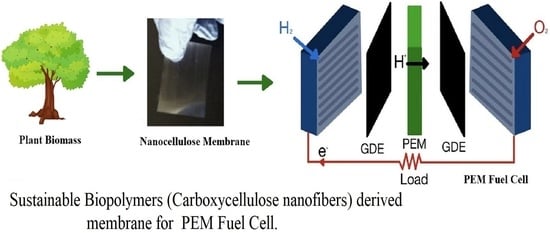
Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells
Abstract
1. Introduction
2. Results and Discussion
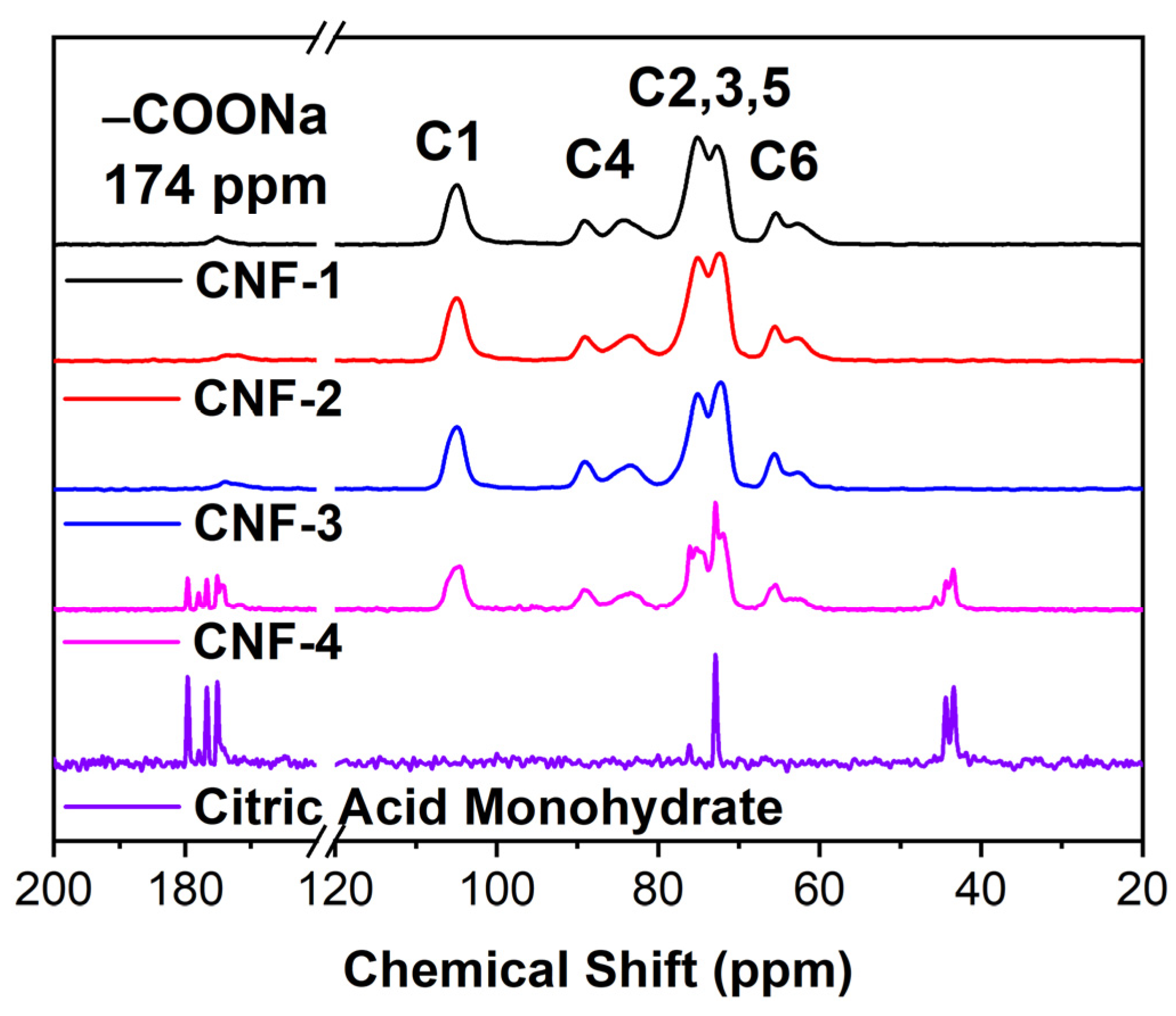
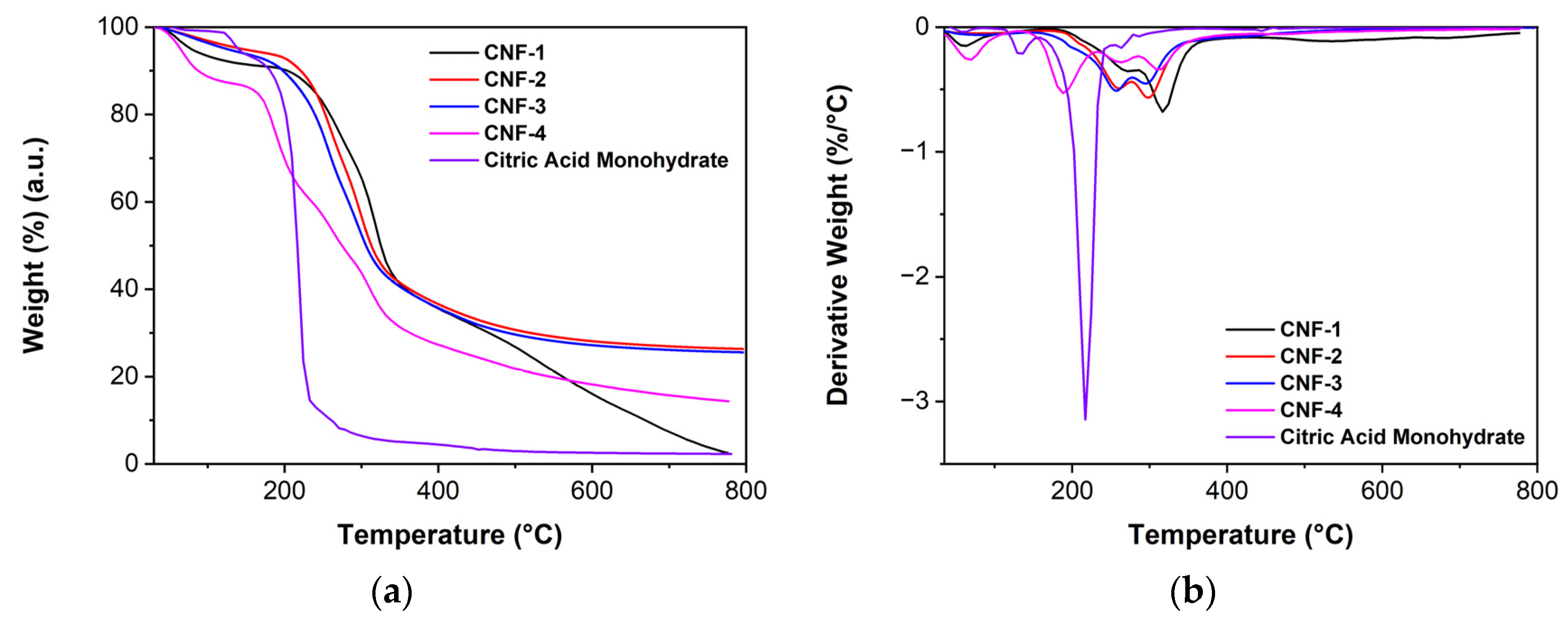
2.1. Citric Acid Cross-Link Characterizations
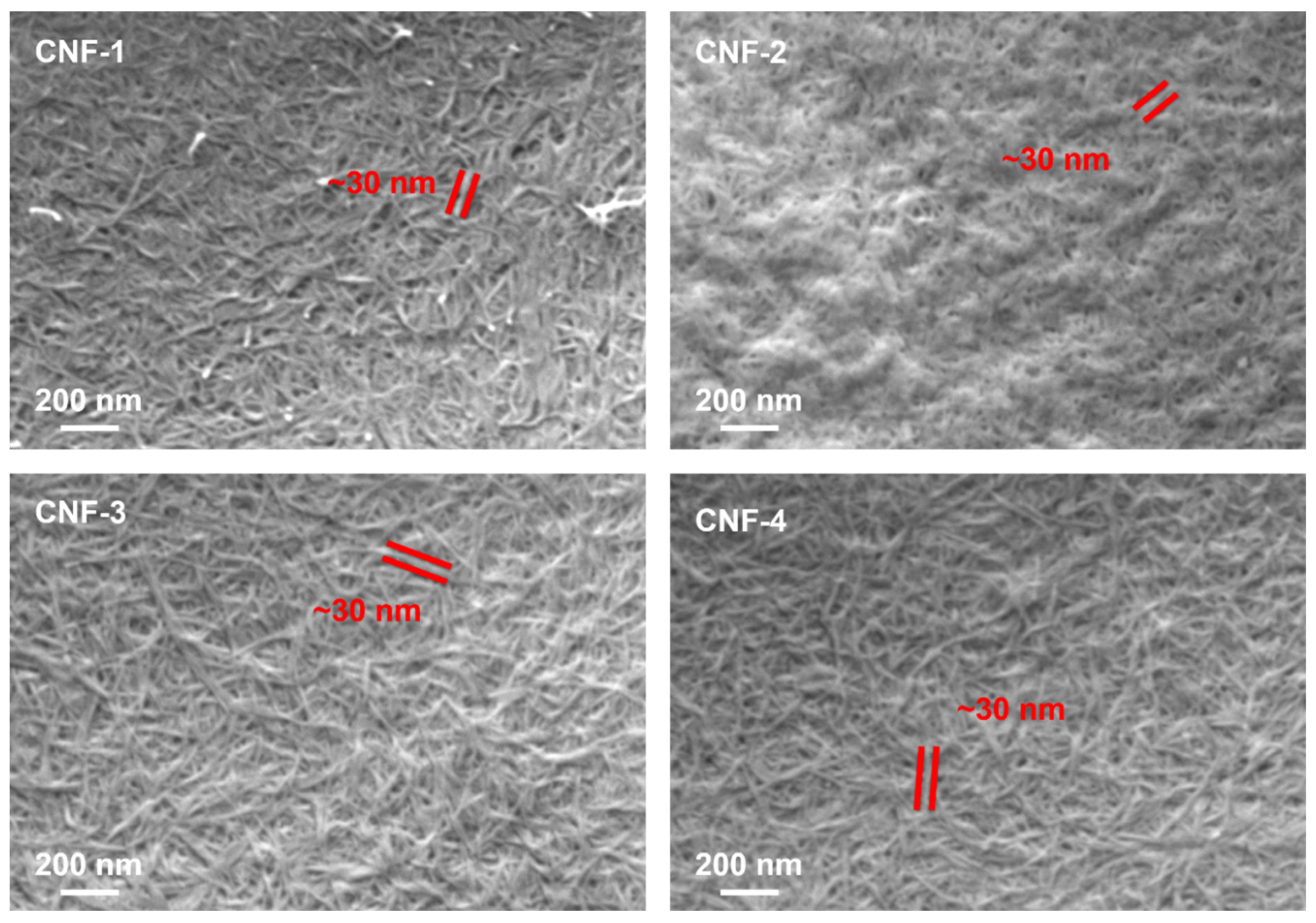
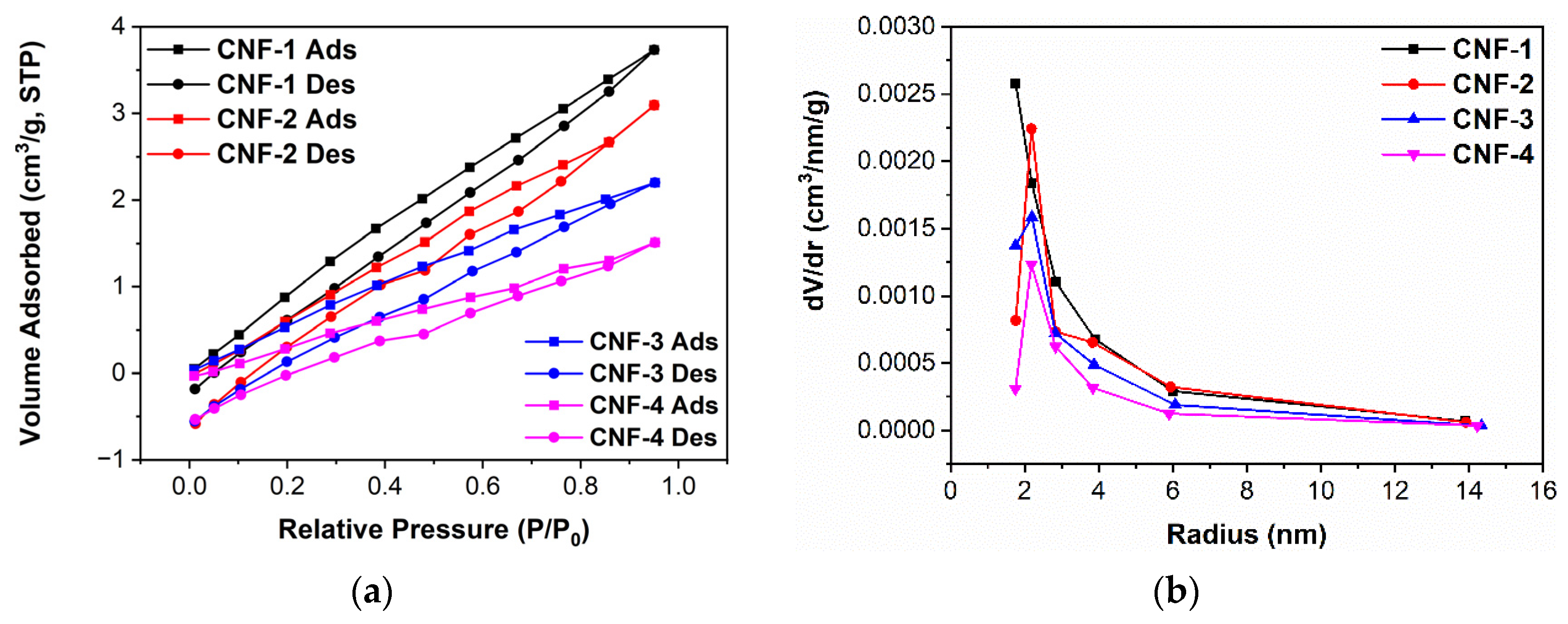
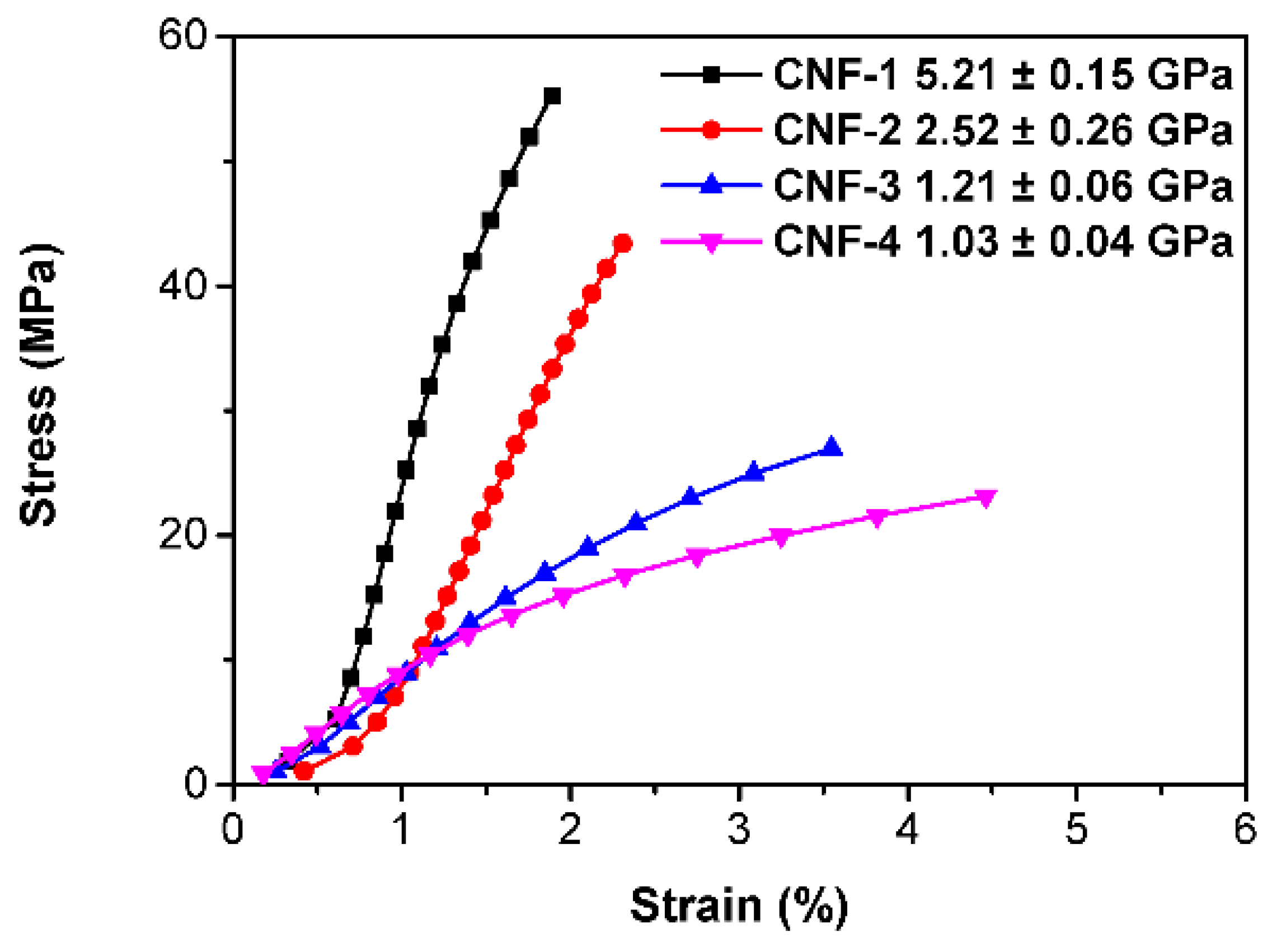
2.2. Morphology Characterizations
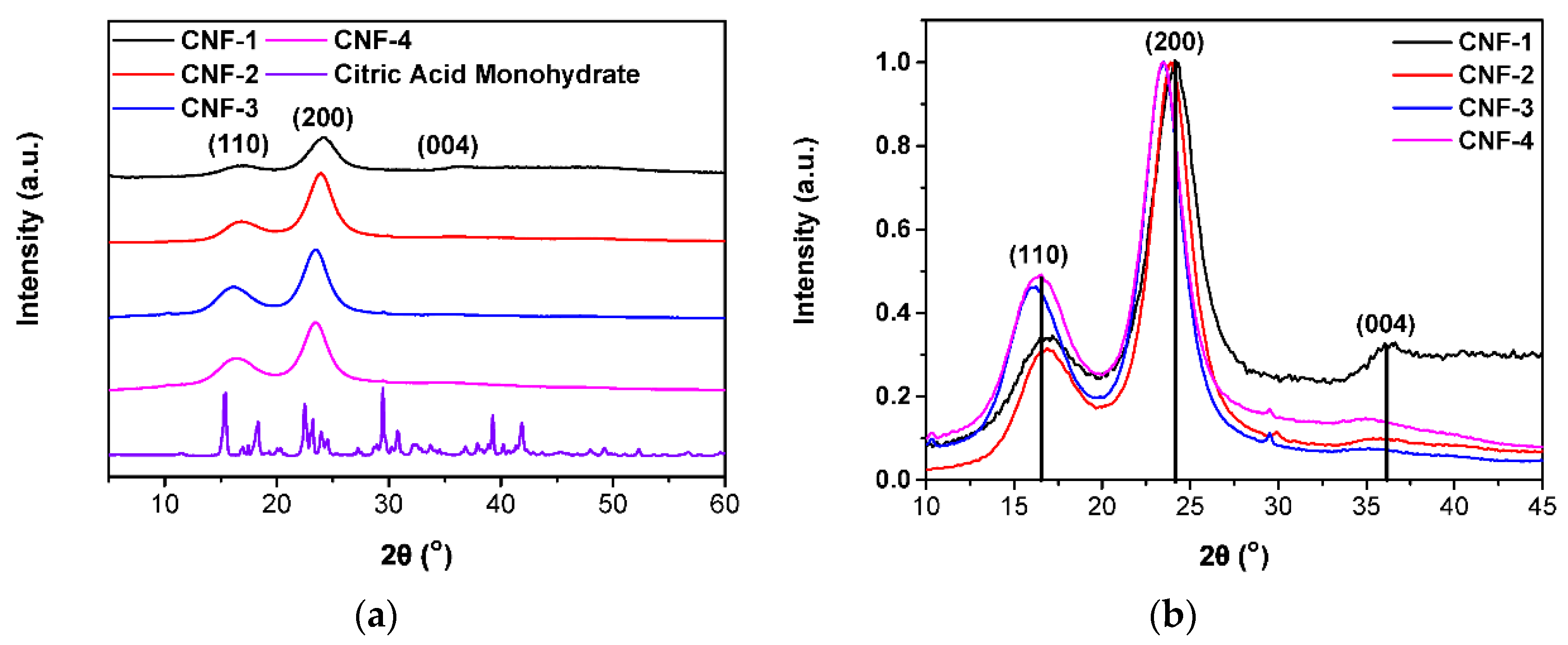
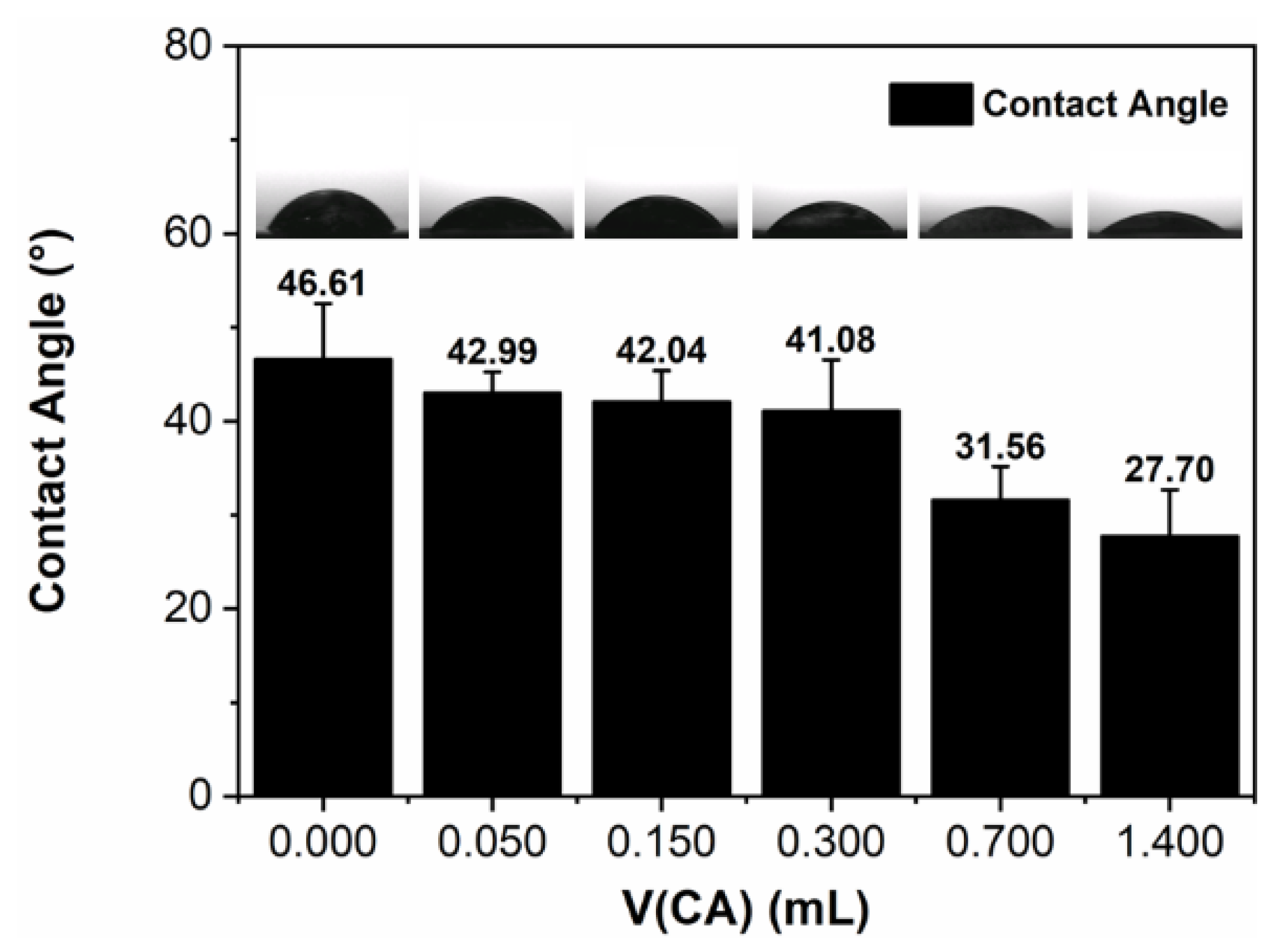
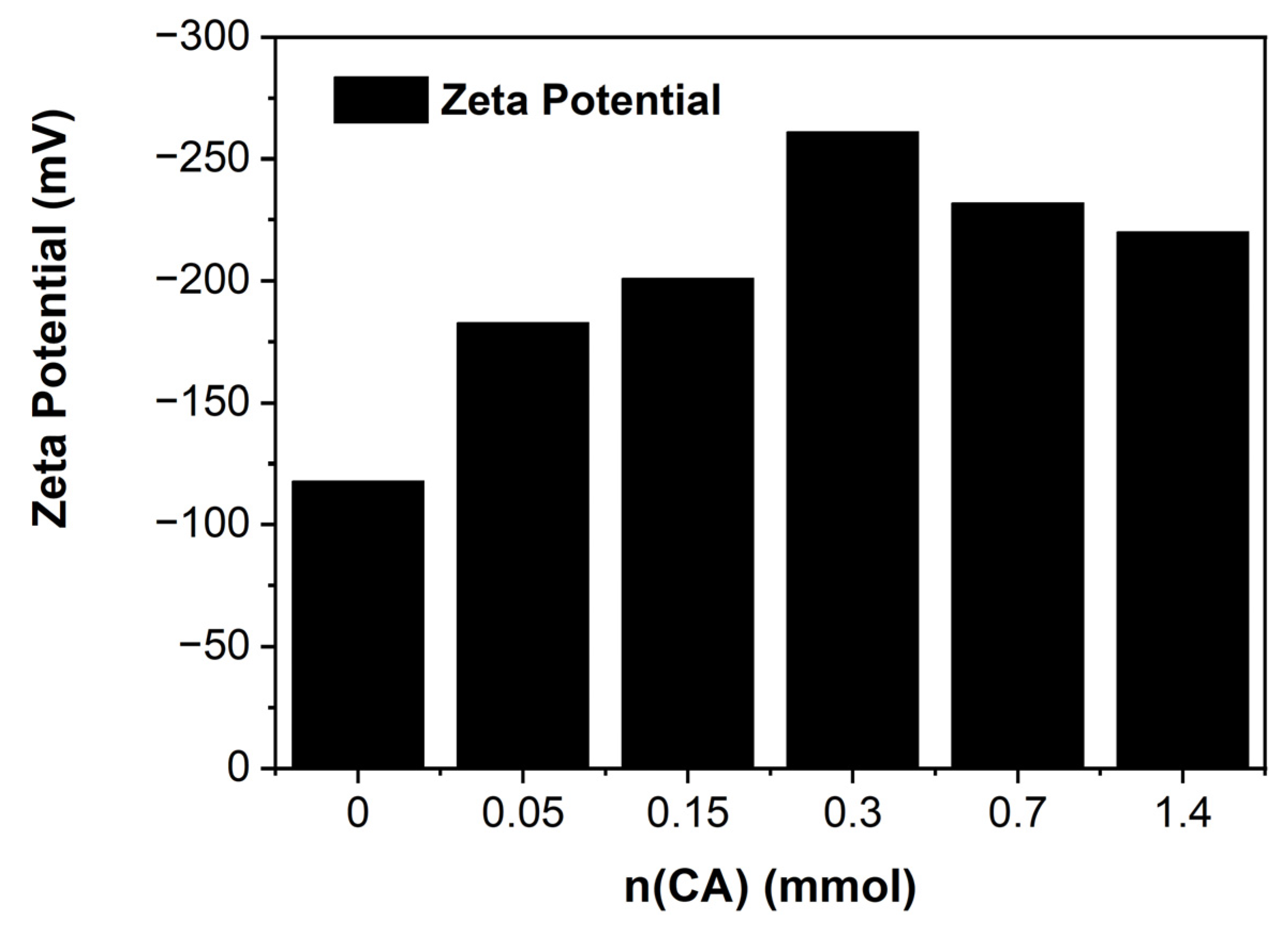
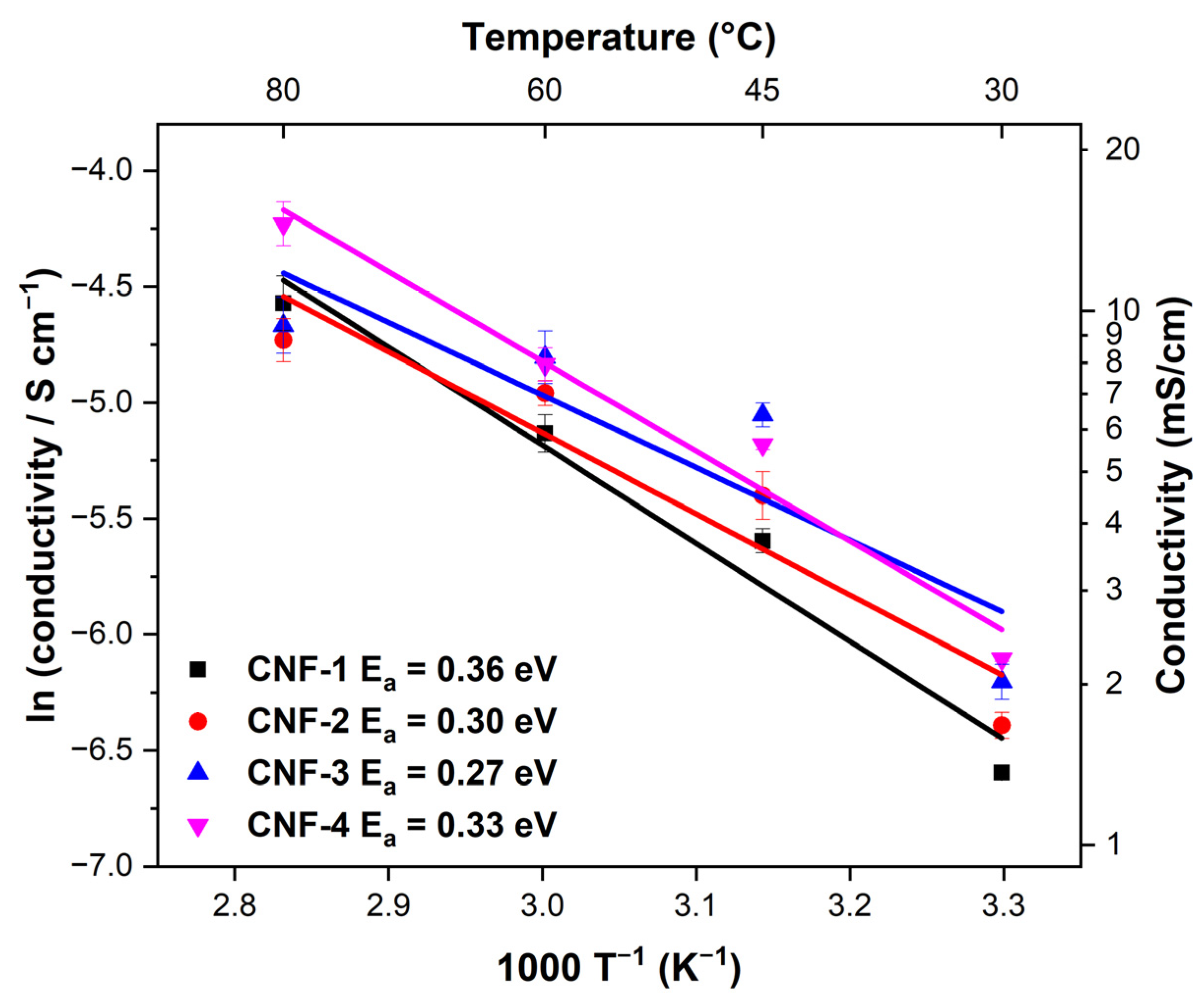
2.3. Surface Characterizations
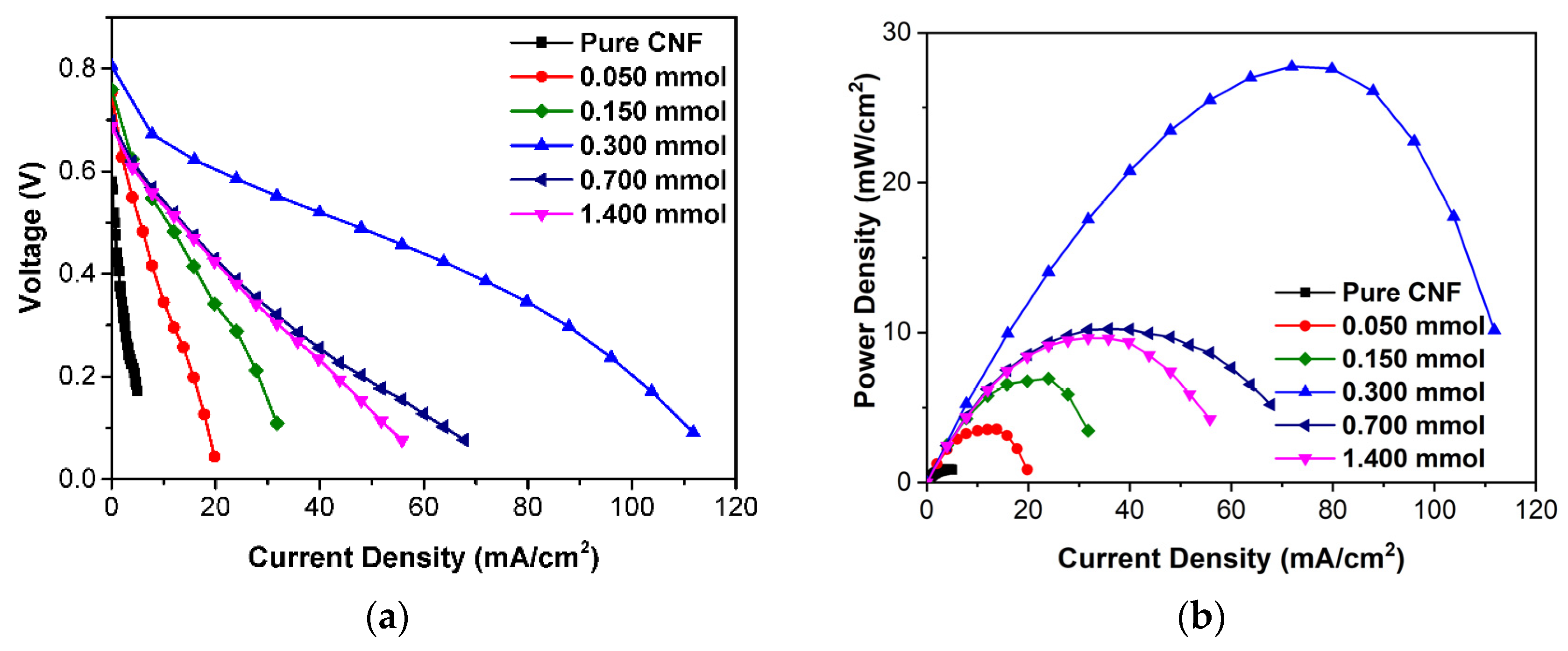
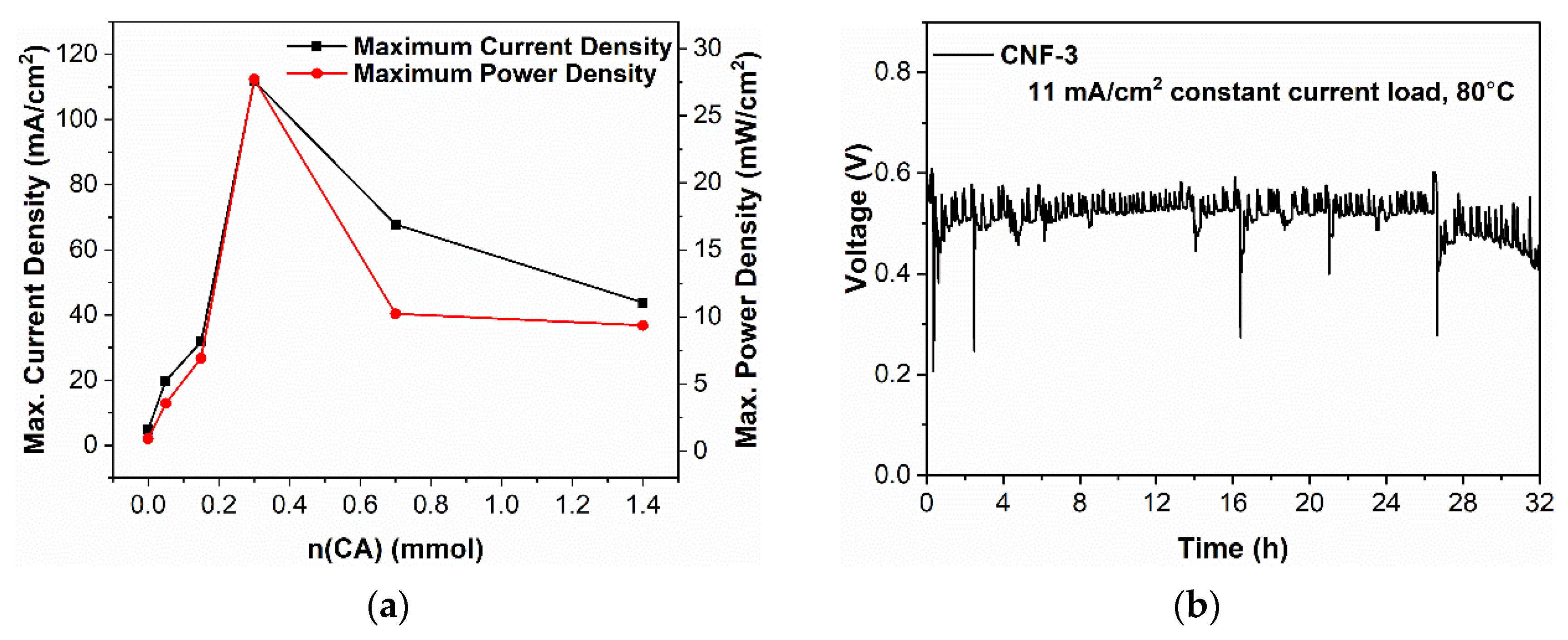
2.4. Fuel Cell Performance
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Carboxycellulose Nanofibers
3.3. Preparation of Citric Acid Cross-Linked Carboxycellulose Nanofiber Membranes
3.4. Fourier-Transform Infrared-Red Spectrometry (FTIR)
3.5. Thermogravimetric Analysis (TGA)
3.6. 13C CPMAS NMR
3.7. X-ray Diffractometry (XRD)
3.8. Focused Ion Beam Scanning Electron Microscopy (FIB/SEM)
3.9. BET Surface Area
3.10. Transmission Electron Microscopy (TEM)
3.11. Surface Scanning Electron Microscopy with Energy Dispersive X-ray Analysis (SEM/EDX)
3.12. Contact Angle
3.13. Fuel Cell Testing
3.14. Dynamic Mechanical Analysis (DMA) of Cross-Linked CNF Membrane
3.15. Zeta Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maness, P.C.; Yu, J.; Eckert, C.; Ghirardi, M.L. Photobiological Hydrogen Production—Prospects and Challenges. Microbe Mag. 2009, 4, 275–280. [Google Scholar] [CrossRef][Green Version]
- REN21. Renewables 2019 Global Status Report; REN21 Secretariat: Paris, France, 2019; ISBN 978-3-9818911-7-1. Available online: https://www.ren21.net/gsr-2019/ (accessed on 1 August 2019).
- Rittmann, B.E. Opportunities for Renewable Bioenergy Using Microorganisms. Biotechnol. Bioeng. 2008, 100, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Breyer, C.; Bogdanov, D.; Gulagi, A.; Aghahosseini, A.; Barbosa, L.S.N.S.; Koskinen, O.; Barasa, M.; Caldera, U.; Afanasyeva, S.; Child, M.; et al. On the Role of Solar Photovoltaics in Global Energy Transition Scenarios. Prog. Photovolt. Res. Appl. 2017, 25, 727–745. [Google Scholar] [CrossRef]
