1. Introduction
Hashimoto’s thyroiditis (HT) is an organ-specific autoimmune disorder, part of the group of heterogeneous autoimmune thyroid diseases (AITDs). It is characterized by abnormal B- and T-cell activation. This pathological reaction is directed against the thyrocites and leads to the destruction of the thyroid and subsequently to hypothyroidism. Together with Graves’ disease (one of the most common causes of chronic hyperthyroidism), these disorders affect up to 15% of adult females, much more commonly than other autoimmune diseases such as type 1 diabetes mellitus and multiple sclerosis [
1,
2,
3]. Most of the cases remain undiagnosed for long periods, but hypothyroidism is associated with various physical and psychological issues. However, no therapeutic approach to prevent HD-induced thyroid destruction has been proven to work and the only treatment is thyroid hormone replacement.
During HT progression, lymphocyte infiltration of overactivated B and T cells in the thyroid gland leads to destruction of thyrocytes by various cell- and antibody-mediated immune mechanisms. The major targets for pathological immune response among the thyroid-specific autoantigens are the thyroglobulin (Tg), the intracellular membrane-bound protein thyroid peroxidase (TPO), and the thyrotropin receptor (TSHR). The breakdown of self-tolerance against the antigens in the thyroid gland is the result from a primary defect in immune recognition and regulation leading to targeting of immune reaction to Tg, TPO, and TSHR [
4,
5]. Tg, the most abundant protein in the thyroid gland, is produced by thyrocytes and then stored in the follicular lumen. The structural analysis of Tg by use of computer algorithms to explore the probable MHC-binding Tg peptides has found many responding epitopes determining the Tg as a T/B-cell attractant, facilitating their activation with subsequent autoimmunity [
6].
The growing importance of HLA class II genes for susceptibility to autoimmune thyroiditis (AITD) has been elucidated in recent years. Some of them, such as HLA-DR3 [
7,
8], -DR4 [
7], and -DR5 [
9] are related to AITD in Caucasians, while others (HLA-DQ2 and -DQ8) are present in half of the patients with AITD [
10,
11].
Autoreactive B and T cells may play diverse roles in the pathology of autoimmunity, but together they form an effector and amplifying pair involved in the development of HT. During HT progression, a diverse array of autoantibodies is generated, those to Tg being frequently the first to be detected. Increased levels of Tg-specific autoantibodies are associated with disease development determining Tg as one of the major autoantigens in HT. Autoantibody production is not the only role of B cells for pathology activation. B lymphocytes are recognized as potential antigen-presenting cells for processing and presentation to T cells [
5,
12]. Due to the specific role that autoreactive B lymphocytes play in the induction of HT, their selective inhibition is a logical goal in the attempt to suppress the manifestations of the disease.
On the other hand, HT-recognizing T cells also play an important role in the development of autoimmunity. HT-specific CD4+ T cells can bind to epitopes of Tg represented by autoreactive B cells and thus amplify B-cell differentiation to plasmablasts and autoantibody-producing plasma cells. Additionally, cytotoxic CD8+ T cells targeted to Tg can destroy the thyrocytes and the thyroid gland by either granule exocytosis or apoptosis [
12,
13].
A possible approach for downregulation of B-cell activity is to engage inhibitory B-cell receptors through their ligands or monoclonal antibodies. A potential candidate for this purpose is the human complement receptor (CR1, CD35), which provides a strong inhibitory signal to B lymphocytes [
14,
15]. We have shown in a previous work how to provide the specificity of B-cell targeting by engineered protein chimeric molecules which crosslink the surface antigen-binding receptors with the inhibitory B-cell receptors [
16,
17,
18]. These engineered molecules contain a monoclonal antibody specific to CD35 conjugated to peptide epitopes derived from basic autoantigens. The chimeras were applied in experimental models of systemic lupus erythematosus and type 1 diabetes mellitus and transduced a strong negative signal to disease-associated B cells through the targeted receptors.
The aim of our study was the selective downmodulation of autoreactive anti-Tg B lymphocytes from HT patients with an engineered protein molecule consisting of an anti-CR1 monoclonal antibody coupled to peptide epitopes derived from human Tg.
