Molecular Characterization of Thymus capitellatus Extracts and Their Antioxidant, Neuroprotective and Anti-Proliferative Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield, Total Phenolic, Total Flavonoid and Ortho-Diphenols Content
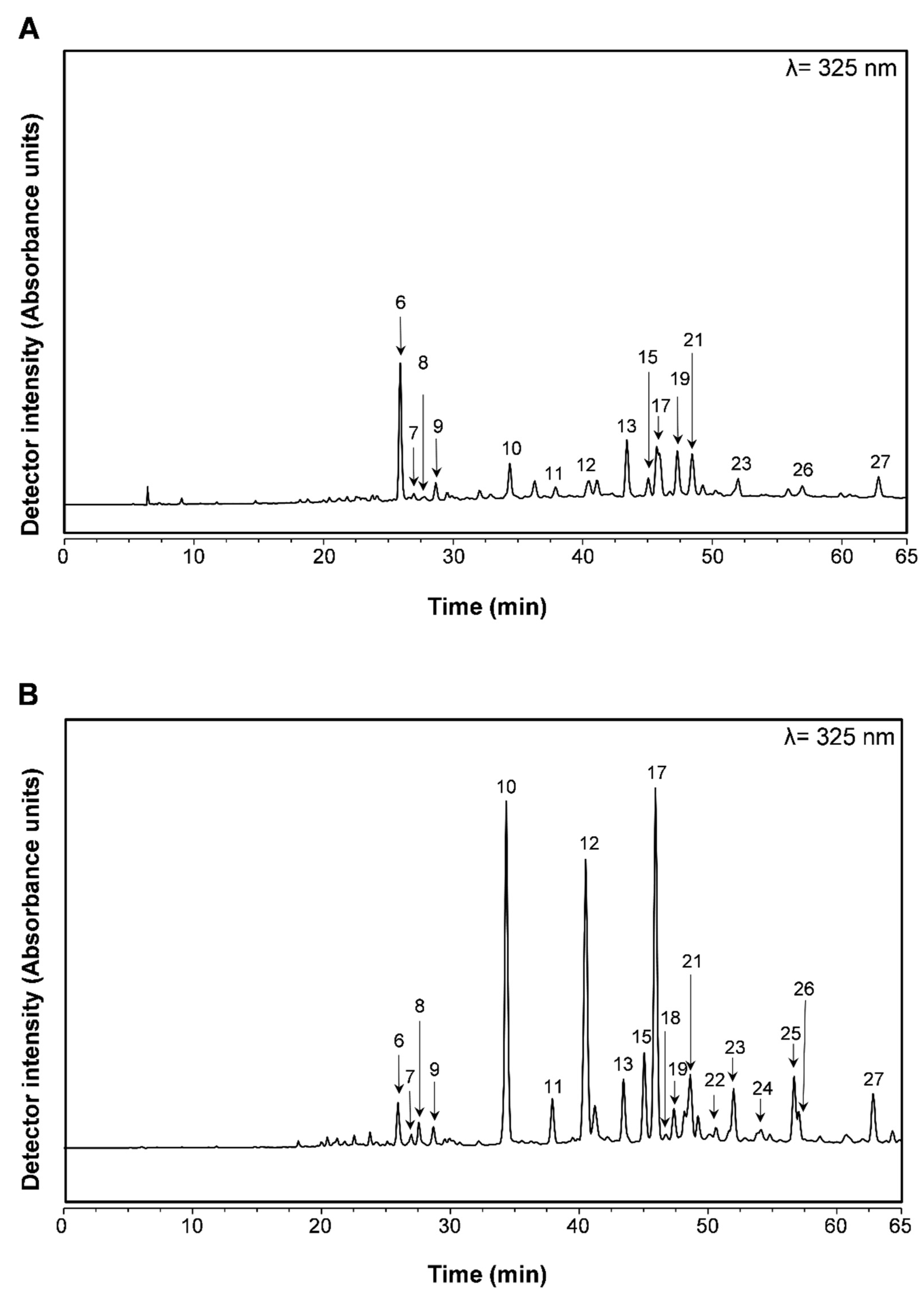
2.2. Profiling and Quantification of Individual Compounds by HPLC-DAD and HPLC-ESI-MSn
2.3. In Vitro Antioxidant Activity Assessment
2.4. T. capitellatus Extracts Inhibit Key Enzymes and Show Therapeutic Potential
2.5. Anti-Proliferative Activity of T. capitellatus Extracts
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material
3.3. Preparation of Extracts
3.4. Total Phenolic Compounds, Ortho-Diphenols and Total Flavonoids Content
3.5. Profiling and Quantification of Individual Phenolic Compounds by HPLC-DAD and HPLC-ESI-MSn
3.6. Quantification of Oleanolic and Ursolic Acids in Hydroethanolic Extracts
3.7. In Vitro Antioxidant Activity Assessment
3.7.1. ABTS Radical Cation (ABTS+•) Scavenging Assay
3.7.2. Hydroxyl Radicals Scavenging Assay
3.7.3. Nitric Oxide Radical Scavenging Assay
3.7.4. Superoxide Radical (O2•−) Scavenging Assay
3.7.5. β-Carotene Bleaching Assay
3.8. In Vitro Enzymatic Inhibition Assays
3.9. Cell Cultures and In Vitro Cell Viability Assay
3.10. Data and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granato, D.; Nunes, D.S.; Barba, F.J. An integrated strategy between food chemistry, biology, nutrition, pharmacology, and statistics in the development of functional foods: A proposal. Trends Food Sci. Technol. 2017, 62, 13–22. [Google Scholar] [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszałek, K.; Eş, I.; Munekata, P.E.S.; Ferreira, I.; Barba, F.J. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: From extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2020-3. 2020. Available online: https://www.iucnredlist.org/ (accessed on 15 June 2022).
- Roxo, M.; Zuzarte, M.; Gonçalves, M.J.; Alves-Silva, J.M.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Antifungal and anti-inflammatory potential of the endangered aromatic plant Thymus albicans. Sci. Rep. 2020, 10, 18859. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Taghouti, M.; Schäfer, J.; Bunzel, M.; Silva, A.M.; Nunes, F.M. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Souto, E.B.; Cosme, F.; Nunes, F.M.; Silva, A.M. Thymus carnosus extracts induce anti-proliferative activity in Caco-2 cells through mechanisms that involve cell cycle arrest and apoptosis. J. Funct. Foods 2019, 54, 128–135. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G.; Trindade, H.; Sanches, J.; Oliveira, C.; Correia, M. Thymus capitellatus Hoffmanns. & Link. Hortofruticult. Floric. Agrotec. 2013, 9, 12–15. [Google Scholar]
