MgADP Promotes Myosin Head Movement toward Actin at Low [Ca2+] to Increase Force Production and Ca2+-Sensitivity of Contraction in Permeabilized Porcine Myocardial Strips
Abstract
:1. Introduction
2. Results
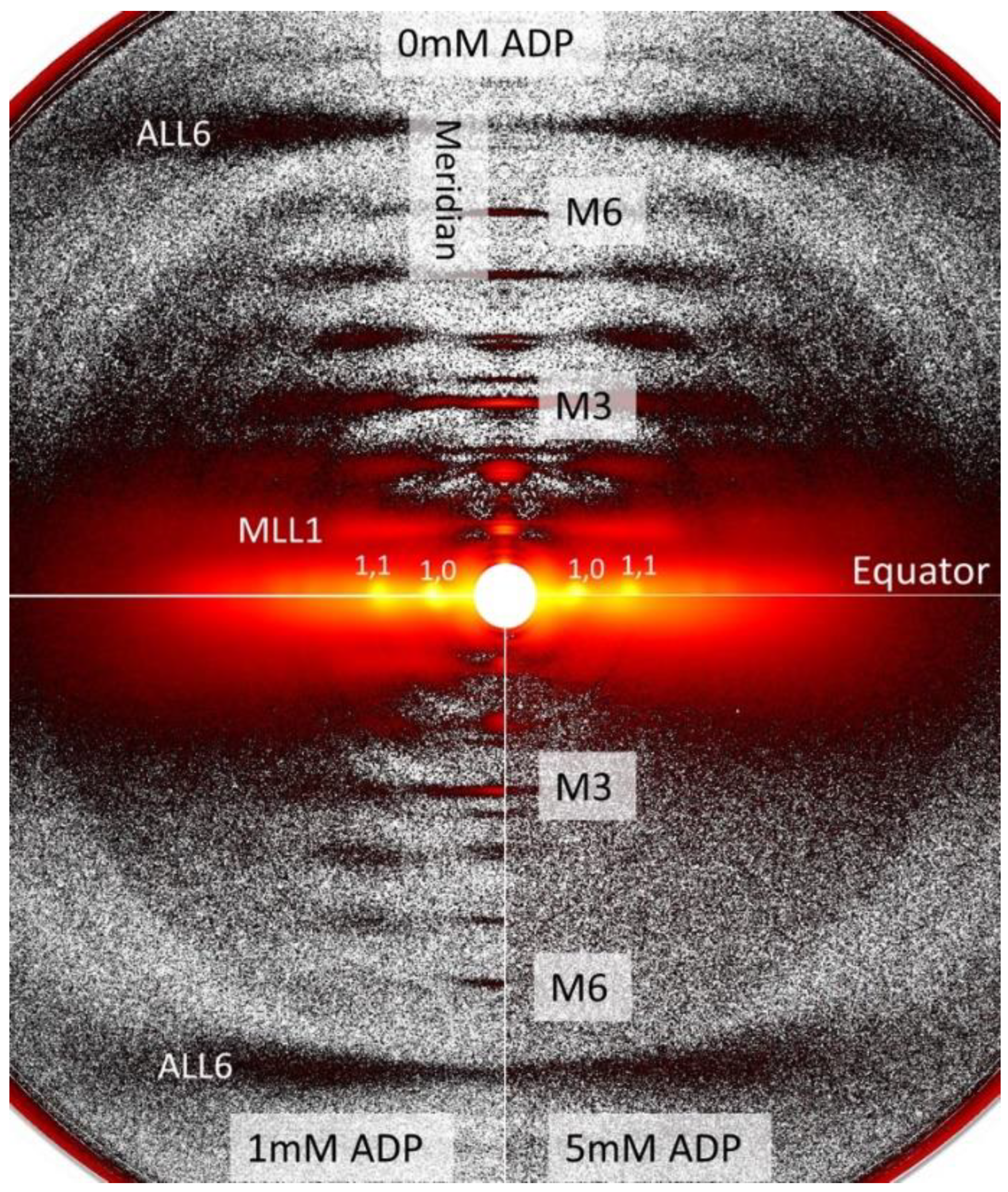
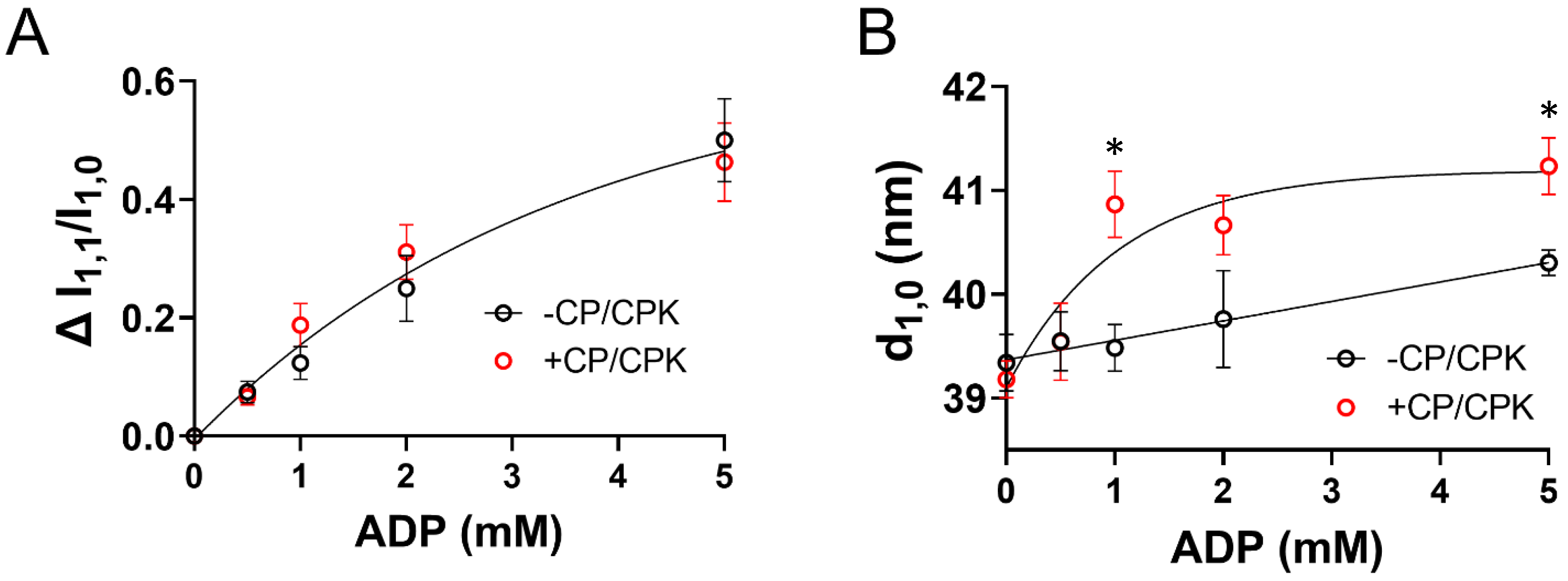
2.1. Effects of ADP on Myofilament Lattice Structure
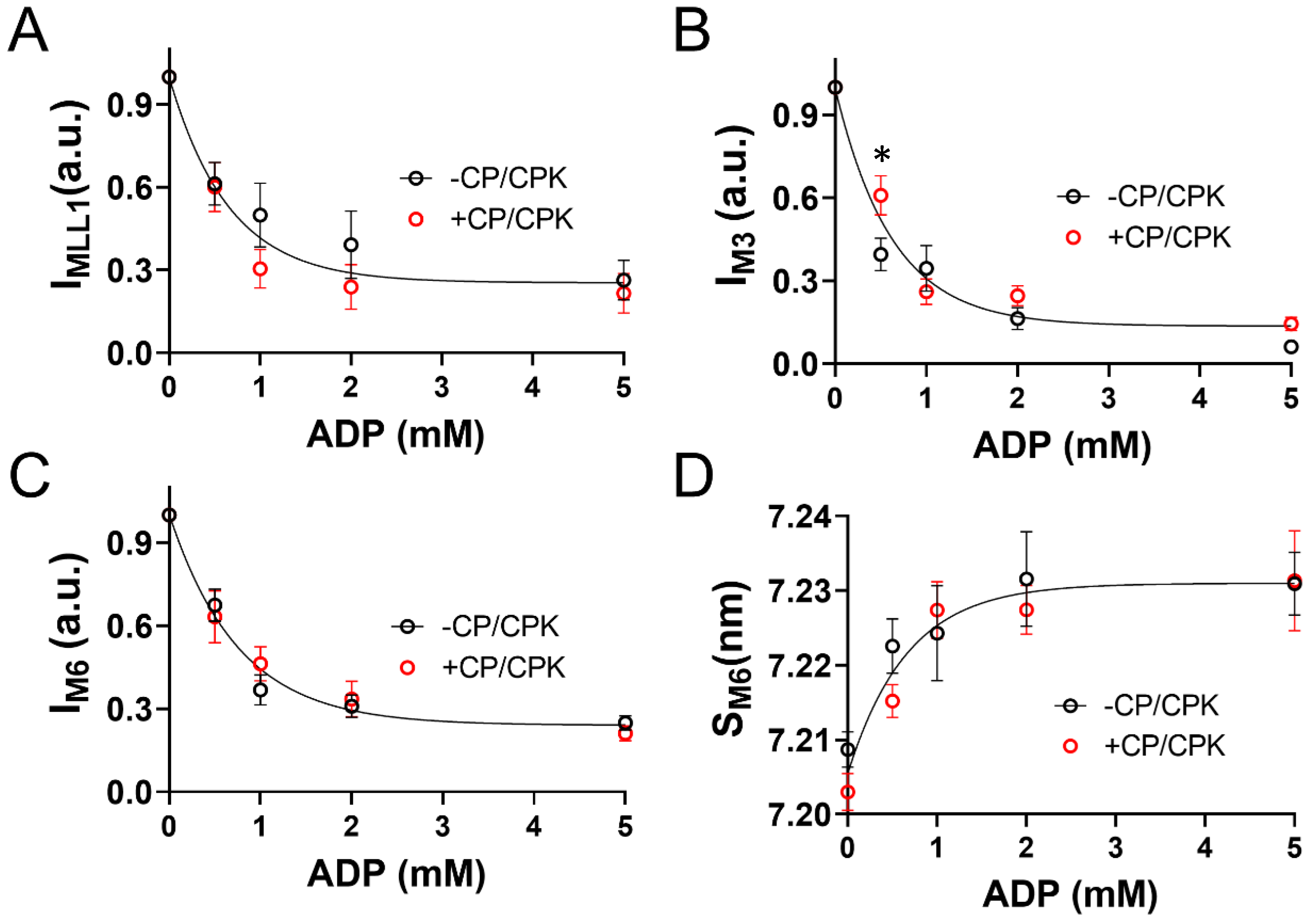
2.2. OFF to ON Transitions of Myosin Heads as a Function of [ADP]
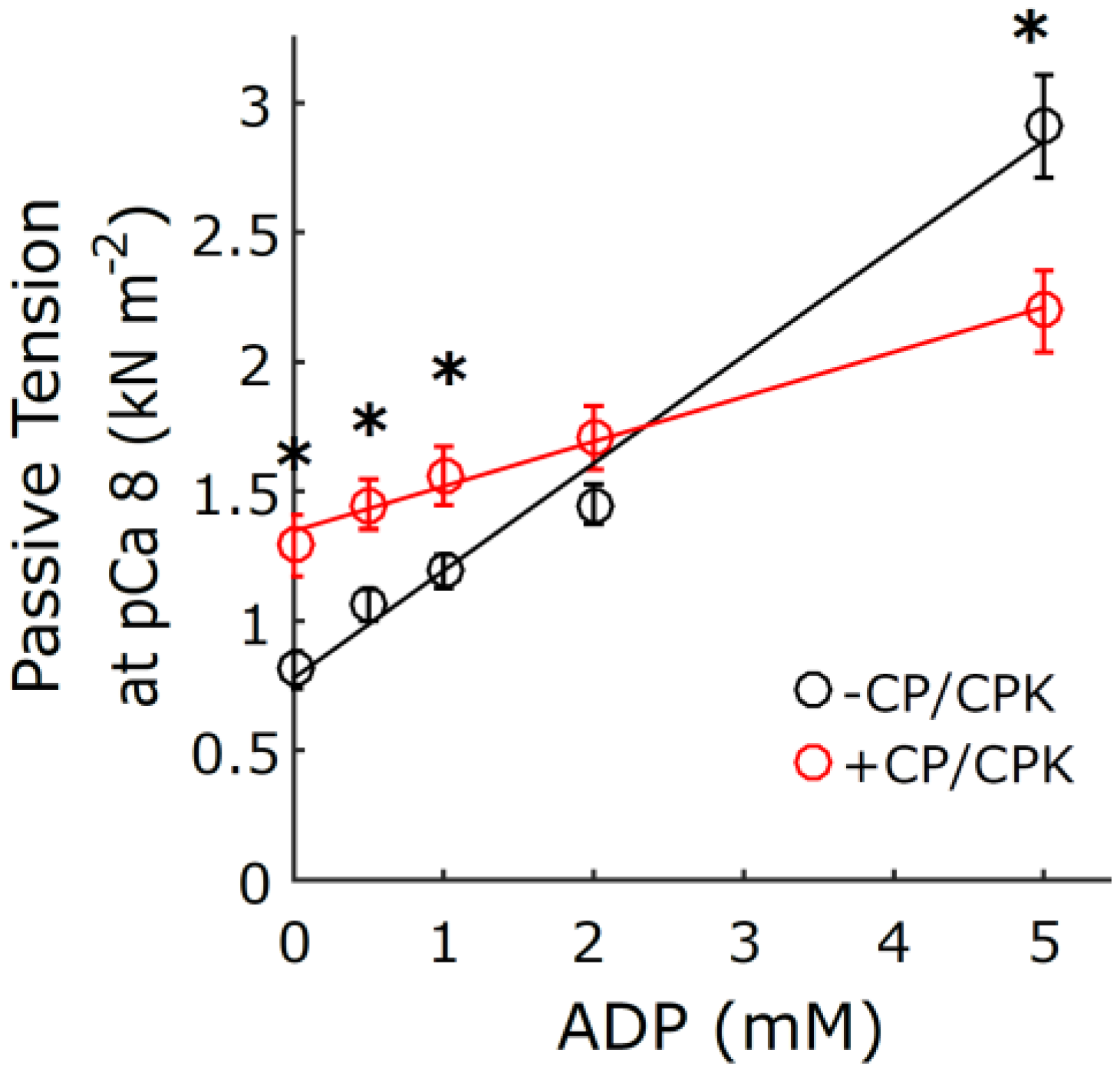
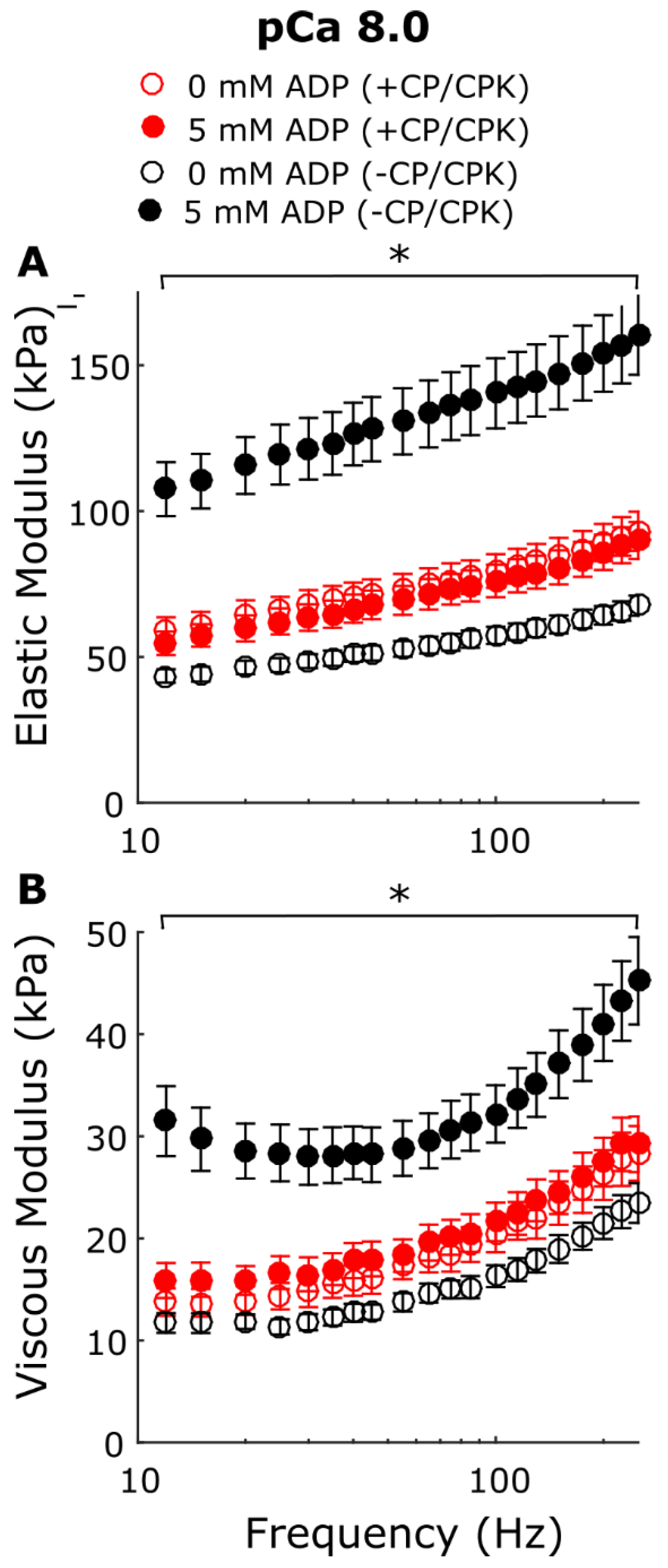
2.3. Myocardial Mechanics at pCa 8.0
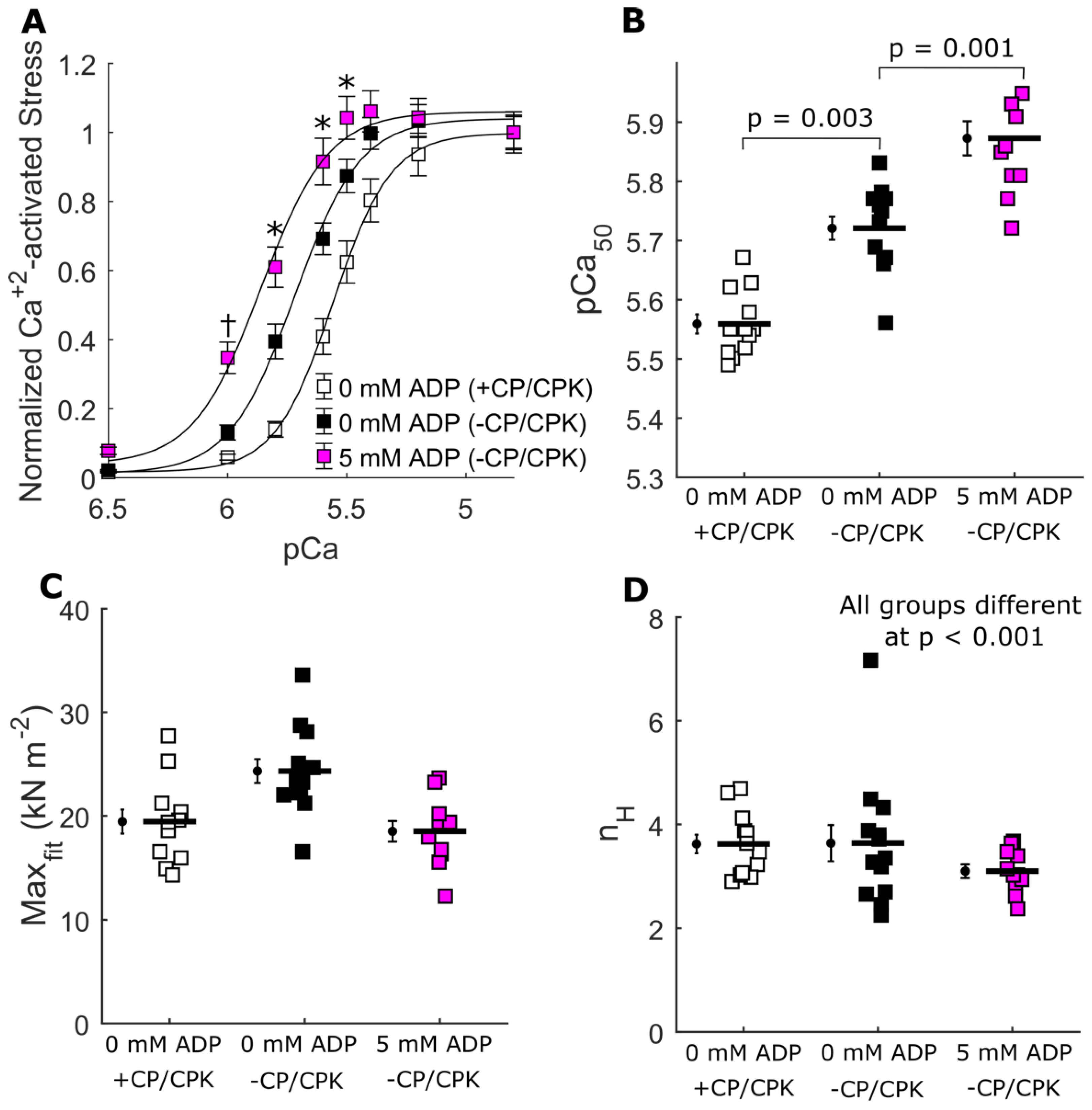
2.4. Ca2+-Activated Myocardial Mechanics
3. Discussion
3.1. Increased Tension and Stiffness with Increased [ADP]
3.2. Increased Calcium Sensitivity with Increased [ADP]
3.3. Increased Nucleotide Concentration Induces Structural Transitions in the Thick filaments
4. Materials and Methods
4.1. Muscle Preparation for X-ray Diffraction
4.2. X-ray Data Analysis
4.3. Muscle Preparation for Mechanics
4.4. Muscle Mechanics
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, D.G.; Orchard, C.H. Myocardial Contractile Function during Ischemia and Hypoxia. Circ. Res. 1987, 60, 153–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, F.; Zhang, J.; Beard, D.A. Experimentally Observed Phenomena on Cardiac Energetics in Heart Failure Emerge from Simulations of Cardiac Metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 