3. Discussion
The disease pathogenesis and secondary markers of tissue damage have been elucidated in most autoimmune diseases, but the main question of which specific antigens are the targets that initiate the autoimmune process still exist. Under normal physiological conditions, small disruptions of the follicles in the thyroid gland result in increased Tg leakage and release into the circulation along with T3 and T4. The Tg molecule exhibits great variability due to the large number of point mutations that do not affect function but might increase Tg immunogenicity. Several pathogenic Tg epitopes have been identified using computer algorithms to predict MHC-binding Tg peptides or elution of human MHC-bound peptides [
5,
6].
B-cell epitopes are basically located away from the cysteine-rich regions of the tandem sequence repetition suggesting the lapse of normal processing and presentation of these peptides to the immune system and involving of additional mechanisms for cryptic epitope presentation. A number of T-cell immunopathogenic nondominant epitope-bearing peptides have also been identified. Most of them overlap the HT-associated pathological B-cell epitopes situated within the acetylcholinesterase-homologous domain of Tg, while the other T-cell epitopes are within cysteine-rich repeats. The features of these epitopes suggest that HT development might be associated with the unmasking of cryptic epitopes, and whose antigen presentation would break the tolerance to Tg [
21]. Similar overlapping of T-cell epitopes within the frame of B-cell epitopes and presentation by autoreactive B cells resulted in blocking or inhibition of antigen-presentation to the respective T-cell clones in a model of human diabetes mellitus type 1 [
18,
22].
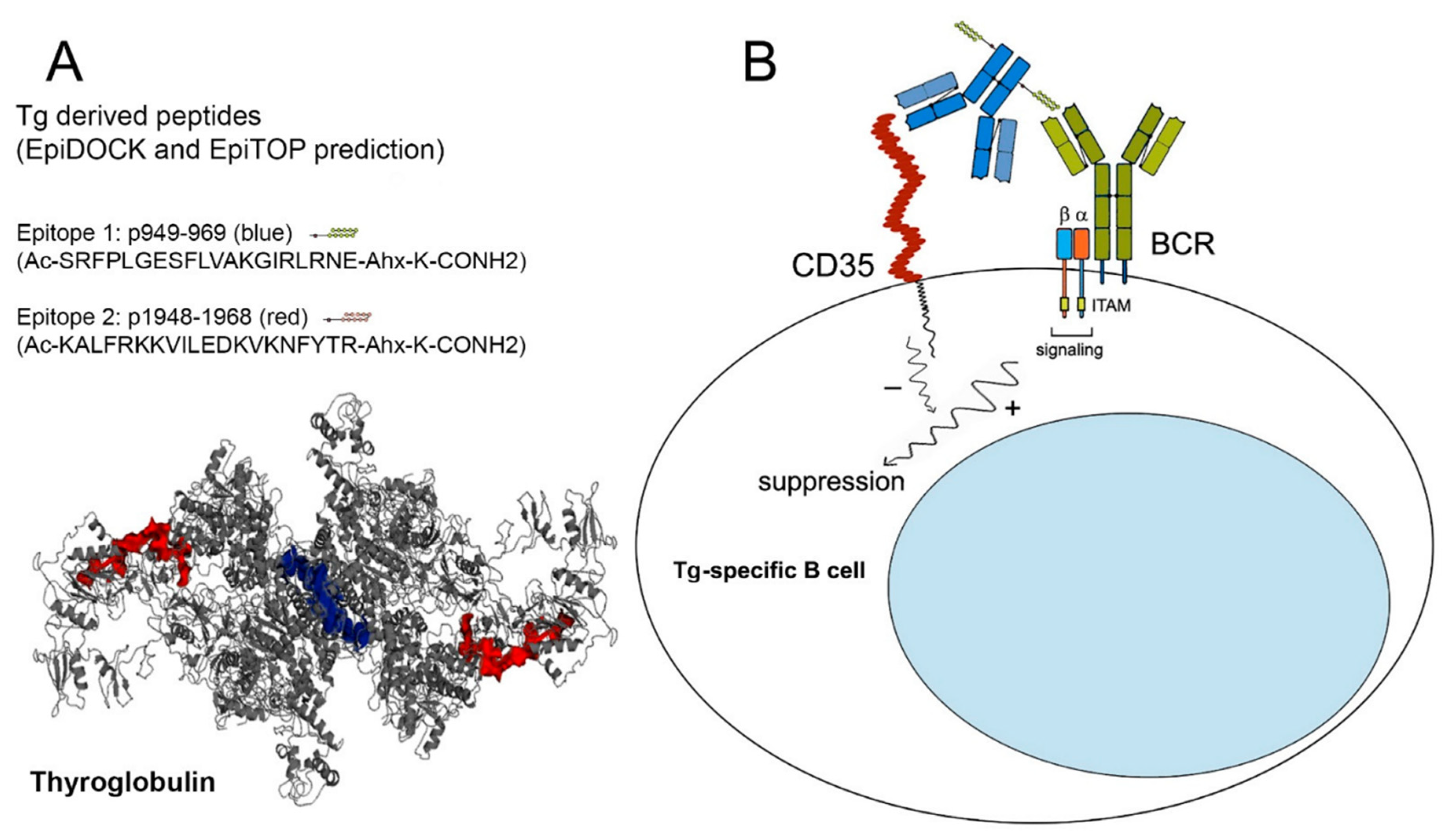
Using the platforms EpiTOP and EpiDOCK, we predicted six T-cell HLA class II protein binding peptides in the sequence of human Tg with possible susceptibility to AITD [
19]. Two of them—p949—Tg1 (eight DR3, DR4, DR5, DQ8 binders) and p1948—Tg2 (six DR4, DR5, DQ2 binders) were selected in the present study for specific targeting to Tg-recognizing B cells. Both peptides were recognized by the sera from HT patients and inhibited the interaction of anti-Tg IgG antibodies from the same sera with thyroid antigens. Indeed, using two Tg peptide epitopes for sera preincubation made it possible to block only the two respective antibody clones, while the real number of potential anti-Tg antibodies recognizing various epitopes is much higher. For this reason, we cannot expect the total suppression of anti-Tg binding.
The lack of effective treatment of HT draws attention to the regulation of the pathological immune response. The activity of B cells involved in the autoimmune process may be affected by binding specific cellular surface molecules. The monoclonal antibody rituximab recognizes CD20 molecules on B cells and contributes to their depletion by complement and Fc receptor-mediated lysis. It has been used with beneficial effect for the treatment of autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and SL, but this therapy is not successful in autoimmune thyroiditis [
