Effects of Avenanthramide on the Small Intestinal Damage through Hsp70-NF-κB Signaling in an Ovalbumin-Induced Food Allergy Model
Abstract
1. Introduction
2. Results
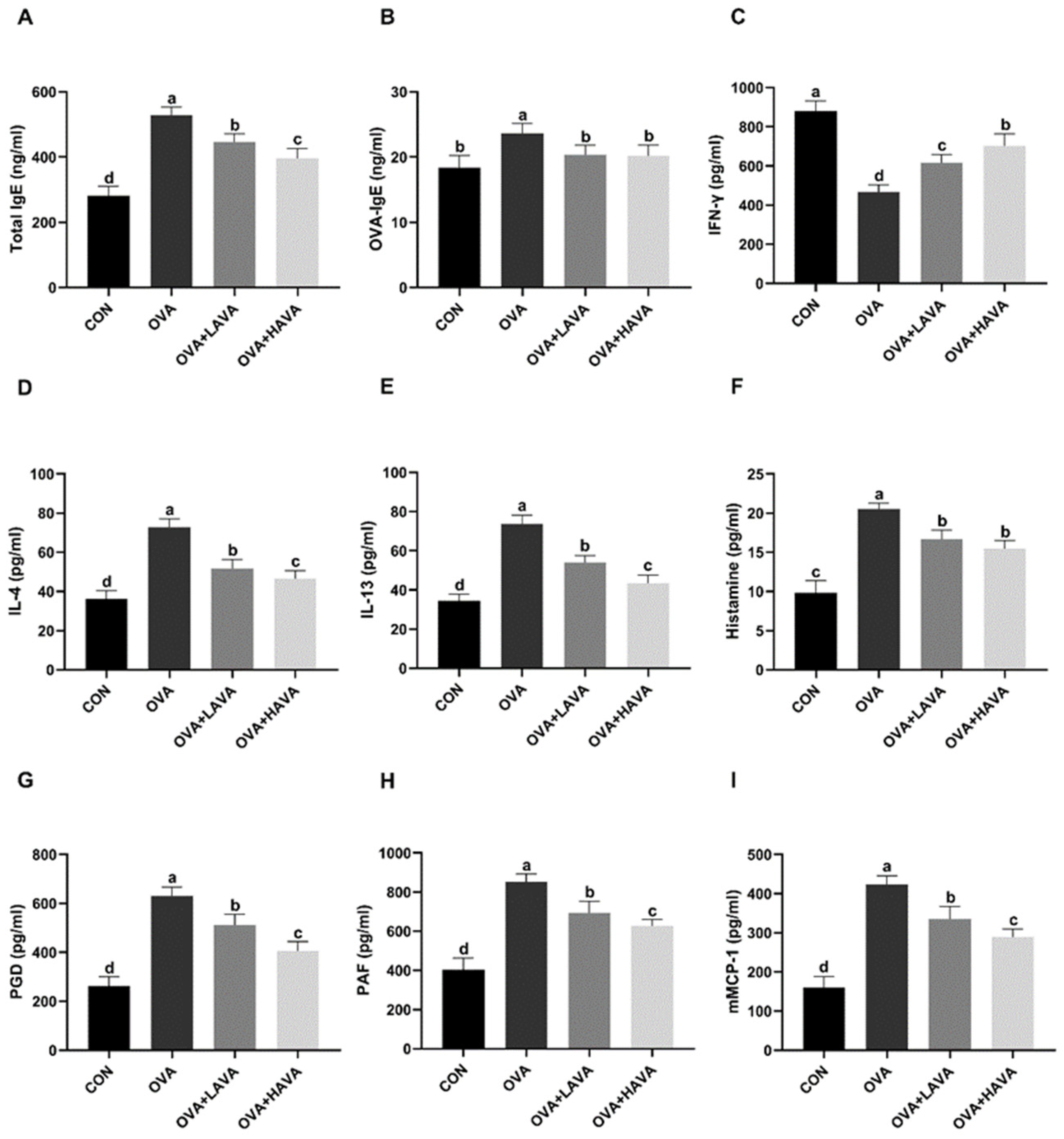
2.1. Effects of AVA on the Allergic and Inflammatory Responses
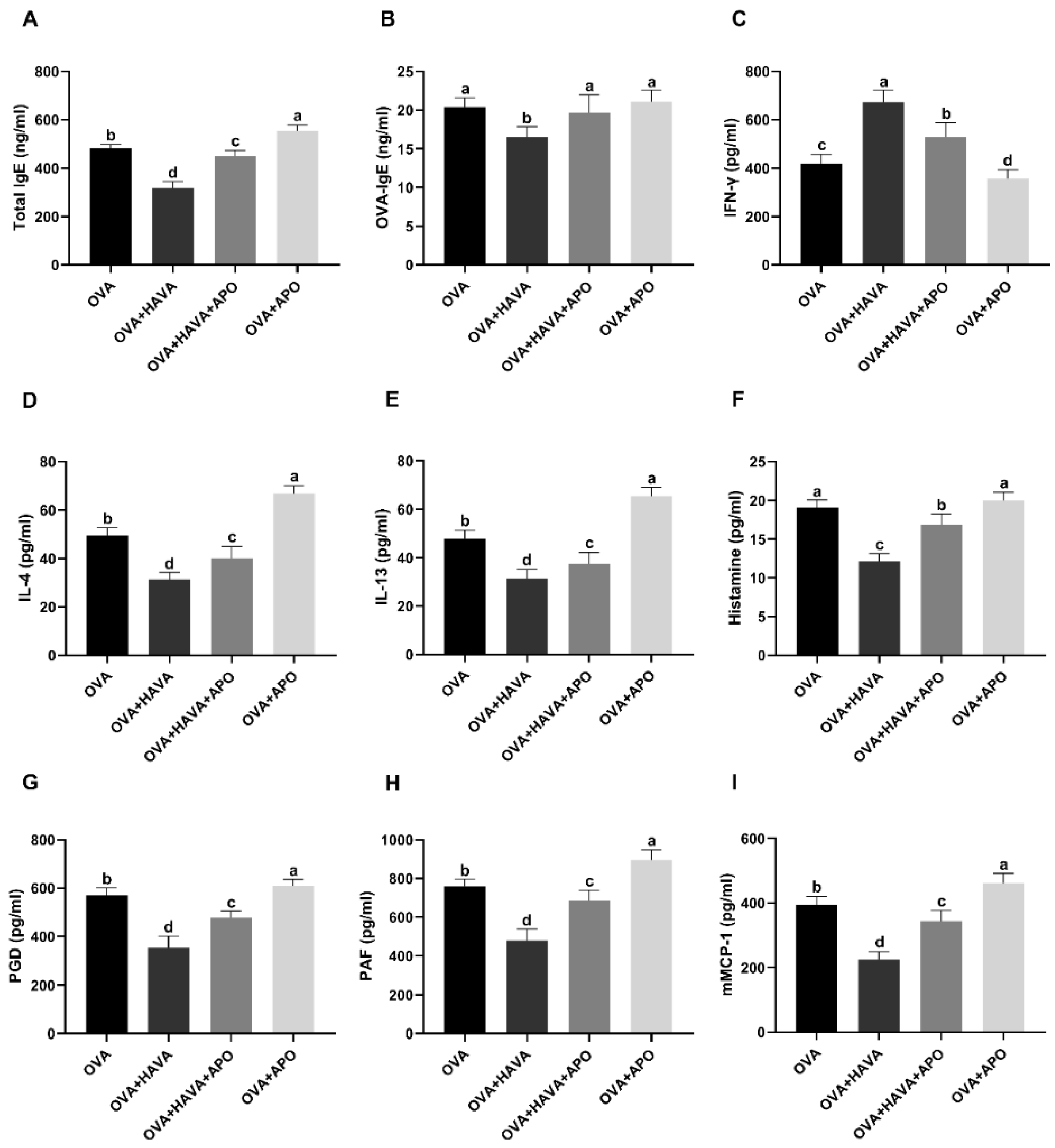
2.2. Effects of AVA on the Allergic and Inflammatory Responses in the Jejunum
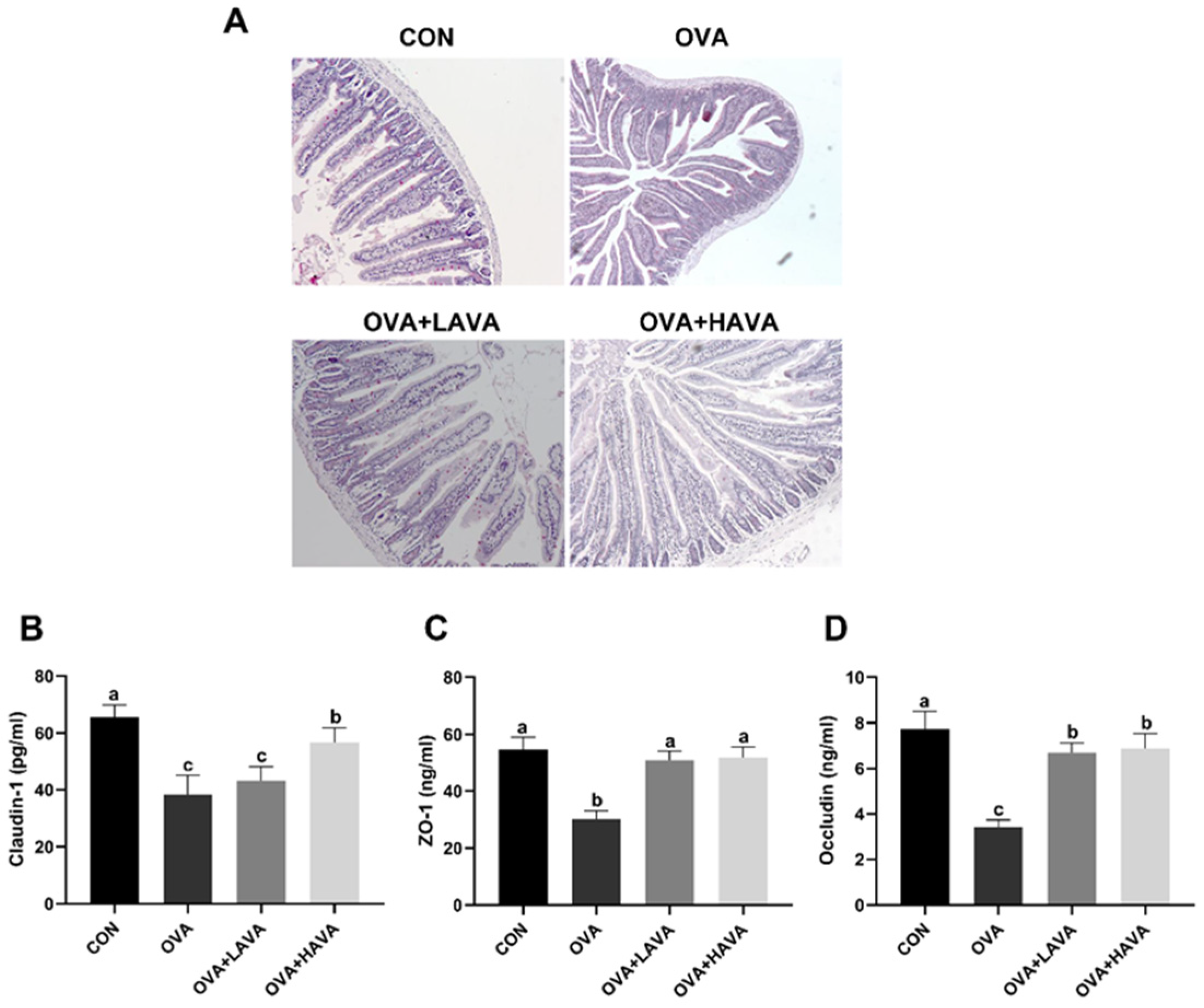
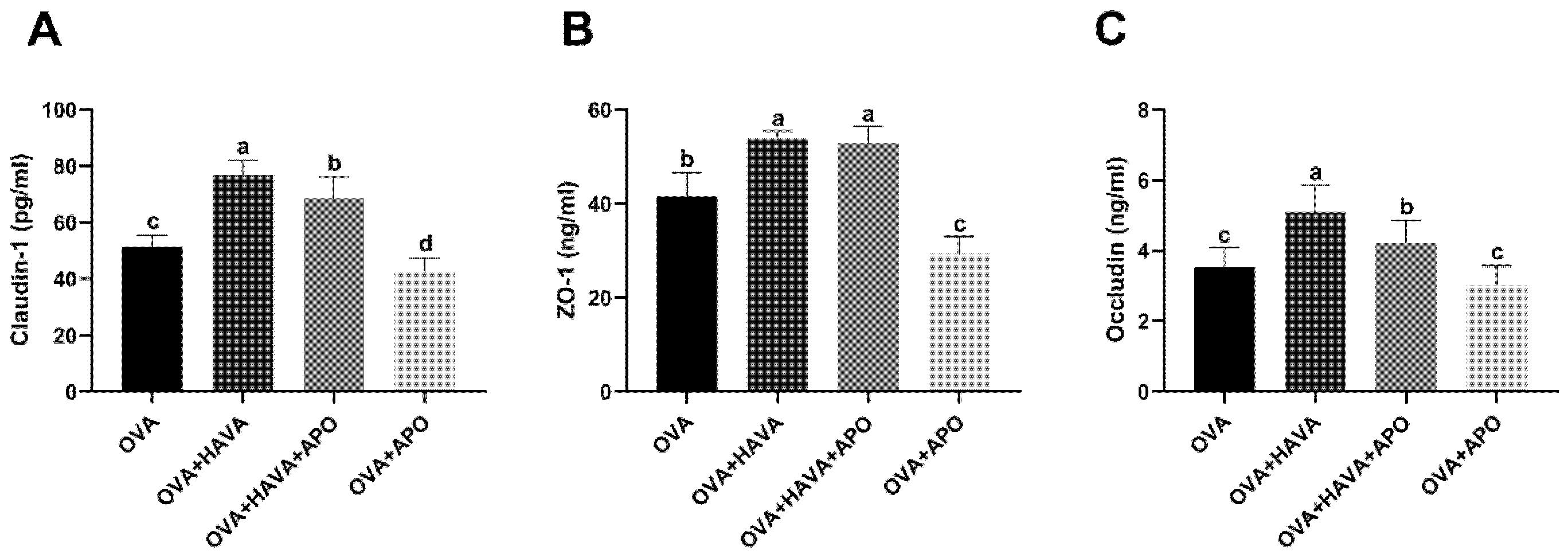
2.3. Effects of AVA on the Small Intestinal Damage Induced by OVA
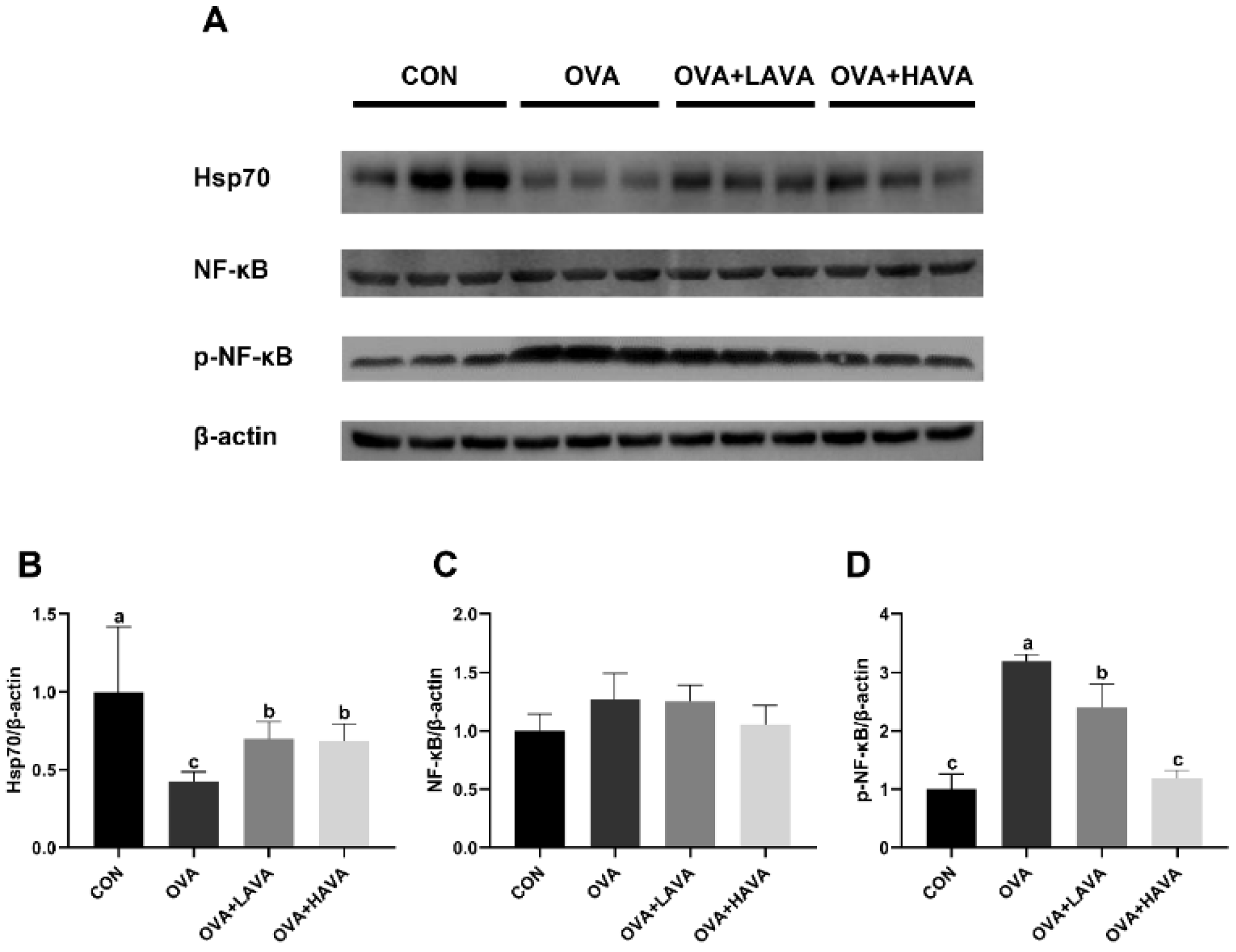
2.4. Effect of AVA on the Intestinal Hsp70-NF-κB Signaling
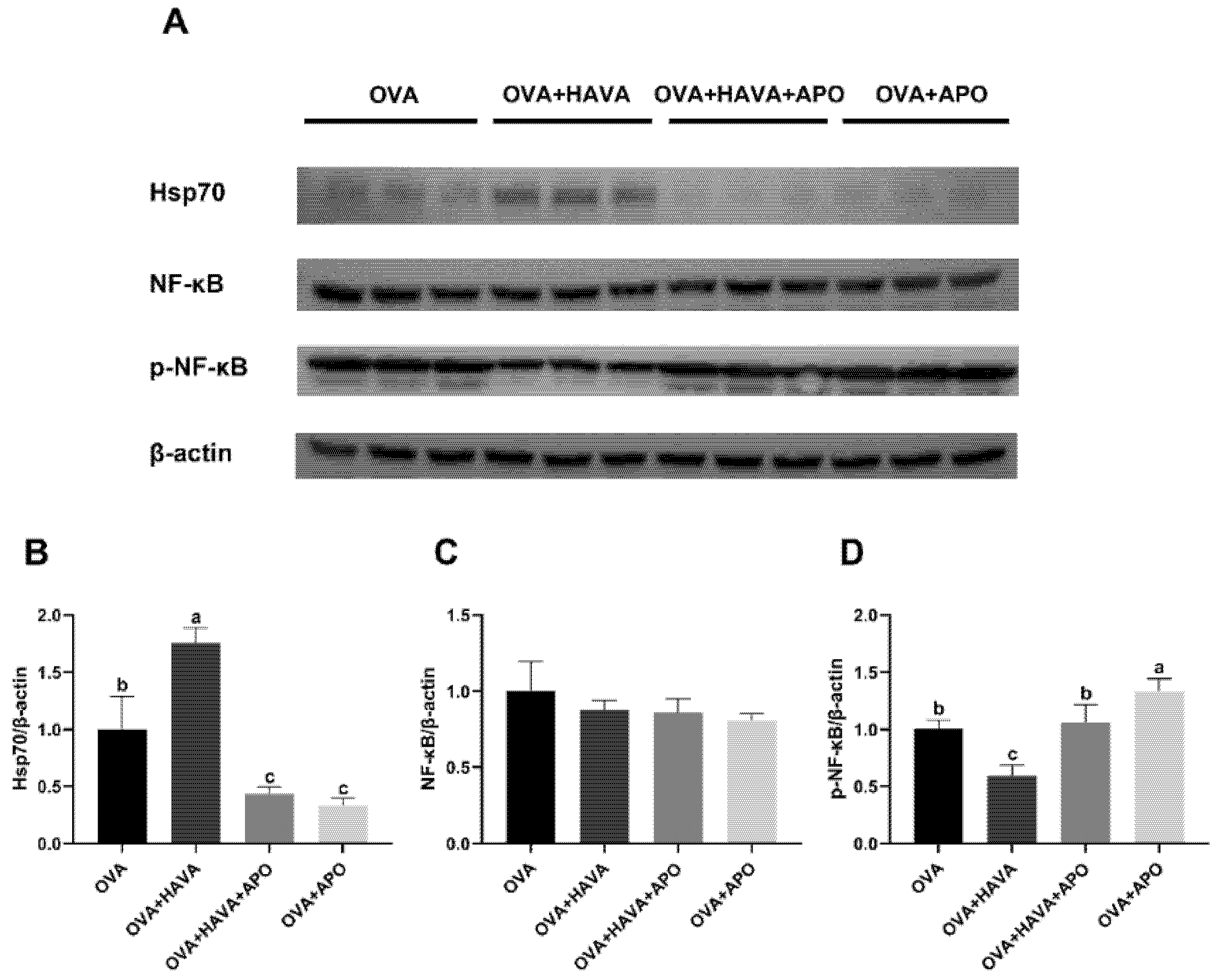
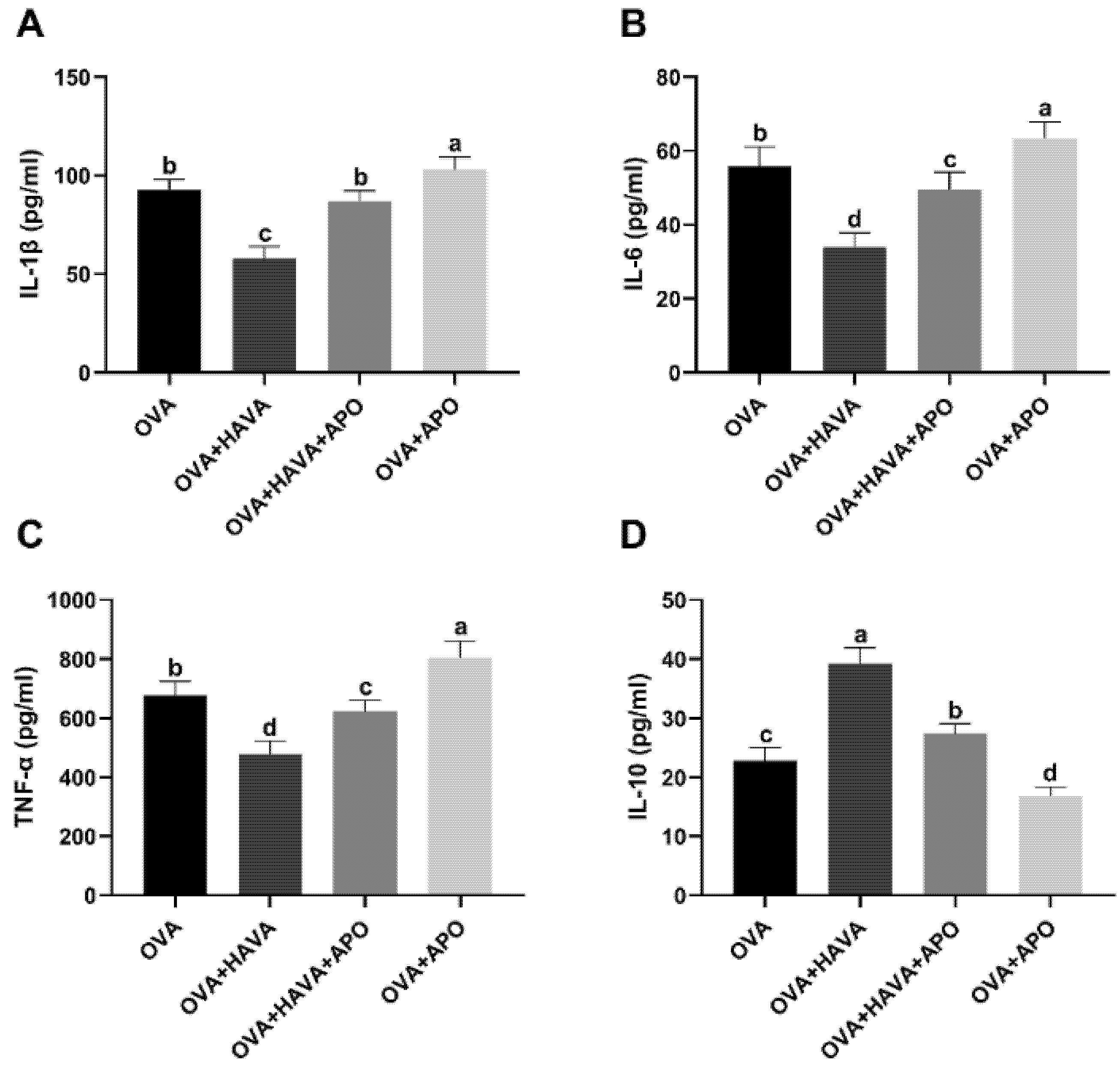
2.5. Effect of Hsp70 Activity Inhibition on the Function of AVA in the Allergic Symptoms and the Small Intestinal Damage Induced by OVA
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Evaluation of Immunoglobulins and Cytokines
4.3. Western Blot
4.4. Histological Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Florsheim, E.B.; Sullivan, Z.A.; Khoury-Hanold, W.; Medzhitov, R. Food allergy as a biological food quality control system. Cell 2021, 184, 1440–1454. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Sicherer, S. Food allergy: Epidemiology, pathogenesis, diagnosis, prevention, and treatment. Curr. Opin. Immunol. 2020, 66, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Joubert, G.; de Agüero, M.G.; Gouanvic, M.; Goubier, A.; Kaiserlian, D. Sequential role of plasmacytoid dendritic cells and regulatory T cells in oral tolerance. Gastroenterology 2009, 137, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.M.; Weiner, H.L. History and mechanisms of oral tolerance. Semin. Immunol. 2017, 30, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rachid, R.; Stephen-Victor, E.; Chatila, T.A. The microbial origins of food sllergy. J. Allergy Clin. Immunol. 2021, 147, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef]
