Ex Vivo Antioxidant and Cholinesterase Inhibiting Effects of a Novel Galantamine–Curcumin Hybrid on Scopolamine-Induced Neurotoxicity in Mice
Abstract
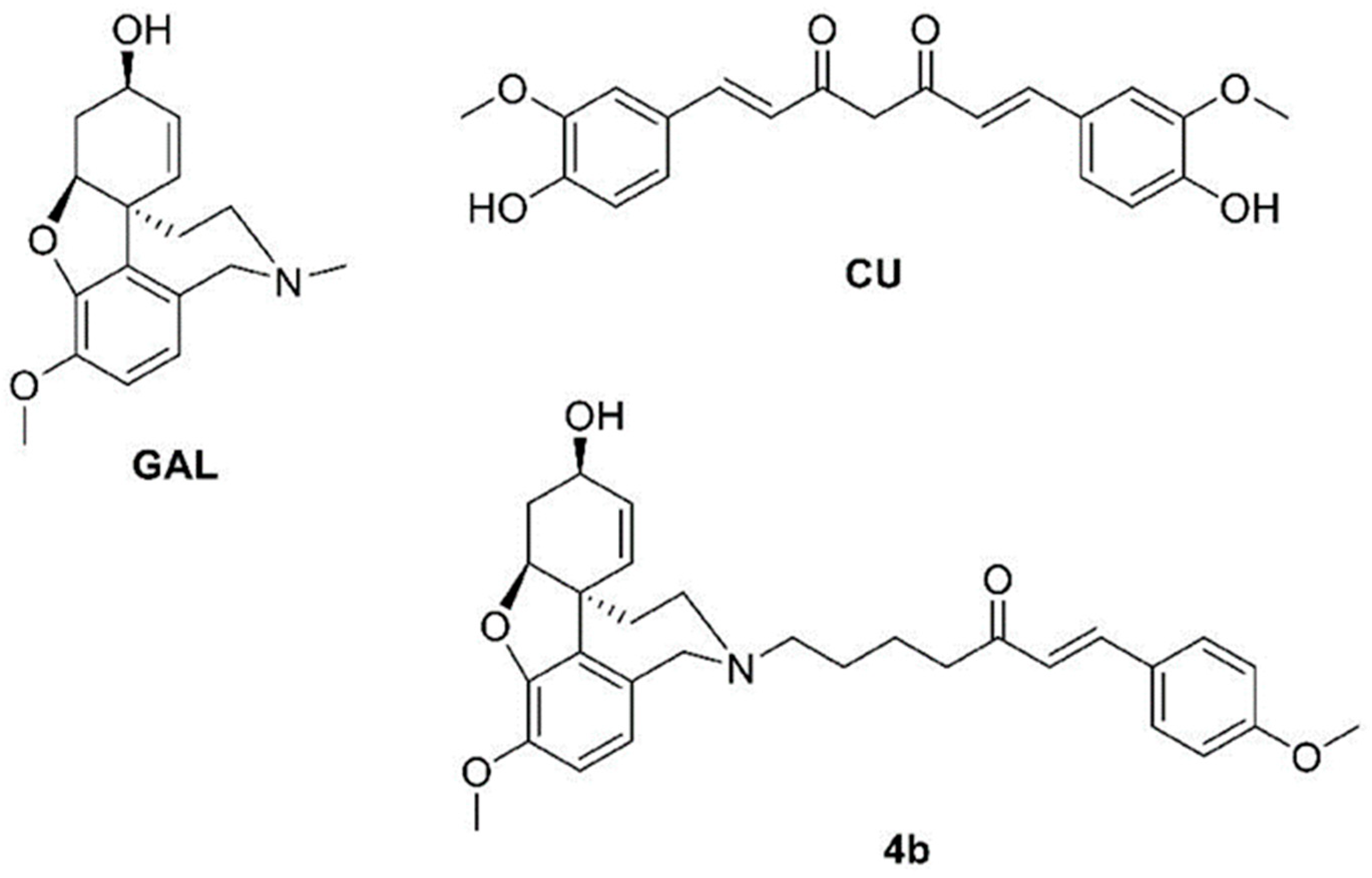
:1. Introduction
2. Results
2.1. Antioxidant Activities on Enzymes and Biochemical Markers of Oxidative Stress
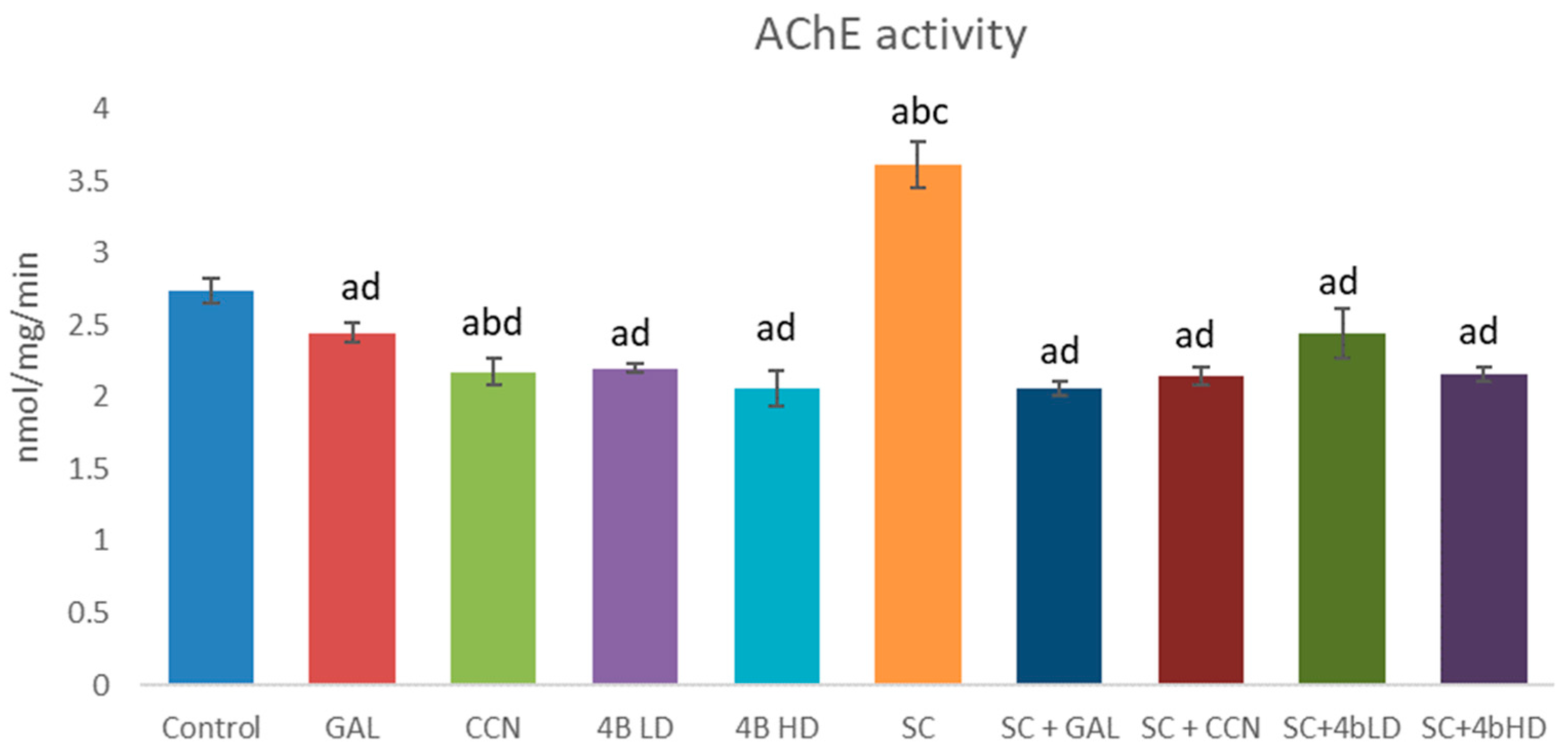
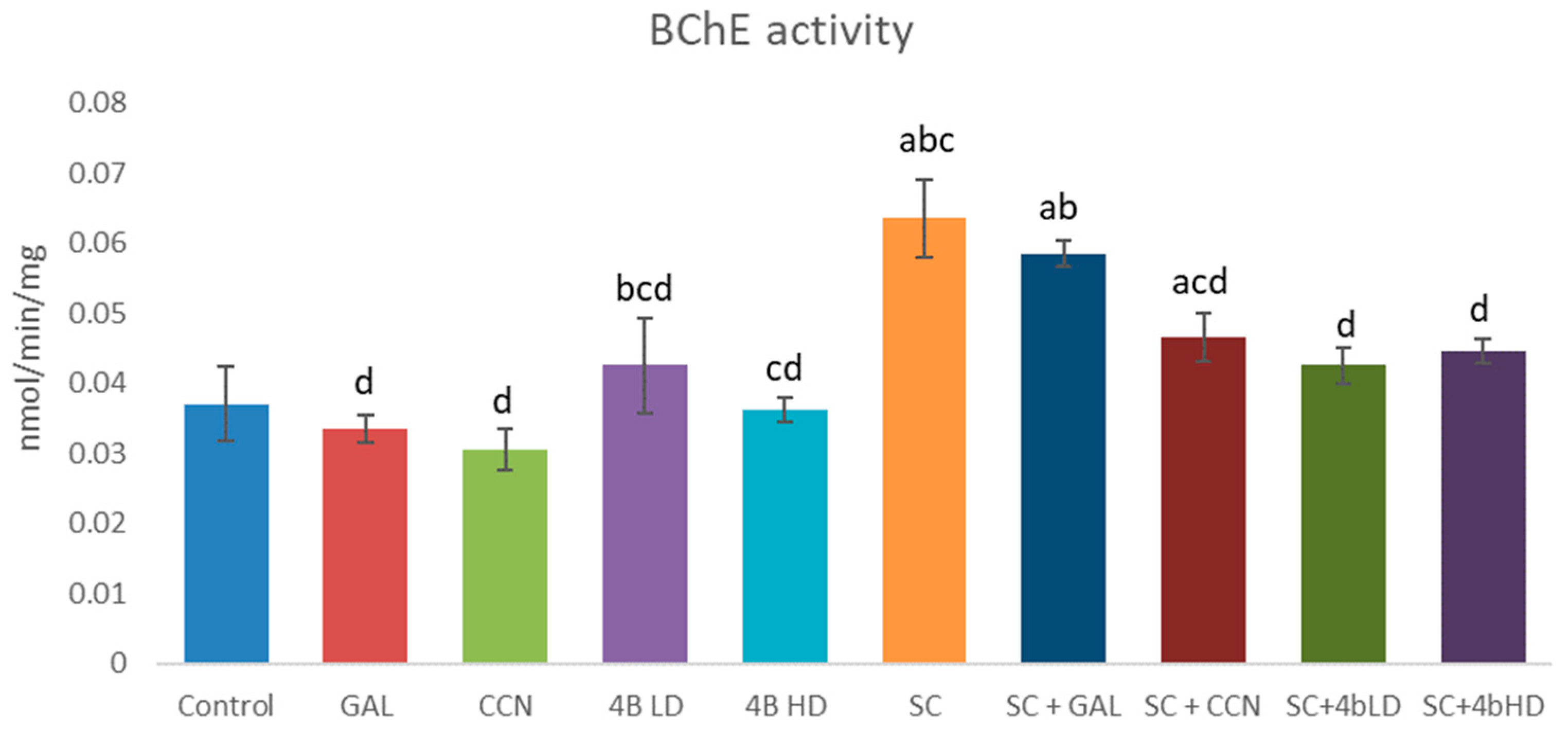
2.2. Assessment of Cholinesterase Inhibition
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Design of the Experiment
- Group 1—control mice, treated with 0.9 % saline po;
- Group 2—animals treated with GAL alone as a positive control (3 mg/kg po);
- Group 3—animals treated with CCN alone as a positive control (25 mg/kg po);
- Group 4—animals treated with 4b alone (2.5 mg/kg po), (low dose, LD);
- Group 5—animals treated with 4b alone (5 mg/kg po) (high dose, HD);
- Group 6—animals treated with SC alone (3 mg/kg ip);
- Group 7—animals treated with GAL (3 mg/kg po) and SC (3 mg/kg ip 30 min after GAL medication);
- Group 8—animals treated with CCN (25 mg/kg po) and SC (3 mg/kg ip 30 min after CCN medication);
- Group 9—animals treated with 4b (2.5 mg/kg po) and SC (3 mg/kg ip 30 min after 4b medication);
- Group 10—animals treated with 4b (5 mg/kg po) and SC (3 mg/kg ip 30 min after 4b medication).