23,
24,
25,
26,
27]. Rituximab is only used to treat selected patients with thyroid-associated orbitopathy, suggesting participation of antibody presentation by B cells [
28]. Moreover, the application of rituximab is limited due to the lack of expression on B-cell precursors and long-term plasma cells [
29].
Another possible option is B-cell suppression by either anti-CD22 antibody (epratuzumab) or antibody blocking of the Lympho-Stat-B, B-cell Activating Factor (BAFF) or a proliferation-inducing ligand (APRIL), which are under investigation for patients with rheumatoid arthritis and lupus but are without application to HT yet [
30,
31,
32,
33]. These nonspecific approaches for total B-cell depletion may result in partial immune deficiency and subsequent emergence of new-onset autoimmune disorders. To increase the specificity of B-cell downregulation, a crosslinking of surface immunoglobulin receptors (BCR) with inhibitory B-cell receptors could be accomplished using artificial chimeric molecules.
We have shown earlier the selective suppression of pathologic human B cells in other autoimmune diseases such as systemic lupus (SLE) and type 1 diabetes mellitus (T1DM) by protein chimeric molecules. These engineered antibodies consist of multiple peptide epitopes recognized in SLE and T1DM and coupled to an anti-human CD35 monoclonal antibody that crosslinks their inhibitory CD35 with BCRs. The administration of SLE- or T1DM-chimeric molecules resulted in a significant reduction in anti-dsDNA or anti-GAD65 IgG antibody-producing plasma cells, inhibition of disease-associated cell proliferation, induction of B- and T-lymphocyte apoptosis as well as reduction in proteinuria and glomerular deposition of human IgG immune complexes in humanized immunodeficient SCID mice reconstituted with PBMCs from SLE patients [
16,
17,
18].
In the present study, we explored the ability of two newly generated protein chimeras consisting of Tg-derived peptide epitopes (Tg1 and Tg2) conjugated to a human anti-CD35 monoclonal antibody to suppress Tg-specific HT-associated B cells producing anti-Tg antibodies.
We needed to prove the functional activity of both elements of the designed chimeric constructs by FACS and ELISA since the antibody affinity or epitope availability could be affected as a result of peptide conjugation. We expected specific binding preferences and higher recognition avidity of the constructed chimeric molecules to Tg-specific B cells due to the additive affinity to both cell receptors (CD35 and BCR) rather than single binding with any protein chimeric element. Indeed, the Tg1/2 chimeras were able to crosslink both targeting receptors and bound preferentially to Tg-specific CD19+ B cells isolated from HT patients. The inhibition effect to CD35-FITC binding or positive cell binding (as % bound cells) was the same as unconjugated anti-CD35 antibody. No specific binding was observed within CD3+ cells either from HT patients or healthy donors.