- Niewiem, M.; Grzybowska-Chlebowczyk, U. Intestinal barrier permeability in allergic diseases. Nutrients 2022, 14, 1893. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front. Immunol. 2020, 11, 3222. [Google Scholar] [CrossRef]
- Chen, C.Y.; Milbury, P.E.; Kwak, H.K.; Collins, F.W.; Samuel, P.; Blumberg, J.B. Avenanthramides and phenolic acids from oats are bioavailable and act synergistically with Vitamin C to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2004, 134, 1459–1466. [Google Scholar] [CrossRef]
- Chen, C.Y.; Milbury, P.E.; Collins, F.W.; Blumberg, J.B. Avenanthramides are bioavailable and have antioxidant activity in humans after acute consumption of an enriched mixture from oats. J. Nutr. 2007, 137, 1375–1382. [Google Scholar] [CrossRef]
- Dhakal, H.; Yang, E.J.; Lee, S.; Kim, M.J.; Baek, M.C.; Lee, B.; Park, P.H.; Kwon, T.K.; Khang, D.; Song, K.S.; et al. Avenanthramide C from germinated oats exhibits anti-allergic inflammatory effects in mast cells. Sci. Rep. 2019, 9, 6884. [Google Scholar] [CrossRef]
- Liu, L.; Zubik, L.; Collins, F.W.; Marko, M.; Meydani, M. The antiatherogenic potential of oat phenolic compounds. Atherosclerosis 2004, 175, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Nie, L.; Wu, D.; Wise, M.L.; Collins, F.W.; Meydani, S.N.; Meydani, M. Avenanthramides inhibit proliferation of human colon cancer cell lines in vitro. Nutr. Cancer 2010, 62, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wise, M.L.; Collins, F.W.; Meydani, M. Avenanthramides, polyphenols from oats, inhibit IL-1 beta-induced NF-kappaB activation in endothelial cells. Free Radic. Biol. Med. 2008, 44, 415–429. [Google Scholar] [CrossRef]
