Nanocomposites of Nitrogen-Doped Graphene Oxide and Manganese Oxide for Photodynamic Therapy and Magnetic Resonance Imaging
Abstract
:1. Introduction
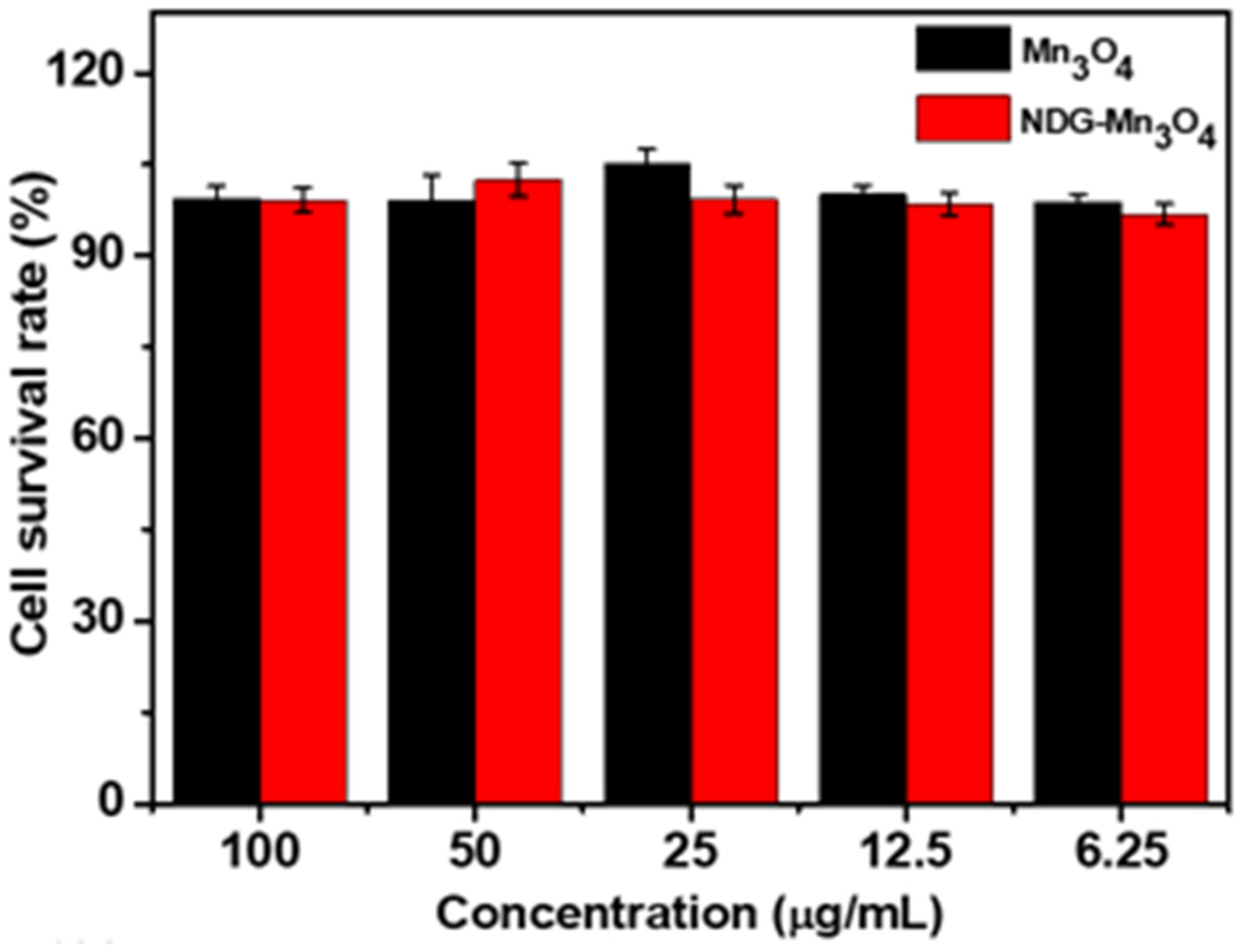
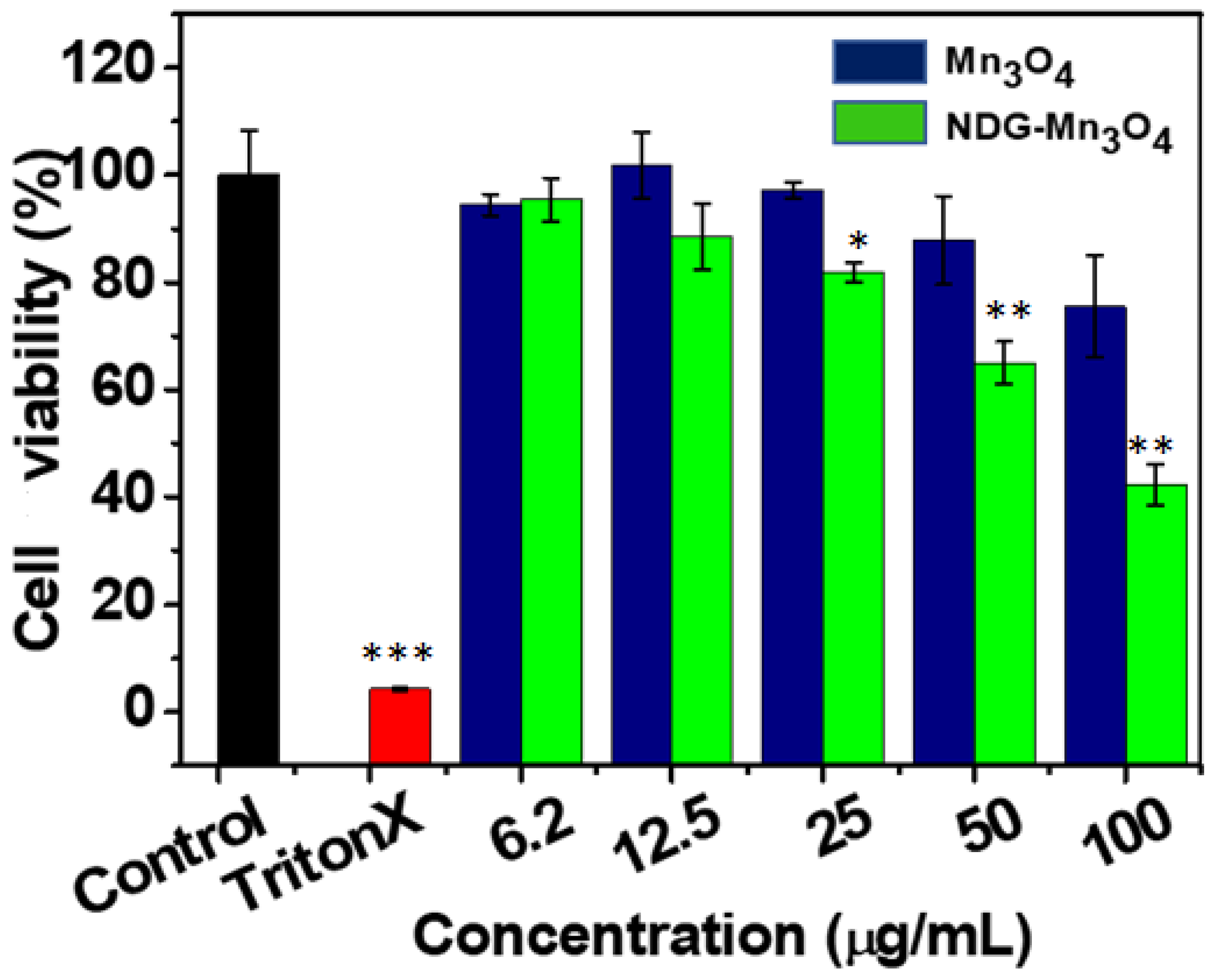
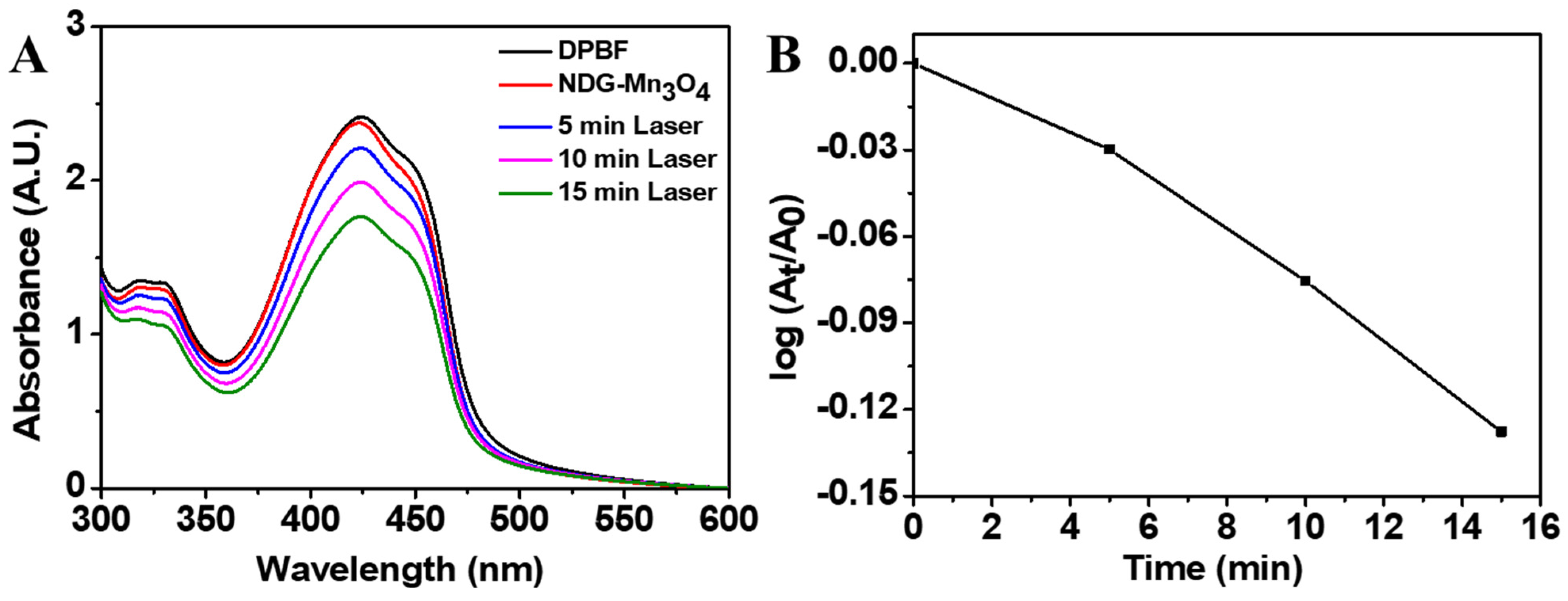
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Mn3O4 Nanoparticles
4.3. Preparation of Nitrogen-Doped Graphene Oxide (NDG)
4.4. Preparation of Nanocomposites of NDG and Mn3O4 (NDG-Mn3O4)
4.5. Characterization of Nanoparticles
4.6. Cell Viability Analysis
4.7. In-Vitro Photodynamic Therapy
4.8. Analysis of Singlet Oxygen Generation
4.9. MRI Relaxivity Analysis
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avci, P.; Erdem, S.S.; Hamblin, M.R. Photodynamic therapy: One step ahead with self-assembled nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 1937–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Kormakov, S.; Liu, Y.; Huang, Y.; Wu, D.; Yang, Z. Recent progress in metal-based nanoparticles mediated photodynamic therapy. Molecules 2018, 23, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.A.; Akhtar, S.; Rauf, M.A.; Alomary, M.N.; AlYahya, S.; Alghamdi, S.; Almessiere, M.; Baykal, A.; Khan, F.; Adil, S.F. Sol–gel synthesis of dy-substituted Ni0.4Cu0.2Zn0.4 (Fe2-xDyx)O4 nano spinel ferrites and evaluation of their antibacterial, antifungal, antibiofilm and anticancer potentialities for biomedical application. Int. J. Nanomed. 2021, 16, 5633. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Aker, W.G.; Huang, M.-J.; D. Watts, J.; Hwang, H.-M. Metal oxide nanomaterials in nanomedicine: Applications in photodynamic therapy and potential toxicity. Curr. Top. Med. Chem. 2015, 15, 1887–1900. [Google Scholar] [CrossRef]
- Wang, D.; Fei, B.; Halig, L.V.; Qin, X.; Hu, Z.; Xu, H.; Wang, Y.A.; Chen, Z.; Kim, S.; Shin, D.M. Targeted iron-oxide nanoparticle for photodynamic therapy and imaging of head and neck cancer. ACS Nano 2014, 8, 6620–6632. [Google Scholar] [CrossRef]
- Li, H.; Cai, X.; Yi, T.; Zeng, Y.; Ma, J.; Li, L.; Pang, L.; Li, N.; Hu, H.; Zhan, Y. Tumor microenvironment responsive Mn3O4 nanoplatform for in vivo real-time monitoring of drug resistance and photothermal/chemodynamic synergistic therapy of gastric cancer. J. Nanobiotechnol. 2022, 20, 1–22. [Google Scholar] [CrossRef]
