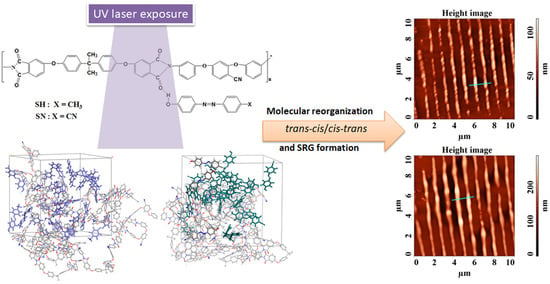
The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics
Abstract
1. Introduction
2. Results and Discussion
2.1. Colorimetry Study
2.2. Thermal Stability
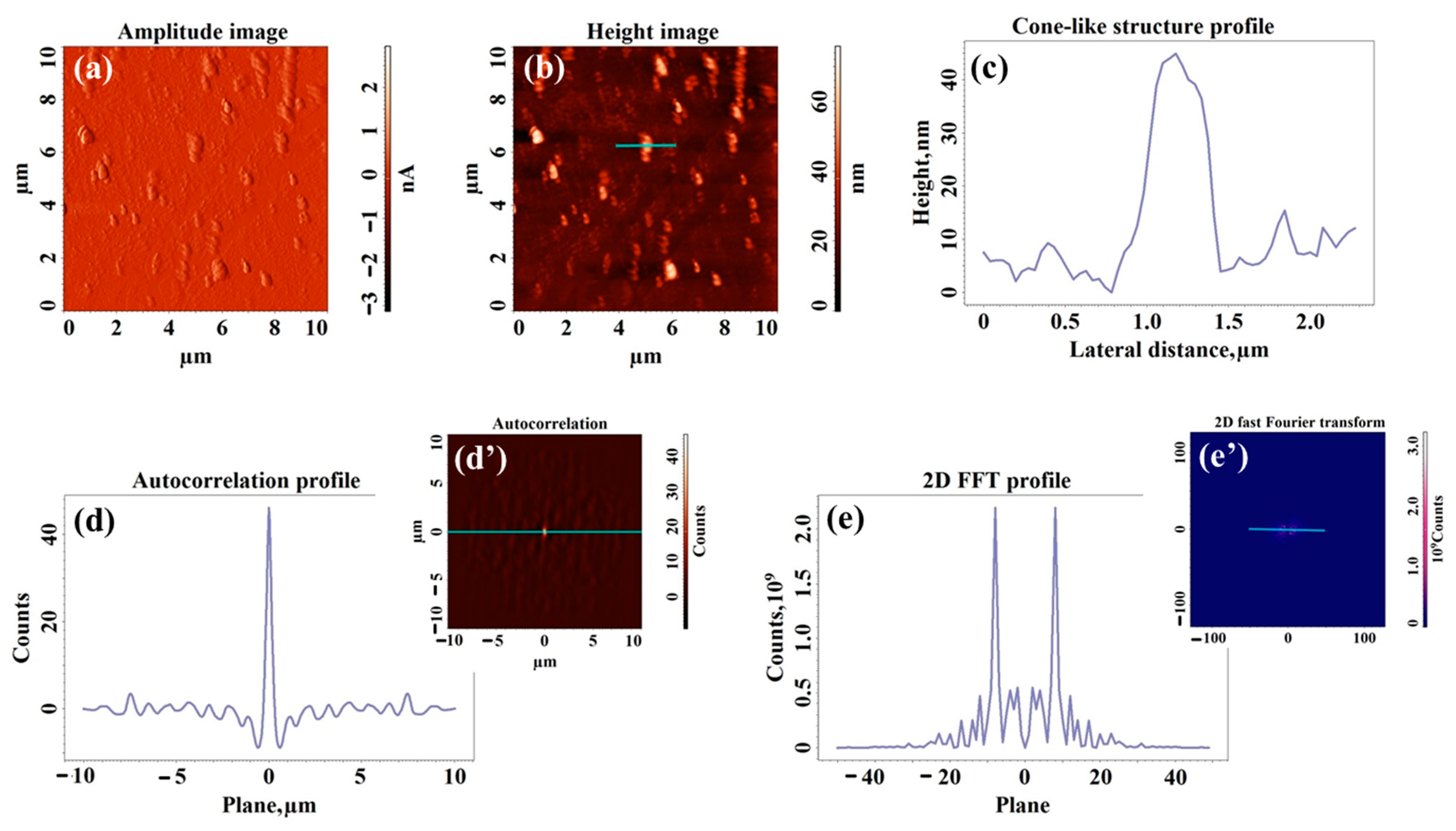
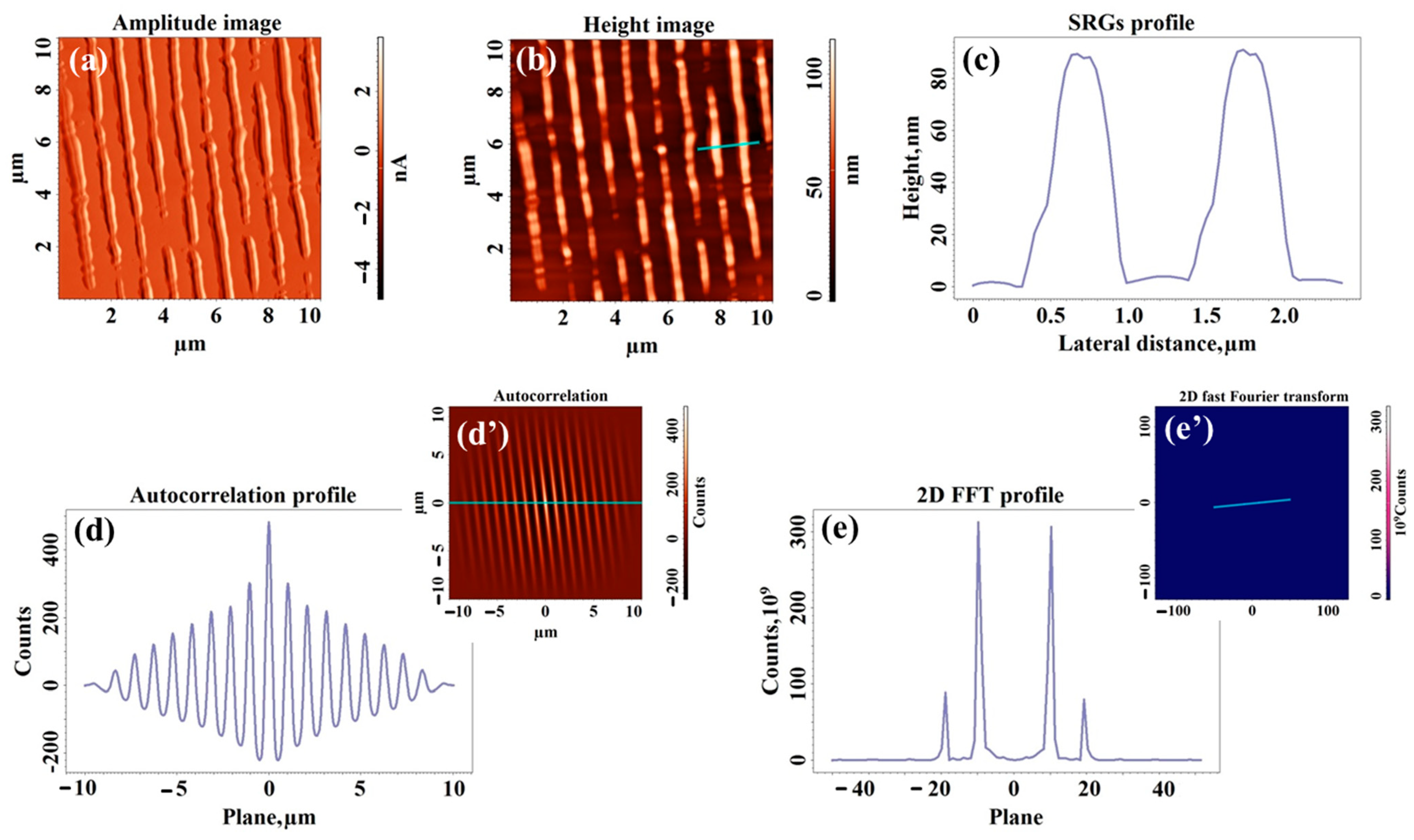
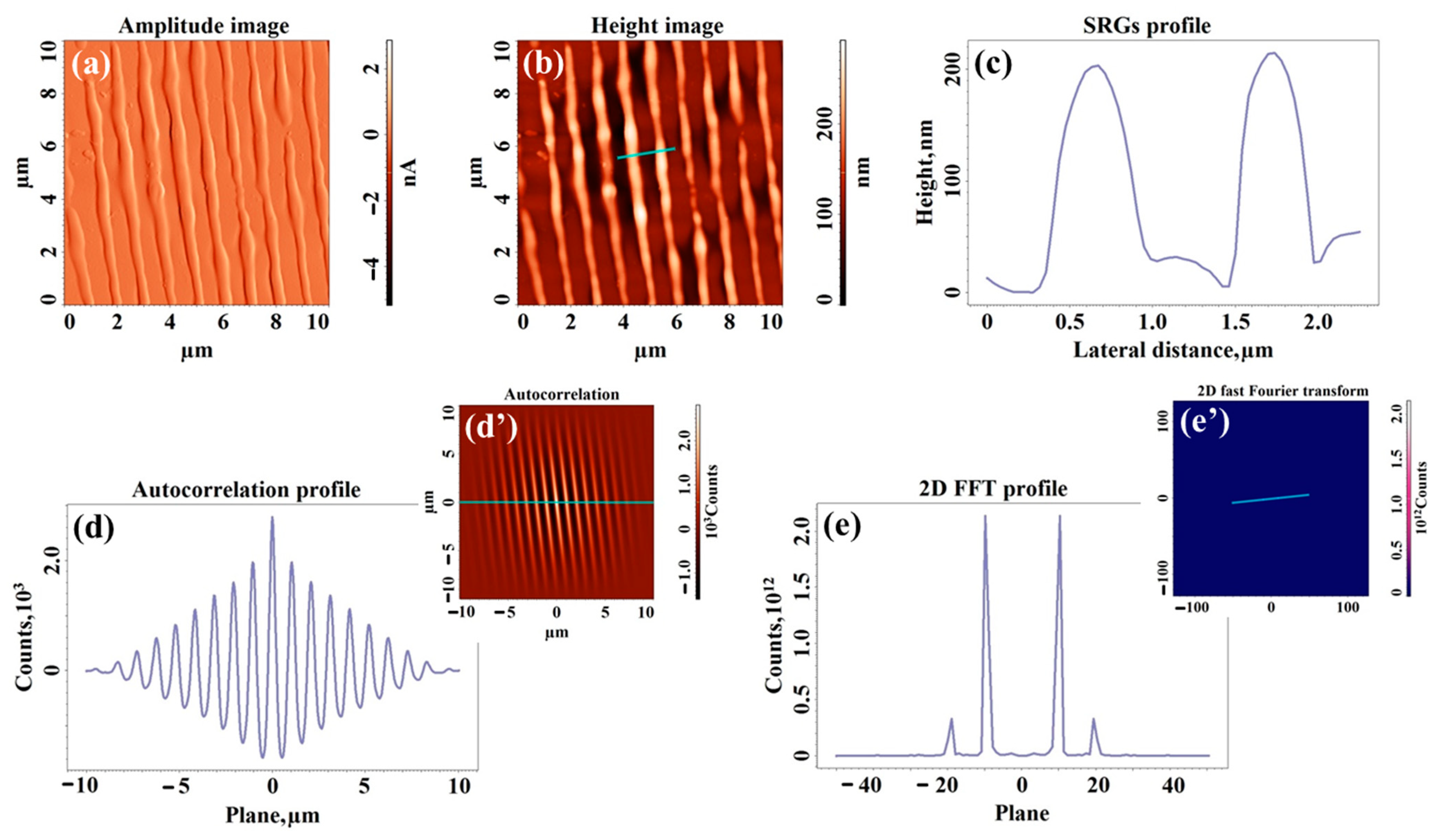
2.3. Morphological Study of UV-Laser Induced Modulations
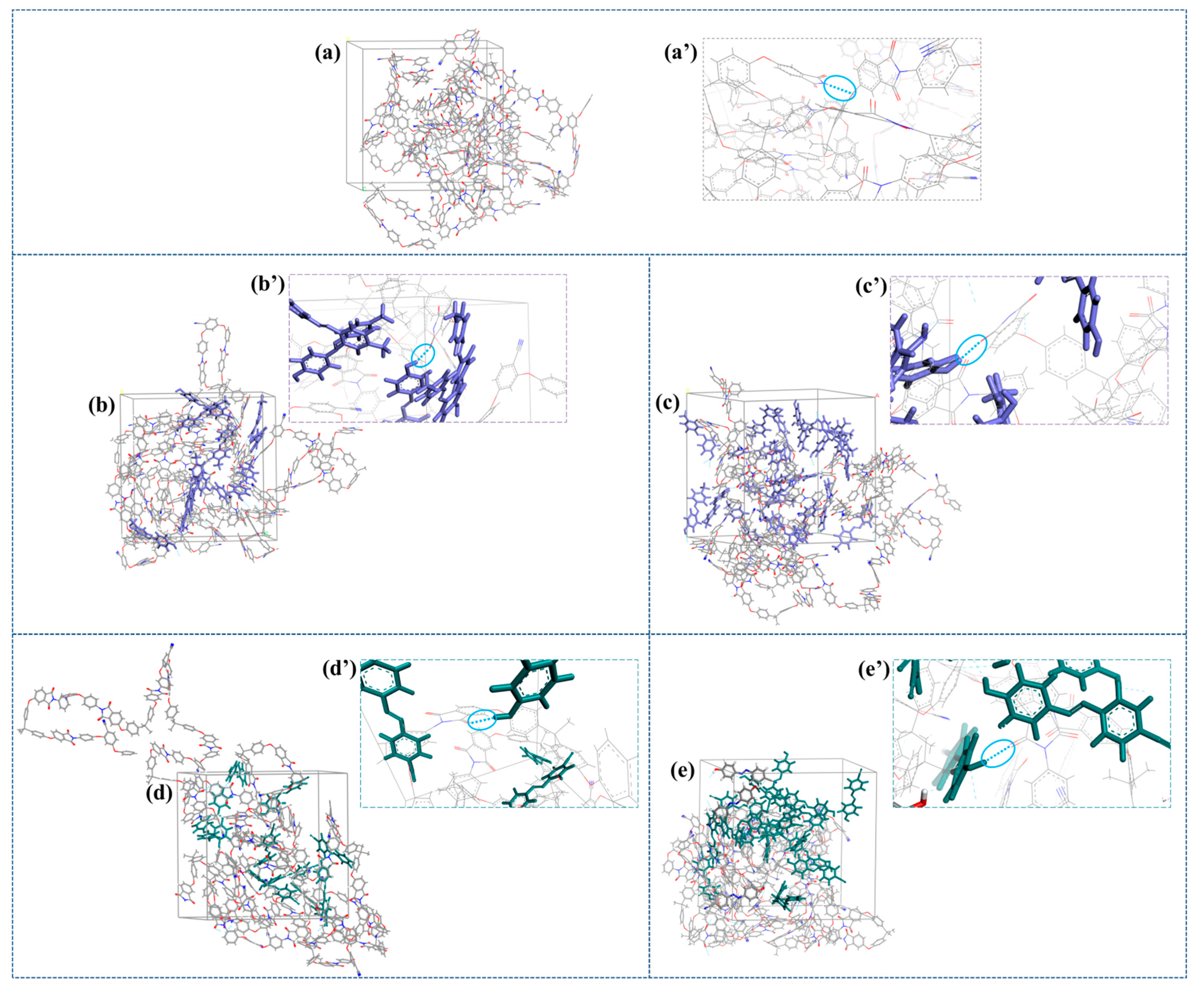
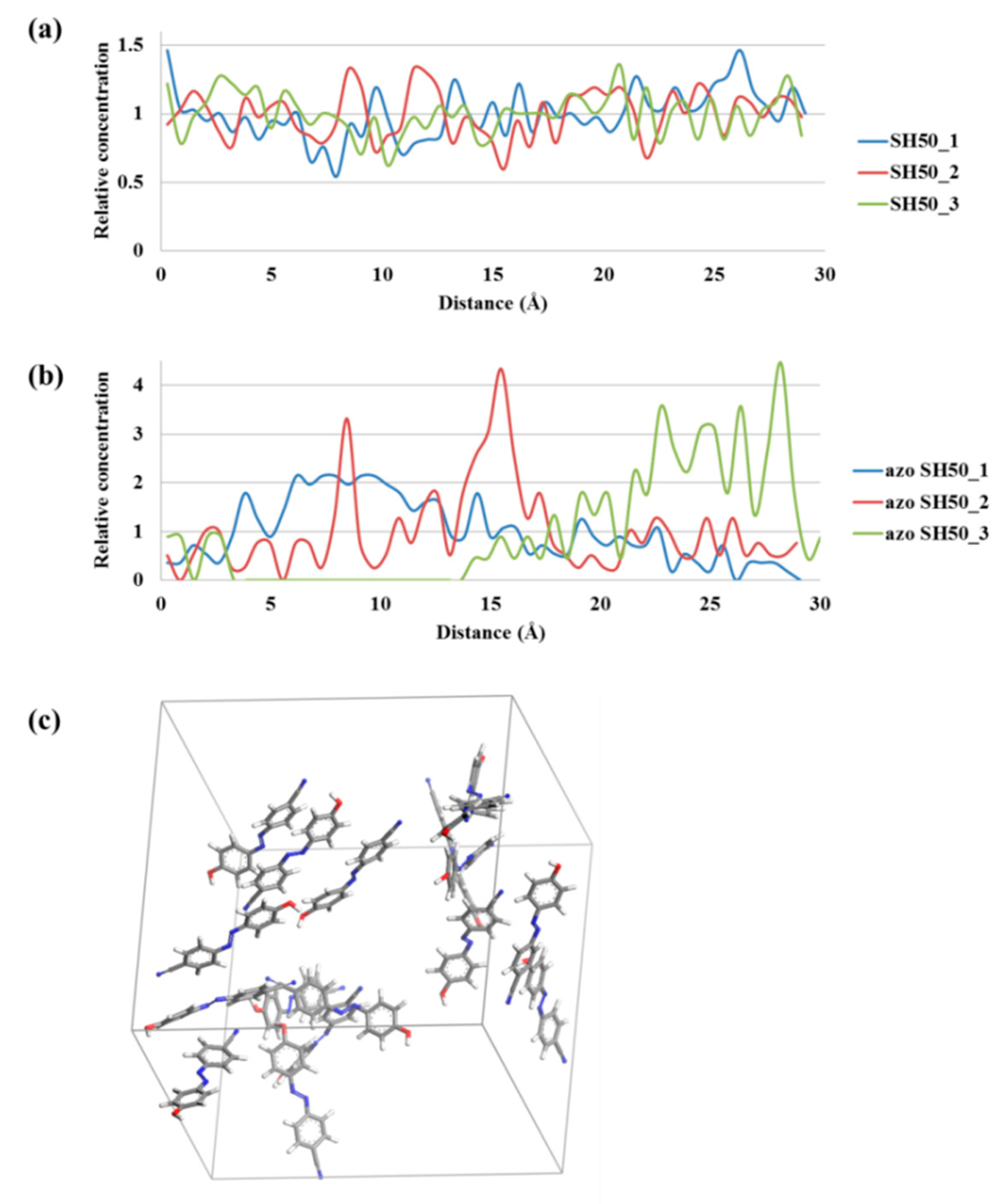
2.4. Molecular Modeling
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Polyamidic Acid (PAA)
3.3. Synthesis of Polyimide-Based Supramolecular Systems
3.4. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B

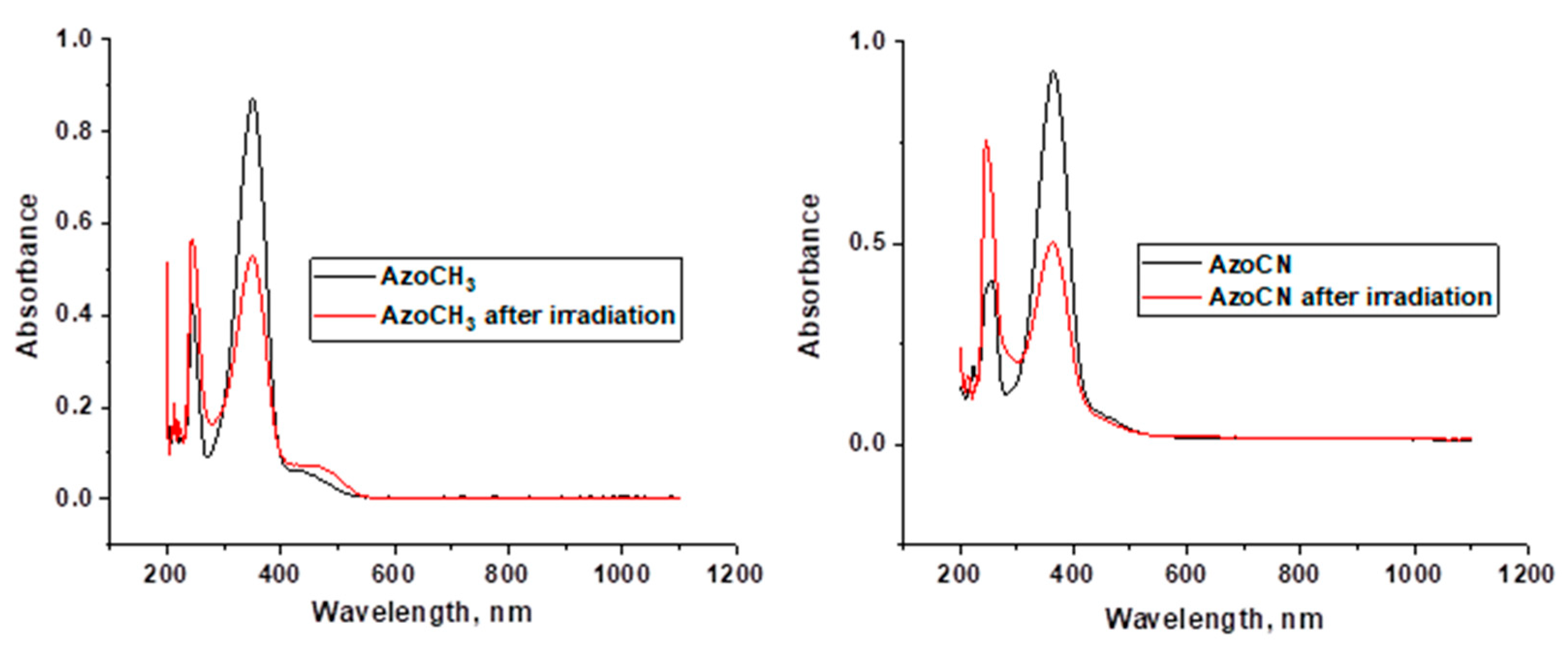
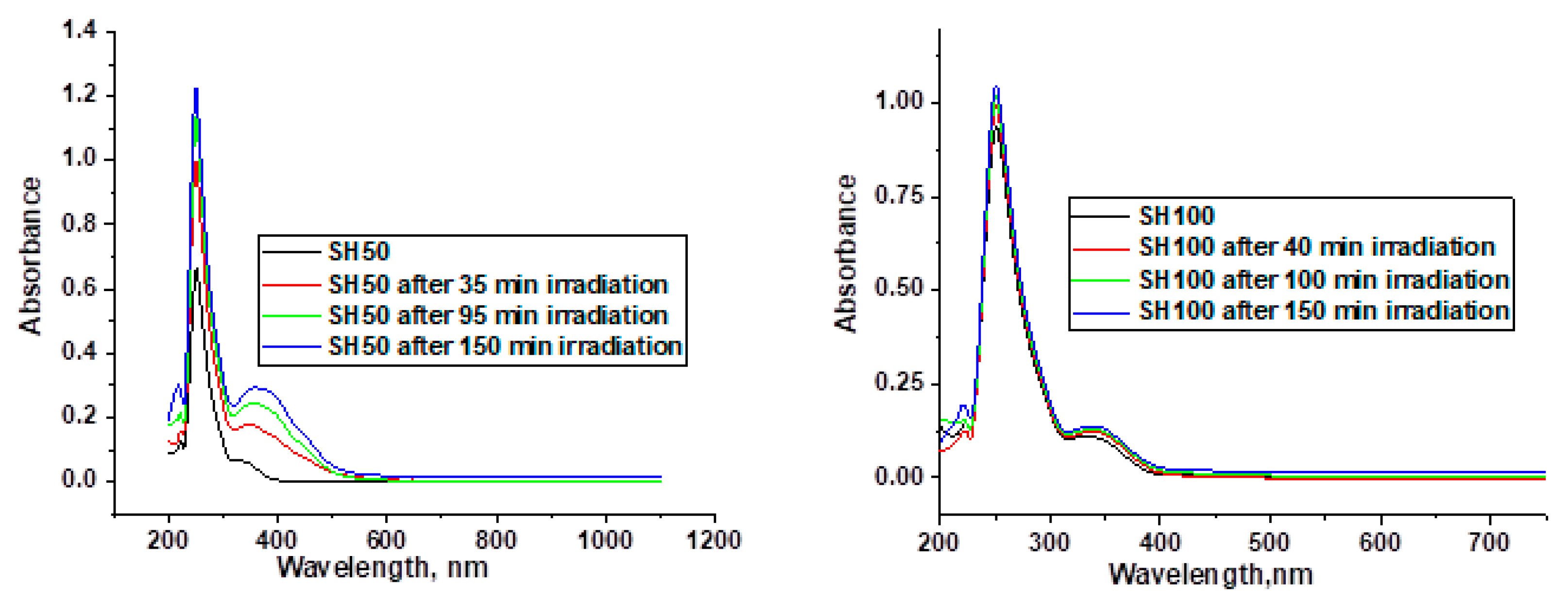
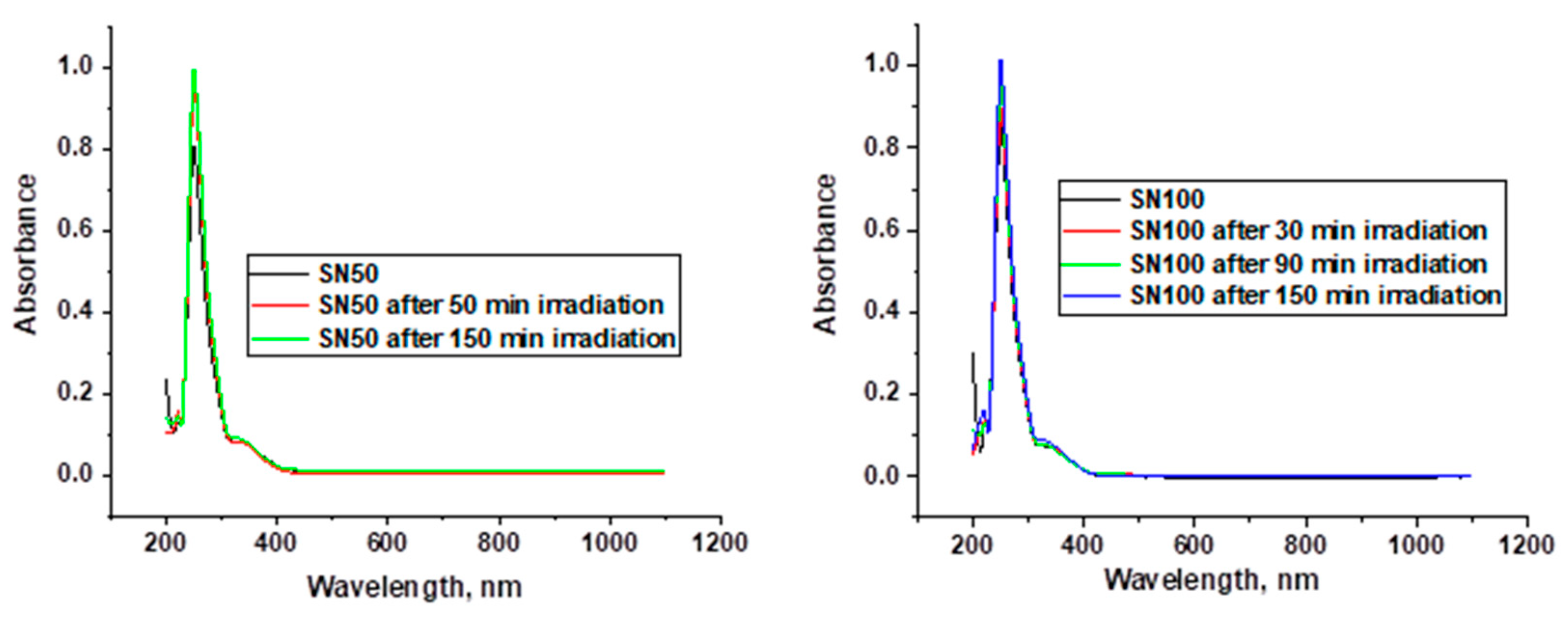
Appendix C
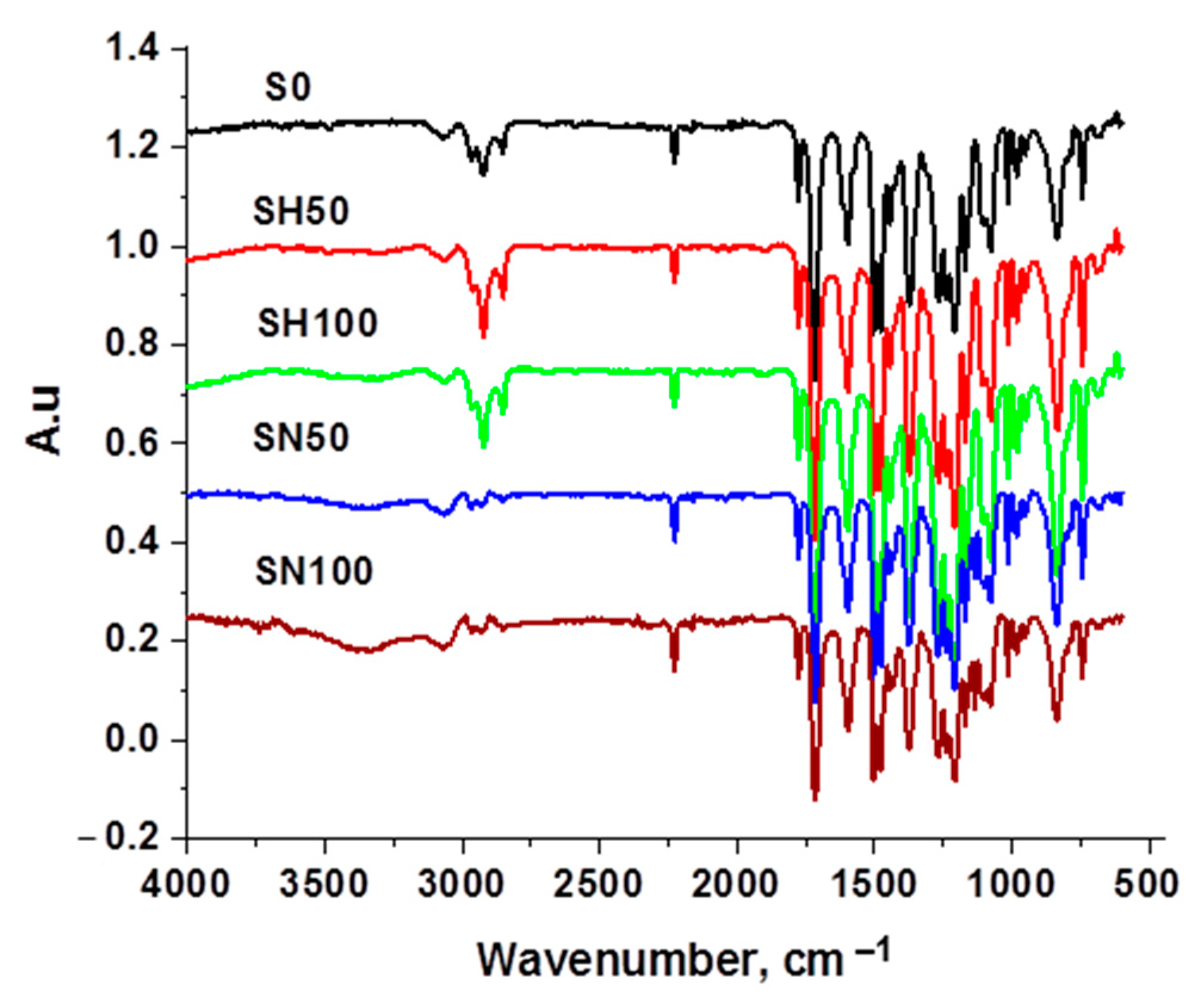

Appendix D



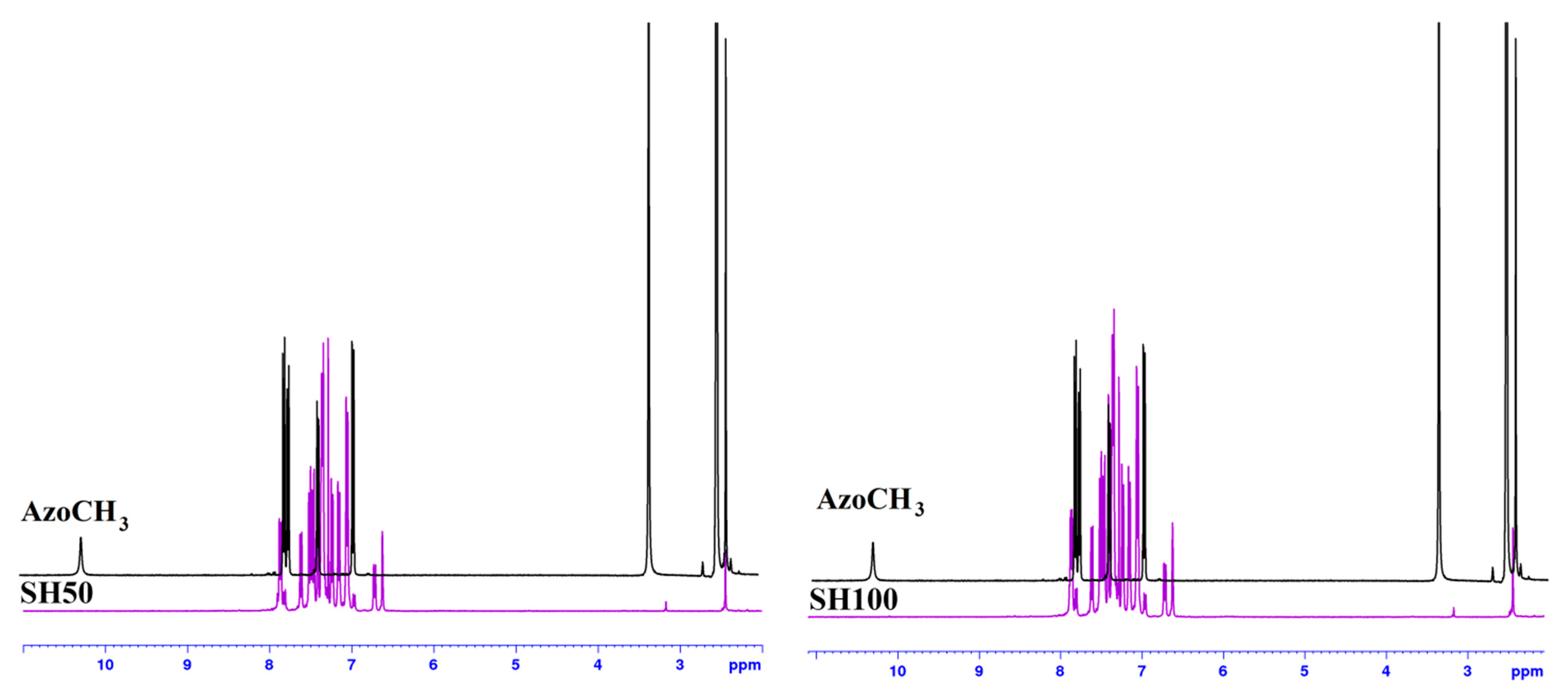
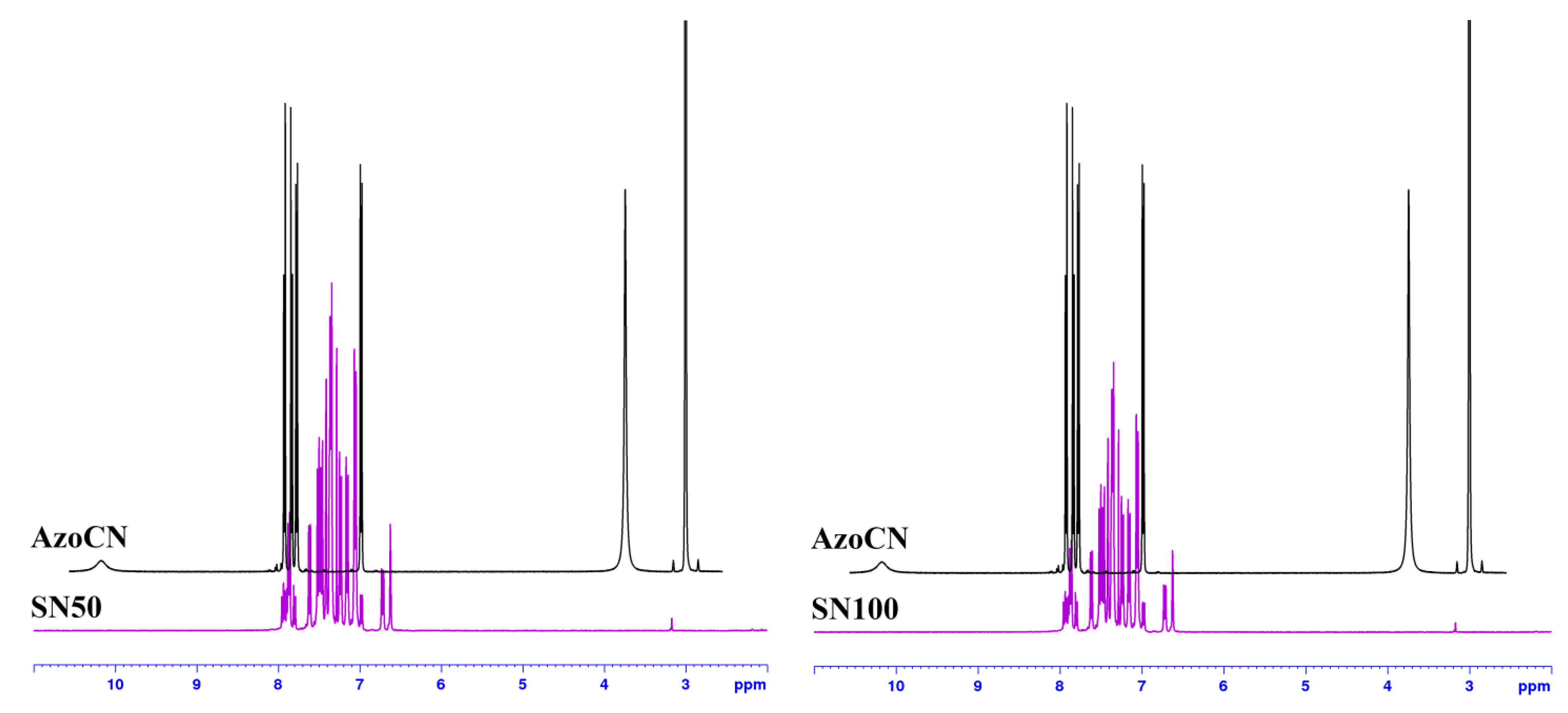
References
- Blom, P.W.M. Polymer Electronics: To Be or Not to Be? Adv. Mater. Technol. 2020, 5, 2000144. [Google Scholar] [CrossRef]
- Onorato, J.; Pakhnyuk, V.; Luscombe, C.K. Structure and design of polymers for durable, stretchable organic electronics. Polym. J. 2017, 49, 41–60. [Google Scholar] [CrossRef]
- Chang, M.; Lim, G.; Park, B.; Reichmanis, E. Control of Molecular Ordering, Alignment, and Charge Transport in Solution-Processed Conjugated Polymer Thin Films. Polymers 2017, 9, 212. [Google Scholar] [CrossRef]
- Kong, D.; Yang, M.; Zhang, X.; Du, Z.; Fu, Q.; Gao, X.; Gong, J. Control of Polymer Properties by Entanglement: A Review. Macromol. Mater. Eng. 2021, 306, 2100536. [Google Scholar] [CrossRef]
