Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats
Abstract
1. Introduction
2. Results
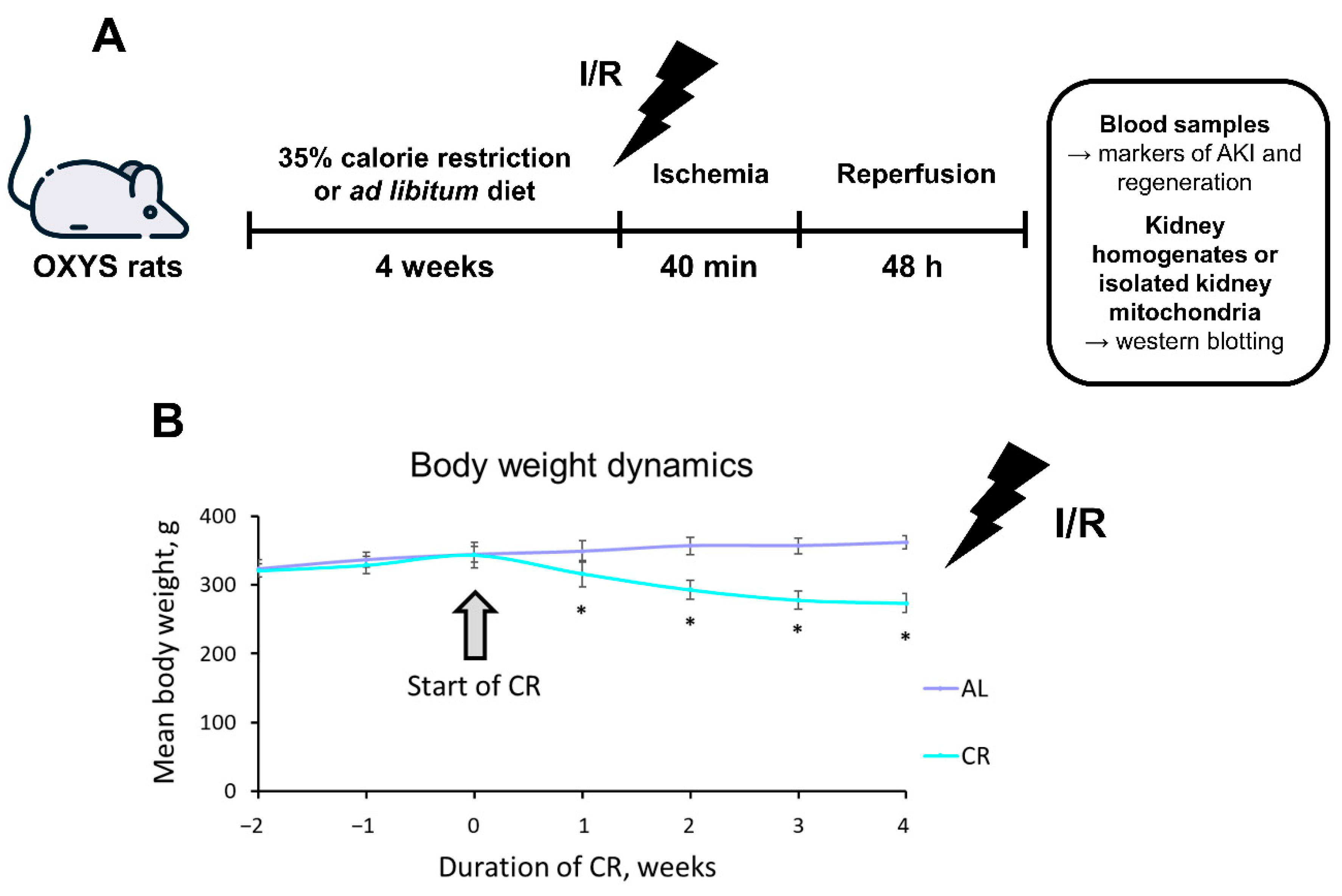
2.1. CR Causes Significant Weight Loss in OXYS Rats
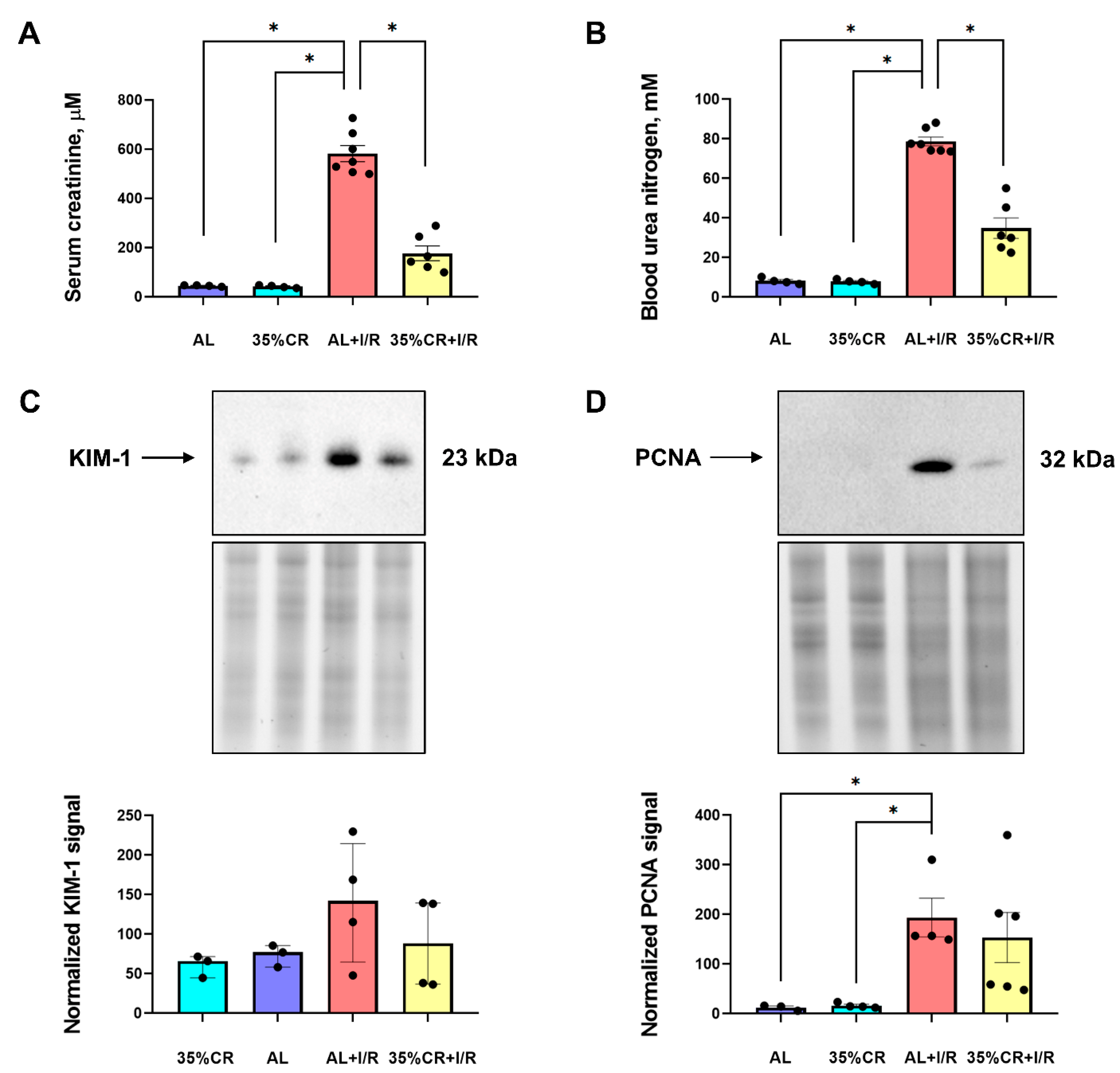
2.2. CR Reduces AKI Severity in OXYS Rats
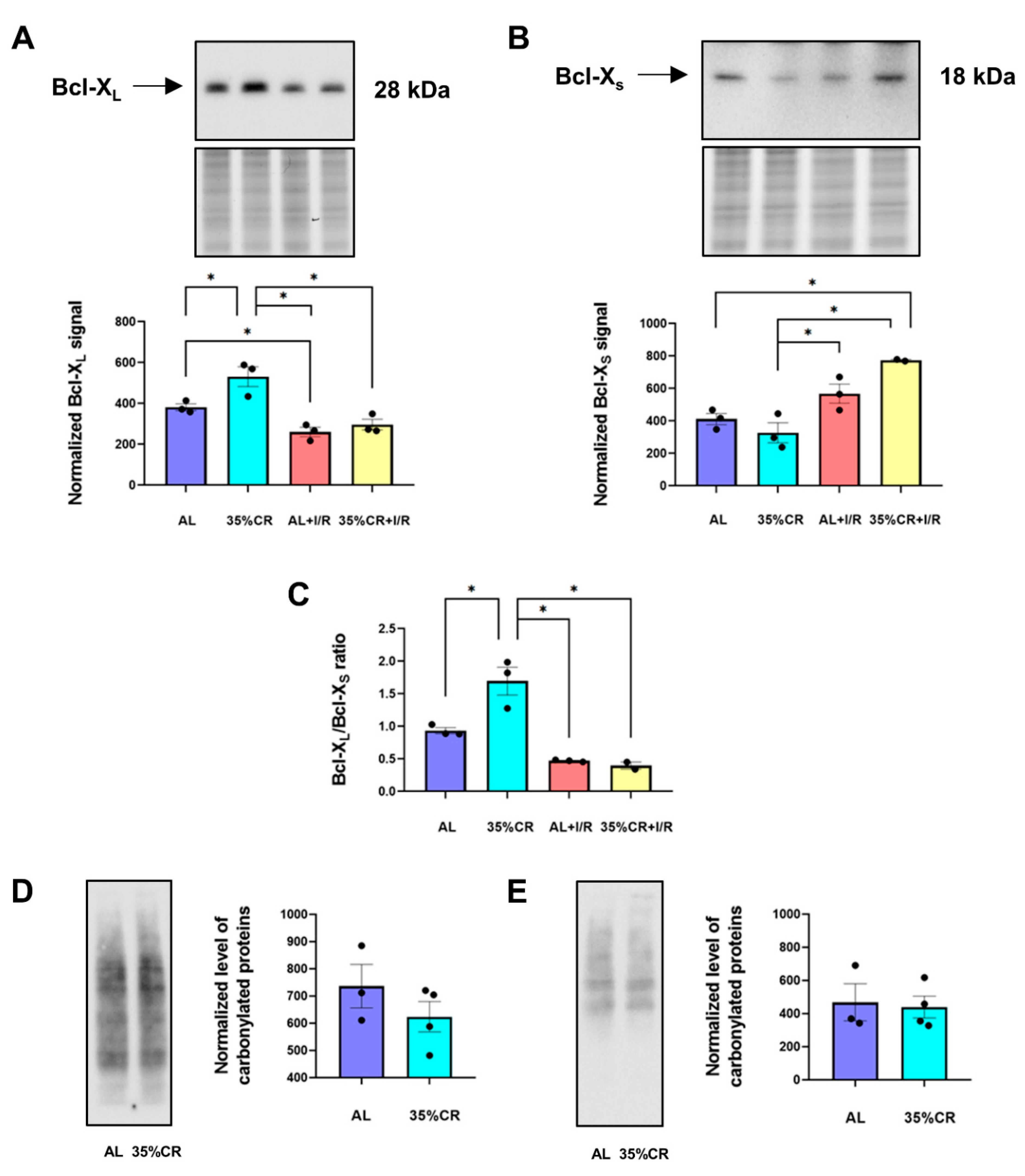
2.3. CR Affects Apoptotic Pathways and the Level of Oxidative Stress in OXYS Rats
2.4. CR Leads to Metabolic Shifts after I/R in OXYS Rats
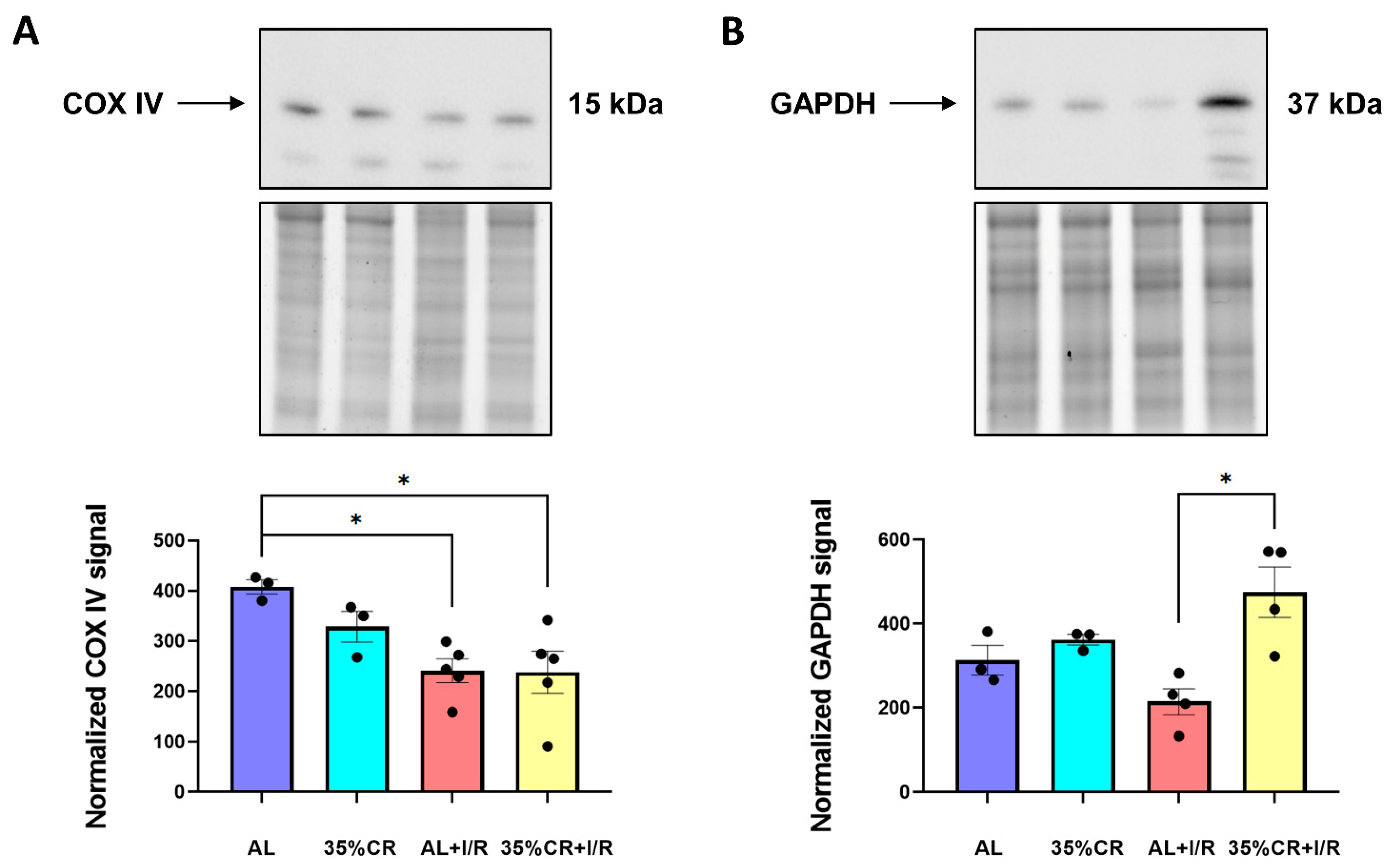
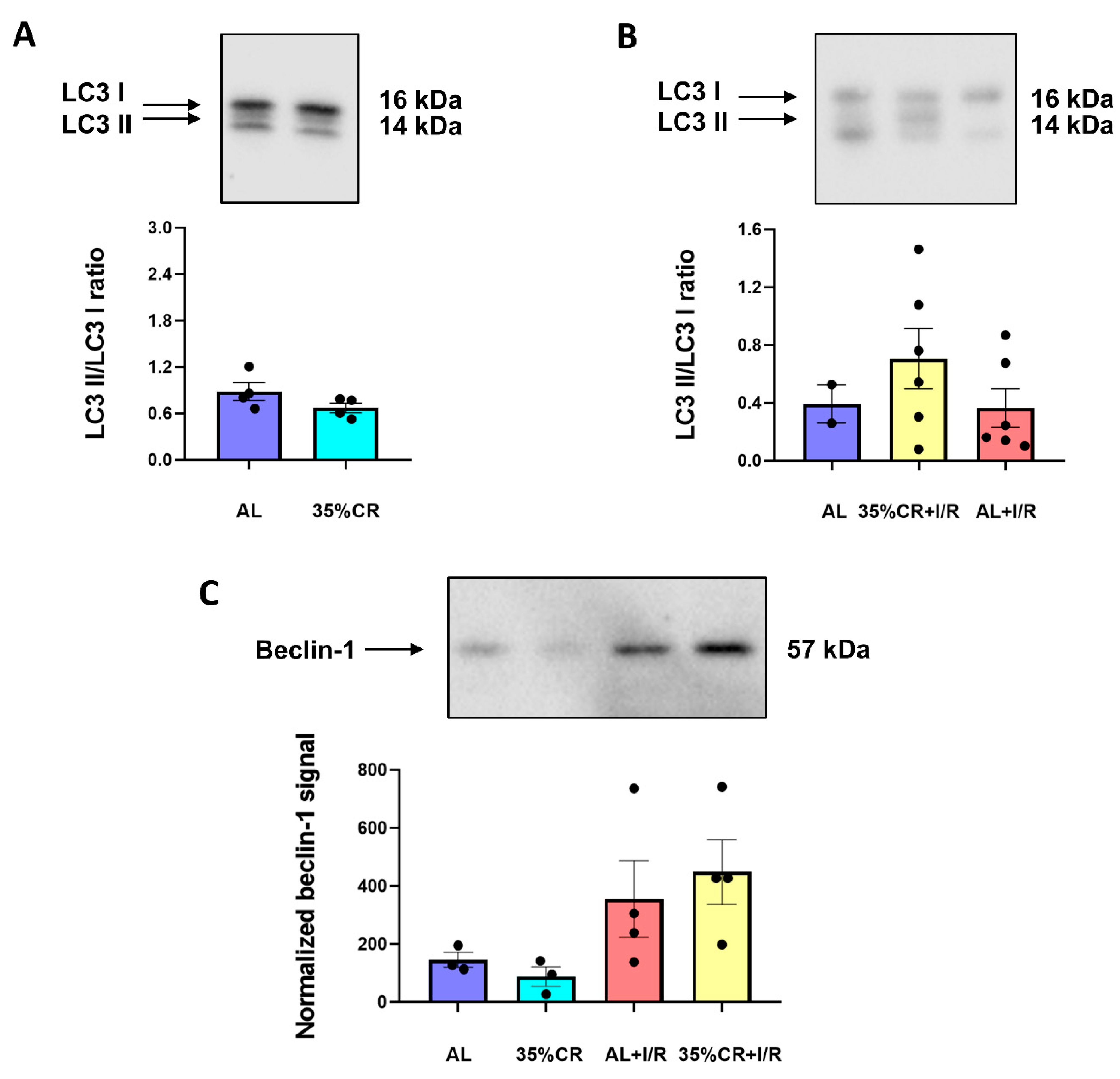
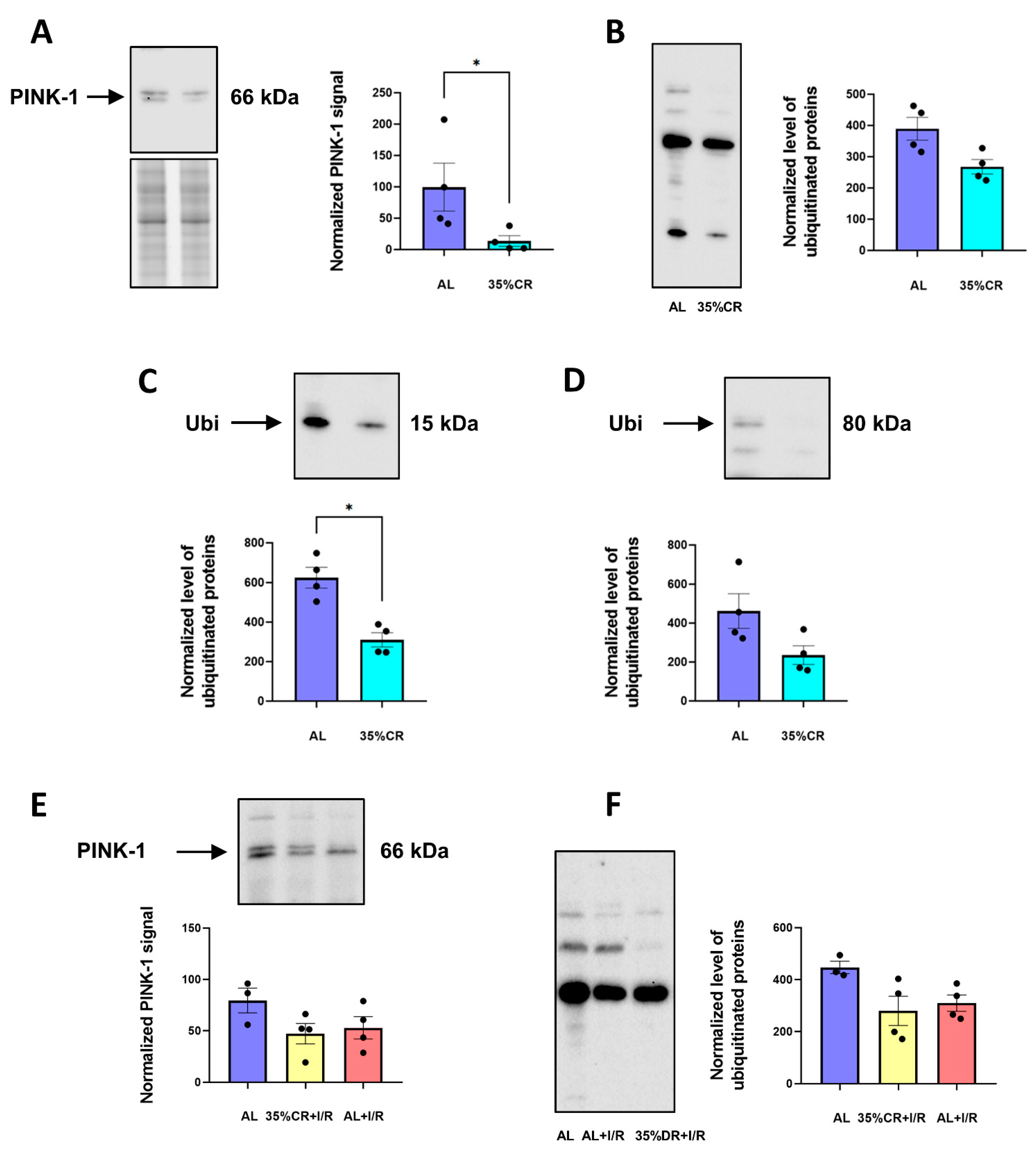
2.5. CR Does Not Activate Autophagy in OXYS Rats but Causes Signs of Mitochondria Renewal
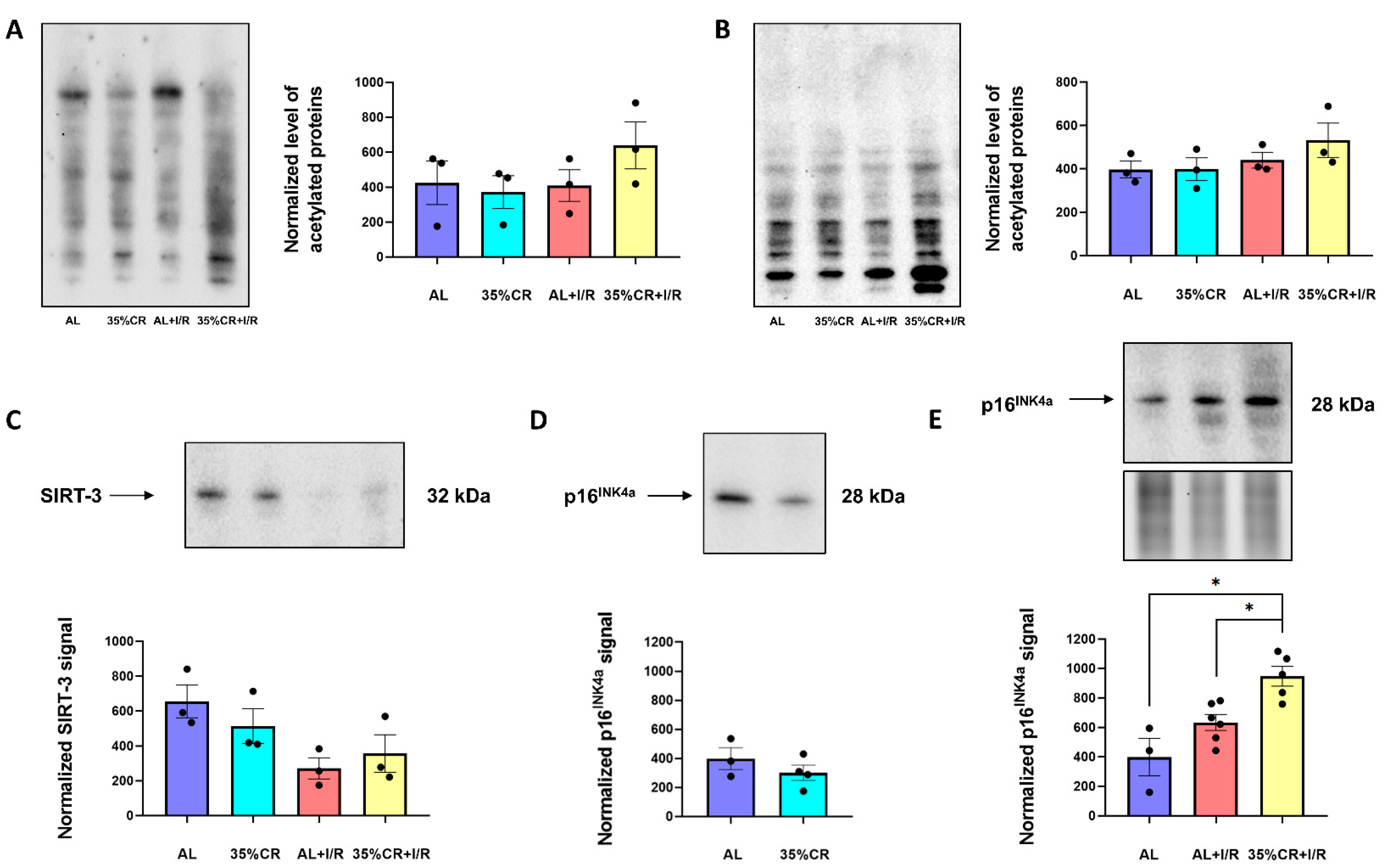
2.6. CR Effects on Protein Acetylation and Cellular Senescence in OXYS Rats
3. Discussion
4. Materials and Methods
4.1. OXYS Rats
4.2. Dietary Protocol
4.3. Renal I/R Protocol
4.4. Western Blotting of Kidney Homogenates
4.5. Mitochondria Isolation and Western Blotting
4.6. Biochemical Analysis and Western Blotting of Serum
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, A.W.; Ogunwole, S.U.; Blakeslee, L.; Rabe, M.A. The Population 65 Years and Older in the United States: 2016; US Department of Commerce, Economics and Statistics Administration: Washington, DC, USA, 2018.
- Mercado, M.G.; Smith, D.K.; Guard, E.L. Acute Kidney Injury: Diagnosis and Management. Am. Fam. Physician 2019, 100, 687–694. [Google Scholar] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bragg-Gresham, J.; Balkrishnan, R.; Bhave, N.; Dietrich, X.; Ding, Z.; Eggers, P.W.; et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2019, 73, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.; McCulloch, C.E.; Lin, F.; Banerjee, T.; Bragg-Gresham, J.L.; Eberhardt, M.S.; Morgenstern, H.; Pavkov, M.E.; Saran, R.; Powe, N.R.; et al. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann. Intern. Med. 2016, 165, 473–481. [Google Scholar] [CrossRef] [PubMed]
- da Silveira Santos, C.G.; Romani, R.F.; Benvenutti, R.; Ribas Zahdi, J.O.; Riella, M.C.; Mazza do Nascimento, M. Acute Kidney Injury in Elderly Population: A Prospective Observational Study. Nephron 2018, 138, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M. Program to Improve Care in Acute Renal Disease Spectrum of Acute Renal Failure in the Intensive Care Unit: The PICARD Experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N. The Pathophysiology of Acute Renal Failure. Crit. Care Clin. 2005, 21, 197–210. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute Kidney Injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Moore, P.K.; Hsu, R.K.; Liu, K.D. Management of Acute Kidney Injury: Core Curriculum 2018. Am. J. Kidney Dis. 2018, 72, 136–148. [Google Scholar] [CrossRef]