7143–7148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingwall, J.S.; Kramer, M.F.; Fifer, M.A.; Lorell, B.H.; Shemin, R.; Grossman, W.; Allen, P.D. The Creatine Kinase System in Normal and Diseased Human Myocardium. N. Engl. J. Med. 1985, 313, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jakovljevic, D.G.; Beard, D.A. Cardiac Metabolic Limitations Contribute to Diminished Performance of the Heart in Aging. Biophys. J. 2019, 117, 2295–2302. [Google Scholar] [CrossRef]
- Ogut, O.; Brozovich, F.V. Creatine Phosphate Consumption and the Actomyosin Crossbridge Cycle in Cardiac Muscles. Circ. Res. 2003, 93, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, R.; Nascimben, L.; Ingwall, J.S.; Lorell, B.H. Failure to Maintain a Low ADP Concentration Impairs Diastolic Function in Hypertrophied Rat Hearts. Circulation 1997, 96, 1313–1319. [Google Scholar] [CrossRef]
- Sequeira, V.; Najafi, A.; Mcconnell, M.; Fowler, E.D.; Bollen, I.A.E.; Wüst, R.C.I.; Dos Remedios, C.; Helmes, M.; White, E.; Stienen, G.J.M.; et al. Synergistic Role of ADP and Ca2+ in Diastolic Myocardial Stiffness. J. Physiol. 2015, 593, 3899–3916. [Google Scholar] [CrossRef] [Green Version]
- Siemankowski, R.F.; Wiseman, M.O.; White, H.D. ADP Dissociation from Actomyosin Subfragment 1 Is Sufficiently Slow to Limit the Unloaded Shortening Velocity in Vertebrate Muscle. Proc. Natl. Acad. Sci. USA 1985, 82, 658–662. [Google Scholar] [CrossRef] [Green Version]
- Tyska, M.J.; Warshaw, D.M. The Myosin Power Stroke. Cell Motil. Cytoskelet. 2002, 51, 1–15. [Google Scholar] [CrossRef]