- Huang, B.-Y.; Yu, K.-Y.; Huang, S.-Y.; Kuo, C.-T. The investigation of the two-dimensional surface relief grating on dye-doped polymer film. Opt. Mater. Express 2014, 4, 308. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X. Amphiphilic azo polymers: Molecular engineering, self-assembly and photoresponsive properties. Prog. Polym. Sci. 2013, 38, 271–301. [Google Scholar] [CrossRef]
- Yang, B.; Yu, M.; Yu, H. Azopolymer-Based Nanoimprint Lithography: Recent Developments in Methodology and Applications. Chempluschem 2020, 85, 2166–2176. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Schab-Balcerzak, E. Supramolecular azopolymers based on hydrogen bonds. Polimery 2015, 60, 425–434. [Google Scholar] [CrossRef]
- Cimrová, V.; Neher, D.; Kostromine, S.; Bieringer, T. Optical Anisotropy in Films of Photoaddressable Polymers. Macromolecules 1999, 32, 8496–8503. [Google Scholar] [CrossRef]
- Vapaavuori, J.; Bazuin, C.G.; Priimagi, A. Supramolecular design principles for efficient photoresponsive polymer-azobenzene complexes. J. Mater. Chem. C 2018, 6, 2168–2188. [Google Scholar] [CrossRef]
- Priimagi, A.; Shevchenko, A. Azopolymer-based micro- and nanopatterning for photonic applications. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 163–182. [Google Scholar] [CrossRef]
- Luca, A.R.; Moleavin, I.-A.; Hurduc, N.; Hamel, M.; Rocha, L. Mass transport in low Tg azo-polymers: Effect on the surface relief grating induction and stability of additional side chain groups able to generate physical interactions. Appl. Surf. Sci. 2014, 290, 172–179. [Google Scholar] [CrossRef]
- Oliveira, O.N.; Li, L.; Kumar, J.; Tripathy, S.K. Surface-Relief Gratings on Azobenzene-Containing Films. In Photoreactive Organic Thin Films; Elsevier: Amsterdam, The Netherlands, 2002; p. 429-I. [Google Scholar]
