IL-5 Serum and Appendicular Lavage Fluid Concentrations Correlate with Eosinophilic Infiltration in the Appendicular Wall Supporting a Role for a Hypersensitivity Type I Reaction in Acute Appendicitis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Study Population
4.2. Ethical Considerations
4.3. Appendicular Lavage Fluid (ALF)
4.4. Pathologic Analysis
4.5. IL-5 Determinations
4.6. Eosinophil Blood Count Determination
4.7. Other Data
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsubsiee, I.F.N.; Alsubsiee, A.F.N. Appendicectomy for Uncomplicated Simple Appendicitis: Is It Always Required? Surg. Res. Pract. 2021, 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Prystowsky, J.B.; Pugh, C.M.; Nagle, A.P. Appendicitis. Curr. Probl. Surg. 2005, 42, 688–742. [Google Scholar] [CrossRef] [PubMed]
- Businco, L. Allergic appendicitis. Allergy 1959, 13, 503–507. [Google Scholar]
- Aravindan, K.P. Eosinophils in acute appendicitis: Possible significance. Indian J. Pathol. Microbiol. 1997, 40, 491–498. [Google Scholar] [PubMed]
- Carvalho, N.; Barros, A.; Coelho, H.O.; Moita, C.F.; Costa, A.N.; Pedroso, D.; Borges, F.; Moita, L.F.; Costa, P.M. A Th2 Cytokine Profile in Appendicular Lavage Fluid Suggests Allergy as a Possible Etiology for Acute Appendicitis. Mediat. Inflamm. 2019, 2019, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, N.; Barros, A.; Coelho, H.; Cóias, A.; Botelho, P.; Cismasiu, B.; Moita, L.; Costa, P. Increased IgE Deposition in Appendicular Tissue Specimens Is Compatible with a Type I Hypersensitivity Reaction in Acute Appendicitis. Mediat. Inflamm. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Carvalho, N.; Borges, F.; Costa, B.; Costa, P. The aetiology of acute appendicitis. Is allergy the missing link? A narrative review. Rev. Port. Cir. 2022, 52, 1–10. [Google Scholar]
- Harlak, A.; Güleç, M.; Mentes, O.; Kilbas, Z.; Onguru, O.; Acikel, C.; Caliskaner, Z.; Erel, F. Atopy is a Risk Factor for Acute Appendicitis? A Prospective Clinical Study. J. Gastrointest. Surg. 2008, 12, 1251–1256. [Google Scholar] [CrossRef]
- Salö, M.; Gudjonsdottir, J.; Omling, E.; Hagander, L.; Stenström, P. Association of IgE-Mediated Allergy With Risk of Complicated Appendicitis in a Pediatric Population. JAMA Pediatr. 2018, 172, 943. [Google Scholar] [CrossRef]
- Javanmard-Emamghissi, H.; Hollyman, M.; Boyd-Carson, H.; Doleman, B.; Adiamah, A.; Lund, J.N.; Moler-Zapata, S.; Grieve, R.; Moug, S.J.; Tierney, G.M.; et al. Antibiotics as first-line alternative to appendicectomy in adult appendicitis: 90-day follow-up from a prospective, multicentre cohort study. Br. J. Surg. 2021, 108, 1351–1359. [Google Scholar] [CrossRef]