- Nyitrai, M.; Geeves, M.A. Adenosine Diphosphate and Strain Sensitivity in Myosin Motors. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 1867–1877. [Google Scholar] [CrossRef]
- Kawai, M.; Zhao, Y. Cross-Bridge Scheme and Force per Cross-Bridge State in Skinned Rabbit Psoas Muscle Fibers. Biophys. J. 1993, 65, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Kawai, M.; Halvorson, H.R. Role of {MgATP} and {MgADP} in the Cross-Bridge Kinetics in Chemically Skinned Rabbit Psoas Fibers. Study of a Fast Exponential Process {C}. Biophys. J. 1989, 55, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.; Pate, E. The Effects of ADP and Phosphate on the Contraction of Muscle Fibers. Biophys. J. 1985, 48, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Best, P.M.; Donaldson, S.K.; Kerrick, W.G. Tension in Mechanically Disrupted Mammalian Cardiac Cells: Effects of Magnesium Adenosine Triphosphate. J. Physiol. 1977, 265, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, N.; Fujita, H.; Fujita, T.; Ishiwata, S. Regulatory Roles of MgADP and Calcium in Tension Development of Skinned Cardiac Muscle. J. Muscle Res. Cell Motil. 1998, 19, 909–921. [Google Scholar] [CrossRef]
- Fukuda, N.; Kajiwara, H.; Ishiwata, S.; Kurihara, S. Effects of MgADP on Length Dependence of Tension Generation in Skinned Rat Cardiac Muscle. Circ. Res. 2000, 86, E1–E6. [Google Scholar] [CrossRef] [Green Version]