Further on, we exploited the accessibility of the Tg-derived peptides comprising T-cell epitopes as part of the engineered chimeric molecules for interaction with anti-Tg antibodies. As expected, both chimeric molecules interacted primarily with the HT patients’ sera and not the healthy donors’ sera. The control peptide as part of the Control chimera was recognized with lower intensity from either IgG antibodies in the sera of patients or healthy donors. This can be explained by the generation of a large set of poly-specific antibodies characteristic of autoimmune diseases.
The coupled action of disease-associated B and T lymphocytes could be disturbed by the Tg1/2 chimeras through several mechanisms: interruption of B/T-cell communication with blocking of antigen presentation from Tg-specific B lymphocytes; inhibition of B-cell differentiation to plasmacytes; or restriction of antibody production from the plasma cells. In silico prediction of these potential epitopes and the generation of bivalent engineered molecules allows for a selective approach for the elimination of the persisting Tg-specific B lymphocytes without nonspecific suppression of the remaining B-cell pool. In fact, the in vitro treatment of TMCs isolated from HT patients with Tg 1/2 chimeras significantly suppressed the total cell proliferation in a dose-dependent manner with significance in higher doses, while the Control chimera did not exhibit any inhibitory effect. In contrast, the same treatment suppressed significantly the number of IgG anti-Tg antibody-producing plasma cells with lowest Tg 1/2 chimera concentration, while a nonspecific suppressive effect was found using the highest concentration of the Control chimera. The silencing of pathological Tg-specific B cells by the Tg 1/2 chimeric antibodies is expected to bring down the activation state as well as the numbers of respective Tg-specific T cells.
It was very important to find the minimal treatment dose affecting all potential targets—Tg-specific autoreactive B cells. Using the minimal dose of Tg1/2 chimera, enough for bi-specific engagement of pathological Tg-specific B cells only, we can observe the suppressive effect on them. An increase in Tg1/2 chimera quantity with excess over the Tg-specific B cells allows nonspecific chimera binding to all CR1-expressing cells, resulting in Tg1/2 epitope presentation and opposite disease-stimulating effects.
No effect was observed in MTT and ELISpot assays after the exposure of PBMCs from healthy donors to the Tg 1/2 or Control chimeric molecules.
A number of B-cell abnormalities such as overactivation and lymphopenia are found in systemic lupus [
34] and T1DM [
35]. The phenotype analysis of Tg-specific B cells in the peripheral blood of AITD patients showed reduced numbers of anergic Tg-specific B cells in blood and increased expression of CD86 compared to healthy controls, suggesting over-activation and increased capacity for antigen-presentation to Tg-specific autoreactive T cells [
36]. The co-culturing of patients’ PBMCs with Tg1/2 chimeras increased significantly the late apoptosis levels (Annexin V/PI-double positive) of B cells, while the Control chimera had no effect on the same cells. The opposite effect was observed on the early apoptosis rate, suggesting a specific interaction of Tg-specific B cells.
Tg 1/2 or Control chimeras did not bind to CD3+ T lymphocytes, but a nonsignificantly increased percentage of late apoptosis was found within CD3+ cells after exposure of PBMCs from HT patients to chimeric molecules. The apoptosis of Tg 1/2 chimera-treated patients’ T cells might be explained by an inhibited antigen presentation by the suppressed Tg-specific B cells, while the effect on the same cells co-cultured with the Control chimera could be explained by a nonspecific binding to various overactivated B cells under in vitro conditions and suppressed signals to T cells.
The significant difference observed by us at low concentrations on the studied chimeras may be indicative of the specificity of the chimeras and the efficient receptor co-ligation. At low concentrations, the chimeric constructs bind more and predominantly to autoreactive B cells. With an excess of chimeras at higher concentrations, specificity and selectivity of binding was probably lost and the probability of simultaneous engagement of multiple CR1 on the same B cell and suppression of its activity increased. Neither B nor T cells from healthy donors were significantly affected by chimera treatment.