- Kim, K.-H.; Jeong, Y.-C. One-step fabrication of hierarchical multiscale surface relief gratings by holographic lithography of azobenzene polymer. Opt. Express 2018, 26, 5711. [Google Scholar] [CrossRef]
- Sava, I.; Stoica, I.; Topala, I.; Mihaila, I.; Barzic, A.I. Photodesign and fabrication of surface relief gratings on films of polyimide-based supramolecular systems obtained using host-guest strategy. Polymer 2022, 249, 124829. [Google Scholar] [CrossRef]
- Ikeda, T.; Tsutsumi, O. Optical Switching and Image Storage by Means of Azobenzene Liquid-Crystal Films. Science 1995, 268, 1873–1875. [Google Scholar] [CrossRef]
- Siitonen, S.; Pietarinen, J.; Laakkonen, P.; Jefimovs, K.; Kuittinen, M.; Alajoki, T.; Mönkkönen, K.; Pääkkönen, E.J.; Tervonen, A. Replicated Polymer Light Guide Interconnector with Depth Modified Surface Relief Grating Couplers. Opt. Rev. 2007, 14, 304–309. [Google Scholar] [CrossRef]
- Hagen, R.; Bieringer, T. Photoaddressable Polymers for Optical Data Storage. Adv. Mater. 2001, 13, 1805–1810. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, L.-N.; Wei, W.-H.; Guan, W.; Dai, Y.-Z.; Sun, X.-C.; Gao, B.-R. Shape memory of a polymer grating surface fabricated by two-beam interference lithography. Appl. Opt. 2022, 61, 792. [Google Scholar] [CrossRef]
- Atmah, N.R.A.; Caliman, W.R.; Pawlicka, A.; Sabat, R.G.; Nunzi, J.-M. Surface Relief Grating on Chitosan-N,N-dimethyl-4-(2-pyridylazo)aniline Thin Film. Polymers 2022, 14, 791. [Google Scholar] [CrossRef]
