TRPV4 Promotes Metastasis in Melanoma by Regulating Cell Motility through Cytoskeletal Rearrangement
Abstract
:1. Introduction
2. Results
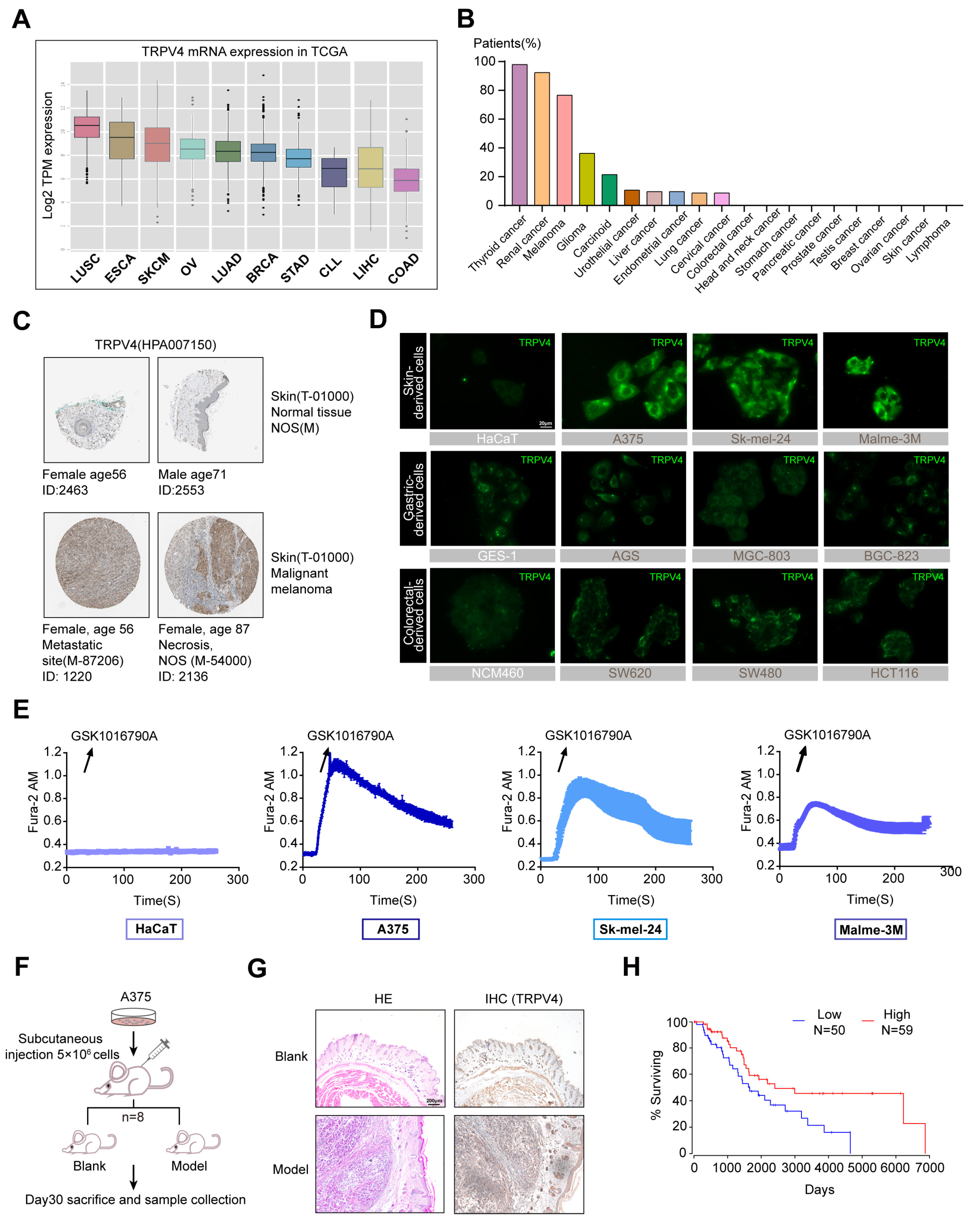
2.1. High TRPV4 Expression in Melanoma
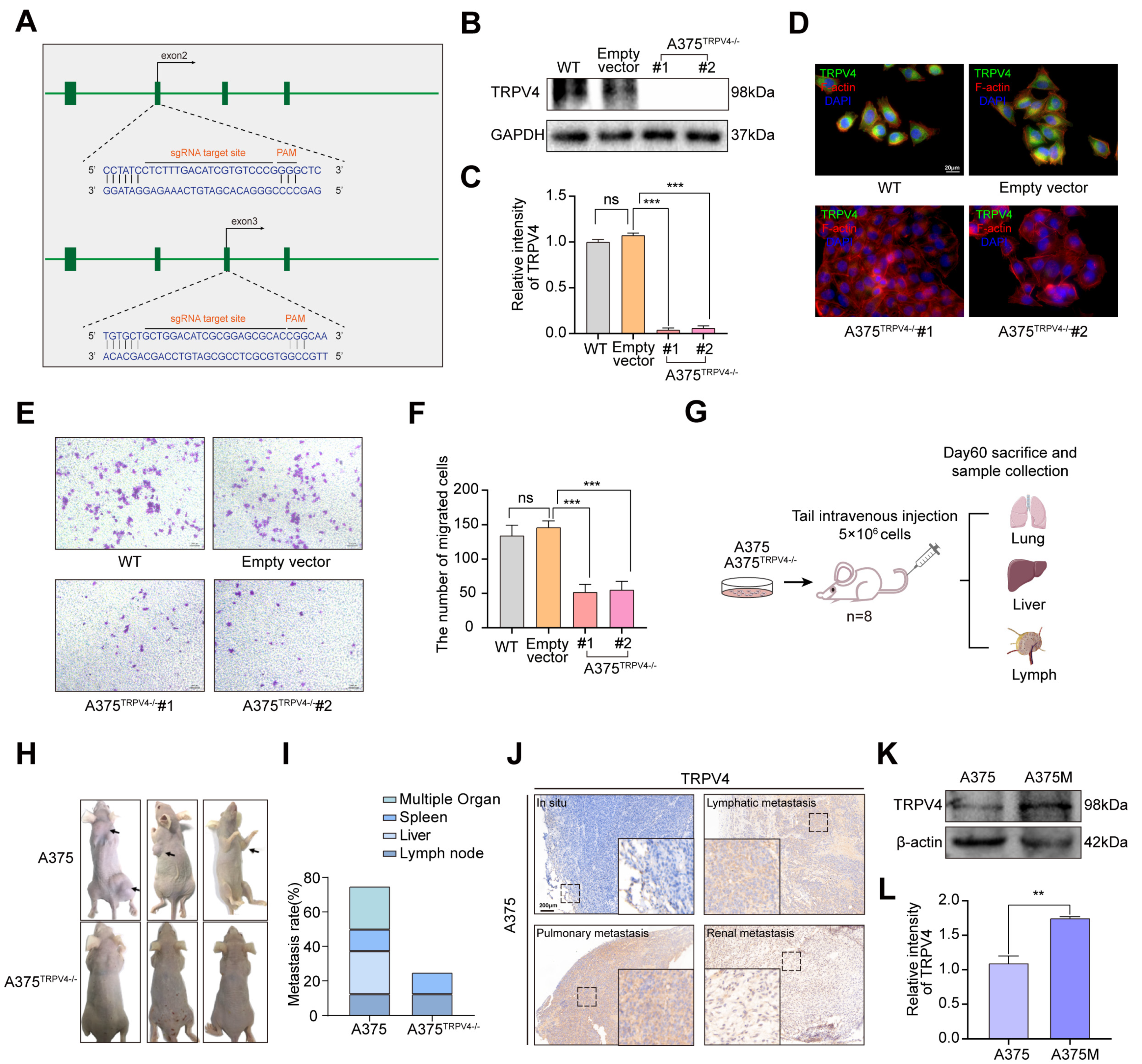
2.2. Functional TRPV4 Is Required for A375 Metastasis
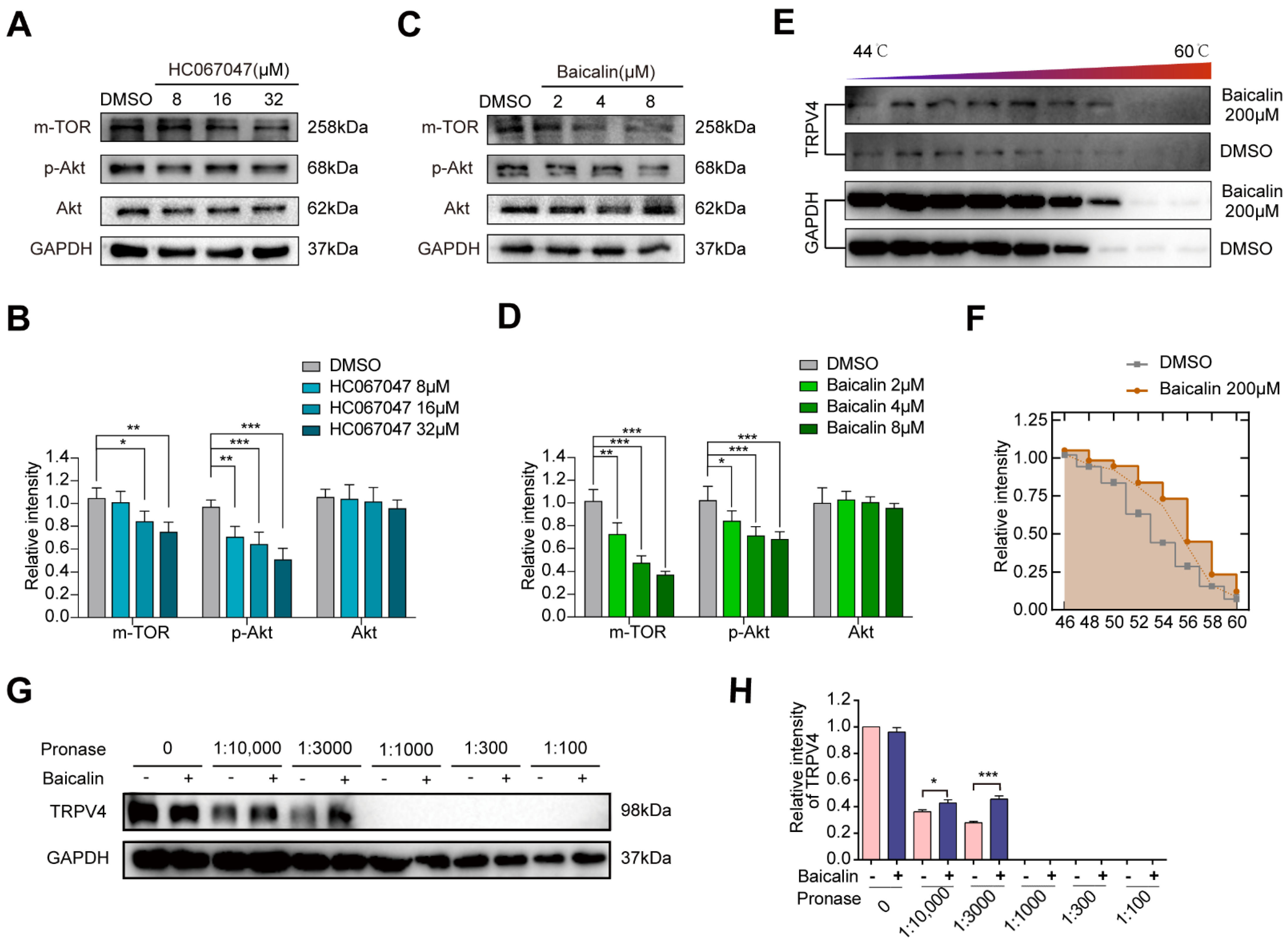
2.3. A Potent TRPV4 Inhibitor from the Natural Product Library: Baicalin
2.4. In Vitro and In Vivo Effects of Baicalin on Melanoma Metastasis
2.5. TRPV4 Promotes Melanoma Metastasis by Regulating Cell Motility
2.6. TRPV4 Regulates Melanoma Metastasis by Inducing Morphological Changes in Cells through the Src-Cofilin Axis
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Animals
4.3. Experimental Metastasis Model and Treatment Studies
4.4. Ca2+ Imaging
4.5. Cellular Thermal Shift Assay (CETSA)
4.6. Drug Affinity Responsive Target Stability Assay (DARTS)
4.7. Immunofluorescence
4.8. Wound-Healing Assay
4.9. Trans-Well Assay
4.10. Immunohistochemical Staining and Histopathological Analysis
4.11. Inverted Invasion Assay
4.12. SDS-PAGE and Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Sethi, N.; Kang, Y. Unravelling the complexity of metastasis—Molecular understanding and targeted therapies. Nat. Rev. Cancer 2011, 11, 735–748. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Esposito, M.; Ganesan, S.; Kang, Y. Emerging strategies for treating metastasis. Nat. Cancer 2021, 2, 258–270. [Google Scholar] [CrossRef]
- Turner, N.; Ware, O.; Bosenberg, M. Genetics of metastasis: Melanoma and other cancers. Clin. Exp. Metastasis 2018, 35, 379–391. [Google Scholar] [CrossRef]