This study has several limitations. We cannot expect to suppress the whole spectrum of autoreactive lymphocytes during HT progression with engineered molecules comprising only two Tg epitopes, even with the antigen-spreading phenomenon, but the precise suppression of B cells with these epitope specificities provides an opportunity for future multi-epitope delivery. We can expect some weak nonsignificant inhibition in the control samples. The reason for this result is that in both Tg1/2 chimeras as well as in the Control chimera, the anti-CR1 antibody can bind any CR1-positive cell. The presence of Tg-specific autoreactive B cells would engage the Tg1/2 chimeras for both active parts and crosslink CR1 and BCR, providing the specificity of binding.
Another issue that cannot be addressed by the study design is the frequency of point mutations in the Tg molecule and the nonpredictability of epitope variability in the particular patient. Nevertheless, moving to in vivo experiments, we might enhance the effectiveness of targeting the specific Tg-recognizing B cells by Tg1/2 chimeras in real conditions through their bivalency. Furthermore, other thyroid antigens such as thyroid peroxidase, for instance, are involved in thyroid autoimmunity and should be addressed as well.
4. Materials and Methods
4.1. Monoclonal Antibodies
Mouse anti-human CD35 monoclonal IgG1 antibody (clone 3D9) was prepared as described [
17]. FITC-conjugated mouse IgG1 Isotype Control (eBioscience, Frankfurt, Germany) was used for FACS experiments. Anti-human CD35-FITC (clone 3D9, kindly provided by Dr. J. Prechl, Diagnosticum zrt, Budapest, Hungary), anti-human CD19-eFlour450, CD3-PeCy5, CD3-PeCy7 (eBioscience), and anti-mouse IgG-FITC (Invitrogen, Waltham, Massachusetts, USA) antibodies were used for FACS experiments. Mouse IgG1 kappa-eFluor 450 and IgG1 kappa-PE-Cy7 Isotype Controls (eBioscience) were used also for FACS experiments.
4.2. Prediction of Peptide Binding to MHC
The servers EpiTOP and EpiDOCK were used for prediction of the Tg peptides binding to HLA-DR3 (DRB1*03:01), HLA-DR4 (DRB1*04:01, DRB1*04:04 and DRB1*04:05), HLA-DR5 (DRB1*11:01 and DRB1*12:01), HLA-DQ2 (DQA1*05:01/DQB1*02:01), and HLA-DQ8 (DQA1*03:01/DQB1*03:02) as described [
19]. The proteochemometrics-based peptide binding prediction server EpiTOP [
37,
38] could predict binding to 24 most frequent human HLA-DR, -DQ, and -DP alleles [
39] and is freely accessible at
http://www.ddg-pharmfac.net/EpiTOP3 (22 March 2020). The resultant affinity is presented as pIC
50 (−logIC
50), wherein the IC50 is the concentration that inhibits 50% of the binding of a radiolabeled standard peptide to MHC molecules.
Another server for peptide binding prediction, EpiDOCK, predicts binding to 23 most frequent HLA-DR, -DQ, and -DP alleles based on quantitative matrices derived by molecular docking of peptide combinatorial libraries on HLA class II proteins [
40]. The predicted affinity is evaluated as a score assessing the free energy of binding. Both servers predict the peptide binding core consisted of nine successive amino acid residues.
4.3. HT Patients and Healthy Donors
Blood and Thyroid Tissue Samples
Patients diagnosed with HT (n = 14) with high titers of anti-Tg and anti-TPO IgG antibodies, typical ultrasound features, and hypothyroidism were included in the study (female to male ratio 13:1; age range 25–60). The control samples were obtained from five age- and sex-matched healthy blood donors. The study (grant KP-06-H33/15/2019 from the National Science Fund, Bulgaria) was approved by the Local institutional ethics committee at USBALE “Acad. Ivan Penchev” (Standpoint 10A, Protocol 1/11 March 2020) and all subjects signed an informed consent.
Venous blood was collected from HT patients and healthy individuals in sterile blood tubes (BD Vacutainer, Franklin Lakes, NJ, USA). Serum samples of 5 mL were collected from each subject using serum separator tubes (Vacutainer BD-Plymouth.PL67BP.UK, 5 mL) and stored at −80 °C.
Purified PBMCs were obtained from heparinized venous blood of both HT patients and healthy volunteers by Histopaque (PAN Biotech, Aidenbach, Germany) gradient separation at 800× g for 20 min at 20 °C and were then cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich) supplemented with 10% FCS, NaHCO3, antibiotics, and 4 mM L-glutamine at 37 °C in 5% CO2.