- Gorga, J.A.; Fishbaugher, D.E.; VanBuren, P. Activation of the Calcium-Regulated Thin Filament by Myosin Strong Binding. Biophys. J. 2003, 84, 2484–2491. [Google Scholar] [CrossRef] [Green Version]
- Bremel, R.D.; Weber, A. Cooperation within Actin Filament in Vertebrate Skeletal Muscle. Nat. New Biol. 1972, 238, 97–101. [Google Scholar] [CrossRef]
- Campbell, K. Rate Constant of Muscle Force Redevelopment Reflects Cooperative Activation as Well as Cross-Bridge Kinetics. Biophys. J. 1997, 72, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimons, D.P.; Moss, R.L. Cooperativity in the Regulation of Force and the Kinetics of Force Development in Heart and Skeletal Muscles: Cross-Bridge Activation of Force. Adv. Exp. Med. Biol. 2007, 592, 177–189. [Google Scholar]
- Chaplain, R.A.; Gergs, U. Calcium- and ATP-Dependent Changes in Myosin Mass Distribution of Glycerinated Rabbit Psoas Muscle. Biochem. Biophys. Res. Commun. 1974, 61, 517–524. [Google Scholar] [CrossRef]
- Ma, W.; Gong, H.; Irving, T. Myosin Head Configurations in Resting and Contracting Murine Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2643. [Google Scholar] [CrossRef] [Green Version]
- Huxley, H.E. Structural Difference between Resting and Rigor Muscle; Evidence from Intensity Changes in the Low-Angle Equatorial X-ray Diagram. J. Mol. Biol. 1968, 37, 507–520. [Google Scholar] [CrossRef]
- Huxley, H.E.; Brown, W. The Low-Angle X-ray Diagram of Vertebrate Striated Muscle and Its Behaviour during Contraction and Rigor. J. Mol. Biol. 1967, 30, 383–434. [Google Scholar] [CrossRef]
