Effects of Fhb1, Fhb2 and Fhb5 on Fusarium Head Blight Resistance and the Development of Promising Lines in Winter Wheat
Abstract
:1. Introduction
2. Results
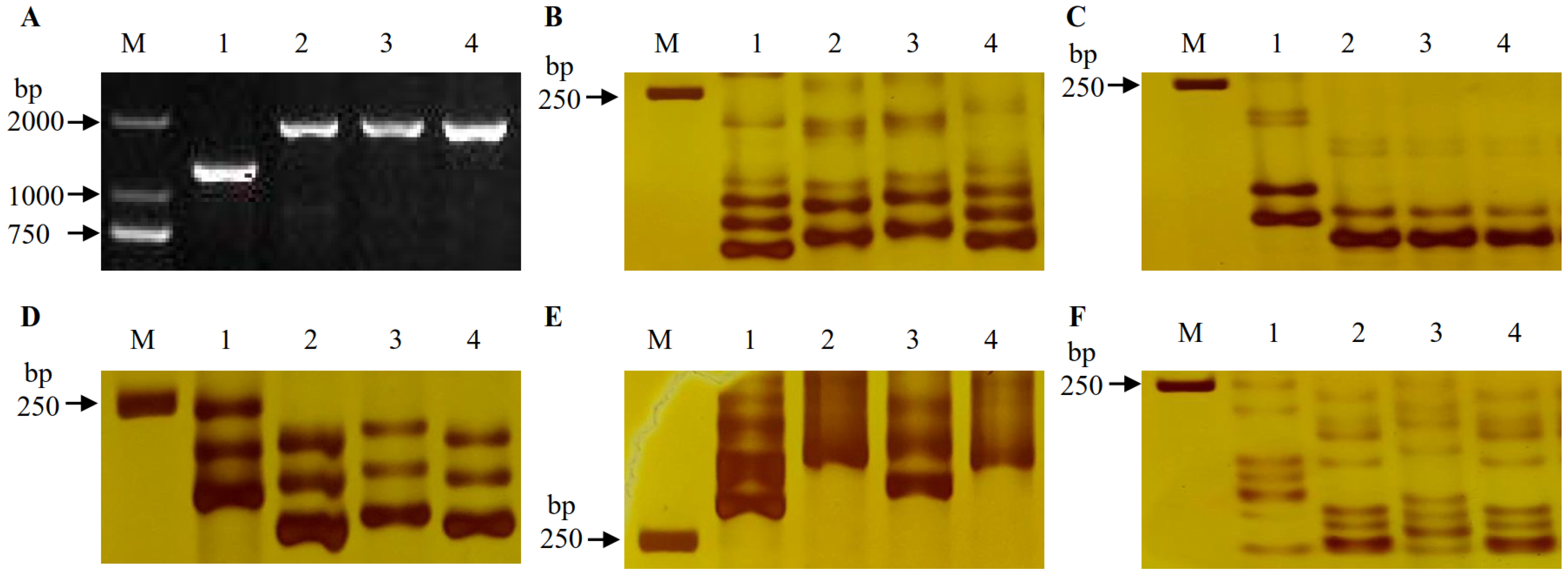
2.1. Identification of Fhb1, Fhb2 and Fhb5 in Parents and DH Lines
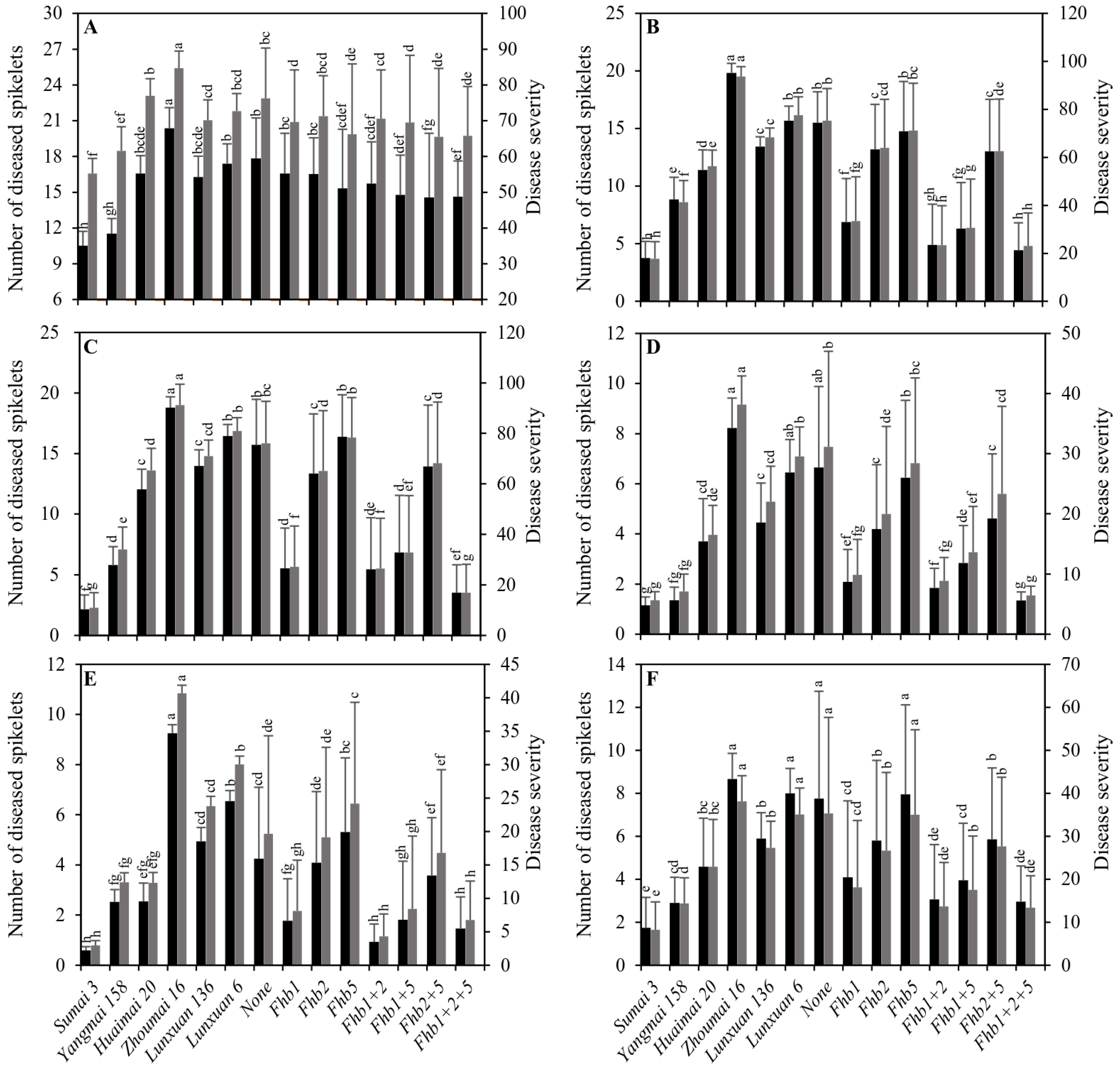
2.2. Effects of a Single Gene and Gene Combinations of Fhb1, Fhb2 and Fhb5 on the FHB Resistance
2.3. Performance of Main Agronomic Traits
2.4. Molecular Marker and Genomic Composition Analyses of the Promising DH Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Molecular Marker Detection of Genes for Resistance to FHB
4.3. Assessments of FHB Resistance
4.4. Observation of Agronomic Traits
4.5. Genomic Composition Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, G.H.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, M.; Sterner, B.; Wagner, C.; Schwarz, P.; Brugger, K.; Barabaschi, D.; Volante, A.; Giampiero, V.; Cattivelli, L.; Buerstmayr, H. High-resolution mapping of the pericentromeric region on wheat chromosome arm 5AS harboring the Fusarium head blight resistance QTL Qfhs.ifa-5A. Plant Biotech. J. 2018, 16, 1046–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.Q.; Xie, Q.; Li, G.Q.; Jia, H.Y.; Zhou, J.Y.; Kong, Z.X.; Li, N.; Yuan, Y. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020, 133, 1541–1568. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Dreisigacker, S.; Singh, R.P.; Singh, P.K. Genetics for low correlation between Fusarium head blight disease and deoxynivalenol (DON) content in a bread wheat mapping population. Theor. Appl. Genet. 2019, 132, 2401–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, F.; Zhang, D.D.; Bockus, W.; Baenziger, P.S.; Carver, B.; Bai, G.H. Fusarium head blight resistance in U.S. winter wheat cultivars and elite breeding lines. Crop Sci. 2013, 53, 2006–2013. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Kistler, H.C.; Ma, Z.H. Fusarium graminearum trichothecene mycotoxins: Biosynthesis, regulation and management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.Q.; Zhang, J.Y.; Jia, H.Y.; Guo, Z.X.; Fan, M.; Luo, Y.J.; Zhao, P.T.; Xue, S.L.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rice calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nat. Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.Y.; Zhou, J.Y.; Xue, S.L.; Li, G.Q.; Yan, H.S.; Ran, C.F.; Zhang, Y.D.; Shi, J.X.; Jia, L.; Wang, X.; et al. A journey to understand wheat Fusarium head blight resistance in Chinese wheat landrace Wangshuibai. Crop J. 2018, 6, 48–59. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.Q.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterizaiton of alien introgression with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef]