- Hoffmann, E.K. Ion channels involved in cell volume regulation: Effects on migration, proliferation, and programmed cell death in non adherent EAT cells and adherent ELA cells. Cell. Physiol. Biochem. 2011, 28, 1061–1078. [Google Scholar] [CrossRef]
- Wang, R.; Gurguis, C.I.; Gu, W.; Ko, E.A.; Lim, I.; Bang, H.; Zhou, T.; Ko, J.H. Ion channel gene expression predicts survival in glioma patients. Sci. Rep. 2015, 5, 11593. [Google Scholar] [CrossRef] [Green Version]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion channels and the hallmarks of cancer. Trends Mol. Med. 2010, 16, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.G.; Sage, S.O. TRP-Na(+)/Ca(2+) Exchanger Coupling. Adv. Exp. Med. Biol. 2016, 898, 67–85. [Google Scholar]
- Banner, K.H.; Igney, F.; Poll, C. TRP channels: Emerging targets for respiratory disease. Pharmacol. Ther. 2011, 130, 371–384. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perna, A.; Sellitto, C.; Komici, K.; Hay, E.; Rocca, A.; Blasiis, P.D.; Lucariello, A.; Moccia, F.; Guerra, G. Transient Receptor Potential (TRP) Channels in Tumor Vascularization. Int. J. Mol. Sci. 2022, 23, 14253. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Szallasi, A. Transient receptor potential channels as drug targets: From the science of basic research to the art of medicine. Pharmacol. Rev. 2014, 66, 676–814. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Duncton, M.A. TRPV4 agonists and antagonists. Curr. Top. Med Chem. 2011, 11, 2216–2226. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Huang, S.; Ding, Y.; Wang, W.; Wang, A.; Lu, Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.H.; Choong, L.Y.; Mon, N.N.; Lu, S.; Lin, Q.; Pang, B.; Yan, B.; Krishna, V.S.; Singh, H.; Tan, T.Z.; et al. TRPV4 Regulates Breast Cancer Cell Extravasation, Stiffness and Actin Cortex. Sci. Rep. 2016, 6, 27903. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 promote metastasis by regulating cytoskeleton through the RhoA/ROCK1 pathway in endometrial cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Wang, X.; Mao, J.; Li, W.; Sun, Y.; Yuan, Y.; Ben, Q.; Hua, L.; Qian, A. TRPV4 Overexpression Promotes Metastasis Through Epithelial-Mesenchymal Transition in Gastric Cancer and Correlates with Poor Prognosis. Onco Targets Ther. 2020, 13, 8383–8394. [Google Scholar] [CrossRef]
- Xie, R.; Xu, J.; Xiao, Y.; Wu, J.; Wan, H.; Tang, B.; Liu, J.; Fan, Y.; Wang, S.; Wu, Y.; et al. Calcium Promotes Human Gastric Cancer via a Novel Coupling of Calcium-Sensing Receptor and TRPV4 Channel. Cancer Res. 2017, 77, 6499–6512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, J.; Zhang, H.; Liu, X.Y. Identification of TRPV4 as a novel target in invasiveness of colorectal cancer. BMC Cancer 2021, 21, 1264. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [Green Version]
- Gardel, M.L.; Schneider, I.C.; Aratyn-Schaus, Y.; Waterman, C.M. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 2010, 26, 315–333. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Cordero, J.J.; Magalhaes, M.A.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, B.W.; Bamburg, J.R. ADF/cofilin: A functional node in cell biology. Trends Cell Biol. 2010, 20, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Huang, T.Y.; Bokoch, G.M. Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol. Biol. Cell 2009, 20, 2650–2660. [Google Scholar] [CrossRef] [Green Version]