- Machado, M.; Dinis, A.M.; Santos-Rosa, M.; Alves, V.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.C. Activity of Thymus capitellatus volatile extract, 1,8-cineole and borneol against Leishmania species. Vet. Parasitol. 2014, 200, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Salgueiro, L.R.; Pinto, E.; Gonçalves, M.J.; Costa, I.; Palmeira, A.; Cavaleiro, C.; Pina-Vaz, C.; Rodrigues, A.G.; Martinez-de-Oliveira, J. Antifungal activity of the essential oil of Thymus capitellatus against Candida, Aspergillus and dermatophyte strains. Flavour Fragr. J. 2006, 21, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Pais, M.S.S.; Scheffer, J.J.C. The essential oils of two endemic Portuguese thyme species: Thymus capitellatus Hoffmanns. & Link and T. lotocephalus G. López & R. Morales. Flavour Fragr. J. 1993, 8, 53–57. [Google Scholar] [CrossRef]
- Adzet, T.; Vila, R.; Canigueral, S. Chromatographic analysis of polyphenols of some Iberian Thymus. J. Ethnopharmacol. 1988, 24, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.M.; Tomás-Barberán, F.A.; Tomás-Lorente, F. A chemotaxonomic study of free flavone aglycones from some Iberian Thymus species. Biochem. Syst. Ecol. 1987, 15, 61–67. [Google Scholar] [CrossRef]
- Husain, S.Z.; Markham, K.R. The glycoflavone vicenin-2 its distribution in related genera within the Labiatae. Phytochemistry 1981, 20, 1171–1173. [Google Scholar] [CrossRef]
- Tavares, L.; Fortalezas, S.; Tyagi, M.; Barata, D.; Serra, A.T.; Martins Duarte, C.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R.; Espírito-Santo, M.D.; et al. Bioactive compounds from endemic plants of Southwest Portugal: Inhibition of acetylcholinesterase and radical scavenging activities. Pharm. Biol. 2012, 50, 239–246. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Chemical Characterization and Bioactivity of Extracts from Thymus mastichina: A Thymus with a Distinct Salvianolic Acid Composition. Antioxidants 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Taghouti, M.; Martins-Gomes, C.; Félix, L.M.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Polyphenol composition and biological activity of Thymus citriodorus and Thymus vulgaris: Comparison with endemic Iberian Thymus species. Food Chem. 2020, 331, 127362. [Google Scholar] [CrossRef]
- Lim, J.; Zhang, X.; Ferruzzi, M.G.; Hamaker, B.R. Starch digested product analysis by HPAEC reveals structural specificity of flavonoids in the inhibition of mammalian α-amylase and α-glucosidases. Food Chem. 2019, 288, 413–421. [Google Scholar] [CrossRef]
- Rover, M.R.; Brown, R.C. Quantification of total phenols in bio-oil using the Folin–Ciocalteu method. J. Anal. Appl. c 2013, 104, 366–371. [Google Scholar] [CrossRef]