- Gollapudi, S.K.; Yu, M.; Gan, Q.F.; Nag, S. Synthetic Thick Filaments: A New Avenue for Better Understanding the Myosin Super-Relaxed State in Healthy, Diseased, and Mavacamten-Treated Cardiac Systems. J. Biol. Chem. 2021, 296, 100114. [Google Scholar] [CrossRef]
- Hooijman, P.; Stewart, M.A.; Cooke, R. A New State of Cardiac Myosin with Very Slow ATP Turnover: A Potential Cardioprotective Mechanism in the Heart. Biophys. J. 2011, 100, 1969–1976. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.A.; Franks-Skiba, K.; Chen, S.; Cooke, R. Myosin ATP Turnover Rate Is a Mechanism Involved in Thermogenesis in Resting Skeletal Muscle Fibers. Proc. Natl. Acad. Sci. USA 2010, 107, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Craig, R.; Padrón, R. Structural Basis of the Super-and Hyper-Relaxed States of Myosin II. J. Gen. Physiol. 2022, 154, e202113012. [Google Scholar] [CrossRef]
- Nag, S.; Trivedi, D.V. To Lie or Not to Lie: Super-Relaxing with Myosins. Elife 2021, 10, e63703. [Google Scholar] [CrossRef]
- Ma, W.; Irving, T.C. Small Angle X-ray Diffraction as a Tool for Structural Characterization of Muscle Disease. Int. J. Mol. Sci. 2022, 23, 3052. [Google Scholar] [CrossRef]
- Ma, W.; Gong, H.; Qi, L.; Nag, S.; Irving, T.C. Cardiac Myosin Filaments Are Directly Regulated by Calcium. J. Gen. Physiol. 2022, 154, e202213213. [Google Scholar] [CrossRef] [PubMed]
