Duckweed Is a Promising Feedstock of Biofuels: Advantages and Approaches
Abstract
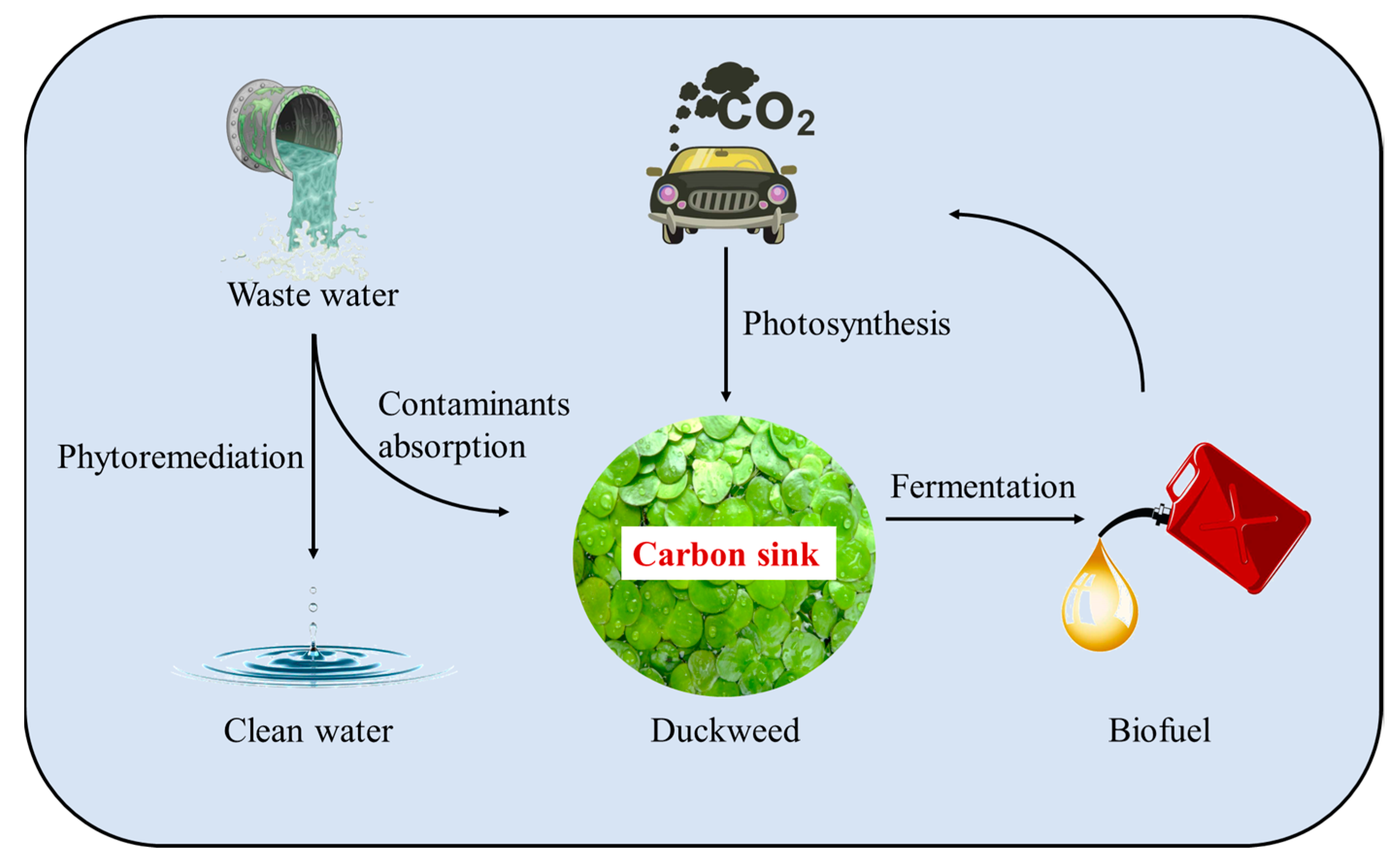
:1. Introduction
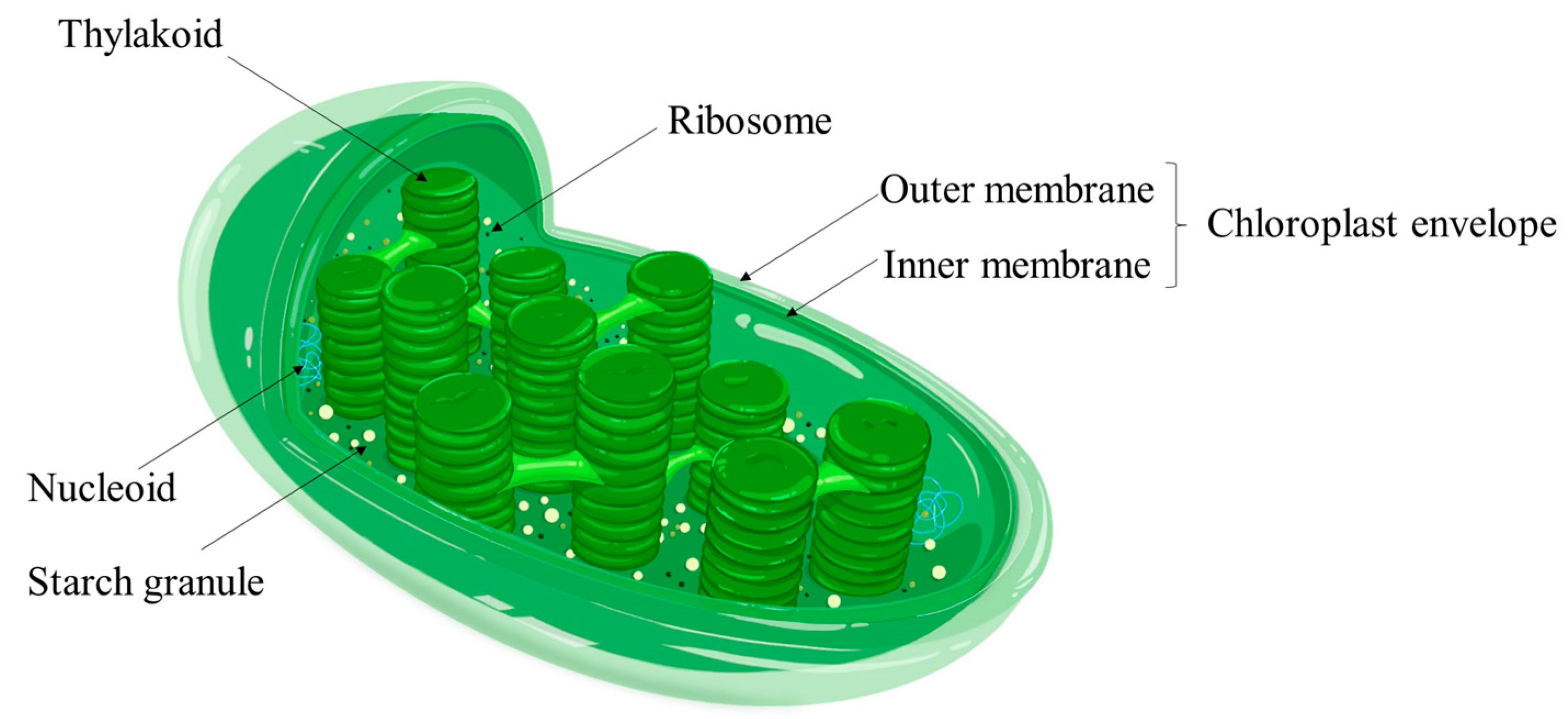
2. Biological Properties of Duckweed
2.1. Advantages of Duckweed as an Energy Source
2.2. High Growth Rate and Low Environmental Requirements
2.3. No Competition with Food Crops for Land
2.4. High Starch Content and Low Cellulose Content
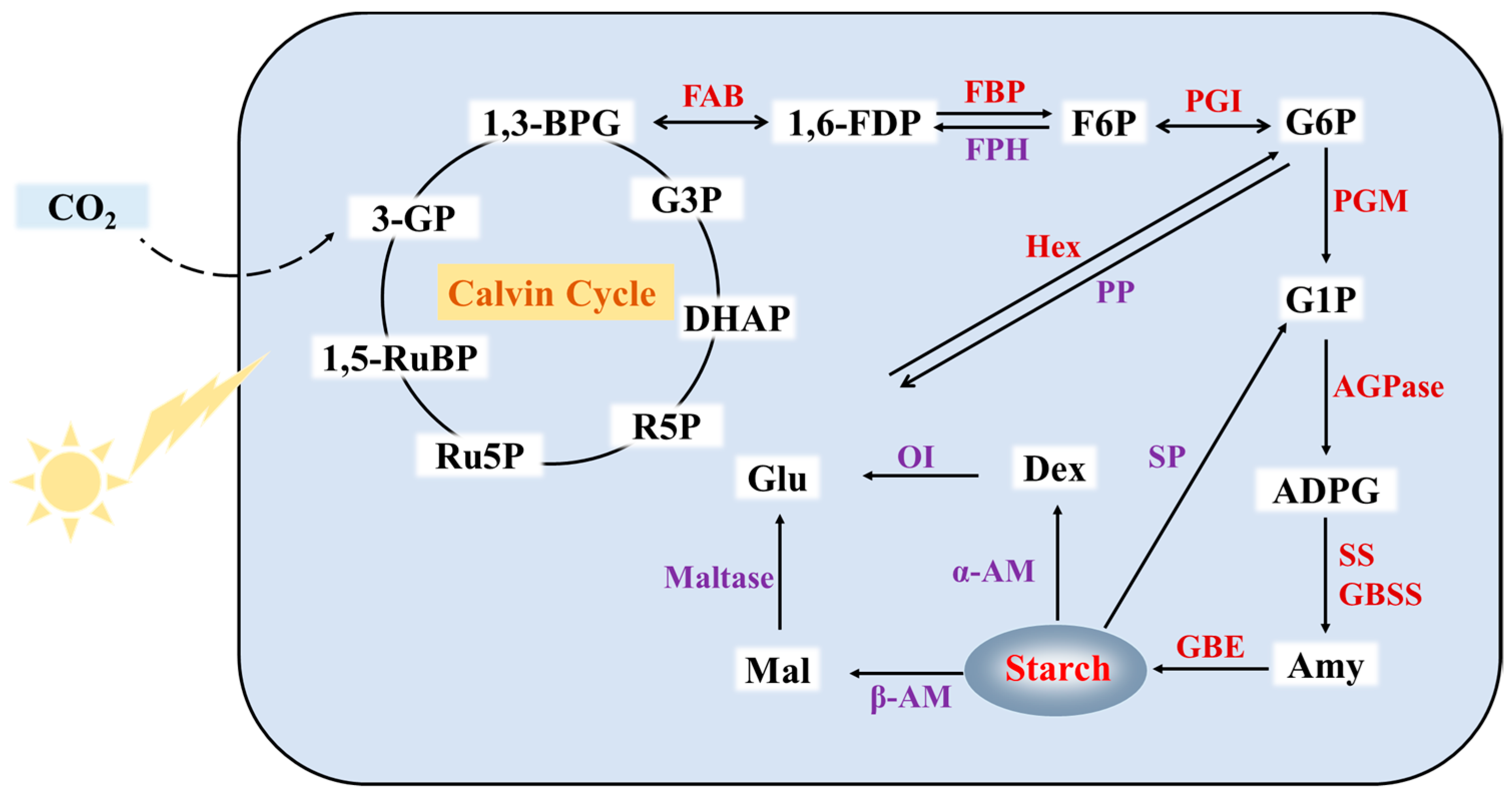
3. Metabolism Pathway of Duckweed Starch
3.1. Synthetic Pathway of Duckweed Starch
3.2. Degradation Pathway of Duckweed Starch
4. Pathways to Promote the Accumulation of Duckweed Starch
4.1. Nutritional Regulation Promotes Duckweed Starch Accumulation
4.2. Application of Exogenous Hormones to Promote Starch Accumulation in Duckweed
| Phytohormone | Species | Starch Content (DW) | Refs |
|---|---|---|---|
| 6-BA | Le. minor | 69% | [54] |
| La. punctata | 4.59~5.03% | [61] | |
| ABA | Le. minor | 100 µg g−1~600 µg g−1 (FW) | [55] |
| S. polyrhiza | 60% | [56] | |
| 125~35.3% | [57] | ||
| La. punctata | 2.29~46.18% | [58] | |
| 4.78~13.73% | [61] | ||
| IAA | La. punctata | 4.5~7.5% | [60] |
| Uniconazole | La. punctata | 3.16~48.01% | [47,59] |
| 2,4-D | La. punctata | 4.39~6.72% | [61] |
| GA3, | La. punctata | 4.83~5.24% | [61] |
| BL | La. punctata | 4.81~4.89% | [61] |
4.3. CO2 Supplementation Promotes Starch Accumulation in Duckweed
4.4. Light Source Regulation Promotes Duckweed Starch Accumulation
4.5. Turion Induction Promotes Starch Accumulation in Duckweed
4.6. Other Stress Factors That Promote the Accumulation of Starch
| Stress Factor | Species | Starch Content (DW) | Refs |
|---|---|---|---|
| Cu2+ | La. punctata Le. minor | 2.34~13.37% 3.74~8.41% | [76] |
| Co2+ | Le. minor | Up to 40.5% | [74] |
| Co2++Ni2+ | La. punctata | Up to 53.3% | [75] |
| NaCl | S. polyrhiza | 12% | [78] |
| NH4+-N | S. polyrhiza, Le. aequinoctialis La. punctata | Up to 32% | [41,80] |
| NO3−-N | La. punctata | 4~8% | [80] |
| MC-LR | La. punctata | Up to 29.8% | [18] |
| NAP | La. punctata | Up to 29.8% | [18] |
5. Study on the Biomass Transformation of High-Starch Duckweed
6. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T.H. Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 2008, 319, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.R. Global Biomass Energy Potential. Mitigat. Adapt. Strateg. Glob. Chang. 2006, 11, 313–342. [Google Scholar] [CrossRef]
- Xue, J.; Hou, D.; Wang, W.; Zhang, J.; Zhang, J. Research on Current Conditions and Development Trends of Global Biomass Energy Industry and Technology. Petrol. Sci.Technol. Forum 2020, 39, 25–35. [Google Scholar]
- Xue, H.; Dong, Z.; Fang, Y.; Jin, Y.; Zhao, H. Producing fuel ethanol from energy hygrophyte duckweed. Renew. Energ. Resourc. 2013, 31, 55–59. [Google Scholar]
- Landis, D.A.; Gardiner, M.M.; Werf, W.; Swinton, S.M. Increasing corn for biofuel production reduces biocontrol services in agricultural landscapes. Proc. Natl. Acad. Sci. USA 2009, 105, 20552–20557. [Google Scholar] [CrossRef] [Green Version]
- Halford, N.G.; Karp, A. Challenges and opportunities for using wheat for biofuel production. Energ. Crop. 2010, 13–26. [Google Scholar] [CrossRef]