- Silva, A.M.; Félix, L.M.; Teixeira, I.; Martins-Gomes, C.; Schäfer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange thyme: Phytochemical profiling, in vitro bioactivities of extracts and potential health benefits. Food Chem. X 2021, 12, 100171. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus x citriodorus using a validated HPLC–UV and ESI–MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Francescato, L.N.; Debenedetti, S.L.; Schwanz, T.G.; Bassani, V.L.; Henriques, A.T. Identification of phenolic compounds in Equisetum giganteum by LC–ESI-MS/MS and a new approach to total flavonoid quantification. Talanta 2013, 105, 192–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Kim, I.-S.; Yang, M.-R.; Lee, O.-H.; Kang, S.-N. Antioxidant Activities of Hot Water Extracts from Various Spices. Int. J. Mol. Sci. 2011, 12, 4120. [Google Scholar] [CrossRef]
- Ozen, T.; Demirtas, I.; Aksit, H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011, 124, 58–64. [Google Scholar] [CrossRef]
- Ertas, A.; Boga, M.; Yilmaz, M.A.; Yesil, Y.; Tel, G.; Temel, H.; Hasimi, N.; Gazioglu, I.; Ozturk, M.; Ugurlu, P. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymus nummularius (Anzer tea): Rosmarinic acid. Ind. Crops Prod. 2015, 67, 336–345. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Neto, R.T.; Silva, A.M.S.; Cardoso, S.M. Health-Promoting Effects of Thymus herba-barona, Thymus pseudolanuginosus, and Thymus caespititius Decoctions. Int. J. Mol. Sci. 2017, 18, 1879. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Lamiaceae often used in Portuguese folk medicine as a source of powerful antioxidants: Vitamins and phenolics. LWT-Food Sci. Technol. 2010, 43, 544–550. [Google Scholar] [CrossRef]
- Jaouadi, R.; Silva, A.M.S.; Boussaid, M.; Yahia, I.B.H.; Cardoso, S.M.; Zaouali, Y. Differentiation of Phenolic Composition Among Tunisian Thymus algeriensis Boiss. et Reut. (Lamiaceae) Populations: Correlation to Bioactive Activities. Antioxidants 2019, 8, 515. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Rahul; Jyoti, S.; Naz, F.; Ashafaq, M.; Shahid, M.; Siddique, Y.H. Therapeutic potential of luteolin in transgenic Drosophila model of Alzheimer’s disease. Neurosci. Lett. 2019, 692, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Loesche, A.; Köwitsch, A.; Lucas, S.D.; Al-Halabi, Z.; Sippl, W.; Al-Harrasi, A.; Csuk, R. Ursolic and oleanolic acid derivatives with cholinesterase inhibiting potential. Bioorg. Chem. 2019, 85, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Şenol, F.S.; Gülpinar, A.R.; Kartal, M.; Şekeroglu, N.; Deveci, M.; Kan, Y.; Şener, B. Acetylcholinesterase inhibitory and antioxidant properties of Cyclotrichium niveum, Thymus praecox subsp. caucasicus var. caucasicus, Echinacea purpurea and E. pallida. Food Chem. Toxicol. 2009, 47, 1304–1310. [Google Scholar] [CrossRef]