- Oser, M.; Yamaguchi, H.; Mader, C.C.; Bravo-Cordero, J.J.; Arias, M.; Chen, X.; Desmarais, V.; van Rheenen, J.; Koleske, A.J.; Condeelis, J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J. Cell Biol. 2009, 186, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Grace, M.S.; Dubuis, E.; Birrell, M.A.; Belvisi, M.G. Pre-clinical studies in cough research: Role of Transient Receptor Potential (TRP) channels. Pulm. Pharmacol. Ther. 2013, 26, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Wetsel, W.C. Sensing hot and cold with TRP channels. Int. J. Hyperth. 2011, 27, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fang, J.; Wang, H.; Fei, M.; Tang, T.; Liu, K.; Niu, W.; Zhou, Y. Baicalin suppresses proliferation, migration, and invasion in human glioblastoma cells via Ca2+-dependent pathway. Drug Des. Devel. Ther. 2018, 2, 3247–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Meena, A.; Luqman, S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Ding, Y.; Huang, S.; Wang, W.; Wu, Y.; Wang, F.; Wang, A.; Han, Y.; Sun, Z.; et al. Exploring the ‘cold/hot’ properties of traditional Chinese medicine by cell temperature measurement. Pharm. Biol. 2020, 58, 208–218. [Google Scholar] [CrossRef] [Green Version]
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef] [Green Version]
- Thorneloe, K.S.; Sulpizio, A.C.; Lin, Z.; Figueroa, D.J.; Clouse, A.K.; McCafferty, G.P.; Chendrimada, T.P.; Lashinger, E.S.; Gordon, E.; Evans, L.; et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J. Pharmacol. Exp. Ther. 2008, 326, 432–442. [Google Scholar]
- Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016, 2, e67. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo Velasco-Herrera, M.; van der Weyden, L.; Nsengimana, J.; Speak, A.O.; Sjöberg, M.K.; Bishop, D.T.; Jönsson, G.; Newton-Bishop, J.; Adams, D.J. Comparative genomics reveals that loss of lunatic fringe (LFNG) promotes melanoma metastasis. Mol. Oncol. 2018, 12, 239–255. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2016; Volume 10, pp. 1–3. [Google Scholar]