- Jelken, J.; Henkel, C.; Santer, S. Formation of half-period surface relief gratings in azobenzene containing polymer films. Appl. Phys. B 2020, 126, 149. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Huang, B.-Y.; Hung, W.-C.; Yu, K.-Y.; Cheng, W.-S.; Kuo, C.-T. Temperature and orientation dependence of surface relief gratings based on dye-doped polymer film with the interface of nematic liquid crystals. Opt. Commun. 2011, 284, 934–937. [Google Scholar] [CrossRef]
- Kitamura, I.; Hara, M.; Nagano, S.; Seki, T. Photo-triggered surface relief formation of polystyrene films based on the Marangoni flow driven by a surface photoresponsive skin layer. Mol. Cryst. Liq. Cryst. 2021, 727, 52–64. [Google Scholar] [CrossRef]
- Ubukata, T.; Seki, T.; Ichimura, K. Surface relief grating in hybrid films composed of azobenzene polymer and liquid crystal molecule. Colloids Surf. A Physicochem. Eng. Asp. 2002, 198–200, 113–117. [Google Scholar] [CrossRef]
- Sava, I.; Burescu, A.; Stoica, I.; Musteata, V.; Cristea, M.; Mihaila, I.; Pohoata, V.; Topala, I. Properties of some azo-copolyimide thin films used in the formation of photoinduced surface relief gratings. RSC Adv. 2015, 5, 10125–10133. [Google Scholar] [CrossRef]
- Barzic, A.I.; Neha Kanwar Rawat, A.K.H. (Eds.) Imidic Polymers and Green Polymer Chemistry New Technology and Developments in Process and Product, 1st ed.; Apple Academic Press: New York, NY, USA, 2021; ISBN 9781774637678. [Google Scholar] [CrossRef]
- Ghosh, M. Polyimides: Fundamentals and Applications; Ghosh, M.K., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 1996; ISBN 0-8247-9466-4. [Google Scholar]
- Chen, J.P.; Labarthet, F.L.; Natansohn, A.; Rochon, P. Highly Stable Optically Induced Birefringence and Holographic Surface Gratings on a New Azocarbazole-Based Polyimide. Macromolecules 1999, 32, 8572–8579. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Lu, Q. Effect of irradiation history on the preparation of laser induced periodic microstructure on polyimide surface. Appl. Surf. Sci. 2007, 253, 3690–3695. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Sobolewska, A.; Stumpe, J.; Hamryszak, L.; Bujak, P. Surface relief gratings in azobenzene supramolecular systems based on polyimides. Opt. Mater. 2012, 35, 155–167. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Konieczkowska, J.; Siwy, M.; Sobolewska, A.; Wojtowicz, M.; Wiacek, M. Comparative studies of polyimides with covalently bonded azo-dyes with their supramolecular analoges: Thermo-optical and photoinduced properties. Opt. Mater. 2014, 36, 892–902. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Flakus, H.; Jarczyk-Jedryka, A.; Konieczkowska, J.; Siwy, M.; Bijak, K.; Sobolewska, A.; Stumpe, J. Photochromic supramolecular azopolyimides based on hydrogen bonds. Opt. Mater. 2015, 47, 501–511. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Janeczek, H.; Małecki, J.; Trzebicka, B.; Szmigiel, D.; Kozanecka-Szmigiel, A.; Schab-Balcerzak, E. Noncovalent azopoly(ester imide)s: Experimental study on structure-property relations and theoretical approach for prediction of glass transition temperature and hydrogen bond formation. Polymer 2017, 113, 53–66. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Bujak, K.; Nocoń, K.; Schab-Balcerzak, E. The large and stable photomechanical effect in the glassy guest-host azopolymers. Dye. Pigment. 2019, 171, 107659. [Google Scholar] [CrossRef]
- Bujak, K.; Orlikowska, H.; Sobolewska, A.; Schab-Balcerzak, E.; Janeczek, H.; Bartkiewicz, S.; Konieczkowska, J. Azobenzene vs. azopyridine and matrix molar masses effect on photoinduced phenomena. Eur. Polym. J. 2019, 115, 173–184. [Google Scholar] [CrossRef]