- Gülçin, İ.; Scozzafava, A.; Supuran, C.T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingöl, Z.; Huyut, Z.; Alwasel, S.H. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J. Enzym. Inhib. Med. Chem. 2016, 31, 1698–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Taslimi, P.; Gulçin, İ. Antidiabetic potential: In vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylase enzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956. [Google Scholar] [CrossRef]
- Angelis, I.D.; Turco, L. Caco-2 Cells as a Model for Intestinal Absorption. Curr. Protoc. Toxicol. 2011, 47, 20-6. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Dehn, P.F.; White, C.M.; Conners, D.E.; Shipkey, G.; Cumbo, T.A. Characterization of the human hepatocellular carcinoma (hepg2) cell line as an in vitro model for cadmium toxicity studies. Vitr. Cell. Dev. Biol. Anim. 2004, 40, 172–182. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Ramos, A.A.; Pereira-Wilson, C.; Collins, A.R. Protective effects of Ursolic acid and Luteolin against oxidative DNA damage include enhancement of DNA repair in Caco-2 cells. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2010, 692, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [CrossRef]
- Machado, M.; Felizardo, C.; Fernandes-Silva, A.A.; Nunes, F.M.; Barros, A. Polyphenolic compounds, antioxidant activity and l-phenylalanine ammonia-lyase activity during ripening of olive cv. “Cobrançosa” under different irrigation regimes. Food Res. Int. 2013, 51, 412–421. [Google Scholar] [CrossRef]
- Sreejayan; Rao, M.N.A. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Tao, H.; Zhou, J.; Wu, T.; Cheng, Z. High-Throughput Superoxide Anion Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2014, 62, 9266–9272. [Google Scholar] [CrossRef]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.; Fangueiro, J.F.; Fernandes, L.; Doktorovova, S.; Santos, D.L.; Garcia, M.L.; Gremiao, M.P.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sanchez-Lopez, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef]


| Thymus capitellatus | ||||
|---|---|---|---|---|
| AD | HE | E.M.E. | ||
| Extraction yield (%, w/w) | 15.82 ± 2.30 | 16.84 ± 2.43 | n.s. | |
| Chemical composition | ||||
| Total phenols content (mg caffeic acid equivalent/g) | Ext. | 150.29 ± 5.29 | 140.81 ± 5.42 | n.s. |
| D.P. | 23.77 ± 0.84 | 23.72 ± 0.91 | n.s. | |
| Ortho-diphenols content (mg caffeic acid equivalent/g) | Ext. | 134.01 ± 5.08 | 146.02 ± 3.62 | * |
| D.P. | 21.36 ± 0.80 | 24.60 ± 0.61 | * | |
| Total flavonoids content (mg catechin equivalent/g) | Ext. | 174.26 ± 11.58 | 188.84 ± 7.81 | n.s. |
| D.P. | 27.56 ± 1.83 | 31.81 ± 1.31 | * | |