- Ernudd, I.; Älgå, A.; Sandblom, G.; Dahlberg, M.; Mantel, Ä. Treatment Strategies and Perforation Rate of Acute Appendicitis During the Early Phase of the COVID-19 Pandemic: A Swedish Cohort Study. J. Surg. Res. 2022, 280, 450–458. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Increased risk of appendectomy due to appendicitis after tonsillectomy in women: A longitudinal follow-up study using a national sample cohort. Medicine 2019, 98, e15579. [Google Scholar] [CrossRef]
- Khasawneh, R.; Hiary, M.; Abadi, B.; Salameh, A.; Moman, S. Total and Specific Immunoglobulin E for Detection of Most Prevalent Aeroallergens in a Jordanian Cohort. Med. Arch. 2019, 73, 272–275. [Google Scholar] [CrossRef]
- Toyoshima, S.; Okayama, Y. Neuro-allergology: Mast cell–nerve cross-talk. Allergol. Int. 2022, 71, 288–293. [Google Scholar] [CrossRef]
- Haddad, E.-B.; Cyr, S.L.; Arima, K.; McDonald, R.A.; Levit, N.A.; Nestle, F.O. Current and Emerging Strategies to Inhibit Type 2 Inflammation in Atopic Dermatitis. Dermatol. Ther. 2022, 12, 1501–1533. [Google Scholar] [CrossRef]
- Wynn, T. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Šokić, M.K.; Rijavec, M.; Korošec, P.; Bidovec-Stojkovič, U.; Kern, I.; Vantur, R.; Škrgat, S. Heterogeneous Response of Airway Eosinophilia to Anti-IL-5 Biologics in Severe Asthma Patients. J. Pers. Med. 2022, 12, 70. [Google Scholar] [CrossRef]
- Vandezande, L.M.; Wallaert, B.; Desreumaux, P.; Tsicopoulos, A.; Lamblin, C.; Tonnel, A.B.; Janin, A. Interleukin-5 immunoreactivity and mRNA expression in gut mucosa from patients with food allergy. Clin. Exp. Allergy 1999, 29, 695–702. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, K.H.; Lee, H.B.; Rhee, Y.K. Serum Levels of Interleukins (IL)-4, IL-5, IL-13, and Interferon-γ in Acute Asthma. J. Asthma 2001, 38, 665–671. [Google Scholar] [CrossRef]
- Gurtner, A.; Gonzalez-Perez, I.; Arnold, I.C. Intestinal eosinophils, homeostasis and response to bacterial intrusion. Semin. Immunopathol. 2021, 43, 295–306. [Google Scholar] [CrossRef]
- Reismann, J.; Romualdi, A.; Kiss, N.; Minderjahn, M.I.; Kallarackal, J.; Schad, M.; Reismann, M. Diagnosis and classification of pediatric acute appendicitis by artificial intelligence methods: An investigator-independent approach. PLoS ONE 2019, 14, e0222030. [Google Scholar] [CrossRef] [PubMed]
- Peeters, T.; Penders, J.; Smeekens, S.P.; Galazzo, G.; Houben, B.; Netea, M.G.; Savelkoul, P.H.; Gyssens, I.C. The fecal and mucosal microbiome in acute appendicitis patients: An observational study. Future Microbiol. 2019, 14, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Bom, W.J.; Scheijmans, J.C.G.; Ubels, S.; van Geloven, A.A.W.; Gans, S.L.; Tytgat, K.M.A.J.; van Rossem, C.C.; Koens, L.; Stoker, J.; Bemelman, W.A.; et al. Optimising diagnostics to discriminate complicated from uncomplicated appendicitis: A prospective cohort study protocol. BMJ Open 2022, 12, e054304. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.; Søreide, K.; Di Saverio, S.; Assarsson, J.H.; Drake, F.T. Acute appendicitis: Modern understanding of pathogenesis, diagnosis, and management. Lancet 2015, 386, 1278–1287, Erratum in Lancet 2017, 390, 1736. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ahanchi, S.; Pisaneschi, M.; Lin, I.; Walter, R. Can acute appendicitis be treated by antibiotics alone? Am. Surg. 2007, 73, 1161–1165. [Google Scholar] [CrossRef]
- Gran, M.V.; Kjønås, D.; Gunnarsson, U.; Strigård, K.; Revhaug, A.; Aahlin, E.K. Antibiotic treatment for ap-pendicitis in Norway and Sweden: A nationwide survey on treatment practices. BMC Surg. 2022, 22, 229. [Google Scholar] [CrossRef]
- Herrod, P.J.J.; Kwok, A.T.; Lobo, D.N. Randomized clinical trials comparing antibiotic therapy with ap-pendicectomy for uncomplicated acute appendicitis: Meta-analysis. BJS Open 2022, 6, zrac100. [Google Scholar] [CrossRef]
- Ielpo, B.; Podda, M.; Pellino, G.; Pata, F.; Caruso, R.; Gravante, G.; Di Saverio, S.; ACIE Appy Study Collaborative. Global atttudes in the management of acute appendicitis during COVID-19 pandemic: ACIE Appy Study. Br. J. Surg. 2021, 108, 717–726. [Google Scholar] [CrossRef]
- Tsuji, M.; McMahon, G.; Reen, D.; Puri, P. New insights into the pathogenesis of appendicitis based on immunocytochemical analysis of early immune response. J. Pediatr. Surg. 1990, 25, 449–452. [Google Scholar] [CrossRef]
- De Costa, A. The appendix-mucosal immunity and tolerance in the gut: Consequences for the syndromes of appendicitis and its epidemiology. ANZ J. Surg. 2022, 92, 653–660. [Google Scholar] [CrossRef]
- Romagnani, S. Cytokines and chemoattractants in allergic inflammation. Mol. Immunol. 2002, 38, 881–885. [Google Scholar] [CrossRef]
- Spencer, L.A.; Szela, C.T.; Perez, S.A.; Kirchhoffer, C.L.; Neves, J.S.; Radke, A.L.; Weller, P.F. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J. Leukoc. Biol. 2009, 85, 117–123. [Google Scholar] [CrossRef]
- Di Saverio, S.; Birindelli, A.; Kelly, M.D.; Catena, F.; Weber, D.G.; Sartelli, M.; Sugrue, M.; De Moya, M.; Gomes, C.A.; Bhangu, A.; et al. WSES Jerusalem guidelines for diagnosis and treatment of acute appendicitis. World J. Emerg. Surg. 2016, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013, 2013, 434010. [Google Scholar] [CrossRef] [Green Version]
- Rubér, M.; Berg, A.; Ekerfelt, C.; Olaison, G.; Andersson, R.E. Different cytokine profiles in patients with a history of gangrenous or phlegmonous appendicitis. Clin. Exp. Immunol. 2006, 143, 117–124. [Google Scholar] [CrossRef]