- Wang, A.; Guo, M.; Wang, N.; Zhao, J.; Qi, W.; Muhammad, F.; Chen, L.; Guo, Y.; Nguyen, N.-T.; Zhu, G. Redox-mediated dissolution of paramagnetic nanolids to achieve a smart theranostic system. Nanoscale 2014, 6, 5270–5278. [Google Scholar] [CrossRef] [Green Version]
- Na, H.B.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.H.; Kim, S.T.; Kim, S.H.; Kim, S.W.; et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. 2007, 46, 5397–5401. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, A.; Das, M.; Darbar, S.; Alharbi, A.; Ahmed, S.A.; Bhattacharya, S.S.; Pal, D.; Pal, S.K. Novel one pot synthesis and spectroscopic characterization of a folate-Mn3O4 nanohybrid for potential photodynamic therapeutic application. RSC Adv. 2019, 9, 30216–30225. [Google Scholar] [CrossRef]
- Xing, C.; Liu, L.; Tang, H.; Feng, X.; Yang, Q.; Wang, S.; Bazan, G.C. Design guidelines for conjugated polymers with light-activated anticancer activity. Adv. Funct. Mater. 2011, 21, 4058–4067. [Google Scholar] [CrossRef]
- Molaei, M.J. Two-dimensional (2D) materials beyond graphene in cancer drug delivery, photothermal and photodynamic therapy, recent advances and challenges ahead: A review. J. Drug Deliv. Sci. Technol. 2021, 61, 101830. [Google Scholar] [CrossRef]
- Khan, M.; Tahir, M.N.; Adil, S.F.; Khan, H.U.; Siddiqui, M.R.H.; Al-warthan, A.A.; Tremel, W. Graphene based metal and metal oxide nanocomposites: Synthesis, properties and their applications. J. Mater. Chem. A 2015, 3, 18753–18808. [Google Scholar] [CrossRef] [Green Version]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Cheng, J.; Li, Y.; Xi, P.; Chen, F.; Liu, H.; Hou, F.; Shi, Y.; Huang, L.; Xu, Z. Fluorescent graphene oxide composites synthesis and its biocompatibility study. J. Mater. Chem. 2012, 22, 9308–9314. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety considerations for graphene: Lessons learnt from carbon nanotubes. Acc. Chem. Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Zhong, H.; Zhang, S.; Chen, S. N-doped graphene/carbon composite as non-precious metal electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2012, 81, 313–320. [Google Scholar] [CrossRef]