- Tenenbaum, D.J. Food vs. fuel: Diversion of crops could cause more hunger. Environ. Health Perspect. 2008, 116, A254–A257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arefin, M.A.; Rashid, F.; Islam, A. A review of biofuel production from floating aquatic plants: An emerging source of bio-renewable energy. Biofuels Bioprod. Biorefin. 2021, 15, 574–591. [Google Scholar] [CrossRef]
- Suali, E.; Sarbatly, R. Conversion of microalgae to biofuel. Renew. Sust. Energ. Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Bog, M.; Appenroth, K.J.; Sree, K.S. Key to the determination of taxa of Lemnaceae: An update. Nord. J. Bot. 2020, 38. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J.; Keurentjes, J. Relative in vitro growth rates of duckweeds (Lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2014, 17, 33–41. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Huang, M.; Peng, M.; Bog, M.; Sree, K.S.; Appenroth, K.J.; Zhang, J. Species distribution, genetic diversity and barcoding in the duckweed family (Lemnaceae). Hydrobiologia 2015, 743, 75–87. [Google Scholar] [CrossRef]
- Cheng, J.J.; Stomp, A.M. Growing Duckweed to Recover Nutrients from Wastewaters and for Production of Fuel Ethanol and Animal Feed. Clean Soil Air Water 2010, 37, 17–26. [Google Scholar] [CrossRef]
- Xu, J.L.; Cui, W.H.; Cheng, J.J.; Stomp, A.M. Production of high-starch duckweed and its conversion to bioethanol. Biosyst. Eng. 2011, 110, 67–72. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, Y.; Zhang, G.; Fang, Y.; Zhao, H. Improving Production of Bioethanol from Duckweed (Landoltia punctata) by Pectinase Pretreatment. Energies 2012, 5, 3019–3032. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.L.; Yang, M.X.; Lv, S.M.; Tan, A.J. The effect of chelating agents on iron plaques and arsenic accumulation in duckweed (Lemna minor). J. Hazard. Mater. 2021, 419, 126410. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; Huang, M.J.; Tan, A.J.; Lv, S.M. Joint effects of naphthalene and microcystin-LR on physiological responses and toxin bioaccumulation of Landoltia punctata. Aquat. Toxicol. 2021, 231, 105710. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, W.; Yang, J.; Zhao, X.; Chen, Y.; Yao, L.; Hou, H. Enhanced biomass production and pollutant removal by duckweed in mixotrophic conditions. Bioresourc. Technol. 2020, 317, 124029. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cheng, J.J. Growing duckweed for biofuel production: A review. Plant Biol. 2015, 17, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Radić, S.; Stipaničev, D.; Cvjetko, P.; Mikelić, I.L.; Rajčić, M.M.; Širac, S.; Pevalek-Kozlina, B.; Pavlica, M. Ecotoxicological assessment of industrial effluent using duckweed (Lemna minor L.) as a test organism. Ecotoxicology 2010, 19, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of common duckweed (Lemna minor) in phytoremediation of chemicals in the environment: State and future perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, X.; Li, G.; Hu, S.; Hou, H. Frond Architecture of The Rootless Duckweed Wolffia Globosa. BMC Plant Biol. 2021, 21, 387. [Google Scholar] [CrossRef]