- Wang, Y.; Reen, D.J.; Puri, P. Is a histologically normal appendix following emergency appendicectomy I normal? Lancet 1996, 347, 1076–1079. [Google Scholar] [CrossRef]
- Cunha, L.; Marcelino, G.; Carvalho, N.; Antunes, C.; Brito, M.J. Eosinophils and C-reactive protein are di-agnostic and severity markers in acute appendicitis. Rev. Port. Cir. 2018, 44, 19–24. [Google Scholar]
- Abidi, K.; Khoudri, I.; Belayachi, J.; Madani, N.; Zekraoui, A.; Zeggwagh, A.A.; Abouqal, R. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit. Care 2008, 12, R59. [Google Scholar] [CrossRef] [Green Version]
- Marcelino, G.; Carvalho, N.; Oliveira, G.; Marialva, C.; Campanha, R.; Albergaria, D.; Santos, C.; Lebre, R.; Corte-Real, J. Neutro-phil-to-eosinophil ratio and c-reactive protein are predictors of surgery in acute diverticulitis. Rev. Port. Cir. 2015, 33, 11–19. [Google Scholar]
- Rogers, M.B.; Brower-Sinning, R.; Firek, B.; Zhong, D.; Morowitz, M.J. Acute Appendicitis in Children Is Associated with a Local Expansion of Fusobacteria. Clin. Infect Dis. 2016, 63, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Guo, X.; Liu, T.; Iqbal, M.; Jiang, K.; Zhu, L.; Chen, X.; Wang, B.M. An Update on Eosinophilic Esophagitis: Etiological Factors, Coexisting Diseases, and Complications. Digestion 2021, 102, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Boom, A.L.V.D.; De Wijkerslooth, E.M.L.; Mauff, K.A.L.; Dawson, I.; van Rossem, C.C.; Toorenvliet, B.R.; Wijnhoven, B.P.L. Interobserver variability in the classification of appendicitis during laparoscopy. Br. J. Surg. 2018, 105, 1014–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolur, A.; Patil, A.M.; Agarwal, V.; Yendigiri, S.; Sajjanar, B.B. The Significance of Mast Cells and Eosinophils Counts in Surgically Resected Appendix. J. Interdiscipl. Histopathol. 2014, 2, 150–153. [Google Scholar] [CrossRef]
- Lamps, L.W. Beyond acute inflammation a review of appendicitis and infections of the appendix. Diagn. Histopathol. 2008, 14, 68–77. [Google Scholar] [CrossRef]
- Hornby, S.T.; Shahtahmassebi, G.; Lynch, S.; Ladwa, N.; Stell, D.A. Delay to surgery does not influence the pathological outcome of acute appendicitis. Scand. J. Surg. 2013, 103, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Omiyale, A.O.; Adjepong, S. Histopathological correlations of appendectomies: A clinical audit of a single center. Ann. Transl. Med. 2015, 3, 119. [Google Scholar] [CrossRef]
- Butler, C. Surgical Pathology of Acute Appendicitis. Hum. Pathol. 1981, 12, 870–878. [Google Scholar] [CrossRef]
- Mizumoto, R.; Cristaudo, A.T.; Lai, N.K.; Premaratne, G.; Hendahewa, R. Dilemma of mucosal appendicitis: A clinic-pathological entity? A retrospective cohort study. ANZ J. Surg. 2018, 88, E284–E288. [Google Scholar] [CrossRef]