- Lee-Manion, A.M.; Price, R.K.; Strain, J.J.; Dimberg, L.H.; Sunnerheim, K.; Welch, R.W. In vitro antioxidant activity and antigenotoxic effects of avenanthramides and related compounds. J. Agric. Food Chem. 2009, 57, 10619–10624. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Snooks, H.D.; Sang, S. The chemistry and health benefits of dietary phenolamides. J. Agric. Food Chem. 2020, 68, 6248–6267. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Jeong, S.O.; Koo, B.S.; Ha, H.Y.; Lee, K.M.; Chung, H.T. Tranilast, an orally active anti-allergic drug, up-regulates the anti-inflammatory heme oxygenase-1 expression but down-regulates the pro-inflammatory cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW264.7 macrophages. Biochem. Biophys. Res. Commun. 2008, 371, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Liao, L.X.; Lv, H.N.; Liu, D.; Dong, W.; Zhu, J.; Chen, J.F.; Shi, M.L.; Fu, G.; Song, X.M.; et al. Highly selective activation of heat shock protein 70 by allosteric regulation provides an insight into efficient neuroinflammation inhibition. eBioMedicine 2017, 23, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chou, P.C.; Chung, F.T.; Lin, H.C.; Huang, K.H.; Kuo, H.P. Heat shock protein70 is implicated in modulating NF-κB activation in alveolar macrophages of patients with active pulmonary tuberculosis. Sci. Rep. 2017, 7, 1214. [Google Scholar] [CrossRef]
- Lyu, Q.; Wawrzyniuk, M.; Rutten, V.; van Eden, W.; Sijts, A.; Broere, F. Hsp70 and NF-kB mediated control of innate inflammatory responses in a canine macrophage cell line. Int. J. Mol. Sci. 2020, 21, 6464. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.; Nigam, A.; Grote, D.; Liebel, F.; Southall, M.D. Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch. Dermatol. Res. 2008, 300, 569–574. [Google Scholar] [CrossRef]
- Gueders, M.M.; Paulissen, G.; Crahay, C.; Quesada-Calvo, F.; Hacha, J.; van Hove, C.; Tournoy, K.; Louis, R.; Foidart, J.M.; Noël, A.; et al. Mouse models of asthma: A comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm. Res. 2009, 58, 845–854. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Gilfillan, A.M. Molecular regulation of mast-cell activation. J. Allergy Clin. Immunol. 2006, 117, 1214–1225. [Google Scholar] [CrossRef]