4.4. Methods
4.4.1. Measurement of AChE and BChE Inhibition in Brain Homogenate
4.4.2. Antioxidant Enzyme Activity Measurement
- Catalase Activity (CAT)
- Superoxide Dismutase Activity (SOD).
- Glutathione Peroxidase Activity (GPx).
4.4.3. Measurement of Malondialdehyde (MDA) Levels in Brain Homogenate
4.4.4. Measurement of Reduced Glutathione (GSH) Levels in Brain Homogenate
4.4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, C.M. Alzheimer’s disease: Addressing a twenty-first century plague. Rend. Fis. Acc. Lincei 2015, 26, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Alzheimer’s Disease. Mayo Clinic Health Information. 2022. Available online: https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/ (accessed on 20 October 2020).
- Swomley, A.M.; Butterfield, D.A. Oxidative stress in Alzheimer’s disease and mild cognitive impairment: Evidence from human data provided by redox proteomics. Arch. Toxicol. 2015, 89, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kolar, V.; Rhodes, C.J.; Valko, M. Free radical targeting in human oxidative stress-related diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Ballard, C.G. Advances in the treatment of Alzheimer’s disease: Benefits of dual cholinesterase inhibition. Eur. Neurol. 2002, 47, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Foyet, H.S.; Keugong, W.E.; Ngatanko, A.H.H.; Assongalem, E.A.; Eyong, G.O.O.D. AChE and antioxidant potential of hydromethanolic leaf extract of Ziziphus mucronata (Rhamnaceae) on scopolamine-induced memory and cognitive dysfunctions in mice. Evid.-Based Suppl. Altern. Med. 2019, 2019, 4568401. [Google Scholar]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Erdogan Orhan, I.; Eren, G.; Gunduz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive facilitation and antioxidant effects of an essential oil mixture on scopolamine-induced amnesia in rats: Molecular modeling of in vitro and in vivo approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef] [Green Version]
- Akinyemi, A.J.; Oboh, G.; Oyeleye, S.I.; Ogunsuyi, O. Antiamnestic effect of curcumin in combination with donepezil, an anticholinester drug: Involvement of the cholinergic system. Neurotox Res. 2017, 31, 560–569. [Google Scholar] [CrossRef]
- Rahimzadegan, M.; Soodi, M. Comparison of memory impairment and oxidative stress after single or multiple doses of scopolamine in the rat hippocampus. Basic Clin. Neurosci. 2018, 9, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Stavrakov, G.; Philipova, I.; Lukarski, A.; Atanasova, M.; Zheleva, D.; Zhivkova, Z.D.; Ivanov, S.; Atanasova, T.; Konstantinov, S.; Doytchinova, I. Galantamine-curcumin hybrids as dual-site binding acetylcholinesterase inhibitors. Molecules 2020, 25, 3341. [Google Scholar] [CrossRef]
- Simeonova, R.; Zheleva, D.; Valkova, I.; Stavrakov, G.; Philipova, I.; Atanasova, M.; Doytchinova, I. A novel galantamine-curcumin hybrid as a potential multi-target agent against neurodegenerative disorders. Molecules 2021, 26, 1865. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218–224. [Google Scholar] [CrossRef]