- Stoica, I.; Sava, I.; Epure, E.-L.; Tiron, V.; Konieczkowska, J.; Schab-Balcerzak, E. Advanced morphological, statistical and molecular simulations analysis of laser-induced micro/nano multiscale surface relief gratings. Surf. Interf. 2022, 29, 101743. [Google Scholar] [CrossRef]
- Stoica, I.; Epure, E.-L.; Constantin, C.-P.; Damaceanu, M.-D.; Ursu, E.-L.; Mihaila, I.; Sava, I. Evaluation of Local Mechanical and Chemical Properties via AFM as a Tool for Understanding the Formation Mechanism of Pulsed UV Laser-Nanoinduced Patterns on Azo-Naphthalene-Based Polyimide Films. Nanomaterials 2021, 11, 812. [Google Scholar] [CrossRef]
- Bujak, K.; Sava, I.; Stoica, I.; Tiron, V.; Topala, I.; Węgłowski, R.; Schab-Balcerzak, E.; Konieczkowska, J. Photoinduced properties of “T-type” polyimides with azobenzene or azopyridine moieties. Eur. Polym. J. 2020, 126, 109563. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Rusu, R.-D.; Olaru, M.; Stoica, I.; Bruma, M. Nanostructured polyimide films by UV excimer laser irradiation. Rom. J. Inf. Sci. Technol. 2010, 13, 368–377. [Google Scholar]
- Damaceanu, M.-D.; Rusu, R.-D.; Olaru, M.A.; Timpu, D.; Bruma, M. KrF Pulsed Laser Ablation of Thin Films Made from Fluorinated Heterocyclic Poly(naphthyl-imide)s. Microsc. Microanal. 2012, 18, 545–557. [Google Scholar] [CrossRef]
- Obilor, A.F.; Pacella, M.; Wilson, A.; Silberschmidt, V.V. Micro-texturing of polymer surfaces using lasers: A review. Int. J. Adv. Manuf. Technol. 2022, 120, 103–135. [Google Scholar] [CrossRef]
- Sdr Parameters and its Applications in Characterizing Surface Data. Available online: https://www.azonano.com/article.aspx?ArticleID=5137 (accessed on 31 October 2022).