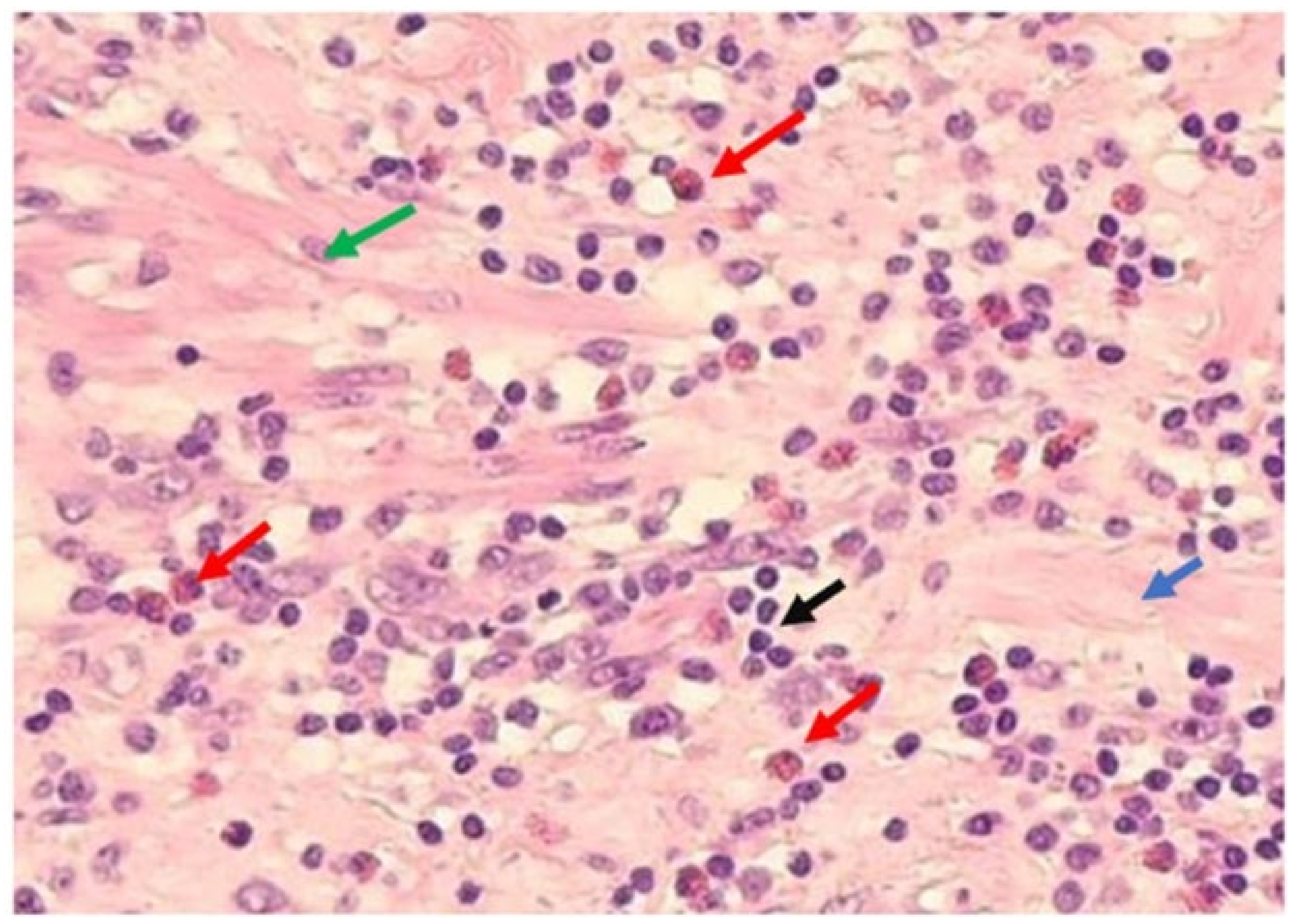
| NPA | APA | AGA | ||
|---|---|---|---|---|
| N (%) | 18 (21.7) | 38 (45.8) | 27(32.5) | p Value |
| Age (y) | 31.5(27–40) | 37(24–48) | 38(26–48) | 0.611 * |
| Gender (M/F) | 14/4 | 25/13 | 14/13 | 0.196 ** |
| Allergy (N/Y) | 15/3 | 30/6 | 23/4 | 0.999 *** |
| BMI | 22.7(21.40–25.85) | 24.5(22–27.80) | 25.30(22.30–28.60) | 0.237 * |
| NPA | APA | AGA | ||
|---|---|---|---|---|
| p Value | ||||
| Eosinophils (PB) | 0.13(0.004–0.22) | 0.08(0.03–0.15) | 0.03(0.02–0.07) | 0.004 * |
| Eosinophils (AW) | 47.5(24–62) | 1(37.5–95.5) | 21(9–34) | 0.000 * |
| IL-5 (PB) | 14.38(12–20.52) | 11.67(9.43–18.00) | 13.05(10.76–18.79) | 0.239 |
| IL-5 (ALF) | 8(6.67–13.6) | 29.22(9.06–115.16) | 8.67(6.67–46.67) | 0.056 |
| Eosinophiles—Wall | IL5—PB (pg/mL) | IL5—ALF (pg/mL) | |
|---|---|---|---|
| Eosinophiles—PB | 0.291 **(a) | 0.161 | 0.238 |
| Eosinophiles—Wall | 0.246 *(b) | 0.374 *(c) | |
| IL5—PB (pg/mL) | 0.139 (d) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, N.; Carolino, E.; Coelho, H.; Cóias, A.; Trindade, M.; Vaz, J.; Cismasiu, B.; Moita, C.; Moita, L.; Costa, P.M. IL-5 Serum and Appendicular Lavage Fluid Concentrations Correlate with Eosinophilic Infiltration in the Appendicular Wall Supporting a Role for a Hypersensitivity Type I Reaction in Acute Appendicitis. Int. J. Mol. Sci. 2022, 23, 15086. https://doi.org/10.3390/ijms232315086
Carvalho N, Carolino E, Coelho H, Cóias A, Trindade M, Vaz J, Cismasiu B, Moita C, Moita L, Costa PM. IL-5 Serum and Appendicular Lavage Fluid Concentrations Correlate with Eosinophilic Infiltration in the Appendicular Wall Supporting a Role for a Hypersensitivity Type I Reaction in Acute Appendicitis. International Journal of Molecular Sciences. 2022; 23(23):15086. https://doi.org/10.3390/ijms232315086
Chicago/Turabian StyleCarvalho, Nuno, Elisabete Carolino, Hélder Coelho, Ana Cóias, Madalena Trindade, João Vaz, Brigitta Cismasiu, Catarina Moita, Luis Moita, and Paulo Matos Costa. 2022. "IL-5 Serum and Appendicular Lavage Fluid Concentrations Correlate with Eosinophilic Infiltration in the Appendicular Wall Supporting a Role for a Hypersensitivity Type I Reaction in Acute Appendicitis" International Journal of Molecular Sciences 23, no. 23: 15086. https://doi.org/10.3390/ijms232315086
APA StyleCarvalho, N., Carolino, E., Coelho, H., Cóias, A., Trindade, M., Vaz, J., Cismasiu, B., Moita, C., Moita, L., & Costa, P. M. (2022). IL-5 Serum and Appendicular Lavage Fluid Concentrations Correlate with Eosinophilic Infiltration in the Appendicular Wall Supporting a Role for a Hypersensitivity Type I Reaction in Acute Appendicitis. International Journal of Molecular Sciences, 23(23), 15086. https://doi.org/10.3390/ijms232315086