- Pai, M.Y.; Lomenick, B.; Hwang, H.; Schiestl, R.; McBride, W.; Loo, J.A.; Huang, J. Drug Affinity Responsive Target Stability (DARTS) for Small Molecule Target Identification. Methods Mol. Biol. 2015, 1263, 287–298. [Google Scholar]
- Martinez Molina, D.; Jafari, R.; Ignatushchenko, M.; Seki, T.; Larsson, E.A.; Dan, C.; Sreekumar, L.; Cao, Y.; Nordlund, P. Monitoring Drug Target Engagement in Cells and Tissues Using the Cellular Thermal Shift Assay. Science 2013, 341, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, A.J.; Kon, T.; Knight, P.J.; Sutoh, K.; Burgess, S.A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Georgouli, M.; Herraiz, C.; Crosas-Molist, E.; Fanshawe, B.; Maiques, O.; Perdrix, A.; Pandya, P.; Rodriguez-Hernandez, I.; Ilieva, K.M.; Cantelli, G.; et al. Regional Activation of Myosin II in Cancer Cells Drives Tumor Progression via a Secretory Cross-Talk with the Immune Microenvironment. Cell 2019, 176, 757–774. [Google Scholar] [CrossRef]
- Giannoni, E.; Chiarugi, P. Redox circuitries driving Src regulation. Antioxid. Redox Signal. 2014, 20, 2011–2025. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Celià-Terrassa, T.; Kang, Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016, 30, 892–908. [Google Scholar] [CrossRef] [Green Version]
- Lehuédé, C.; Dupuy, F.; Rabinovitch, R.; Jones, R.G.; Siegel, P.M. Metabolic Plasticity as a Determinant of Tumor Growth and Metastasis. Cancer Res. 2016, 76, 5201–5208. [Google Scholar] [CrossRef] [Green Version]