- ISO 25178-2:2012; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/42785.html (accessed on 31 October 2022).
- Lagugné Labarthet, F.; Buffeteau, T.; Sourisseau, C. Molecular Orientations in Azopolymer Holographic Diffraction Gratings as Studied by Raman Confocal Microspectroscopy. J. Phys. Chem. B 1998, 102, 5754–5765. [Google Scholar] [CrossRef]
- Hurduc, N.; Donose, B.C.; Macovei, A.; Paius, C.; Ibanescu, C.; Scutaru, D.; Hamel, M.; Branza-Nichita, N.; Rocha, L. Direct observation of athermal photofluidisation in azo-polymer films. Soft Matter 2014, 10, 4640–4647. [Google Scholar] [CrossRef]
- Damian, V.; Resmerita, E.; Stoica, I.; Ibanescu, C.; Sacarescu, L.; Rocha, L.; Hurduc, N. Surface relief gratings induced by pulsed laser irradiation in low glass-transition temperature azopolysiloxanes. J. Appl. Polym. Sci. 2014, 131, 41015. [Google Scholar] [CrossRef]
- Heath, D.R.; Wirth, J.G. Aminophenoxy Benzonitriles. US Patent 3,763,211, 2 October 1973. [Google Scholar]
- Bruma, M.; Schulz, B.; Mercer, F.W. Polyamide Copolymers Containing Hexafluoroisopropylene Groups. J. Macromol. Sci. Part A 1995, 32, 259–286. [Google Scholar] [CrossRef]
- Saxena, A.; Prabhakaran, P.; Rao, V.L.; Ninan, K. Synthesis and characterization of polyamides and poly(amide-imide)s derived from 2,6-bis(3-aminophenoxy)benzonitrile or 2,6-bis(4-aminophenoxy)benzonitrile. Polym. Int. 2005, 54, 544–552. [Google Scholar] [CrossRef]
- Helmut Ringsdorf, H.-W.S. Electro-optical effects of azo dye containing liquid crystalline copolymers. Die Makromol. Chemie 1984, 185, 1327–1334. [Google Scholar] [CrossRef]
- Sava, I.; Köpnick, T. Synthesis and characterization of new diamines containing side substituted azobenzene groups. Rev. Roum. Chim. 2014, 59, 585–592. [Google Scholar]
- Materials Studio 4.0. DMol3, Synthia, Discover, Forcite and Amorphous Cell Module; Accelrys Software Inc.: San Diego, CA, USA, 2005. [Google Scholar]
- Singh, M.; Sethi, S.K.; Manik, G. Pressure-sensitive adhesives based on acrylated epoxidized linseed oil: A computational approach. Int. J. Adhes. Adhes. 2022, 112, 103031. [Google Scholar] [CrossRef]
- Bujak, K.; Orlikowska, H.; Małecki, J.G.; Schab-Balcerzak, E.; Bartkiewicz, S.; Bogucki, J.; Sobolewska, A.; Konieczkowska, J. Fast dark cis-trans isomerization of azopyridine derivatives in comparison to their azobenzene analogues: Experimental and computational study. Dye. Pigment. 2019, 160, 654–662. [Google Scholar] [CrossRef]
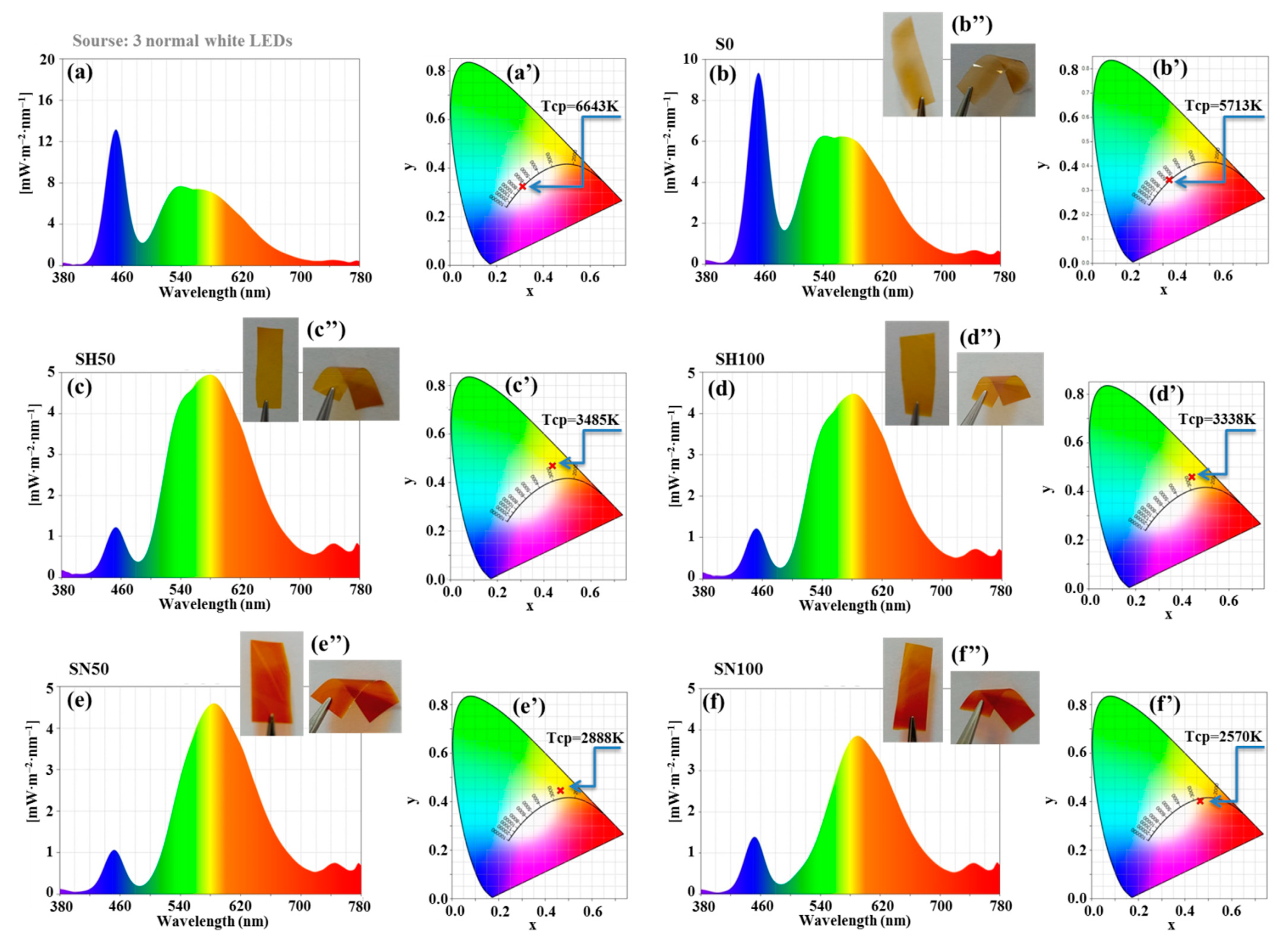




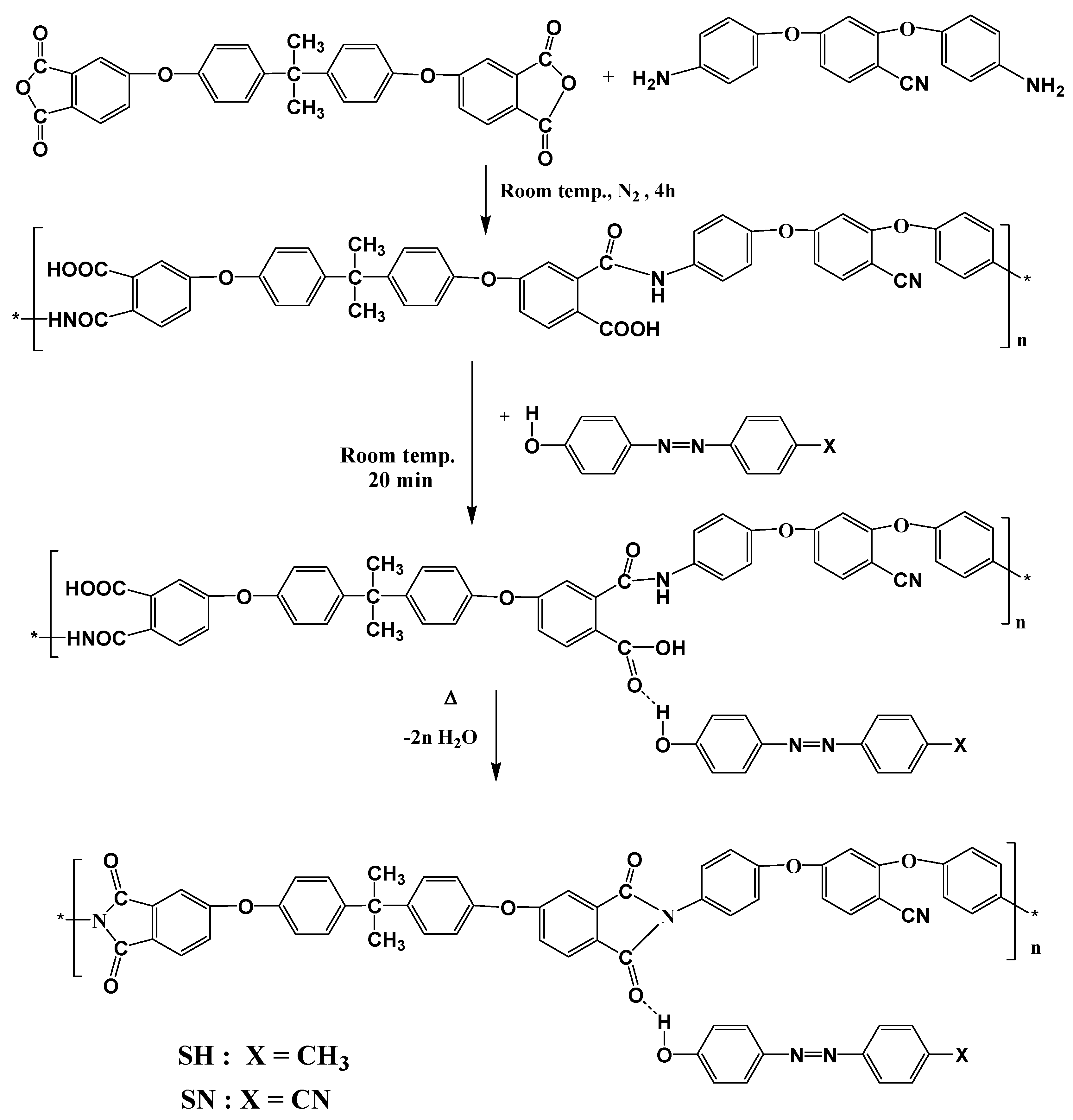
| Sample | λpk (nm) | Tcp (K) | Ev (lx) | T (%) |
|---|---|---|---|---|
| three normal light LEDs | 486 | 6643 | 465 | - |
| S0 | 452 | 5713 | 388 | 83 |
| SH50 | 579 | 3485 | 278 | 59 |
| SH100 | 582 | 3338 | 246 | 52 |
| SN50 | 585 | 2888 | 224 | 48 |
| SN100 | 590 | 2570 | 170 | 36 |
| Sample | Tonset, °C | T5, °C | Tmax, °C |