- Ighodaroab, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Yumei, F.; Chen, A. De novo glutathione synthesis is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic. Biol. Med. 2007, 43, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Izuo, N.; Nojiri, H.; Uchiyama, S.; Noda, Y.; Kawakami, S.; Kojima, S.; Sasaki, T.; Shirasawa, T.; Shimizu, T. Brain-specific superoxide dismutase 2 deficiency causes perinatal death with spongiform encephalopathy in mice. Oxid Med. Cell Longev. 2015, 2015, 238914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual role in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traikova, M.; Traikov, T.; Hadzhimitova, V.; Krikoryan, K.; Boyadzhieva, N. Antioxidant properties of galantamine hydrobromide. Z. Naturforsch. C 2003, 58, 361–365. [Google Scholar] [CrossRef]
- Simeonova, R.; Vitcheva, V.; Kostadinova, I.; Valkova, I.; Philipova, I.; Stavrakov, G.; Danchev, N.; Doytchinova, I. Biochemical studies on a novel potent acetylcholinesterase inhibitor with dual-site binding for treatment of Alzheimer’s disease. Comptes Rendus Acad. Bulg. Sci. 2021, 74, 219–225. [Google Scholar]
- Triana-Vidal, L.E.; Carvajal-Varona, S.M. Protective effect of galantamine against oxidative damage using human lymphocytes: A novel in vitro model. Arch. Med. Res. 2013, 44, 85–92. [Google Scholar] [CrossRef]
- Tsvetkova, D.; Obreshkova, D.; Zheleva-Dimitrova, D.; Saso, L. Antioxidant activity of galantamine and some of its derivatives. Curr. Med. Chem. 2013, 20, 4595–4608. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. The role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant therapy in oxidative stress-induced neurodegenerative diseases: Role of nanoparticle-based drug delivery systems in clinical translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Doghri, R.; Ellefi, A.; Degrach, I.; Srairi-Abid, N.; Gati, A. Curcumin attenuated neurotoxicity in sporadic animal model of Alzheimer’s disease. Molecules 2021, 26, 3011. [Google Scholar]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant potential of curcumin—A meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Adedayo, B.C.; Jesubowale, O.S.; Adebayo, A.A.; Oboh, G. Effect of Andrographis paniculata leaves extract on neurobehavioral and biochemical indices in scopolamine-induced amnesic rats. J. Food Biochem. 2021, 45, e13280. [Google Scholar] [CrossRef]
- Ownby, R.L.; Crocco, E.; Acevedo, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 2006, 63, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Janowsky, D.S.; el-Yousef, M.K.; Davis, J.M. Acetylcholine and depression. Psychosom. Med. 1974, 36, 248–257. [Google Scholar] [CrossRef]
- Mitić, M.; Lazarević-Pašti, T. Does the application of acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease lead to depression? Expert Opin. Drug Metab. Toxicol. 2021, 17, 841–856. [Google Scholar] [CrossRef]
- Auro-Galantamine. Product Monograph; AuroPharma Inc.: Woodbridge, ON, Canada, 2017. [Google Scholar]
- ChemlDpus Datadase, US National Library of Medicine. Available online: https://chem.nlm.nih.gov/chemidplus/ (accessed on 20 October 2020).