- Colmenar-Santos, A.; Perera-Perez, J.; Borge-Diez, D.; DePalacio-Rodríguez, C. Offshore Wind Energy: A Review of the Current Status, Challenges and Future Development in Spain. Renew. Sustain. Energy Rev. 2016, 64, 1–18. [Google Scholar] [CrossRef]
- Shortall, R.; Davidsdottir, B.; Axelsson, G. Geothermal Energy for Sustainable Development: A Review of Sustainability Impacts and Assessment Frameworks. Renew. Sustain. Energy Rev. 2015, 44, 391–406. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Biomass Combustion Systems: A Review on the Physical and Chemical Properties of the Ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A Mini-Review on Anion Exchange Membranes for Fuel Cell Applications: Stability Issue and Addressing Strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Advances in the High Performance Polymer Electrolyte Membranes for Fuel Cells. Chem. Soc. Rev. 2012, 41, 2382–2394. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Zhao, Q.; Wei, Y.; Ni, C.; Wang, L.; Liu, B.; Liu, J.; Zhang, M.; Men, Y.; Sun, Z.; Xie, H.; et al. Effect of Aminated Nanocrystal Cellulose on Proton Conductivity and Dimensional Stability of Proton Exchange Membranes. Appl. Surf. Sci. 2019, 466, 691–702. [Google Scholar] [CrossRef]
- Tsai, J.-C.; Cheng, H.-P.; Kuo, J.-F.; Huang, Y.-H.; Chen, C.-Y. Blended Nafion®/SPEEK Direct Methanol Fuel Cell Membranes for Reduced Methanol Permeability. J. Power Sources 2009, 189, 958–965. [Google Scholar] [CrossRef]
- Barbir, F.; Gómez, T. Efficiency and Economics of Proton Exchange Membrane (PEM) Fuel Cells. Int. J. Hydrogen Energy 1997, 22, 1027–1037. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khalid, M.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Walvekar, R.; Kadhum, A.A.H. Additives in Proton Exchange Membranes for Low- and High-Temperature Fuel Cell Applications: A Review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Massinelli, L.; Bauer, B. Polymeric Proton Conducting Membranes for Medium Temperature Fuel Cells (110–160 °C). J. Memb. Sci. 2001, 185, 73–81. [Google Scholar] [CrossRef]
- Yee, R.S.L.; Rozendal, R.A.; Zhang, K.; Ladewig, B.P. Cost Effective Cation Exchange Membranes: A Review. Chem. Eng. Res. Des. 2012, 90, 950–959. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Ren, S.; Lei, T. Comparison of Highly Transparent All-Cellulose Nanopaper Prepared Using Sulfuric Acid and TEMPO-Mediated Oxidation Methods. Cellulose 2015, 22, 1123–1133. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Chi, K.; Fung, E.; Aubrecht, K.; Keroletswe, N.; Chigome, S.; Hsiao, B.S. Nitro-Oxidized Carboxycellulose Nanofibers from Moringa Plant: Effective Bioadsorbent for Mercury Removal. Cellulose 2021, 28, 8611–8628. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead Removal from Water Using Carboxycellulose Nanofibers Prepared by Nitro-Oxidation Method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Zhan, C.; Li, Y.; Sharma, P.R.; He, H.; Sharma, S.K.; Wang, R.; Hsiao, B.S. A Study of TiO2 Nanocrystal Growth and Environmental Remediation Capability of TiO2/CNC Nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Sharma, S.K.; Hsiao, B.S. Chapter 5—Nanocellulose in Membrane Technology for Water Purification. In Separation Science and Technology. Volume 15—Separations of Water Pollutants with Nanotechnology; Ahuja, S., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 69–85. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Hsiao, B.S. Efficient Removal of UO22+ from Water Using Carboxycellulose Nanofibers Prepared by the Nitro-Oxidation Method. Ind. Eng. Chem. Res. 2017, 56, 13885–13893. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Das, R.; Lindström, T.; Sharma, P.R.; Chi, K.; Hsiao, B.S. Nanocellulose for Sustainable Water Purification. Chem. Rev. 2022, 122, 8936–9031. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Borges, W.; Chen, H.; Hsiao, B.S. Remediation of UO22+ from Water by Nitro-Oxidized Carboxycellulose Nanofibers: Performance and Mechanism. In Contaminants in Our Water: Identification and Remediation Methods; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1352, pp. 269–283. ISBN 9780841298941. [Google Scholar]
- Sharma, S.K.; Sharma, P.R.; Chen, H.; Johnson, K.; Zhan, C.; Wang, R.; Hsiao, B. Cellulose-Supported Nanosized Zinc Oxide: Highly Efficient Bionanomaterial for Removal of Arsenic from Water. In Contaminants in Our Water: Identification and Remediation Methods; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1352, pp. 253–267. ISBN 9780841298941. [Google Scholar]