- Les, D.H.; Crawford, D.J.; Landolt, E.; Gabel, J.D.; Rettig, J.H. Phylogeny and Systematics of Lemnaceae, the Duckweed Family. Syst. Bot. 2002, 27, 221–240. [Google Scholar]
- Dekock, P.C.; Hall, D.V. Effect of Abscisic Acid and Benzyl Adenine on the Inorganic and Organic Composition of the Duckweed, Lemna gibba L. New Phytol. 1978, 81, 505–511. [Google Scholar] [CrossRef]
- Huang, M.; Xu, Y.; Kanjana, K.; Sun, X.; Zhang, J. Flower-Induction of Lemna gibba SH0204 by Salicylic Acid. Plant Physiol. Commun. 2015, 51, 559–565. [Google Scholar]
- Fu, L.; Huang, M.; Han, B.; Sun, X.; Sree, K.S.; Appenroth, K.J.; Zhang, J. Flower induction, microscope-aided cross-pollination, and seed production in the duckweed Lemna gibba with discovery of a male-sterile clone. Sci. Rep. 2017, 7, 3047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Baig, M.A. Nitrogen and phosphorous removal from leachate by duckweed (Duckweed minor). Environ. Protect. Eng. 2017, 43, 123–134. [Google Scholar] [CrossRef]
- Wang, B.; Peng, L.; Zhu, L.; Ren, P. Protective effect of total flavonoids from Spirodela polyrrhiza (L.) Schleid on human umbilical vein endothelial cell damage induced by hydrogen peroxide. Colloid. Surf. B Biointerf. 2007, 60, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Pagliuso, D.; Jara, C.E.P.; Grandis, A.; Lam, E.; Ferreira, M.J.P.; Buckeridge, M.S. Flavonoids from duckweeds: Potential applications in the human diet. RSC Adv. 2020, 10, 44981–44988. [Google Scholar] [CrossRef]
- De Beukelaar, M.F.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R. Duckweed as human food. The influence of meal context and information on duckweed acceptability of Dutch consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Busi, M.V.; Barchiesi, J.; Martín, M.; Gomez-Casati, D.F. Starch metabolism in green algae. Starch Stärke 2014, 66, 28–40. [Google Scholar] [CrossRef]
- Huang, M.; Fang, Y.; Xiao, Y.; Sun, J.; Jin, Y.; Tao, X.; Ma, X.; He, K.; Zhao, H. Proteomic analysis to investigate the high starch accumulation of duckweed (Landoltia punctata) under nutrient starvation. Indust. Crops 2014, 59, 299–308. [Google Scholar] [CrossRef]
- Guo, L.; Jin, Y.; Xiao, Y.; Tan, L.; Tian, X.; Ding, Y.; He, K.; Du, A.; Li, J.; Yi, Z. Energy-efficient and environmentally friendly production of starch-rich duckweed biomass using nitrogen-limited cultivation. J. Clean. Prod. 2020, 251, 119726. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.-J.; Wang, M.-L.; Cui, L.; Zhao, H.; Zhao, Y. Effect of nitrogen and phosphorus deficiency on transcriptional regulation of genes encoding key enzymes of starch metabolism in duckweed (Landoltia punctata). Plant Physiol. Biochem. 2015, 86, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kruger, K.; Chen, L.D.; He, B.B. Nutrient starvation and light deprivation effects on starch accumulation in Landoltia punctata cultivated on anaerobically digested dairy manure. J. Environ. Qual. 2020, 49, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Frédéric, M.; Samir, L.; Louise, M.; Abdelkrim, A. Comprehensive modeling of mat density effect on duckweed (Lemna minor) growth under controlled eutrophication. Water Res. 2006, 40, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Borisjuk, N.; Chu, P.; Gutierrez, R.; Zhang, H.; Acosta, K.; Friesen, N.; Sree, K.S.; Garcia, C.; Appenroth, K.J.; Lam, E. Assessment, validation and deployment strategy of a two-barcode protocol for facile genotyping of duckweed species. Plant Biol. 2015, 17, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, X.; Lian, Y.; Xu, L.; Chen, Y. Reduction of ammonia volatilization from urea by a floating duckweed in flooded rice fields. Soil Sci. Soc. Am. J. 2009, 73, 1890–1895. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, M.; Tian, Y.; Zhao, M.; Zhang, B.; Zhao, M.; Zeng, K.; Yin, B. Duckweed (Spirodela polyrhiza) as green manure for increasing yield and reducing nitrogen loss in rice production. Field Crops Res. 2017, 214, 273–282. [Google Scholar] [CrossRef]
- Xiao, Y.; Fang, Y.; Jin, Y.; Zhang, G.; Zhao, H. Culturing duckweed in the field for starch accumulation. Ind. Crops Prod. 2013, 48, 183–190. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.J.; Stomp, A.M. Growing Spirodela polyrrhiza in swine wastewater for the production of animal feed and fuel ethanol: A pilot study. CLEAN–Soil Air Water 2012, 40, 760–765. [Google Scholar] [CrossRef]
- Cui, W.; Xu, J.; Cheng, J.; Stomp, A. Starch accumulation in duckweed for bioethanol production. Biol. Eng. Trans. 2011, 3, 187–197. [Google Scholar] [CrossRef]
- Tetlow, I.J.; Morell, M.K.; Emes, M.J. Recent developments in understanding the regulation of starch metabolism in higher plants. J. Exp. Bot. 2004, 55, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhao, X.; Qi, G.; Bai, Z.; Wang, Y.; Wang, S.; Ma, Y.; Liu, Q.; Hu, R.; Zhou, G. Integrated analysis of transcriptome and metabolites reveals an essential role of metabolic flux in starch accumulation under nitrogen starvation in duckweed. Biotechnol. Biofuels 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Li, X.; Gao, X.; Sun, J.; Ji, X.; Feng, G.; Shen, G.; Xiang, B.; Wang, Y. Molecular mechanism underlying the effect of maleic hydrazide treatment on starch accumulation in S. polyrrhiza 7498 fronds. Biotechnol. Biofuels 2021, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.; Huang, M.; Jin, Y.; Sun, J.; Tao, X.; Zhang, G.; He, K.; Zhao, Y.; Zhao, H. Uniconazole-induced starch accumulation in the bioenergy crop duckweed (Landoltia punctata) II: Transcriptome alterations of pathways involved in carbohydrate metabolism and endogenous hormone crosstalk. Biotechnol. Biofuel. 2015, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Reid, M.; Bieleski, R. Response of Spirodela oligorrhiza to phosphorus deficiency. Plant Physiol. 1970, 46, 609–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-M.; Du, A.-P.; Liu, P.-H.; Tian, X.-P.; Jin, Y.-L.; Yi, Z.-L.; He, K.-Z.; Fang, Y.; Zhao, H. High starch accumulation mechanism and phosphorus utilization efficiency of duckweed (Landoltia punctata) under phosphate starvation. Ind. Crops Prod. 2021, 167, 113529. [Google Scholar] [CrossRef]
- Zhao, Z.; Yao, G.B.; Zhang, Y.Q.; Zhao, Y. The analysis of the biomass and starch content of duckweed growing in swine wastewater. J. Sichuan Univ. Nat. Sci. Ed. 2012, 49, 693–698. [Google Scholar]