- Fusar-Poli, L.; Vozza, L.; Gabbiadini, A.; Vanella, A.; Concas, I.; Tinacci, S.; Petralia, A.; Signorelli, M.S.; Aguglia, E. Curcumin for depression: A meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 2643–2653. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. OJEU 2010, L 276, 33–79.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andre, J.V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1974; pp. 673–684. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Tappel, A.L. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978, 52, 506–513. [Google Scholar] [PubMed]
- Deby, C.; Goutier, R. New perspectives on the biochemistry of superoxide anion and the efficiency of superoxide dismutases. Biochem. Pharmacol. 1990, 39, 399–405. [Google Scholar] [CrossRef]
- Bump, E.A.; Taylor, Y.C.; Brown, J.M. Role of glutathione in the hypoxic cell cytotoxicity of misonidazole. Cancer Res. 1983, 43, 997–1002. [Google Scholar]
| Parameters | Control | GAL | CCN | 4b LD | 4b HD | SC | SC + GAL | SC + CCN | SC + 4b LD | SC + 4b HD |
|---|---|---|---|---|---|---|---|---|---|---|
| CAT nmol/mg/min | 8.50 ± 0.41 | 7.70 ± 0.42 d | 9.35 ± 0.68 d | 7.80 ± 0.32 cd | 7.10 ± 0.70 cd | 5.35 ± 0.52 abc | 7.20 ± 0.43 d | 8.55 ± 0.34 d | 8.15 ± 0.28 d | 8.40 ± 0.36 d |
| SOD nmol/mg/min | 0.35 ± 0.05 | 0.31 ± 0.04 d | 0.34 ± 0.01 d | 0.44 ± 0.03 bcd | 0.41 ± 0.03 bcd | 0.22 ± 0.03 abc | 0.31 ± 0.02 d | 0.34 ± 0.01 d | 0.33 ± 0.01 d | 0.34 ± 0.02 d |
| GPx nmol/mg/min | 0.43 ± 0.01 | 0.39 ± 0.03 d | 0.40 ± 0.04 d | 0.44 ± 0.03 d | 0.46 ± 0.02 d | 0.21 ± 0.02 abc | 0.34 ± 0.03 ad | 0.39 ± 0.05 d | 0.41 ± 0.03 d | 0.44 ± 0.02 d |
| GSH nmol/g tissue | 1.59 ± 0.03 | 1.73 ± 0.07 d | 2.04 ± 0.12 abd | 2.06 ± 0.07 abd | 2.22 ± 0.08 abd | 1.09 ± 0.13 abc | 1.66 ± 0.12 | 1.83 ± 0.03 cd | 1.44 ± 0.08 d | 1.59 ± 0.04 d |
| MDA nmol/g tissue | 1.41 ± 0.27 | 1.75 ± 0.13 d | 1.45 ± 0.09 d | 1.62 ± 0.30 d | 1.76 ± 0.12 d | 2.39 ± 0.1 abc | 1.79 ± 0.08 d | 1.64 ± 0.05 d | 1.76 ± 0.03 d | 1.65 ± 0.16 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonova, R.; Atanasova, M.; Stavrakov, G.; Philipova, I.; Doytchinova, I. Ex Vivo Antioxidant and Cholinesterase Inhibiting Effects of a Novel Galantamine–Curcumin Hybrid on Scopolamine-Induced Neurotoxicity in Mice. Int. J. Mol. Sci. 2022, 23, 14843. https://doi.org/10.3390/ijms232314843
Simeonova R, Atanasova M, Stavrakov G, Philipova I, Doytchinova I. Ex Vivo Antioxidant and Cholinesterase Inhibiting Effects of a Novel Galantamine–Curcumin Hybrid on Scopolamine-Induced Neurotoxicity in Mice. International Journal of Molecular Sciences. 2022; 23(23):14843. https://doi.org/10.3390/ijms232314843
Chicago/Turabian StyleSimeonova, Rumyana, Mariyana Atanasova, Georgi Stavrakov, Irena Philipova, and Irini Doytchinova. 2022. "Ex Vivo Antioxidant and Cholinesterase Inhibiting Effects of a Novel Galantamine–Curcumin Hybrid on Scopolamine-Induced Neurotoxicity in Mice" International Journal of Molecular Sciences 23, no. 23: 14843. https://doi.org/10.3390/ijms232314843
APA StyleSimeonova, R., Atanasova, M., Stavrakov, G., Philipova, I., & Doytchinova, I. (2022). Ex Vivo Antioxidant and Cholinesterase Inhibiting Effects of a Novel Galantamine–Curcumin Hybrid on Scopolamine-Induced Neurotoxicity in Mice. International Journal of Molecular Sciences, 23(23), 14843. https://doi.org/10.3390/ijms232314843