- Elgarahy, A.M.; Elwakeel, K.Z.; Akhdhar, A.; Hamza, M.F. Recent Advances in Greenly Synthesized Nanoengineered Materials for Water/Wastewater Remediation: An Overview. Nanotechnol. Environ. Eng. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Ahmed, M.M.; Akhdhar, A.; Sulaiman, M.G.M.; Khan, Z.A. Recent Advances in Alginate-Based Adsorbents for Heavy Metal Retention from Water: A Review. Desalination Water Treat. 2022, 272, 50–74. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Functional Nanoparticles Obtained from Cellulose: Engineering the Shape and Size of 6-Carboxycellulose. Chem. Commun. 2013, 49, 8818–8820. [Google Scholar] [CrossRef]
- Sharma, P.R.; Varma, A.J. Functionalized Celluloses and Their Nanoparticles: Morphology, Thermal Properties, and Solubility Studies. Carbohydr. Polym. 2014, 104, 135–142. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in Cellulose Nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Nelson, K.; Retsina, T.; Iakovlev, M.; van Heiningen, A.; Deng, Y.; Shatkin, J.A.; Mulyadi, A. American Process: Production of Low Cost Nanocellulose for Renewable, Advanced Materials Applications. In Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2016; Volume 224, pp. 267–302. [Google Scholar]
- Jiang, G.; Zhang, J.; Qiao, J.; Jiang, Y.; Zarrin, H.; Chen, Z.; Hong, F. Bacterial Nanocellulose/Nafion Composite Membranes for Low Temperature Polymer Electrolyte Fuel Cells. J. Power Sources 2015, 273, 697–706. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Vilela, C.; Loureiro, F.J.A.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nafion® and Nanocellulose: A Partnership for Greener Polymer Electrolyte Membranes. Ind. Crops Prod. 2016, 93, 212–218. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Nasseri, R.; Karkhaneh, A.; Majedi, F.S.; Mokarram, N.; Renaud, P.; Jacob, K.I. Cellulose Nanowhiskers to Regulate the Microstructure of Perfluorosulfonate Ionomers for High-Performance Fuel Cells. J. Mater. Chem. A 2014, 2, 11334–11340. [Google Scholar] [CrossRef]
- Ni, C.; Wei, Y.; Zhao, Q.; Liu, B.; Sun, Z.; Gu, Y.; Zhang, M.; Hu, W. Novel Proton Exchange Membranes Based on Structure-Optimized Poly(Ether Ether Ketone Ketone)s and Nanocrystalline Cellulose. Appl. Surf. Sci. 2018, 434, 163–175. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, X.; Raut, A.; Isseroff, R.; Xue, Y.; Zhou, Y.; Sandhu, B.; Schein, T.; Zeliznyak, T.; Sharma, P.; et al. Operation of Proton Exchange Membrane (PEM) Fuel Cells Using Natural Cellulose Fiber Membranes. Sustain. Energy Fuels 2019, 3, 2725–2732. [Google Scholar] [CrossRef]