- Späth, M.R.; Koehler, F.C.; Hoyer-Allo, K.J.R.; Grundmann, F.; Burst, V.; Müller, R.-U. Preconditioning Strategies to Prevent Acute Kidney Injury. F1000Research 2020, 9, 237. [Google Scholar] [CrossRef]
- Speakman, J.R.; Mitchell, S.E. Caloric Restriction. Mol. Aspects Med. 2011, 32, 159–221. [Google Scholar] [CrossRef]
- Singh, G.; Krishan, P. Dietary Restriction Regimens for Fighting Kidney Disease: Insights from Rodent Studies. Exp. Gerontol. 2019, 128, 110738. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Navas, P. Calorie Restriction as an Intervention in Ageing. J. Physiol. 2016, 594, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Cai, G.-Y.; Chen, X.-M. Energy Restriction in Renal Protection. Br. J. Nutr. 2018, 120, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, N.G.; Stefanova, N.A.; Korbolina, E.E.; Fursova, A.Z.; Kozhevnikova, O.S. The senescence-accelerated oxys rats--a genetic model of premature aging and age-dependent degenerative diseases. Adv. Gerontol. 2014, 27, 336–340. [Google Scholar] [PubMed]
- Stefanova, N.A.; Kozhevnikova, O.S.; Vitovtov, A.O.; Maksimova, K.Y.; Logvinov, S.V.; Rudnitskaya, E.A.; Korbolina, E.E.; Muraleva, N.A.; Kolosova, N.G. Senescence-Accelerated OXYS Rats: A Model of Age-Related Cognitive Decline with Relevance to Abnormalities in Alzheimer Disease. Cell Cycle 2014, 13, 898–909. [Google Scholar] [CrossRef]
- Telegina, D.V.; Suvorov, G.K.; Kozhevnikova, O.S.; Kolosova, N.G. Mechanisms of Neuronal Death in the Cerebral Cortex during Aging and Development of Alzheimer’s Disease-Like Pathology in Rats. Int. J. Mol. Sci. 2019, 20, 5632. [Google Scholar] [CrossRef]
- Rudnitskaya, E.A.; Kozlova, T.A.; Burnyasheva, A.O.; Kolosova, N.G.; Stefanova, N.A. Alterations of Hippocampal Neurogenesis during Development of Alzheimer’s Disease-like Pathology in OXYS Rats. Exp. Gerontol. 2019, 115, 32–45. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Ofitserov, E.N.; Tikhonov, V.P.; Kolosova, N.G. Efficacy of Glucosamine Alendronate Alone & in Combination with Dihydroquercetin for Treatment of Osteoporosis in Animal Model. Indian J. Med. Res. 2012, 135, 221–227. [Google Scholar]
- Devyatkin, V.A.; Redina, O.E.; Muraleva, N.A.; Kolosova, N.G. Single-Nucleotide Polymorphisms (SNPs) Both Associated with Hypertension and Contributing to Accelerated-Senescence Traits in OXYS Rats. Int. J. Mol. Sci. 2020, 21, 3542. [Google Scholar] [CrossRef]