- Santos, J.; Bayarri, C.; Saperas, E.; Nogueiras, C.; Antolín, M.; Mourelle, M.; Cadahia, A.; Malagelada, J.R. Characterisation of immune mediator release during the immediate response to segmental mucosal challenge in the jejunum of patients with food allergy. Gut 1999, 45, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Minai-Fleminger, Y.; Levi-Schaffer, F. Mast cells and eosinophils: The two key effector cells in allergic inflammation. Inflamm. Res. 2009, 58, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, A.; Sajib, M.S.; Lim, J.H.; Alves, T.C.; Das, A.; Witt, A.; Hagag, E.; Androulaki, N.; Grossklaus, S.; Gerlach, M.; et al. Glycolysis is integral to histamine-induced endothelial hyperpermeability. FASEB J. 2021, 35, e21425. [Google Scholar] [CrossRef] [PubMed]
- Rosam, A.C.; Wallace, J.L.; Whittle, B.J. Potent ulcerogenic actions of platelet-activating factor on the stomach. Nature 1986, 319, 54–56. [Google Scholar] [CrossRef]
- Matsuoka, T.; Hirata, M.; Tanaka, H.; Takahashi, Y.; Murata, T.; Kabashima, K.; Sugimoto, Y.; Kobayashi, T.; Ushikubi, F.; Aze, Y.; et al. Prostaglandin D2 as a mediator of allergic asthma. Science 2000, 287, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Arima, M.; Cheng, G.; Taki, S.; Hirata, H.; Eda, F.; Fukushima, F.; Yamaguchi, B.; Hatano, M.; Tokuhisa, T.; et al. Prostaglandin D2 reinforces Th2 type inflammatory responses of airways to low-dose antigen through bronchial expression of macrophagederived chemokine. J. Exp. Med. 2003, 198, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Burton, O.T.; Medina Tamayo, J.; Stranks, A.J.; Miller, S.; Koleoglou, K.J.; Weinberg, E.O.; Oettgen, H.C. IgE promotes type 2 innate lymphoid cells in murine food allergy. Clin. Exp. Allergy 2018, 48, 288–296. [Google Scholar] [CrossRef]