- Smolarkiewicz, I.; Rachocki, A.; Pogorzelec-Glasser, K.; Pankiewicz, R.; Ławniczak, P.; Łapiński, A.; Jarek, M.; Tritt-Goc, J.; Pogorzelec-Glaser, K.; Pankiewicz, R.; et al. Proton-Conducting Microcrystalline Cellulose Doped with Imidazole. Thermal and Electrical Properties. Electrochim. Acta 2015, 155, 38–44. [Google Scholar] [CrossRef]
- Bideau, B.; Cherpozat, L.; Loranger, E.; Daneault, C. Conductive Nanocomposites Based on TEMPO-Oxidized Cellulose and Poly(N-3-Aminopropylpyrrole-Co-Pyrrole). Ind. Crops Prod. 2016, 93, 136–141. [Google Scholar] [CrossRef]
- Jiang, G.; Qiao, J.; Hong, F. Application of Phosphoric Acid and Phytic Acid-Doped Bacterial Cellulose as Novel Proton-Conducting Membranes to PEMFC. Int. J. Hydrogen Energy 2012, 37, 9182–9192. [Google Scholar] [CrossRef]
- Jankowska, I.; Pankiewicz, R.; Pogorzelec-Glaser, K.; Ławniczak, P.; Łapiński, A.; Tritt-Goc, J. Comparison of Structural, Thermal and Proton Conductivity Properties of Micro- and Nanocelluloses. Carbohydr. Polym. 2018, 200, 536–542. [Google Scholar] [CrossRef]
- Guccini, V.; Carlson, A.; Yu, S.; Lindbergh, G.; Lindström, R.W.; Salazar-Alvarez, G. Highly Proton Conductive Membranes Based on Carboxylated Cellulose Nanofibres and Their Performance in Proton Exchange Membrane Fuel Cells. J. Mater. Chem. A Mater. 2019, 7, 25032–25039. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, P.R.; Wang, L.; Pagel, M.; Borges, W.; Johnson, K.I.; Raut, A.; Gu, K.; Bae, C.; Rafailovich, M.; et al. Nitro-Oxidized Carboxylated Cellulose Nanofiber Based Nanopapers and Their PEM Fuel Cell Performance. Sustain. Energy Fuels 2022, 6, 3669–3680. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Kontou-Vrettou, C.; Moates, G.K.; Wellner, N.; Cross, K.; Pereira, P.H.F.; Waldron, K.W. Wheat Straw Hemicellulose Films as Affected by Citric Acid. Food Hydrocoll. 2015, 50, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Armentrout, A.A.; Li, J.; Tekinalp, H.L.; Nanda, J.; Ozcan, S. A Cellulose Nanocrystal-Based Composite Electrolyte with Superior Dimensional Stability for Alkaline Fuel Cell Membranes. J. Mater. Chem. A Mater. 2015, 3, 13350–13356. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel Superabsorbent Cellulose-Based Hydrogels Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of Citric Acid and Curing on Moisture Sorption, Diffusion and Permeability of Starch Films. Carbohydr. Polym. 