- Obukhova, L.A.; Skulachev, V.P.; Kolosova, N.G. Mitochondria-Targeted Antioxidant SkQ1 Inhibits Age-Dependent Involution of the Thymus in Normal and Senescence-Prone Rats. Aging 2009, 1, 389–401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bobko, A.A.; Sergeeva, S.V.; Bagryanskaya, E.G.; Markel, A.L.; Khramtsov, V.V.; Reznikov, V.A.; Kolosova, N.G. 19F NMR Measurements of NO Production in Hypertensive ISIAH and OXYS Rats. Biochem. Biophys. Res. Commun. 2005, 330, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A.; Ershov, N.I.; Maksimova, K.Y.; Muraleva, N.A.; Tyumentsev, M.A.; Kolosova, N.G. The Rat Prefrontal-Cortex Transcriptome: Effects of Aging and Sporadic Alzheimer’s Disease-Like Pathology. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Tyumentsev, M.A.; Stefanova, N.A.; Muraleva, N.A.; Rumyantseva, Y.V.; Kiseleva, E.; Vavilin, V.A.; Kolosova, N.G. Mitochondrial Dysfunction as a Predictor and Driver of Alzheimer’s Disease-Like Pathology in OXYS Rats. J. Alzheimers. Dis. 2018, 63, 1075–1088. [Google Scholar] [CrossRef]
- Kozhevnikova, O.S.; Telegina, D.V.; Devyatkin, V.A.; Kolosova, N.G. Involvement of the Autophagic Pathway in the Progression of AMD-like Retinopathy in Senescence-Accelerated OXYS Rats. Biogerontology 2018, 19, 223–235. [Google Scholar] [CrossRef]
- Korbolina, E.E.; Zhdankina, A.A.; Fursova, A.Z.; Kozhevnikova, O.S.; Kolosova, N.G. Genes of Susceptibility to Early Neurodegenerative Changes in the Rat Retina and Brain: Analysis by Means of Congenic Strains. BMC Genet. 2016, 17, 153. [Google Scholar] [CrossRef][Green Version]
- Kozhevnikova, O.S.; Korbolina, E.E.; Ershov, N.I.; Kolosova, N.G. Rat Retinal Transcriptome: Effects of Aging and AMD-like Retinopathy. Cell Cycle 2013, 12, 1745–1761. [Google Scholar] [CrossRef]
- Stefanova, N.A.; Ershov, N.I.; Kolosova, N.G. Suppression of Alzheimer’s Disease-Like Pathology Progression by Mitochondria-Targeted Antioxidant SkQ1: A Transcriptome Profiling Study. Oxid. Med. Cell. Longev. 2019, 2019, 3984906. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Pevzner, I.B.; Andrianova, N.V.; Zorova, L.D.; Popkov, V.A.; Silachev, D.N.; Kolosova, N.G.; Plotnikov, E.Y.; Zorov, D.B. The Age-Associated Loss of Ischemic Preconditioning in the Kidney Is Accompanied by Mitochondrial Dysfunction, Increased Protein Acetylation and Decreased Autophagy. Sci. Rep. 2017, 7, 44430. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in Acute Kidney Injury—Pathophysiological Basis and Clinical Performance. Acta Physiol. 2017, 219, 554–572. [Google Scholar] [CrossRef] [PubMed]