|---|---|---|---|
| S0 | 512 | - | 546 |
| SH100 | 228 | 308 | 537 |
| SN100 | 247 | 308 | 532 |
| Sample | Type of Structuration | H (nm) | W (nm) | Sq (nm) | Sdr (%) | Stdi | Str |
|---|---|---|---|---|---|---|---|
| S0 | Cone-like structures | 45 ± 15 | 667 ± 10 | 6.8 | 0.366 | 0.556 | 0.483 |
| SH50 | Surface relief gratings | 53 ± 2 | 592 ± 55 | 11.7 | 0.672 | 0.286 | 0.074 |
| SH100 | Surface relief gratings | 99 ± 6 | 532 ± 27 | 18.7 | 2.041 | 0.179 | 0.052 |
| SN50 | Surface relief gratings | 88 ± 3 | 670 ± 5 | 22.1 | 1.752 | 0.189 | 0.078 |
| SN100 | Surface relief gratings | 198 ± 11 | 672 ± 7 | 52.9 | 8.866 | 0.162 | 0.076 |
| System | S0 | SH50_1 | SH50_2 | SH50_3 | SN50_1 | SN50_2 | SN50_3 |
|---|---|---|---|---|---|---|---|
| % cis | - | 0 | 30 | 80 | 0 | 20 | 80 |
| Density (g/cm3) | 1.235 | 1.186 | 1.207 | 1.218 | 1.238 | 1.229 | 1.232 |
| System | SH100_1 | SH100_2 | SH100_3 | SN100_1 | SN100_2 | SN100_3 | |
| % cis | 0 | 30 | 80 | 40 | 0 | 75 | |
| Density (g/cm3) | 1.201 | 1.219 | 1.205 | 1.223 | 1.215 | 1.227 |
| System | S0 | SH50_1 | SH50_2 | SH50_3 | SN50_1 | SN50_2 | SN50_3 |
|---|---|---|---|---|---|---|---|
| MSD | 1.71 | 1.03 | 1.46 | 1.84 | 0.73 | 1.27 | 2.94 |
| System | SH100_1 | SH100_2 | SH100_3 | SN100_1 | SN100_2 | SN100_3 | |
| MSD | 1.13 | 0.91 | 1.25 | 0.80 | 1.21 | 3.11 |
| Sample Code | Polyamidic Acid mol (g) | Azo Monomer AzoCH3 mol (g) | Azo Monomer AzoCN mol (g) |
|---|---|---|---|
| SH50 | 0.9768 × 10−4 (0.0818) | 0.4889 × 10−4 (0.010354) | - |
| SH100 | 0.9768 × 10−4 (0.0818) | 0.9768 × 10−4 (0.020708) | - |
| SN50 | 0.9768 × 10−4 (0.0818) | - | 0.4889 × 10−4 (0.01089) |
| SN100 | 0.9768 × 10−4 (0.0818) | - | 0.9768 × 10−4 (0.02178) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, I.; Epure, E.-L.; Barzic, A.I.; Mihaila, I.; Constantin, C.-P.; Sava, I. The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics. Int. J. Mol. Sci. 2022, 23, 15223. https://doi.org/10.3390/ijms232315223
Stoica I, Epure E-L, Barzic AI, Mihaila I, Constantin C-P, Sava I. The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics. International Journal of Molecular Sciences. 2022; 23(23):15223. https://doi.org/10.3390/ijms232315223
Chicago/Turabian StyleStoica, Iuliana, Elena-Luiza Epure, Andreea Irina Barzic, Ilarion Mihaila, Catalin-Paul Constantin, and Ion Sava. 2022. "The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics" International Journal of Molecular Sciences 23, no. 23: 15223. https://doi.org/10.3390/ijms232315223
APA StyleStoica, I., Epure, E.-L., Barzic, A. I., Mihaila, I., Constantin, C.-P., & Sava, I. (2022). The Impact of the Azo-Chromophore Sort on the Features of the Supramolecular Azopolyimide Films Desired to Be Used as Substrates for Flexible Electronics. International Journal of Molecular Sciences, 23(23), 15223. https://doi.org/10.3390/ijms232315223