- Ge, X.; Zhang, N.; Phillips, G.C.; Xu, J. Growing Lemna minor in agricultural wastewater and converting the duckweed biomass to ethanol. Bioresour. Technol. 2012, 124, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yang, F.; Yao, X.; Jin, Y.L.; Ma, X. Comparative transcriptome analysis to investigate the high starch accumulation of duckweed (Landoltia punctata) under nutrient starvation. Biotechnol. Biofuel. 2013, 6, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.N.; Irfan, M.; Shah, A.A.; Hasan, F.; Khan, S.; Ahmed, S.; Adnan, F.; Li, W.; Ju, M.; Badshah, M. Starved Spirodela polyrhiza and Saccharomyces cerevisiae: A potent combination for sustainable bioethanol production. Biomass Convers. Biorefinery 2021, 11, 1665–1674. [Google Scholar]
- Jong, J.; Veldstra, H. Investigations on Cytokinins. I. Effect of 6-Benzylaminopurine on Growth and Starch Content of Lemna minor. Physiol. Plant. 1970, 24, 235–238. [Google Scholar] [CrossRef]
- McLaren, J.S.; Smith, M.L. The Effect of Abscisic Acid on Growth, Photosynthetic Rate and Carbohydrate Metabolism in Lemna minor L. New Phytol. 1975, 76, 11–20. [Google Scholar] [CrossRef]
- Wang, W.; Messing, J. Analysis of ADP-glucose pyrophosphorylase expression during turion formation induced by abscisic acid in Spirodela polyrhiza (greater duckweed). BMC Plant Biol. 2012, 12, 5. [Google Scholar] [CrossRef]
- Wang, X.; Cui, W.; Hu, W.; Feng, C. Abscisic Acid-Induced Starch Accumulation in Bioenergy Crop Duckweed Spirodela polyrrhiza. BioEnerg. Res. 2016, 10, 417–426. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, X.; Fang, Y.; Huang, M.; Guo, L.; Zhang, Y.; Zhao, H. Improving biomass and starch accumulation of bioenergy crop duckweed (Landoltia punctata) by abscisic acid application. Sci. Rep. 2018, 8, 9544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Fang, Y.; Liu, Y.; Jin, Y.; Sun, J.; Tao, X.; Ma, X.; He, K.; Zhao, H. Using proteomic analysis to investigate uniconazole-induced phytohormone variation and starch accumulation in duckweed (Landoltia punctata). BMC Biotechnol. 2015, 15, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, R.; Zhang, T.; Zhang, Y.; Zhao, Q. Effect of Different Concentrations of IAA on the Growth and Starch Accumulation of Duckweed. Acta Bot. Boreali-Occident. Sin. 2018, 38, 1722–1726. [Google Scholar]
- Liu, Y.; Chen, X.; Wang, X.; Fang, Y.; Zhang, Y.; Huang, M.; Zhao, H. The influence of different plant hormones on biomass and starch accumulation of duckweed: A renewable feedstock for bioethanol production. Renew. Energ. 2019, 138, 659–665. [Google Scholar] [CrossRef]
- Jacobs, D.L. An Ecological Life-History of Spirodela Polyrhiza (Greater Duckweed) with Emphasis on the Turion Phase. Ecol. Monogr. 1947, 17, 437–469. [Google Scholar] [CrossRef]
- Pankey, R.D.; Draudt, H.N.; Desrosier, N.W. Characterization of the Starch of Spirodela polyrrhiza. J. Food Sci. 1965, 30, 627–631. [Google Scholar] [CrossRef]
- Li, Q.; Fang, Y.; Xu, Y.; Lai, F.; Su, Y.; Jin, Y.; Zhao, H. Duckweed Landoltia punctata purifies micro-polluted surface water and produces starch. Chin. J. Appl. Environ. Biol. 2018, 24, 1–9. [Google Scholar]