- Licona-Limón, P.; Kim, L.K.; Palm, N.W.; Flavell, R.A. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013, 14, 536–542. [Google Scholar] [CrossRef]
- Shida, K.; Takahashi, R.; Iwadate, E.; Takamizawa, K.; Yasui, H.; Sato, T.; Habu, S.; Hachimura, S.; Kaminogawa, S. Lactobacillus casei strain Shirota suppresses serum immunoglobulin E and immunoglobulin G1 responses and systemic anaphylaxis in a food allergy model. Clin. Exp. Allergy 2002, 32, 563–570. [Google Scholar] [CrossRef]
- Chen, T.; Liu, X.; Li, M.; He, W.; Li, W.; Cao, Y.; Liu, Z. Food allergens affect the intestinal tight junction permeability in inducing intestinal food allergy in rats. Asian Pac. J. Allergy Immunol. 2014, 32, 345–353. [Google Scholar] [CrossRef]
- Knoop, K.A.; McDonald, K.G.; McCrate, S.; McDole, J.R.; Newberry, R.D. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015, 8, 198–210. [Google Scholar] [CrossRef]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef]
- Heuschkel, S.; Wohlrab, J.; Schmaus, G.; Neubert, R.H. Modulation of dihydroavenanthramide release and skin penetration by 1,2-alkanedio. Eur. J. Pharm. Biopharm. 2008, 70, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Geremia, A.; Biancheri, P.; Allan, P.; Corazza, G.R.; Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 2014, 13, 3–10. [Google Scholar] [CrossRef]
- Bemark, M.; Angeletti, D. Know your enemy or find your friend? Induction of IgA at mucosal surfaces. Immunol. Rev. 2021, 303, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Konkel, J.E.; Chen, W.J. Balancing acts: The role of TGF-β in the mucosal immune system. Trends Mol. Med. 2011, 17, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Pavón, D.; Cervantes-García, D.; Bermúdez-Humarán, L.G.; Córdova-Dávalos, L.E.; Quintanar-Stephano, A.; Jiménez, M.; Eva