2013, 94, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Quellmalz, A.; Mihranyan, A. Citric Acid Cross-Linked Nanocellulose-Based Paper for Size-Exclusion Nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Liu, Q.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Characterization of Citric Acid/Glycerol Co-Plasticized Thermoplastic Starch Prepared by Melt Blending. Carbohydr. Polym. 2007, 69, 748–755. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric Acid Crosslinked β-Cyclodextrin/Carboxymethylcellulose Hydrogel Films for Controlled Delivery of Poorly Soluble Drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Dhane, N.S.; Ghorpade, V.S. Citric Acid Crosslinked Carboxymethyl Cellulose-Based Composite Hydrogel Films for Drug Delivery. Indian J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Zheng, B.; Sharma, S.K.; Zhan, C.; Wang, R.; Bhatia, S.R.; Hsiao, B.S. High Aspect Ratio Carboxycellulose Nanofibers Prepared by Nitro-Oxidation Method and Their Nanopaper Properties. ACS Appl. Nano Mater. 2018, 1, 3969–3980. [Google Scholar] [CrossRef]
- Wang, M.; Shao, C.; Zhou, S.; Yang, J.; Xu, F. Preparation of Carbon Aerogels from TEMPO-Oxidized Cellulose Nanofibers for Organic Solvents Absorption. RSC Adv. 2017, 7, 38220–38230. [Google Scholar] [CrossRef]
- Kim, H.; Guccini, V.; Lu, H.; Salazar-Alvarez, G.; Lindbergh, G.; Cornell, A. Lithium Ion Battery Separators Based on Carboxylated Cellulose Nanofibers from Wood. ACS Appl. Energy Mater. 2019, 2, 1241–1250. [Google Scholar] [CrossRef]
- Paleckiene, R.; Sviklas, A.; Slinksiene, R. Reaction of Urea with Citric Acid. Russ. J. Appl. Chem. 2005, 78, 1651–1655. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Meftahi, A.; Khajavi, R.; Rashidi, A.; Rahimi, M.K.; Bahador, A. Preventing the Collapse of 3D Bacterial Cellulose Network via Citric Acid. J. Nanostructure Chem. 2018, 8, 311–320. [Google Scholar] [CrossRef]
- Chen, F.; Kan, Z.; Hua, S.; Liu, Z.; Yang, M. A New Understanding Concerning the Influence of Structural Changes on the Thermal Behavior of Cellulose. J. Polym. Res. 2015, 22, 1–8. [Google Scholar] [CrossRef]
- Wyrzykowski, D.; Hebanowska, E.; Nowak-Wiczk, G.; Makowski, M.; Chmurzyński, L. Thermal Behaviour of Citric Acid and Isomeric Aconitic Acids. J. Anal. Calorim. 2011, 104, 731–735. [Google Scholar] [CrossRef]