| Compound | R.T. (min) | ESI-MS2 | Quantification | |||||
|---|---|---|---|---|---|---|---|---|
| HE | AD | E.M.E. Sig. | ||||||
| mg/g D.P. | mg/g Extract | mg/g D.P. | mg/g Extract | |||||
| 1 | Unknown | 21.73 ± 0.11 | [459]:161 | n.q. | n.q. | n.q. | n.q. | |
| 2 | Unknown | 23.13 ± 0.08 | [509]:463;441;329;295 | n.q. | n.q. | n.q. | n.q. | |
| 3 | Apigenin-(6,8)-C-diglucoside | 23.19 ± 0.08 | [593]:575;503;473;383;353 | n.q. | n.q. | n.q. | n.q. | |
| 4 | Unknown | 23.48 ± 0.02 | [455]:409;387;317;233;173 | n.q. | n.q. | n.q. | n.q. | |
| 5 | Hydroxyjasmonic acid-(?)-O-hexoside | 23.44 ± 0.11 | [387]:369;225;207;163 | n.q. | n.q. | n.q. | n.q. | |
| 6 | Caffeic acid | 23.76 ± 0.08 | [179]:135 | 0.25 ± 0.02 | 1.49 ± 0.13 | 0.63 ± 0.12 | 3.99 ± 0.73 | * |
| 7 | Eriodictyol-(?)-O-hexoside | 24.20 ± 0.14 | [449]:287 | 0.49 ± 0.06 | 2.9 ± 0.38 | 0.25 ± 0.14 | 1.61 ± 0.9 | * |
| 8 | Unknown | 24.27 ± 0.09 | [495]:427;341;333;315 | n.q. | n.q. | n.q. | n.q. | |
| 9 | Eriodictyol-(?)-O-hexoside | 27.16 ± 0.21 | [449]:287 | 0.84 ± 0.1 | 4.99 ± 0.61 | 0.38 ± 0.12 | 2.43 ± 0.79 | * |
| 10 | Quercetin-(?)-O-hexoside | 27.33 ± 0.22 | [463]:301 | 5.91 ± 0.68 | 35.11 ± 4.02 | 0.67 ± 0.44 | 4.23 ± 2.78 | * |
| 11 | Luteolin-(?)-O-hexoside | 29.28 ± 0.12 | [447]:285 | 0.55 ± 0.06 | 3.26 ± 0.35 | 0.16 ± 0.07 | 1.01 ± 0.42 | * |
| 12 | Luteolin-(?)-O-hexoside | 31.17 ± 0.18 | [447]:285 | 3.94 ± 0.44 | 23.38 ± 2.6 | 0.42 ± 0.13 | 2.63 ± 0.84 | * |
| 13 | Salvianolic acid A isomer | 33.84 ± 0.19 | [493]:383;313;295 | 0.32 ± 0.04 | 1.92 ± 0.24 | 0.25 ± 0.04 | 1.6 ± 0.26 | * |
| 14 | Luteolin-(?)-O-hexuronide | 33.87 ± 0.24 | [461]:285 | n.q. | n.q. | n.q. | n.q. | |
| 15 | Quercetin-(?)-O-(caffeoyl)-hexoside | 33.96 ± 0.25 | [625]:463;323;301 | 1.7 ± 0.08 | 10.07 ± 0.49 | 0.56 ± 0.28 | 3.51 ± 1.76 | * |
| 16 | Chrysoeriol-(?)-O-hexoside | 34.83 ± 0.08 | [461]:299 | n.q. | n.q. | n.q. | n.q. | |
| 17 | Rosmarinic acid | 35.19 ± 0.04 | [359]:223;179;161 | 3.66 ± 0.34 | 21.71 ± 2.03 | 1.01 ± 0.61 | 6.37 ± 3.84 | * |
| 18 | Apigenin-(?)-O-hexoside | 35.79 ± 0.06 | [431]:269 | 0.12 ± 0.02 | 0.7 ± 0.13 | 0.1 ± 0.03 | 0.61 ± 0.19 | n.s. |
| 19 | Acetyl-luteolin-(?)-O-hexoside-pentoside | 36.09 ± 0.40 | [621]:579;561;447;327;285 | 0.44 ± 0.02 | 2.59 ± 0.13 | 0.84 ± 0.17 | 5.33 ± 1.1 | * |
| 20 | Unknown | 36.37 ± 0.05 | [549]:531;489;387;207;161 | n.q. | n.q. | n.q. | n.q. | |
| 21 | Salvianolic acid K | 36.95 ± 0.10 | [555]:537;493;359 | 0.83 ± 0.06 | 4.91 ± 0.37 | 0.64 ± 0.2 | 4.06 ± 1.25 | n.s. |
| 22 | Quercetin -(?)-O-hexoside-deoxy-hexoside | 38.17 ± 0.05 | [609]:463;301 | 0.28 ± 0.03 | 1.64 ± 0.15 | 0.08 ± 0.03 | 0.51 ± 0.21 | * |
| 23 | Quercetin-(?)-O-hexoside-hexuronide | 39.07 ± 0.38 | [639]:301 | 1.03 ± 0.05 | 6.09 ± 0.31 | 0.4 ± 0.14 | 2.51 ± 0.91 | * |