- Caremani, M.; Brunello, E.; Linari, M.; Fusi, L.; Irving, T.C.; Gore, D.; Piazzesi, G.; Irving, M.; Lombardi, V.; Reconditi, M. Low Temperature Traps Myosin Motors of Mammalian Muscle in a Refractory State That Prevents Activation. J. Gen. Physiol. 2019, 151, 1272–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caremani, M.; Fusi, L.; Linari, M.; Reconditi, M.; Piazzesi, G.; Irving, T.C.; Narayanan, T.; Irving, M.; Lombardi, V.; Brunello, E. Dependence of Thick Filament Structure in Relaxed Mammalian Skeletal Muscle on Temperature and Interfilament Spacing. J. Gen. Physiol. 2021, 153, e202012713. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Duno-Miranda, S.; Irving, T.; Craig, R.; Padrón, R. Relaxed Tarantula Skeletal Muscle Has Two ATP Energy-Saving Mechanisms. J. Gen. Physiol. 2021, 153, e202012780. [Google Scholar] [CrossRef] [PubMed]
- Millman, B.M. The Filament Lattice of Striated Muscle. Physiol. Rev. 1998, 78, 359–391. [Google Scholar] [CrossRef]
- Williams, C.D.; Salcedo, M.K.; Irving, T.C.; Regnier, M.; Daniel, T.L. The Length-Tension Curve in Muscle Depends on Lattice Spacing. Proc. Biol. Sci. 2013, 280, 20130697. [Google Scholar] [CrossRef] [Green Version]
- Beard, D.A.; Marzban, B.; Li, O.Y.; Campbell, K.S.; Janssen, P.M.L.; Chesler, N.C.; Baker, A.J. Reduced Cardiac Muscle Power with Low ATP Simulating Heart Failure. Biophys. J. 2022, 121, 3213–3223. [Google Scholar] [CrossRef]
