, T.A.; Chesnokov, V.V.; Kiskin, M.A.; Nikolaevskii, S.A.; Garnovskii, D.A.; Uraev, A.I.; Vlasenko, V.G.; et al. Mixed-ligand Zn(II) complexes of 1-phenyl-3-methyl-4-formylpyrazole-5-one and various aminoheterocycles: Synthesis, structure and photoluminescence properties. Synth. Metals 2016, 220, 543–550. [Google Scholar] [CrossRef]
- Gusev, A.N.; Braga, E.V.; Kryukova, M.A.; Lyubomirskii, N.V.; Zamnius, E.A.; Shul’gin, V.F. Photoluminescence of the Coordination Zinc Compounds with 3-Methyl-4-Formyl-1-Phenylpyrazol-5-one Acylhydrazones. Russ. J. Coord. Chem. 2020, 46, 251–259. [Google Scholar] [CrossRef]
- Gusev, A.N.; Kiskin, M.A.; Braga, E.V.; Kryukova, M.A.; Baryshnikov, G.V.; Karaush-Karmazin, N.N.; Minaeva, V.A.; Minaev, B.F.; Ivaniuk, K.; Stakhira, P.; et al. Schiff Base Zinc(II) Complexes as Promising Emitters for Blue Organic Light-Emitting Diodes. ACS Appl. Electron. Mater. 2021, 3, 3436–3444. [Google Scholar] [CrossRef]
- Gusev, A.N.; Kiskin, M.A.; Braga, E.V.; Chapran, M.; Wiosna-Salyga, G.; Baryshnikov, G.V.; Minaeva, V.A.; Minaev, B.F.; Ivaniuk, K.; Stakhira, P.; et al. Novel Zinc Complex with an Ethylenediamine Schiff Base for High-Luminance Blue Fluorescent OLED Applications. J. Phys. Chem. C. 2019, 123, 11850–11859. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Makarova, N.I.; Kiskin, M.A.; Kolodina, A.A.; Garnovskii, D.A.; Trigub, A.L.; Metelitsa, A.V. Chemical and electrochemical synthesis, structure, photoluminescent properties, and biological activity of 4-methyl-N-2-(Z)-2-(2-pyridyl)alkyliminomethylphenylbenzenesulfamide zinc(II) complexes. Appl. Organometal. Chem. 2020, 34, e5302. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Makarova, N.I.; Lyssenko, K.A.; Chesnokov, V.V.; Borodkin, G.S.; Vasilchenko, I.S.; Uraev, A.I.; Garnovskii, D.A.; Metelitsa, A.V. Chemical and electrochemical synthesis, molecular structures, DFT calculations and optical properties of metal-chelates of 8-(2-tosylaminobenzilideneimino)quinolone. Polyhedron 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Milutka, M.S.; Garnovskii, D.A.; Kolodina, A.A.; Zubavichus, Y.V.; Kiskin, M.A. Synthesis, structure, and photoluminescence of Zn(II) and Cd(II) complexes with N-2-(diethylaminoalkyliminomethyl)-phenyl-4-methylbenzenesulfonamides. Polyhedron 2021, 208, 115400. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Milutka, M.S.; Mal’tsev, E.I.; Dmitriev, A.V.; Lypenko, D.A.; Nekrasova, N.V.; Kolodina, A.A.; Makarova, N.I.; et al. Synthesis, structure, and photoluminescent and electroluminescent properties of zinc(II) complexes with bidentate azomethine ligands. Appl. Organomet. Chem. 2021, 35, e6107. [Google Scholar] [CrossRef]