- Al-Sabah, A.; Burnell, S.E.A.; Simoes, I.N.; Jessop, Z.; Badiei, N.; Blain, E.; Whitaker, I.S. Structural and Mechanical Characterization of Crosslinked and Sterilised Nanocellulose-Based Hydrogels for Cartilage Tissue Engineering. Carbohydr. Polym. 2019, 212, 242–251. [Google Scholar] [CrossRef]
- Kaya, M. Super Absorbent, Light, and Highly Flame Retardant Cellulose-Based Aerogel Crosslinked with Citric Acid. J. Appl. Polym. Sci. 2017, 134, 45315. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent Advances in Additive-Enhanced Polymer Electrolyte Membrane Properties in Fuel Cell Applications: An Overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Qiao, J.; Yoshimoto, N.; Morita, M. Proton Conducting Behavior of a Novel Polymeric Gel Membrane Based on Poly(Ethylene Oxide)-Grafted-Poly(Methacrylate). J. Power Sources 2002, 105, 45–51. [Google Scholar] [CrossRef]
- Ludueña, G.A.; Kühne, T.D.; Sebastiani, D. Mixed Grotthuss and Vehicle Transport Mechanism in Proton Conducting Polymers from Ab Initio Molecular Dynamics Simulations. Chem. Mater. 2011, 23, 1424–1429. [Google Scholar] [CrossRef]
- Shigematsu, A.; Yamada, T.; Kitagawa, H. Wide Control of Proton Conductivity in Porous Coordination Polymers. J. Am. Chem. Soc. 2011, 133, 2034–2036. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Liu, Y.; Liu, S.; He, D.; Miao, J.; Wang, X.; Yang, G.; Shi, Z.; Zheng, Z. High Proton Conduction at above 100 °C Mediated by Hydrogen Bonding in a Lanthanide Metal–Organic Framework. J. Am. Chem. Soc. 2014, 136, 12444–12449. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.A.; Kuznetsov, A.M.; Ulstrup, J.; Walbran, S. Mechanisms of Proton Conductance in Polymer Electrolyte Membranes. J. Phys. Chem. B 2001, 105, 3646–3662. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of Advanced Materials for Proton Exchange Membrane Fuel Cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Zhang, S.-D.; Zhang, Y.-R.; Huang, H.-X.; Yan, B.-Y.; Zhang, X.; Tang, Y. Preparation and Properties of Starch Oxalate Half-Ester with Different Degrees of Substitution. J. Polym. Res. 2009, 17, 43. [Google Scholar] [CrossRef]
- Saadiah, M.A.; Tan, H.M.; Samsudin, A.S. Enhancement of Proton Conduction in Carboxymethyl Cellulose-Polyvinyl Alcohol Employing Polyethylene Glycol as a Plasticizer. Bull. Mater. Sci. 2020, 43, 203. [Google Scholar] [CrossRef]