- Fischetti, R.; Stepanov, S.; Rosenbaum, G.; Barrea, R.; Black, E.; Gore, D.; Heurich, R.; Kondrashkina, E.; Kropf, A.J.; Wang, S.; et al. The BioCat Undulator Beamline 18ID: A Facility for Biological Non-Crystalline Diffraction and X-ray Absorption Spectroscopy at the Advanced Photon Source. J. Synchrotron Radiat. 2004, 11, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Jiratrakanvong, J.; Shao, J.; Menendez, M.; Li, X.; Li, J.; Agam, G.; Irving, T. MuscleX: Software Suite for Diffraction X-ray Imaging, v1.13.3; Zenodo: Geneva, Switzerland, 2018. [Google Scholar] [CrossRef]
- Ma, W.; Henze, M.; Anderson, R.L.; Gong, H.; Wong, F.L.; Del Rio, C.L.; Irving, T. The Super-Relaxed State and Length Dependent Activation in Porcine Myocardium. Circ. Res. 2021, 129, 617–630. [Google Scholar] [CrossRef]
- Ma, W.; Gong, H.; Kiss, B.; Lee, E.J.; Granzier, H.; Irving, T. Thick-Filament Extensibility in Intact Skeletal Muscle. Biophys. J. 2018, 115, 1580–1588. [Google Scholar] [CrossRef]

| pCa | CP (mM) | CPK (U/mL) | Solution Recipe ADP (mM) | Measured ADP (mM) |
|---|---|---|---|---|
| 8 | 0 | 0 | 0 | 0.37 ± 0.00 |
| 8 | 0 | 0 | 5 | 5.16 ± 0.42 |
| 8 | 35 | 300 | 0 | 0.40 ± 0.03 |
| 8 | 35 | 300 | 2 | 0.45 ± 0.06 |
| 8 | 35 | 300 | 5 | 0.65 ± 0.13 |
| 4.8 | 0 | 0 | 0 | 0.48 ± 0.06 |
| 4.8 | 0 | 0 | 5 | 5.63 ± 1.49 |