- Strzalka, W.; Ziemienowicz, A. Proliferating Cell Nuclear Antigen (PCNA): A Key Factor in DNA Replication and Cell Cycle Regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; McMahon, A.P.; Valerius, M.T. Recent Genetic Studies of Mouse Kidney Development. Curr. Opin. Genet. Dev. 2004, 14, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Liu, D.; Huang, Q.; Cai, G.; Cui, S.; Sun, X.; Chen, X. GDF11 Improves Tubular Regeneration after Acute Kidney Injury in Elderly Mice. Sci. Rep. 2016, 6, 34624. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Rejuvenation of Aging Hearts. Rejuvenation Res. 2013, 16, 330–332. [Google Scholar] [CrossRef]
- Rochette, L.; Mazini, L.; Meloux, A.; Zeller, M.; Cottin, Y.; Vergely, C.; Malka, G. Anti-Aging Effects of GDF11 on Skin. Int. J. Mol. Sci. 2020, 21, 2598. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Zorov, S.D.; Popkov, V.A.; Jankauskas, S.S.; Zinchenko, V.P.; Sukhikh, G.T.; et al. The Mitochondrion as a Key Regulator of Ischaemic Tolerance and Injury. Heart Lung Circ. 2014, 23, 897–904. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 Family Proteins: Changing Partners in the Dance towards Death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Minn, A.J.; Boise, L.H.; Thompson, C.B. Bcl-x(S) Anatagonizes the Protective Effects of Bcl-x(L). J. Biol. Chem. 1996, 271, 6306–6312. [Google Scholar] [CrossRef]
- Wong, C.M.; Cheema, A.K.; Zhang, L.; Suzuki, Y.J. Protein Carbonylation as a Novel Mechanism in Redox Signaling. Circ. Res. 2008, 102, 310–318. [Google Scholar] [CrossRef]
- Wong, C.M.; Marcocci, L.; Liu, L.; Suzuki, Y.J. Cell Signaling by Protein Carbonylation and Decarbonylation. Antioxid. Redox Signal. 2010, 12, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Ham, P.B., 3rd; Raju, R. Mitochondrial Function in Hypoxic Ischemic Injury and Influence of Aging. Prog. Neurobiol. 2017, 157, 92–116. [Google Scholar] [CrossRef] [PubMed]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The Spotlight for Cellular Stress Responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a Promoter of Longevity: Insights from Model Organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy (4th edition)1. Autophagy 2021, 17, 1–382. [Google Scholar]
- He, L.; Livingston, M.J.; Dong, Z. Autophagy in Acute Kidney Injury and Repair. Nephron Clin. Pract. 2014, 127, 56–60. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Tang, C.; Dong, Z. Mitophagy in Acute Kidney Injury and Kidney Repair. Cells 2020, 9, 338. [Google Scholar] [CrossRef]
- Mehrabani, S.; Bagherniya, M.; Askari, G.; Read, M.I.; Sahebkar, A. The Effect of Fasting or Calorie Restriction on Mitophagy Induction: A Literature Review. J. Cachexia Sarcopenia Muscle 2020, 11, 1447–1458. [Google Scholar] [CrossRef]