- Hasan, M.T.; Gonzalez-Rodriguez, R.; Ryan, C.; Pota, K.; Green, K.; Coffer, J.L.; Naumov, A.V. Nitrogen-doped graphene quantum dots: Optical properties modification and photovoltaic applications. Nano Res. 2019, 12, 1041–1047. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, R.; Wang, C.; Heng, C.-L. Cell biocompatibility of functionalized graphene oxide. Acta Phys. Chim. Sin. 2012, 28, 1520–1524. [Google Scholar]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Nafiujjaman, M.; Khan, H.A.; Lee, Y.-K. Peptide-influenced graphene quantum dots on iron oxide nanoparticles for dual imaging of lung cancer cells. J. Nanosci. Nanotechnol. 2017, 17, 1704–1711. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y.-K. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Cai, X.; Zhu, Q.; Zeng, Y.; Zeng, Q.; Chen, X.; Zhan, Y. Manganese oxide nanoparticles as MRI contrast agents in tumor multimodal imaging and therapy. Int. J. Nanomed. 2019, 14, 8321. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Li, H.; Xiong, Z.; Shen, M.; Conti, P.S.; Shi, X.; Chen, K. Polyethyleneimine-coated manganese oxide nanoparticles for targeted tumor PET/MR imaging. ACS Appl. Mater. Interfaces 2018, 10, 34954–34964. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, C.; Lin, X.; Jiang, H.; Zhang, C.; Liu, G. Dye degradation studies of hausmannite manganese oxide (Mn3O4) nanoparticles synthesized by chemical method. Appl. Phys. A 2021, 127, 1–7. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Durai, M.; Sengottaiyan, C.; Ahn, Y.-H. Effective chemical vapor deposition and characterization of N-doped graphene for high electrochemical performance. J. Nanosci. Nanotechnol. 2021, 21, 3183–3191. [Google Scholar] [CrossRef]
- Wu, M.; Xia, S.; Ding, J.; Zhao, B.; Jiao, Y.; Du, A.; Zhang, H. Growth of MoS2 nanoflowers with expanded interlayer distance onto N-doped graphene for reversible lithium storage. ChemElectroChem 2018, 5, 2263–2270. [Google Scholar] [CrossRef]