| 4.8 | 35 | 300 | 0 | 0.51 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awinda, P.O.; Ma, W.; Turner, K.L.; Zhao, J.; Gong, H.; Thompson, M.S.; Campbell, K.S.; Irving, T.C.; Tanner, B.C.W. MgADP Promotes Myosin Head Movement toward Actin at Low [Ca2+] to Increase Force Production and Ca2+-Sensitivity of Contraction in Permeabilized Porcine Myocardial Strips. Int. J. Mol. Sci. 2022, 23, 15084. https://doi.org/10.3390/ijms232315084
Awinda PO, Ma W, Turner KL, Zhao J, Gong H, Thompson MS, Campbell KS, Irving TC, Tanner BCW. MgADP Promotes Myosin Head Movement toward Actin at Low [Ca2+] to Increase Force Production and Ca2+-Sensitivity of Contraction in Permeabilized Porcine Myocardial Strips. International Journal of Molecular Sciences. 2022; 23(23):15084. https://doi.org/10.3390/ijms232315084
Chicago/Turabian StyleAwinda, Peter O., Weikang Ma, Kyrah L. Turner, Jing Zhao, Henry Gong, Mindy S. Thompson, Kenneth S. Campbell, Thomas C. Irving, and Bertrand C. W. Tanner. 2022. "MgADP Promotes Myosin Head Movement toward Actin at Low [Ca2+] to Increase Force Production and Ca2+-Sensitivity of Contraction in Permeabilized Porcine Myocardial Strips" International Journal of Molecular Sciences 23, no. 23: 15084. https://doi.org/10.3390/ijms232315084
APA StyleAwinda, P. O., Ma, W., Turner, K. L., Zhao, J., Gong, H., Thompson, M. S., Campbell, K. S., Irving, T. C., & Tanner, B. C. W. (2022). MgADP Promotes Myosin Head Movement toward Actin at Low [Ca2+] to Increase Force Production and Ca2+-Sensitivity of Contraction in Permeabilized Porcine Myocardial Strips. International Journal of Molecular Sciences, 23(23), 15084. https://doi.org/10.3390/ijms232315084