- Yin, Y.; Yu, C.; Li, Y.; Zhao, J.; Zhou, G. The influence of light intensity and photoperiod on duckweed biomass and starch accumulation for bioethanol production. Bioresourc. Technol. 2015, 187, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Q.; Zhao, Q. Effect of Different Photoperiod on the Growth and Starch Accumulation of Duckweed. Northern Horticul. 2017, 22, 50–54. [Google Scholar]
- Liu, Y.; Wang, X.; Fang, Y.; Huang, M.; Chen, X.; Zhang, Y.; Zhao, H. The effects of photoperiod and nutrition on duckweed (Landoltia punctata) growth and starch accumulation. Indust. Crops Prod. 2018, 115, 243–249. [Google Scholar] [CrossRef]
- Xu, Y.L.; Tan, L.; Guo, L.; Yang, G.L.; Li, Q.; Lai, F.; He, K.Z.; Jin, Y.L.; Du, A.P.; Fang, Y.; et al. Increasing starch productivity of Spirodela polyrhiza by precisely control the spectral composition and nutrients status. Indust. Crops Prod. 2019, 134, 284–291. [Google Scholar] [CrossRef]
- Mccombs, P.; Ralph, R.K. Protein, nucleic acid and starch metabolism in the duckweed, Spirodela oligorrhiza, treated with cytokinins. Biochem. J. 1972, 129, 403–417. [Google Scholar] [CrossRef]
- Yang, G.L.; Feng, D.; Liu, Y.T.; Lv, S.M.; Zheng, M.M.; Tan, A.J. Research Progress of a Potential Bioreactor: Duckweed. Biomolecules 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Yang, F.; Qi, L.; Yang, G.L.; Ling, G.; Chen, G.K.; Li, T.; He, K.Z.; Jin, Y.L.; Hai, Z. Turion, an innovative duckweed-based starch production system for economical biofuel manufacture. Indust. Crops Prod. 2018, 124, 108–114. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Teller, S.; Horn, M. Photophysiology of turion formation and germination in Spirodela polyrhiza. Biol. Plant. 1996, 38, 95. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.; Zhang, N.; Xiao, G.; Liu, C.; Jin, L. Starch accumulation during the formation of duckweed dormancy. Jiangsu Agric. Sci. 2018, 46, 315–318. [Google Scholar]
- Sree, K.S.; Keresztes, A.; Mueller-Roeber, B.; Brandt, R.; Eberius, M.; Fischer, W.; Appenroth, K.J. Phytotoxicity of cobalt ions on the duckweed Lemna minor—Morphology, ion uptake, and starch accumulation. Chemosphere 2015, 131, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ding, Y.; Xu, Y.; Li, Z.; Jin, Y.; He, K.; Fang, Y.; Zhao, H. Responses of Landoltia punctata to cobalt and nickel: Removal, growth, photosynthesis, antioxidant system and starch metabolism. Aquat. Toxicol. 2017, 190, 87–93. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, H.; Duan, D.; Li, H.; Lei, T.; Wang, M.; Zhao, H.; Zhao, Y. The influence of duckweed species diversity on ecophysiological tolerance to copper exposure. Aquat. Toxicol. 2015, 164, 92–98. [Google Scholar] [CrossRef]
- Cheng, T.S. NaCl-induced responses in giant duckweed. J. Aquat. Plant Manag. 2011, 49, 62–71. [Google Scholar]
- Fu, L.; Ding, Z.; Sun, X.; Zhang, J. Physiological and Transcriptomic Analysis Reveals Distorted Ion Homeostasis and Responses in the Freshwater Plant Spirodela polyrhiza L. under Salt Stress. Genes 2019, 10, 743. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Fang, Y.; Jin, Y.; Yi, Z.; Li, J.; Du, A.; He, K.; Huang, Y.; Zhao, H. Ammonium detoxification mechanism of ammonium-tolerant duckweed (Landoltia punctata) revealed by carbon and nitrogen metabolism under ammonium stress. Environ. Poll. 2021, 277, 116834. [Google Scholar] [CrossRef]