- Nafiujjaman, M.; Nurunnabi, M.; Kang, S.-h.; Reeck, G.R.; Khan, H.A.; Lee, Y.-k. Ternary graphene quantum dot–polydopamine–Mn3O4 nanoparticles for optical imaging guided photodynamic therapy and T 1-weighted magnetic resonance imaging. J. Mater. Chem. B 2015, 3, 5815–5823. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, M.-H.; Min, D.-H. Biocompatible reduced graphene oxide prepared by using dextran as a multifunctional reducing agent. Chem. Commun. 2011, 47, 3195–3197. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of aminodextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef]
- Benov, L. Photodynamic therapy: Current status and future directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The application of graphene oxide in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef]
- Yue, H.; Wei, W.; Yue, Z.; Wang, B.; Luo, N.; Gao, Y.; Ma, D.; Ma, G.; Su, Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials 2012, 33, 4013–4021. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Wate, P.S.; Banerjee, S.S.; Jalota-Badhwar, A.; Mascarenhas, R.R.; Zope, K.R.; Khandare, J.; Misra, R.D.K. Cellular imaging using biocompatible dendrimer-functionalized graphene oxide-based fluorescent probe anchored with magnetic nanoparticles. Nanotechnology 2012, 23, 415101. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Portolés, M.; Marques, P.; Feito, M.; Matesanz, M.; Ramírez-Santillán, C.; Gonçalves, G.; Cruz, S.; Nieto, A.; Vallet-Regi, M. Cell uptake survey of pegylated nanographene oxide. Nanotechnology 2012, 23, 465103. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Kim, J.; Kim, T.-I.; Kim, W.J. Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano 2013, 7, 6735–6746. [Google Scholar] [CrossRef]
- Li, S.; Chang, K.; Sun, K.; Tang, Y.; Cui, N.; Wang, Y.; Qin, W.; Xu, H.; Wu, C. Amplified singlet oxygen generation in semiconductor polymer dots for photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2016, 8, 3624–3634. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic therapy mediated by nontoxic core–shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef] [Green Version]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057. [Google Scholar] [CrossRef]
- Sies, H. Biological redox systems and oxidative stress. Cell. Mol. Life Sci. 2007, 64, 2181–2188. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, Z.; Zhou, Y.; Liu, R. Near-infrared organic fluorescent nanoparticles for long-term monitoring and photodynamic therapy of cancer. Nanotheranostics 2019, 3, 156. [Google Scholar] [CrossRef]