- Milutka, M.S.; Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Makarova, N.I.; Metelitsa, A.V.; Korshunova, E.V.; Trigub, A.L.; Zubenko, A.A.; Klimenko, A.I. Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted Azomethines and Their Zinc(II) Complexes. Russ. J. Gen. Chem. 2021, 91, 1706–1716. [Google Scholar] [CrossRef]
- Milutka, M.S.; Burlov, A.S.; Vlasenko, V.G.; Koshchienko, Y.V.; Korshunova, E.V.; Uraev, A.I.; Trigub, A.L.; Zubenko, A.A.; Klimenko, A.I.; Gusev, A.N. Zinc(II) Complexes with Azomethines of 2,4,6-Trimethylaniline and Halogen-Substituted Salicylaldehyde. Russ. J. Gen. Chem. 2022, 92, 1297–1308. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Oloyede-Akinsulere, A.I.; Babajide, J.O.; Salihu, S.O. Synthesis, Antibacterial and Antioxidant Activities of Some Tridentate Substituted Salicylaldimines. Asian J. Appl. Chem. Res. 2018, 1, 43067. [Google Scholar] [CrossRef]
- Al-Shboul, T.M.A.; El-khateeb, M.; Obeidat, Z.H.; Ababneh, T.S.; Al-Tarawneh, S.S.; Al Zoubi, M.S.; Alshaer, W.; Abu Seni, A.; Qasem, T.; Moriyama, H.; et al. Synthesis, Characterization, Computational and Biological Activity of Some Schiff Bases and Their Fe, Cu and Zn Complexes. Inorganics 2022, 10, 112. [Google Scholar] [CrossRef]
- Krasovitskii, B.M.; Bolotin, B.M. Organicheskie lyuminofory Organic Luminophores; Khimiya: Moscow, Russia, 1984; p. 292. (In Russian) [Google Scholar]
- Gusev, A.; Shul’gin, V.; Braga, E.; Zamnius, E.; Kryukova, M.; Linert, W. Luminescent properties of Zn complexes based on tetradentate N2O2 -donor pyrazolone schiff bases. Dyes Pigm. 2020, 183, 108626–108632. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.A.; Zubavichus, Y.V. Structural Materials Science End-Station at the Kurchatov Synchrotron Radiation Source: Recent Instrumentation Upgrades and Experimental Results. Nucl. Instr. Meth. Phys. Res. A 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Newville, M. EXAFS analysis using FEFF and FEFFIT. J. Synchrotron Rad. 2001, 8, 96–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabinski, S.I.; Rehr, J.J.; Ankudinov, A.; Alber, R.C. Multiple-scattering calculations of x-ray-absorption spectra. Phys. Rev. 1995, 52, 2995–3011. [Google Scholar] [CrossRef] [PubMed]