- Pandya, P.; Orgaz, J.L.; Sanz-Moreno, V. Modes of invasion during tumour dissemination. Mol. Oncol. 2017, 11, 5–27. [Google Scholar] [CrossRef] [Green Version]
- Arnold, T.R.; Stephenson, R.E.; Miller, A.L. Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function. Exp. Cell Res. 2017, 358, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Sinha, S.; Esposito, I.; Schiavon, G.; López-Lago, M.A.; Su, W.; Pratilas, C.A.; Abele, C.; Hernandez, J.M.; Ohara, M.; et al. The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT by restraining Ras-MAPK signalling. Nat. Cell Biol. 2015, 17, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorentzen, A.; Becker, P.F.; Kosla, J.; Saini, M.; Weidele, K.; Ronchi, P.; Klein, C.; Wolf, M.J.; Geist, F.; Seubert, B.; et al. Single cell polarity in liquid phase facilitates tumour metastasis. Nat. Commun. 2018, 9, 887. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Yu, S.; Deng, R.; Liu, H.; Ding, Y.; Sun, Y.; Chen, W.; Wang, A.; Wei, Z.; Lu, Y. TRPV4 Promotes Metastasis in Melanoma by Regulating Cell Motility through Cytoskeletal Rearrangement. Int. J. Mol. Sci. 2022, 23, 15155. https://doi.org/10.3390/ijms232315155
Huang S, Yu S, Deng R, Liu H, Ding Y, Sun Y, Chen W, Wang A, Wei Z, Lu Y. TRPV4 Promotes Metastasis in Melanoma by Regulating Cell Motility through Cytoskeletal Rearrangement. International Journal of Molecular Sciences. 2022; 23(23):15155. https://doi.org/10.3390/ijms232315155
Chicago/Turabian StyleHuang, Shuai, Suyun Yu, Rui Deng, Huan Liu, Yushi Ding, Yifan Sun, Wenxing Chen, Aiyun Wang, Zhonghong Wei, and Yin Lu. 2022. "TRPV4 Promotes Metastasis in Melanoma by Regulating Cell Motility through Cytoskeletal Rearrangement" International Journal of Molecular Sciences 23, no. 23: 15155. https://doi.org/10.3390/ijms232315155