TMCs from HT patients undergoing thyroidectomy were isolated by grinding the gland fragments obtained during surgery (n = 4) through a sterile steel mesh and 70 µm cell strainers and then following the gradient separation protocol for PBMCs.
4.4. Thyroid Gland Lysate Preparation
Human thyroid tissue samples 1–3 cm3 in volume were harvested during thyroidectomy from subjects without HT. The samples were placed immediately in sterile media and transported to the laboratory for processing. The freshly obtained human thyroid gland samples were cut into pieces, which were placed in liquid nitrogen. Later, the tissue was homogenized by mechanical disruption on ice in 500–1000 µL PBS with a 4 mM protease inhibitor (PefaBloc, Roche, Basel, Switzerland), and 1 mM EDTA (Sigma-Aldrich, Taufkirchen, Germany). Tissue extracts were centrifuged at 17,000× g for 5 min at 4 °C and the supernatant was measured spectrophotometrically at 280 nm.
4.5. Assembling the Chimeric Molecules Containing Tg Epitopes and Anti-CR1 Antibody
Two peptides from the human Tg were selected for chimera construction: p949-969 (SRFPLGESFLVAKGIRLRNE) and p1948-1968 (KALFRKKVILEDKVKNFYTR). The peptides were synthesized with >98% purity (Caslo Laboratory, Lyngby, Denmark), and during the process a lysine-carrying linker (-Ahx-K-CONH2) was introduced to the C-end. A control peptide containing the same number of amino acids with nonsense sequence was also synthesized.
The three chimeras were engineered by separate chemical coupling of the peptides to anti-CR1 antibody as previously described [
17,
41]. Briefly, the monoclonal antibody and peptide solutions were mixed at a 20-fold molar excess of the peptides, and a zero-length crosslinking agent EDC (1-ethyl-3-(3′-dimethylaminopropyl) carbodiimide.HCl), (Sigma) was added for initiation of the coupling reaction at a 60-fold molar excess relative to the antibody. The ε-amino groups from the lysine residues of the peptide linker could interact with the available free carboxyl groups from the antibody structure. The engineered chimeras were as follows: a Tg1 chimera, a Tg2 chimera (containing the p949-969 or p1948-1968, respectively), and a Control chimera (containing the control peptide) were purified and concentrated.
4.6. Tg Peptide Epitopes Recognition and Anti-Tg Antibody Binding Inhibition
The thyroid gland lysate was tested for recognition by the anti-Tg antibodies in sera from the HT patients by ELISA. The lysate was diluted to 10 μg/mL in a coating buffer (NaHCO3, pH-9.6) and was then used for coating of immunoplates (Maxisorp, Nunc, Roskilde, Denmark) for 12h/4 °C. Next, the plates were washed with PBS/0.05% Tween 20 (T-PBS) and blocked with 1% bovine serum albumin (BSA) in T-PBS for 2 h at room temperature, followed by three washes. Serum samples from HT patients and healthy donors diluted 1:50 in T-PBS were added and incubated for 1h at RT followed by incubation with alkaline phosphatase-labeled goat anti-human IgG (Sigma-Aldrich). After washing, a phosphatase substrate (1 mg/mL pNPP, Sigma) was added and the absorbance was measured at 405 nm. The obtained results were presented as optical density (OD), corresponding to the titer of anti-Tg IgG antibodies.
Next, a competitive ELISA for epitope recognition was performed. Using the same experimental design, the sera from HT patients and healthy donors were preincubated with Tg1/2 peptide epitope mix (10 mM) or with the same quantity of control peptide for 1h/RT. The ELISA was then performed as described above.
4.7. Flow Cytometry Analysis
A competition binding assay for CD35 was performed using isolated PBMCs from HT patients. The cells (2 × 106 cells/mL) were incubated with either Tg1, Tg2, the Control chimeras, with an unconjugated anti-CD35 MoAb (1 µg/106 cells), or with PBS alone for 30 min at 4 °C, followed by two washes with a FACS buffer (PBS containing 2.5% FCS and 0.05% sodium azide). Subsequently, the lymphocytes were incubated with FITC-conjugated anti-human CD35 antibody combined with anti-human anti-CD19-eFluor450 or with anti-CD3 PE-Cy7 antibodies for 30 min at 4 °C.