- Petersen, F.; Demann, J.; Restemeyer, D.; Ulbrich, A.; Olfs, H.-W.; Westendarp, H.; Appenroth, K.-J. Influence of the Nitrate-N to Ammonium-N Ratio on Relative Growth Rate and Crude Protein Content in the Duckweeds Lemna minor and Wolffiella hyalina. Plants 2021, 10, 1741. [Google Scholar] [CrossRef]
- Djandja, O.S.; Yin, L.; Wang, Z.; Guo, Y.; Duan, P. Progress in thermochemical conversion of duckweed and upgrading of the bio-oil: A critical review. Sci. Total Environ. 2021, 769, 144660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, H.; Yu, C.; Zhou, G. Multifaceted roles of duckweed in aquatic phytoremediation and bioproducts synthesis. GCB Bioenerg. 2021, 13, 70–82. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, M.; Yu, C.; Wang, Y.; Liu, Y.; Li, M.; Sun, Y.; Zhao, J.; Zhou, G. Large-scale screening and characterisation of Lemna aequinoctialis and Spirodela polyrhiza strains for starch production. Plant Biol. 2017, 20, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Bhatt, N. Aquatic weed Spirodela polyrhiza, a potential source for energy generation and other commodity chemicals production. Renew. Energ. 2021, 173, 455–465. [Google Scholar] [CrossRef]
- Yu, C.; Sun, C.; Yu, L.; Zhu, M.; Xu, H.; Zhao, J.; Ma, Y.; Zhou, G. Comparative Analysis of Duckweed Cultivation with Sewage Water and SH Media for Production of Fuel Ethanol. PLoS ONE 2014, 9, e115023. [Google Scholar] [CrossRef] [PubMed]
- Calicioglu, O.; Brennan, R.A. Sequential ethanol fermentation and anaerobic digestion increases bioenergy yields from duckweed. Bioresourc. Technol. 2018, 257, 344–348. [Google Scholar] [CrossRef]
- Calicioglu, O.; Richard, T.L.; Brennan, R.A. Anaerobic bioprocessing of wastewater-derived duckweed: Maximizing product yields in a biorefinery value cascade. Bioresourc. Technol. 2019, 289, 121716. [Google Scholar] [CrossRef]
- Li, X. Fermentation Method of High Ratios of Biobutanol with Landoltia punctata; University of Chinese Academy of Sciences: Beijing, China, 2011. [Google Scholar]
- Shen, N.; Wang, Q.; Zhu, J.; Qin, Y.; Liao, S.; Li, Y.; Zhu, Q.; Jin, Y.; Du, L.; Huang, R. Succinic acid production from duckweed (Landoltia punctata) hydrolysate by batch fermentation of Actinobacillus succinogenes GXAS137. Bioresour. Technol. 2016, 211, 307–312. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Yang, J.; Qi, X. Catalytic production of levulinic acid and ethyl levulinate from uniconazole-induced duckweed (Lemna minor). Bioresour. Technol. 2018, 255, 50–57. [Google Scholar] [CrossRef]
- Mu, D.; Liu, H.; Lin, W.; Shukla, P.; Luo, J. Simultaneous biohydrogen production from dark fermentation of duckweed and waste utilization for microalgal lipid production. Bioresour. Technol. 2020, 302, 122879. [Google Scholar] [CrossRef]
- Lai, F.; Jin, Y.; Tan, L.; He, K.; Guo, L.; Tian, X.; Li, J.; Du, A.; Huang, Y.; Zhao, H. Bioconversion of wastewater-derived duckweed to lactic acid through fed-batch fermentation at high-biomass loading. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, K.; Li, W.; Yan, B.; Yu, Y.; Li, J.; Zhang, Y.; Xia, S.; Cheng, Z.; Lin, F. A review on bioenergy production from duckweed. Biomass Bioenergy 2022, 161, 106468. [Google Scholar] [CrossRef]