- Whitley, B.N.; Engelhart, E.A.; Hoppins, S. Mitochondrial Dynamics and Their Potential as a Therapeutic Target. Mitochondrion 2019, 49, 269–283. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Mariño, G.; Pietrocola, F.; Eisenberg, T.; Kong, Y.; Malik, S.A.; Andryushkova, A.; Schroeder, S.; Pendl, T.; Harger, A.; Niso-Santano, M.; et al. Regulation of Autophagy by Cytosolic Acetyl-Coenzyme A. Mol. Cell 2014, 53, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Perico, L.; Benigni, A. Sirtuins in Renal Health and Disease. J. Am. Soc. Nephrol. 2018, 29, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.-H.; Kim, H.-S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.-X.; Finkel, T. A Role for the Mitochondrial Deacetylase Sirt3 in Regulating Energy Homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf Expression Is a Biomarker of Aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The Effect of Retarded Growth upon the Length of Life Span and upon the Ultimate Body Size. 1935. Nutrition 1989, 5, 155–171; discussion 172. [Google Scholar] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending Healthy Life Span--from Yeast to Humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Rohrbach, S.; Aslam, M.; Niemann, B.; Schulz, R. Impact of Caloric Restriction on Myocardial Ischaemia/reperfusion Injury and New Therapeutic Options to Mimic Its Effects. Br. J. Pharmacol. 2014, 171, 2964–2992. [Google Scholar] [CrossRef]
- Ciobanu, O.; Elena Sandu, R.; Tudor Balseanu, A.; Zavaleanu, A.; Gresita, A.; Petcu, E.B.; Uzoni, A.; Popa-Wagner, A. Caloric Restriction Stabilizes Body Weight and Accelerates Behavioral Recovery in Aged Rats after Focal Ischemia. Aging Cell 2017, 16, 1394–1403. [Google Scholar] [CrossRef]
- Di Daniele, N.; Marrone, G.; Di Lauro, M.; Di Daniele, F.; Palazzetti, D.; Guerriero, C.; Noce, A. Effects of Caloric Restriction Diet on Arterial Hypertension and Endothelial Dysfunction. Nutrients 2021, 13, 274. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Vandendriessche, C.; Libert, C.; Vandenbroucke, R.E. Caloric Restriction: Beneficial Effects on Brain Aging and Alzheimer’s Disease. Mamm. Genome 2016, 27, 300–319. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The Role of Low-Calorie Diets and Intermittent Fasting in the Treatment of Obesity and Type-2 Diabetes. J. Physiol. Pharmacol. 2018, 663–683. [Google Scholar] [CrossRef]
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Recent Results Cancer Res. 2016, 207, 241–266. [Google Scholar] [PubMed]