Salinas, E. Protective effect of Glycomacropeptide on food allergy with gastrointestinal manifestations in a rat model through down-regulation of Type 2 immune response. Nutrients 2020, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial whey products promote intestinal barrier function with glycomacropeptide enhanced activity in downregulating bacterial endotoxin lipopolysaccharides (LPS)-induces inflammation in vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of food allergy development and suppression of established food allergy by neutralization of thymic stromal lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179. [Google Scholar] [CrossRef]
- Borges, T.J.; Wieten, L.; van Herwijnen, M.J.; Broere, F.; van der Zee, R.; Bonorino, C.; van Eden, W. The anti-inflammatory mechanisms of Hsp70. Front. Immunol. 2012, 3, 95. [Google Scholar] [CrossRef]
- Singleton, K.D.; Wischmeyer, P.E. Effects of HSP70.1/3 gene knockout on acute respiratory distress syndrome and the inflammatory response following sepsis. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L956–L961. [Google Scholar] [CrossRef]
- Shi, Y.; Tu, Z.; Tang, D.; Zhang, H.; Liu, M.; Wang, K.; Calderwood, S.K.; Xiao, X. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kB pathway but not the MAPK pathway. Shock 2016, 26, 277–284. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
- Subramanian, N.; Bray, M.A. Interleukin 1 releases histamine from human basophils and mast cells in vitro. J. Immunol. 1987, 138, 271–275. [Google Scholar] [PubMed]
- Vannier, E.; Dinarello, C.A. Histamine enhances interleukin (IL)-1-induced IL-1 gene expression and protein synthesis via H2 receptors in peripheral blood mononuclear cells comparison with IL-1 receptor antagonist. J. Clin. Investig. 1993, 92, 281–287. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petrof, E.O.; Ciancio, M.J.; Chang, E.B. Role and regulation of intestinal epithelial heat shock proteins in health and disease. Chin. J. Dig. Dis. 2004, 5, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Q.; Zhang, Y.; Li, X.L.; Peng, Y.Z.; Huang, Y.S.; Yang, Z.C. HSP70 protects intestinal epithelial cells from hypoxia/reoxygenation injury via a mechanism that involves the mitochondrial pathways. Eur. J. Pharmacol. 2010, 643, 282–288. [Google Scholar] [CrossRef]
- Ko, S.K.; Kim, J.; Na, D.C.; Park, S.; Park, S.H.; Hyun, J.Y.; Shin, I. A small molecule inhibitor of ATPase activity of Hsp70 induces apoptosis and has antitumor activities. Chem. Biol. 2015, 22, 391–403. [Google Scholar] [CrossRef]
- Li, Q.; Tang, X.; Xu, J.; Ren, X.; Wang, R.; Jiang, S. Study on alleviation effect of stachyose on food allergy through TLR2/NF-κB signal pathway in a mouse model. Life Sci. 2021, 286, 120038. [Google Scholar] [CrossRef] [PubMed]

| Variable | Control | OVA | OVA + LAVA | OVA + HAVA | p Value |
|---|---|---|---|---|---|
| IL-4 (pg/mL) | 24.87 ± 4.10 d | 48.67 ± 3.82 a | 44.24 ± 3.80 b | 32.76 ± 2.51 c | <0.01 |
| TNF-α (pg/mL) | 357.7 ± 36.11 d | 686.7 ± 46.93 a | 567.3 ± 24.48 b | 505.4 ± 37.31 c | <0.01 |
| IL-10 (pg/mL) | 40.58 ± 1.78 a | 24.15 ± 2.46 c | 31.09 ± 2.43 b | 33.01 ± 2.25 b | <0.01 |
| IL-25 (pg/mL) | 73.09 ± 4.02 a | 44.72 ± 3.87 d | 52.82 ± 5.24 c | 64.42 ± 6.07 b | <0.01 |
| IL-33 (pg/mL) | 194.9 ± 9.16 a | 100.6 ± 11.46 d | 120.5 ± 7.12 c | 157.6 ± 16.28 b | <0.01 |
| slgA (ng/mL) | 3533 ± 175.3 a | 1804 ± 204.9 d | 2504 ± 263.7 c | 2934 ± 179.6 b | <0.01 |
| TSLP (pg/mL) | 41.42 ± 3.65 a | 23.03 ± 4.21 c | 33.20 ± 3.30 b | 36.50 ± 3.54 b | <0.01 |
| TGF-β (pg/mL) | 656.9 ± 98.91 a | 233.1 ± 55.31 d | 407.8 ± 44.22 c | 538.8 ± 41.11 b | <0.01 |
| Variable | OVA | OVA + HAVA | OVA + HAVA + APO | OVA + APO | p Value |
|---|---|---|---|---|---|
| IL-4 (pg/mL) | 36.2 ± 3.13 b | 23.23 ± 2.24 d | 31.52 ± 5.52 c | 42.41 ± 3.95 a | <0.01 |