- Prajapati, G.K.; Roshan, R.; Gupta, P.N. Effect of Plasticizer on Ionic Transport and Dielectric Properties of PVA–H3PO4 Proton Conducting Polymeric Electrolytes. J. Phys. Chem. Solids 2010, 71, 1717–1723. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, H.; Saito, T.; Iwata, T.; Kumamoto, Y.; Isogai, A. Transparent and High Gas Barrier Films of Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation. Biomacromolecules 2009, 10, 162–165. [Google Scholar] [CrossRef]


| Surface Area (m2/g) | Pore Volume (cc/g) | |
|---|---|---|
| CNF-1 | 1.63720 | 0.0057850 |
| CNF-2 | 1.26727 | 0.0047933 |
| CNF-3 | 0.94770 | 0.0034088 |
| CNF-4 | 0.64708 | 0.0023407 |
| Reference | PEM Material | Maximum Power Density (mW cm−2) |
|---|---|---|
| Jiang et al., 2012 [45] | Phytic acid-incorporated Bacterial Cellulose | 23.0 |
| Phosphoric acid-incorporated Bacterial Cellulose | 17.9 | |
| Bayer et al., 2016 [36] | Cellulose Nanocrystal | 17.2 |
| Cellulose Nanofiber | 0.8 | |
| Wang et al., 2019 [42] | Nafion-impregnated Cellulose Filter Paper | 23 |
| RDP-impregnated Cellulose Filter Paper | 10 | |
| Sharma et al., 2022 [48] | Nitro-oxidized CNF w/Carboxylic Acid Functionalities | 19.1 |
| Nitro-oxidized CNF w/Carboxylate Functionalities | 5.8 | |
| This Study | Citric acid cross-linked CNF | 27.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Cai, G.; Wu, S.; Raut, A.; Borges, W.; Sharma, P.R.; Sharma, S.K.; Hsiao, B.S.; Rafailovich, M. Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells. Int. J. Mol. Sci. 2022, 23, 15245. https://doi.org/10.3390/ijms232315245
Li S, Cai G, Wu S, Raut A, Borges W, Sharma PR, Sharma SK, Hsiao BS, Rafailovich M. Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells. International Journal of Molecular Sciences. 2022; 23(23):15245. https://doi.org/10.3390/ijms232315245
Chicago/Turabian StyleLi, Songtao, George Cai, Songze Wu, Aniket Raut, William Borges, Priyanka R. Sharma, Sunil K. Sharma, Benjamin S. Hsiao, and Miriam Rafailovich. 2022. "Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells" International Journal of Molecular Sciences 23, no. 23: 15245. https://doi.org/10.3390/ijms232315245
APA StyleLi, S., Cai, G., Wu, S., Raut, A., Borges, W., Sharma, P. R., Sharma, S. K., Hsiao, B. S., & Rafailovich, M. (2022). Sustainable Plant-Based Biopolymer Membranes for PEM Fuel Cells. International Journal of Molecular Sciences, 23(23), 15245. https://doi.org/10.3390/ijms232315245