- Cainong, J.C.; Bockus, W.W.; Feng, Y.; Chen, P.; Qi, L.; Sehgal, S.K.; Danilova, T.V.; Koo, D.H.; Friebe, B.; Gill, B.S. Chromosome engineering, mapping, and transferring of resistance to Fusarium head blight disease from Elymus tsukushiensis into wheat. Theor. Appl. Genet. 2015, 128, 1019–1027. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, X.L.; Hou, Y.L.; Cai, J.J.; Shen, X.R.; Zhou, T.T.; Xu, H.H.; Ohm, H.W.; Wang, H.W.; Li, A.F.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.H.; Su, Z.Q.; Cai, J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar] [CrossRef]
- Rawat, N.; Pumphrey, M.O.; Liu, S.; Zhang, X.; Tiwari, V.K.; Ando, K.; Trick, H.N.; Bockus, W.W.; Akhunov, E.; Anderson, J.A.; et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat. Genet. 2016, 48, 1576–1580. [Google Scholar] [CrossRef]
- Su, Z.Q.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.X.; Cai, S.B.; Liu, D.T.; Zhang, D.D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Paudel, B.; Zhuang, Y.; Galla, A.; Dahal, S.; Qiu, Y.; Ma, A.; Raihan, T.; Yen, Y. WFhb1-1 plays an important role in resistance against Fusarium head blight in wheat. Sci. Rep. 2020, 10, 7794. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Sun, S.L.; Ge, W.Y.; Zhao, L.F.; Hou, B.Q.; Wang, K.; Lyu, Z.F.; Chen, L.Y.; Xu, S.S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Li, Z.; Shen, Q.; Shi, H.; Li, H.; Wang, W.; Zou, C.; Shang, H.; Li, H.; Xiao, G. Genome-wide characterization of the WAK gene family and expression analysis under plant hormone treatment in cotton. BMC Genom. 2021, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Giancaspro, A.; Lionetti, V.; Giove, S.L.; Zito, D.; Fabri, E.; Reem, N.; Zabotina, O.A.; Angelis, E.D.; Monaci, L.; Bellincampi, D.; et al. Cell wall features transferred from common into durum wheat to improve Fusarium head blight resistance. Plant Sci. 2018, 274, 121–128. [Google Scholar] [CrossRef]