| TNF-α (pg/mL) | 519.3 ± 37.91 b | 378.6 ± 27.55 d | 440.5 ± 42.27 c | 628.6 ± 35.15 a | <0.01 |
| IL-10 (pg/mL) | 29.33 ± 1.80 b | 35.88 ± 3.17 a | 34.89 ± 1.43 a | 23.58 ± 2.22 c | <0.01 |
| IL-25 (pg/mL) | 55.22 ± 4.85 c | 80.37 ± 4.61 a | 69.18 ± 6.26 b | 47.19 ± 4.64 d | <0.01 |
| IL-33 (pg/mL) | 123.5 ± 15.74 c | 181.6 ± 13.95 a | 163.3 ± 11.14 b | 106.9 ± 8.07 d | <0.01 |
| slgA (ng/mL) | 2173 ± 154.0 c | 3048 ± 232.0 a | 2755 ± 128.8 b | 1901 ± 177.5 d | <0.01 |
| TSLP (pg/mL) | 35.86 ± 3.38 | 37.58 ± 4.33 | 37.01 ± 4.16 | 35.03 ± 2.31 | 0.46 |
| TGF-β (pg/mL) | 406.9 ± 82.60 c | 690.4 ± 45.14 a | 592 ± 28.36 b | 303.2 ± 32.17 d | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Liu, T.; Zhang, M.; Mo, R.; Zhou, W.; Li, D.; Wu, Y. Effects of Avenanthramide on the Small Intestinal Damage through Hsp70-NF-κB Signaling in an Ovalbumin-Induced Food Allergy Model. Int. J. Mol. Sci. 2022, 23, 15229. https://doi.org/10.3390/ijms232315229
Liu P, Liu T, Zhang M, Mo R, Zhou W, Li D, Wu Y. Effects of Avenanthramide on the Small Intestinal Damage through Hsp70-NF-κB Signaling in an Ovalbumin-Induced Food Allergy Model. International Journal of Molecular Sciences. 2022; 23(23):15229. https://doi.org/10.3390/ijms232315229
Chicago/Turabian StyleLiu, Pan, Tianyi Liu, Mingrui Zhang, Ruixia Mo, Weiwei Zhou, Defa Li, and Yi Wu. 2022. "Effects of Avenanthramide on the Small Intestinal Damage through Hsp70-NF-κB Signaling in an Ovalbumin-Induced Food Allergy Model" International Journal of Molecular Sciences 23, no. 23: 15229. https://doi.org/10.3390/ijms232315229
APA StyleLiu, P., Liu, T., Zhang, M., Mo, R., Zhou, W., Li, D., & Wu, Y. (2022). Effects of Avenanthramide on the Small Intestinal Damage through Hsp70-NF-κB Signaling in an Ovalbumin-Induced Food Allergy Model. International Journal of Molecular Sciences, 23(23), 15229. https://doi.org/10.3390/ijms232315229