| 24 | Salvianolic acid K isomer | 40.76 ± 0.14 | [555]:493;359 | 0.16 ± 0.01 | 0.93 ± 0.03 | 0.03 ± 0.01 | 0.16 ± 0.05 | * |
| 25 | Luteolin-(?)-O-hexoside-hexoside | 43.06 ± 0.25 | [609]:447;323;285 | 0.85 ± 0.09 | 5.06 ± 0.53 | 0.22 ± 0.03 | 1.36 ± 0.2 | * |
| 26 | Chrysoeriol-(?)-O-hexoside-hexoside | 43.10 ± 0.15 | [623]:461;323;299;285 | 0.33 ± 0.03 | 1.94 ± 0.19 | 0.23 ± 0.05 | 1.48 ± 0.32 | n.s. |
| 27 | Luteolin | 49.05 ± 0.23 | [285]:241;217;199;75;151 | 0.65 ± 0.11 | 3.85 ± 0.66 | 0.42 ± 0.15 | 2.67 ± 0.93 | n.s. |
| 28 | Oleanolic acid | 6.02 ± 1.35 | 35.77 ± 8.04 | n.d. | n.d. | * | ||
| 29 | Ursolic acid | 4.88 ± 1.07 | 28.97 ± 6.38 | n.d. | n.d. | * | ||
| Total phenolic compounds | 23.07 ± 1.48 | 137.67 ± 8.77 | 7.84 ± 2.14 | 49.55 ± 13.51 | * | |||
| Total flavonoids | 17.68 ± 1.17 | 104.97 ± 6.95 | 5.19 ± 1.33 | 32.8 ± 8.42 | * | |||
| Total phenolic acids | 5.21 ± 0.44 | 30.96 ± 2.63 | 2.65 ± 0.89 | 16.75 ± 5.64 | * | |||
| Total terpenoids | 10.90 ± 2.43 | 64.74 ± 14.4 | n.d. | n.d. | * | |||
| Thymus capitellatus Extracts (mg/mL) | ||||
|---|---|---|---|---|
| AD | HE | E.M.E. | ||
| Acetylcholinesterase | IC50 | 0.79 ± 0.05 | 0.36 ± 0.04 | * |
| 0.5 mg/mL | 34.03 ± 3.83 | 54.22 ± 6.84 | * | |
| 1 mg/mL | 55.72 ± 6.39 | 69.28 ± 10.22 | n.s. | |
| Tyrosinase | 0.5 mg/mL | 16.56 ± 4.26 | 17.58 ± 0.33 | n.s. |
| 1 mg/mL | 22.37 ± 1.29 | 28.17 ± 1.73 | * | |
| Elastase | 0.5 mg/mL | - | - | n.s. |
| 1 mg/mL | 7.16 ± 1.50 | - | * | |
| α-Amylase | 0.5 mg/mL | 3.17 ± 0.5 | 4.92 ± 0.19 | * |
| 1 mg/mL | 4.10 ± 0.18 | 8.71 ± 0.59 | * | |
| α-Glucosidase | 0.5 mg/mL | 16.74 ± 1.01 | 13.30 ± 1.4 | * |
| 1 mg/mL | 24.57 ± 0.24 | 23.02 ± 1.12 | n.s | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins-Gomes, C.; Steck, J.; Keller, J.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Molecular Characterization of Thymus capitellatus Extracts and Their Antioxidant, Neuroprotective and Anti-Proliferative Activities. Int. J. Mol. Sci. 2022, 23, 15187. https://doi.org/10.3390/ijms232315187
Martins-Gomes C, Steck J, Keller J, Bunzel M, Nunes FM, Silva AM. Molecular Characterization of Thymus capitellatus Extracts and Their Antioxidant, Neuroprotective and Anti-Proliferative Activities. International Journal of Molecular Sciences. 2022; 23(23):15187. https://doi.org/10.3390/ijms232315187
Chicago/Turabian StyleMartins-Gomes, Carlos, Jan Steck, Judith Keller, Mirko Bunzel, Fernando M. Nunes, and Amélia M. Silva. 2022. "Molecular Characterization of Thymus capitellatus Extracts and Their Antioxidant, Neuroprotective and Anti-Proliferative Activities" International Journal of Molecular Sciences 23, no. 23: 15187. https://doi.org/10.3390/ijms232315187