- Lazarenko, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Burlov, A.S.; Koshchienko, Y.V.; Vlasenko, V.G.; Khrustalev, V.N. High-throughput small-molecule crystallography at the ‘Belok’ beamline of the Kurchatov synchrotron radiation source: Transition metal complexes with azomethine ligands as a case study. Crystals 2017, 7, 325. [Google Scholar] [CrossRef] [Green Version]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA diffraction beamline for studying crystalline samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Bio. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]

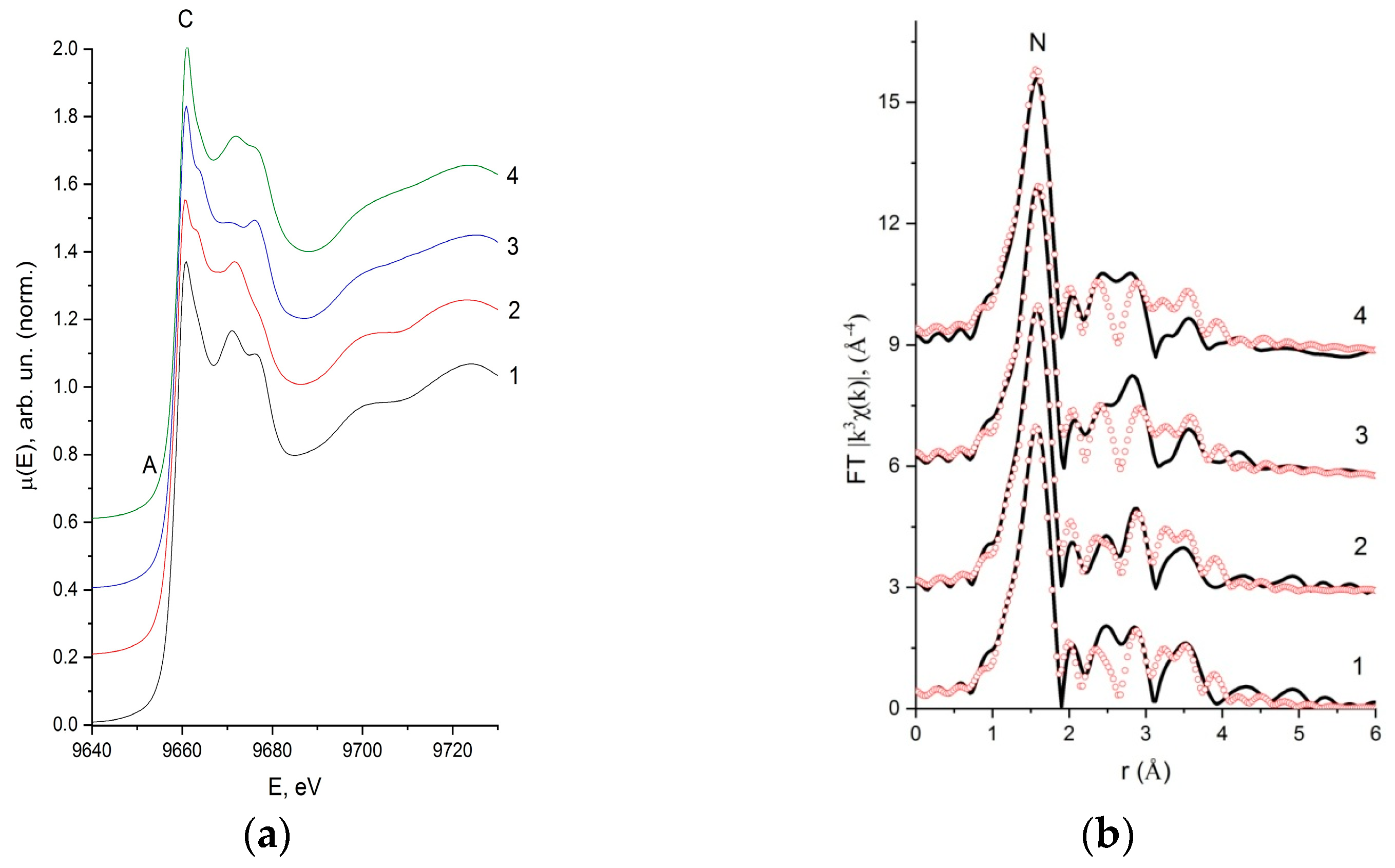
| Compound | Bond | N | R, Å | σ2, Å 2 | Q, % |
|---|---|---|---|---|---|
| 2a | Zn–N Zn–N | 2 2 | 1.98 2.06 | 0.0030 0.0030 | 1.3 |
| 2b | Zn–N Zn–N | 2 2 | 1.98 2.06 | 0.0030 0.0030 | 1.2 |
| 2c | Zn–N Zn–N | 2 2 | 1.98 2.06 | 0.0030 0.0030 | 1.3 |
| 2d | Zn–N Zn–N | 2 2 | 1.99 2.05 | 0.0030 0.0030 | 1.6 |
| No. | Structure | Absorption λmax, nm (ε, 104 M−1cm−1) | Photoluminescence, Solution/Solid State | ||
|---|---|---|---|---|---|
| Excitation λmax, nm | Emission λmax, nm | φ * | |||
| 2a |  | 308 (2.81), 385 (2.04) | 310, 385 | 488/499 | 0.032/0.391 |
| 2b |  | 306 (2.70), 385 (1.78) | 307, 386 | 497/507 | 0.054/0.483 |
| 2c | 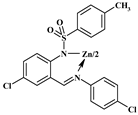 | 312 (2.62), 397 (2.00) | 313, 397 | 500/520 | 0.050/0.214 |
| 2d | 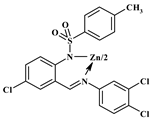 | 307 (2.40), 400 (1.90) | 312, 398 | 511, 586/503 | 0.062/0.047 |
| 2e |  | 306 (2.60), 370 (1.58) | 310, 385 | 485/487 | 0.015/0.113 |
| Compound | Tm, °C | Td, °C | Tg, °C | E(Ox), V | E(Red), V | E(HOMO), eV | E(LUMO), eV |
|---|---|---|---|---|---|---|---|
| 2a | 317 | 340 | 101 | 1.02 | −0.58 | −5.42 | −2.59 |
| 2b | 304 | 305 | - | 1.08 | −0.72 | −5.48 | −2.72 |
| 2c | 311 | 335 | - | 1.04 | −0.62 | −5.44 | −2.68 |
| 2d | 275 | 290 | - | 1.11 | −0.68 | −5.51 | −2.79 |
| Device | EL λmax, nm | CIE | Max. Brigthness (Cd/m2) | Current Efficiency Cd/A | Power Efficiencyl m/W | EQE, % |
|---|---|---|---|---|---|---|
| A | 493 | 0.15:0.36 | 1542 | 2.7 | 1.7 | 2.11 |
| B | 509 | 0.19:0.42 | 854 | 1.9 | 1.5 | 1.20 |
| C | 511 | 0.20:0.44 | 1007 | 2.4 | 1.6 | 1.25 |
| D | 533 | 0.24:0.50 | 501 | 1.7 | 1.4 | 0.90 |
| Compound | Protistocidal Activity | Fungistatic Activity | Antibacterial Activity | |