- Rosner, M.H.; La Manna, G.; Ronco, C. Acute Kidney Injury in the Geriatric Population. Contrib. Nephrol. 2018, 193, 149–160. [Google Scholar]
- Lempiäinen, J.; Finckenberg, P.; Mervaala, E.E.; Sankari, S.; Levijoki, J.; Mervaala, E.M. Caloric Restriction Ameliorates Kidney Ischaemia/reperfusion Injury through PGC-1α-eNOS Pathway and Enhanced Autophagy. Acta Physiol. 2013, 208, 410–421. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Müller, C.; de Jong, M.; et al. Short-Term Dietary Restriction and Fasting Precondition against Ischemia Reperfusion Injury in Mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Jankauskas, S.S.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Mechanisms of Age-Dependent Loss of Dietary Restriction Protective Effects in Acute Kidney Injury. Cells 2018, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Popkov, V.A.; Chernikov, V.P.; Silachev, D.N.; Plotnikov, E.Y.; Zorov, D.B. Resemblance and Differences in Dietary Restriction Nephroprotective Mechanisms in Young and Old Rats. Aging 2020, 12, 18693–18715. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Buyan, M.I.; Bolikhova, A.K.; Zorov, D.B.; Plotnikov, E.Y. Dietary Restriction for Kidney Protection: Decline in Nephroprotective Mechanisms During Aging. Front. Physiol. 2021, 12, 699490. [Google Scholar] [CrossRef]
- Chen, G.; Bridenbaugh, E.A.; Akintola, A.D.; Catania, J.M.; Vaidya, V.S.; Bonventre, J.V.; Dearman, A.C.; Sampson, H.W.; Zawieja, D.C.; Burghardt, R.C.; et al. Increased Susceptibility of Aging Kidney to Ischemic Injury: Identification of Candidate Genes Changed during Aging, but Corrected by Caloric Restriction. Am. J. Physiol. Renal Physiol. 2007, 293, F1272–F1281. [Google Scholar] [CrossRef]
- Podkowka-Sieczka, R.; Wieczorowska-Tobis, K.; Niemir, Z.I.; Styszynski, A.; Breborowicz, A.; Oreopoulos, D.G. The Effect on Renal Structure and Function of Late-Life-Introduced Caloric Restriction (CR) in Rats. Int. Urol. Nephrol. 2009, 41, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bras, G.; Ross, M.H. Kidney disease and nutrition in the rat. Toxicol. Appl. Pharmacol. 1964, 6, 247–262. [Google Scholar] [CrossRef]
- Warner, G.; Hein, K.Z.; Nin, V.; Edwards, M.; Chini, C.C.S.; Hopp, K.; Harris, P.C.; Torres, V.E.; Chini, E.N. Food Restriction Ameliorates the Development of Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Min, K.-J. Caloric Restriction and Its Mimetics. BMB Rep. 2013, 46, 181–187. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Hunt, N.; Jones, B.; Zhu, M.; Jamieson, H.; Hilmer, S.; Cascajo, M.V.; Allard, J.; Ingram, D.K.; Navas, P.; et al. Calorie Restriction Induces Mitochondrial Biogenesis and Bioenergetic Efficiency. Proc. Natl. Acad. Sci. USA 2006, 103, 1768–1773. [Google Scholar] [CrossRef]