In an independent experiment, PBMCs from HT patients were incubated with Tg1/Tg2 chimeras, with unconjugated anti-CD35 MoAb, or with PBS alone for 20 min at 4 °C, followed by incubation with anti-mouse IgG FITC antibody combined with anti-human anti-CD19-eFluor450 or with anti-CD3 PE-Cy7 antibodies for 30 min at 4 °C.
Next, after two washes, 50,000 cells from each sample were analyzed by a BD LSR II flow cytometer (BD Biosciences, Mountain View, CA) using Weasel v3.7.1. software (from Frank Battye).
4.8. ELISA for Tg-Epitope Recognition
To check the availability of Tg 1 and 2 epitopes in the composition of chimeric molecules for B-cell receptors, we performed an ELISA epitope recognition test. Tg1, Tg2, and the Control chimeras (10 µg/mL in coating buffer) were coated in 96-well immunoplates (Maxisorp, Nunc, Roskilde, Denmark) for 12 h at 4 °C. Further, the plates were blocked, washed, and incubated with pre-diluted (1:50 in T-PBS) sera from HT patients or healthy volunteers for 1 h at room temperature. Later, the ELISA development was performed as described above and the Tg1 and Tg2 peptide recognition was assessed spectrophotometrically.
4.9. Suppression of Proliferation Assay
PBMCs and TMCs from HT patients or PBMCs from healthy donors (2 × 106 cells/mL) were incubated with increasing concentrations of the Tg 1/2 chimera mix, or with the Control chimera (8 ng/mL, 40 ng/mL, 200 ng/mL, 1000 ng/mL) in 96-well culture plates for 4 days. The control cells were stimulated with 50 ng/mL PMA (phorbol myristate acetate) plus 0.75 µg/mL Ionomycin, or cultured in a medium only. MTT was then added to the wells and the plates were incubated for further 4 h. The plates were centrifuged at 700 RPM for 10 min, the supernatant was aspirated, and dimethyl sulfoxide was added to each well. The absorbance of the dissolved formazan crystals was measured at 595 nm and the background subtracted at 620 nm.
4.10. ELISpot (Enzyme Linked Immunospot) Assay
To count the anti-Tg IgG antibody-secreting plasma cells we used the same experimental design as in the proliferation assay for the cultivation and treatment of PBMCs isolated from HT patients and healthy volunteers for 24 h. The cells were stimulated with 3 μg/mL CpG (InvivoGen, Toulouse, France) plus 5 μg/mL LPS (from E. coli, Sigma) for 3 additional days. The control samples were cultured in RPMI medium only. Later, the pre-incubated PBMCs were transferred to the respective wells in a 96-well ELISpot plate (Millipore, Bedford, MA, USA) pre-coated with 5 μg/mL Tg1/2 peptides (2.5 μg Tg1 + 2.5 μg Tg2) for 12–16 h at 4 °C and were further cultured for additional 4 h in a humidified 5% CO2 atmosphere at 37 °C. After extensive washing, the samples were incubated with an alkaline phosphatase-conjugated anti-human IgG antibody for 1 h and developed by NBT/BCIP substrate (Sigma). The number of colored spots corresponding to anti-Tg IgG antibody-producing plasmocytes was counted by C.T.L.Immunospot S6 Ultimate UV Image Analyzer (Bonn, Germany). Each donor (patient or healthy volunteer) was tested separately.
4.11. Apoptotic Assay
For apoptosis evaluation of PBMCs from HT patients and healthy individuals, the isolated cells (2 × 106 cells/mL in complete RPMI-1640 mediums) were incubated for 2 days with increasing concentrations of the Tg1/2 chimeras or the Control chimera (8, 40, 200, 1000 ng/mL) at 37 °C/5% CO2. The untreated cells were used as controls. The collected cells were washed and stained with anti-human CD19-eFlour450 or anti-human CD3-PeCy7 antibodies together with Annexin V-FITC/PI Apoptosis kit (eBioscience™ Annexin V-FITC Apoptosis Detection Kit). Subsequently, the cells were washed and the apoptosis levels were evaluated within gated B- or T-cell populations by a BD LSR II flow cytometer counting 50,000 cells from each sample and were analyzed by Diva version 6.1.1. software (BD Biosciences, Mountain View, CA, USA).
4.12. Statistical Analysis
All statistical analyses were performed with GraphPad Prism 5 software (San Diego, CA). The one-way ANOVA test and the two-tailed Student’s t-test were used to determine the differences between each two groups. Values in the figures correspond to mean ± SD. A value of p < 0.05 was considered statistically significant.