- Liu, P.; Xie, X.; Liu, M.; Hu, S.; Ding, J.; Zhou, W. A smart MnO2-doped graphene oxide nanosheet for enhanced chemo-photodynamic combinatorial therapy via simultaneous oxygenation and glutathione depletion. Acta Pharm. Sin. B 2021, 11, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.-J.; Chen, H.-Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jiang, X.; Chen, Y.; Peng, K.; Huang, Y.; Zhao, H.; Chen, Q.; Lv, F.; Liu, L.; Wang, S. Cyclometalated iridium (III) complex nanoparticles for mitochondria-targeted photodynamic therapy. Nanoscale 2020, 12, 14061–14067. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Huang, X.; Wei, G.; Xu, F.; Wang, Y.; Zhou, S. Fenton reaction-assisted photodynamic therapy for cancer with multifunctional magnetic nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 29579–29592. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Semelka, R.C.; Helmberger, T.K. Contrast agents for MR imaging of the liver. Radiology 2001, 218, 27–38. [Google Scholar] [CrossRef]
- Caravan, P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef]
- Idée, J.-M.; Port, M.; Dencausse, A.; Lancelot, E.; Corot, C. Involvement of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: An update. Radiol. Clin. 2009, 47, 855–869. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.A.; Lee, Y.-K.; Shaik, M.R.; Alrashood, S.T.; Ekhzaimy, A.A. Nanocomposites of Nitrogen-Doped Graphene Oxide and Manganese Oxide for Photodynamic Therapy and Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 15087. https://doi.org/10.3390/ijms232315087
Khan HA, Lee Y-K, Shaik MR, Alrashood ST, Ekhzaimy AA. Nanocomposites of Nitrogen-Doped Graphene Oxide and Manganese Oxide for Photodynamic Therapy and Magnetic Resonance Imaging. International Journal of Molecular Sciences. 2022; 23(23):15087. https://doi.org/10.3390/ijms232315087
Chicago/Turabian StyleKhan, Haseeb A., Yong-Kyu Lee, Mohammed Rafi Shaik, Sara T. Alrashood, and Aishah A. Ekhzaimy. 2022. "Nanocomposites of Nitrogen-Doped Graphene Oxide and Manganese Oxide for Photodynamic Therapy and Magnetic Resonance Imaging" International Journal of Molecular Sciences 23, no. 23: 15087. https://doi.org/10.3390/ijms232315087