- Gouspillou, G.; Hepple, R.T. Facts and Controversies in Our Understanding of How Caloric Restriction Impacts the Mitochondrion. Exp. Gerontol. 2013, 48, 1075–1084. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Smith, S.R.; Ravussin, E. Diet, Energy Metabolism and Mitochondrial Biogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 679–687. [Google Scholar] [CrossRef]
- Hess, M.L.; Manson, N.H. Molecular Oxygen: Friend and Foe. The Role of the Oxygen Free Radical System in the Calcium Paradox, the Oxygen Paradox and Ischemia/reperfusion Injury. J. Mol. Cell. Cardiol. 1984, 16, 969–985. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Son, J.M.; Lee, C. Mitochondria: Multifaceted Regulators of Aging. BMB Rep. 2019, 52, 13–23. [Google Scholar] [CrossRef]
- López-Lluch, G.; Santos-Ocaña, C.; Sánchez-Alcázar, J.A.; Fernández-Ayala, D.J.M.; Asencio-Salcedo, C.; Rodríguez-Aguilera, J.C.; Navas, P. Mitochondrial Responsibility in Ageing Process: Innocent, Suspect or Guilty. Biogerontology 2015, 16, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. Biomed. Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef]
- Abe, K.; Kawagoe, J.; Itoyama, Y.; Kogure, K. Isolation of an Ischemia-Induced Gene and Early Disturbance of Mitochondrial DNA Expression after Transient Forebrain Ischemia. Adv. Neurol. 1996, 71, 485–503. [Google Scholar] [PubMed]
- Barbosa, P.R.; Cardoso, M.R.; Daufenbach, J.F.; Gonçalves, C.L.; Machado, R.A.; Roza, C.A.; Scaini, G.; Rezin, G.T.; Schuck, P.F.; Dal-Pizzol, F.; et al. Inhibition of Mitochondrial Respiratory Chain in the Brain of Rats after Renal Ischemia Is Prevented by N-Acetylcysteine and Deferoxamine. Metab. Brain Dis. 2010, 25, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Seidler, N.W. GAPDH and Intermediary Metabolism. Adv. Exp. Med. Biol. 2013, 985, 37–59. [Google Scholar]
- Nicholls, C.; Li, H.; Liu, J.-P. GAPDH: A Common Enzyme with Uncommon Functions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Kosova, A.A.; Khodyreva, S.N.; Lavrik, O.I. Role of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) in DNA Repair. Biochemistry 2017, 82, 643–654. [Google Scholar] [CrossRef]
- Fenton, A.R.; Jongens, T.A.; Holzbaur, E.L.F. Mitochondrial Dynamics: Shaping and Remodeling an Organelle Network. Curr. Opin. Cell Biol. 2021, 68, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial Fusion and Fission: The Fine-Tune Balance for Cellular Homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Putti, R.; Sica, R.; Migliaccio, V.; Lionetti, L. Diet Impact on Mitochondrial Bioenergetics and Dynamics. Front. Physiol. 2015, 6, 109. [Google Scholar] [CrossRef]