- Gadaleta, A.; Colasuonno, P.; Giove, S.L.; Blanco, A.; Giancaspro, A. Map-based cloning of QFhb.mgb-2A identifies a WAK2 gene responsible for Fusarium head blight resistance inwheat. Sci. Rep. 2019, 9, 6929. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.W.; Hao, Y.F.; Mergoum, M.; Bai, G.H.; Humphreys, G.; Cloutier, S.; Xia, X.C.; He, Z.H. Breeding wheat for resistance to Fusarium head blight in the Global North: China, USA, and Canada. Crop J. 2019, 7, 730–738. [Google Scholar] [CrossRef]
- Clark, A.J.; Sarti-Dvorjak, D.; Brown-Guedira, G.; Dong, Y.; Baik, B.K.; van Sanford, D.A. Identifying rare FHB-resistant segregants in intransigent backcross and F2 winter wheat populations. Front. Microbiol. 2016, 7, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Zhang, H.J.; Huang, Y.W.; Su, Z.Q.; Deng, Y.; Liu, H.W.; Mai, C.Y.; Yu, G.J.; Li, H.L.; Yu, L.Q.; et al. Effects of the Fhb1 gene on Fusarium head blight resistance and agronomic traits of winter wheat. Crop J. 2019, 7, 799–808. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2020, 139, 429–454. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Yang, Z.B.; Ma, H.C.; Huang, L.Y.; Ding, F.; Du, Y.Y.; Jia, H.Y.; Li, G.Q.; Kong, Z.X.; Ran, C.F.; et al. Pyramiding of Fusarium head blight resistance quantitative trait loci, Fhb1, Fhb4, and Fhb5, in modern Chinese wheat cultivars. Front. Plant Sci. 2021, 12, 694023. [Google Scholar] [CrossRef]
- Dai, X.R.; Huang, Y.W.; Li, T.; Deng, Y.; Su, Y.; Mai, C.Y.; Yu, L.Q.; Li, H.L.; Liu, H.W.; Yang, L.; et al. Improvement of resistance to Fusarium head blight by Fhb1 molecular marker-assisted backcrossing in the Huang-Huang River Valley Winter Wheat Region. J. Triticeae Crops 2021, 41, 1081–1089. [Google Scholar]
- Zhang, H.J.; Su, Z.Q.; Bai, G.H.; Zhang, X.; Ma, H.Q.; Li, T.; Deng, Y.; Mai, C.Y.; Yu, L.Q.; Liu, H.W.; et al. Improvement of resistance of wheat cultivars to Fusarium head blight in the Yellow-Huai River Valleys Winter Wheat Zone with functional marker selection of Fhb1 gene. Acta Agron. Sin. 2018, 44, 505–511. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.J.; Wang, C.Y.; Li, H.J.; Mai, C.Y.; Yang, L.; Liu, H.W.; Yu, L.Q.; Yu, G.J.; Liu, B.H. Dwarf-male-sterile wheat and its application in breeding program for Fusarium head blight resistance in Southern Yellow and Huai River Valleys Winter Wheat Region. Acta Agron. Sin. 2022, 48, 2683–2690. [Google Scholar]