| Plants | Advantages or Disadvantages | Species | Refs |
|---|---|---|---|
| duckweed | rapid growth rate | All species | [12] |
| high content of starch | La. punctata | [32] | |
| Le. aequinoctialis | [33] | ||
| S. polyrhiza | [34] | ||
| Le. minor | [35] | ||
| Le. perpusilla | [35] | ||
| S. polyrhiza | [36] | ||
| low environmental requirements | Le. minor | [37] | |
| Le. japonica | [37] | ||
| La. punctata | [37] | ||
| high adsorption and transfer capacity of nitrogen, phosphorus, organic matter | Le. minor | [28] | |
| Le. gibba | [28] | ||
| La. punctata | [28] | ||
| S. polyrhiza | [28] | ||
| high adsorption capacity of heavy metals | Le. minor | [22] | |
| Le. gibba | [22] | ||
| La. punctata | [22] | ||
| S. polyrhiza | [22] | ||
| Le. japonica | [22] | ||
| no competition with people for food and land | All species | [38] | |
| low cellulose content | S. polyrhiza | [15] | |
| corn | Slow growth rate | [12] | |
| High nutrient condition | [12] | ||
| competition with people for land | [12] |
| Nutritional Conditions | Species | Starch Content (DW) | Refs |
|---|---|---|---|
| Nitrogen starvation | Le. aequinoctialis | 20~60% | [33] |
| La. punctata | 16.97~52.37% | [32] | |
| 8.86~32.5% | [49] | ||
| Phosphorus starvation | S. polyrhiza | 75% | [34] |
| La. punctata | 2.14~38.05% | [48] | |
| 8.86~23% | [49] | ||
| Nutrient starvation | Le. minor | 32.9%, | [35] |
| 10~36% | [50] | ||
| Le. perpusilla | 38.0% | [35] | |
| La. punctata | 3~45.4% | [44,51] | |
| 30% | [52] | ||
| S. polyrhiza | 44.4% | [35] | |
| S. polyrhiza | 64.9% | [15] | |
| 35.5% | [35] | ||
| 20~45.8% | [14] | ||
| 78% | [36] |
| Condition | Parameter | Species | Starch Content (DW) | Refs |
|---|---|---|---|---|
| Light intensity (µmol m−2 s−1) | 20, 50, 80, 110, 200 and 400 | Le. aequinoctialis | 3.86~62.24% | [65] |
| Photoperiod (L/D) | 12:12, 16:8 and 24:0 | Le. aequinoctialis | 3.86~62.24% | [65] |
| 12:12, 16:8, 20:4 and 24:0 | La. punctata | 7~19.75% | [66] | |
| 8:16, 12:12 and 16:8 | S. polyrhiza | 15~67.5% | [43] | |
| 24:0 and 16:8 | La. punctata | 15.7~60.03% | [67] | |
| Spectrum (R/B) | 1/0,0/1,1/2,1/1,2/1 and 4/1 | S. polyrhiza | 65~77.01%(turion) 4.82%~23.19%(frond) | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.-L. Duckweed Is a Promising Feedstock of Biofuels: Advantages and Approaches. Int. J. Mol. Sci. 2022, 23, 15231. https://doi.org/10.3390/ijms232315231
Yang G-L. Duckweed Is a Promising Feedstock of Biofuels: Advantages and Approaches. International Journal of Molecular Sciences. 2022; 23(23):15231. https://doi.org/10.3390/ijms232315231
Chicago/Turabian StyleYang, Gui-Li. 2022. "Duckweed Is a Promising Feedstock of Biofuels: Advantages and Approaches" International Journal of Molecular Sciences 23, no. 23: 15231. https://doi.org/10.3390/ijms232315231
APA StyleYang, G.-L. (2022). Duckweed Is a Promising Feedstock of Biofuels: Advantages and Approaches. International Journal of Molecular Sciences, 23(23), 15231. https://doi.org/10.3390/ijms232315231