|---|---|---|---|---|
| Colpoda steinii, µg/mL | Penicillium italicum, Inhibition Zone Diameter, mm | Staphylococcus aureus 6538 P, Inhibition Zone Diameter, mm | Escherichia coli F50, Inhibition Zone Diameter, mm | |
| 1a | 500 | 0 | 0 | 8 |
| 1b | 250 | 0 | 9 | 0 |
| 1c | 500 | 0 | 0 | 0 |
| 1d | 250 | 0 | 9 | 0 |
| 1e | 15.6 | 0 | 15 | 15 |
| 2a | >500 | 0 | 8 | 0 |
| 2b | >500 | 0 | 0 | 0 |
| 2c | 31.25 | 0 | 9 | 12 |
| 2d | >500 | 0 | 8 | 10 |
| 2e | 500 | 0 | 15 | 14 |
| Baycox (Toltrazuril) | 62.5 | - | - | - |
| Fundazol | - | 40 | - | - |
| Furazolidone | 20 | 18 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlov, A.S.; Vlasenko, V.G.; Milutka, M.S.; Koshchienko, Y.V.; Makarova, N.I.; Lazarenko, V.A.; Trigub, A.L.; Kolodina, A.A.; Zubenko, A.A.; Metelitsa, A.V.; et al. Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted N-[2-(Phenyliminomethyl)phenyl]-4-methylbenzenesulfamide and Their Zinc(II) Complexes. Int. J. Mol. Sci. 2022, 23, 15259. https://doi.org/10.3390/ijms232315259
Burlov AS, Vlasenko VG, Milutka MS, Koshchienko YV, Makarova NI, Lazarenko VA, Trigub AL, Kolodina AA, Zubenko AA, Metelitsa AV, et al. Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted N-[2-(Phenyliminomethyl)phenyl]-4-methylbenzenesulfamide and Their Zinc(II) Complexes. International Journal of Molecular Sciences. 2022; 23(23):15259. https://doi.org/10.3390/ijms232315259
Chicago/Turabian StyleBurlov, Anatolii S., Valery G. Vlasenko, Maxim S. Milutka, Yurii V. Koshchienko, Nadezhda I. Makarova, Vladimir A. Lazarenko, Alexander L. Trigub, Alexandra A. Kolodina, Alexander A. Zubenko, Anatoly V. Metelitsa, and et al. 2022. "Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted N-[2-(Phenyliminomethyl)phenyl]-4-methylbenzenesulfamide and Their Zinc(II) Complexes" International Journal of Molecular Sciences 23, no. 23: 15259. https://doi.org/10.3390/ijms232315259
APA StyleBurlov, A. S., Vlasenko, V. G., Milutka, M. S., Koshchienko, Y. V., Makarova, N. I., Lazarenko, V. A., Trigub, A. L., Kolodina, A. A., Zubenko, A. A., Metelitsa, A. V., Garnovskii, D. A., Gusev, A. N., & Linert, W. (2022). Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted N-[2-(Phenyliminomethyl)phenyl]-4-methylbenzenesulfamide and Their Zinc(II) Complexes. International Journal of Molecular Sciences, 23(23), 15259. https://doi.org/10.3390/ijms232315259