- Weir, H.J.; Yao, P.; Huynh, F.K.; Escoubas, C.C.; Goncalves, R.L.; Burkewitz, K.; Laboy, R.; Hirschey, M.D.; Mair, W.B. Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab. 2017, 26, 884–896.e5. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the Regulation of Metabolism: Mechanisms and Consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- López-Lluch, G.; Irusta, P.M.; Navas, P.; de Cabo, R. Mitochondrial Biogenesis and Healthy Aging. Exp. Gerontol. 2008, 43, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.A.; Reichert, A.S. Impaired Quality Control of Mitochondria: Aging from a New Perspective. Exp. Gerontol. 2010, 45, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Horiike, K.; Chin-Kanasaki, M.; Isshiki, K.; Araki, S.-I.; Sugimoto, T.; Haneda, M.; Kashiwagi, A.; Koya, D. Calorie Restriction Enhances Cell Adaptation to Hypoxia through Sirt1-Dependent Mitochondrial Autophagy in Mouse Aged Kidney. J. Clin. Investig. 2010, 120, 1043–1055. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- De Gaetano, A.; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and Oxidative Stress: The Role of Aging. Antioxidants 2021, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-C.; Bergamini, C.; Fato, R.; Pon, L.A.; Pallotti, F. Isolation of Mitochondria from Cells and Tissues. Methods Cell Biol. 2020, 155, 3–31. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Kolosova, N.G.; Plotnikov, E.Y.; Zorov, D.B. Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats. Int. J. Mol. Sci. 2022, 23, 15224. https://doi.org/10.3390/ijms232315224
Andrianova NV, Zorova LD, Pevzner IB, Kolosova NG, Plotnikov EY, Zorov DB. Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats. International Journal of Molecular Sciences. 2022; 23(23):15224. https://doi.org/10.3390/ijms232315224
Chicago/Turabian StyleAndrianova, Nadezda V., Ljubava D. Zorova, Irina B. Pevzner, Nataliya G. Kolosova, Egor Y. Plotnikov, and Dmitry B. Zorov. 2022. "Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats" International Journal of Molecular Sciences 23, no. 23: 15224. https://doi.org/10.3390/ijms232315224
APA StyleAndrianova, N. V., Zorova, L. D., Pevzner, I. B., Kolosova, N. G., Plotnikov, E. Y., & Zorov, D. B. (2022). Calorie Restriction Provides Kidney Ischemic Tolerance in Senescence-Accelerated OXYS Rats. International Journal of Molecular Sciences, 23(23), 15224. https://doi.org/10.3390/ijms232315224