- Brar, G.S.; Brûlé-Babel, A.L.; Ruan, Y.F.; Henriquez, M.A.; Pozniak, C.J.; Kutcher, H.R.; Hucl, P.J. Genetic factors affecting Fusarium head blight resistance improvement from introgression of exotic Sumai 3 alleles (including Fhb1, Fhb2, and Fhb5) in hard red spring wheat. BMC Plant Biol. 2019, 19, 179. [Google Scholar] [CrossRef]
- Hu, W.J.; Fu, L.P.; Cao, D.R.; Li, D.S.; Liao, S.; Lu, C.B. Marker-assisted selection to pyramid Fusarium head blight resistance loci Fhb1 and Fhb2 in a high-quality soft wheat cultivar Yangmai 15. J. Integr. Agr. 2022. [CrossRef]
- Rudd, J.C.; Horsley, R.D.; McKendry, A.L.; Elias, E.M. Host plant resistance genes for Fusarium head blight: Sources, mechanisms, and utility in conventional breeding systems. Crop Sci. 2001, 41, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Brar, G.S.; Pozniak, C.J.; Kutcher, H.R.; Hucl, P.J. Evaluation of Fusarium head blight resistance genes Fhb1, Fhb2, and Fhb5 introgressed into elite Canadian hard red spring wheats: Effect on agronomic and end-use quality traits and implications for breeding. Mol. Breed. 2019, 39, 44. [Google Scholar] [CrossRef]
- Su, Z.Q.; Jin, S.J.; Zhang, D.D.; Bai, G.H. Development and validation of diagnostic markers for Fhb1 region, a major QTL for Fusarium head blight resistance in wheat. Theor. Appl. Genet. 2018, 131, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, P.A.; Somers, D.J.; Brulé-Babel, A. Mapping of Fhb2 on chromosome 6BS: A gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.L.; Xu, F.; Tang, M.Z.; Zhou, Y.; Li, G.Q.; An, X.; Lin, F.; Xu, H.B.; Jia, H.Y.; Zhang, L.X.; et al. Precise mapping Fhb5, a major QTL conditionaing resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 123, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Xue, X.H.; Guo, J.; Huang, Y.W.; Dai, X.R.; Li, T.; Hu, J.H.; Qu, Y.F.; Yu, L.Q.; Mai, C.Y.; et al. Association of the recessive allele vrn-D1 with winter frost tolerance in bread wheat. Front. Plant Sci. 2022, 13, 879768. [Google Scholar] [CrossRef]
- Bai, G.H.; Shaner, G. Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis. 1996, 80, 975–979. [Google Scholar] [CrossRef]
- Bai, G.H.; Kolb, F.L.; Shaner, G.; Domier, L.L. Amplified fragment length polymorphism markers linked to a major quantitative trait locus controlling scab resistance in wheat. Phytopathology 1999, 89, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Jiang, P.; Ye, R.Y.; Wu, L.; Zhang, Y.; Ma, H.X. Evaluation and source tracing of resistance of Fusarium head blight in wheat variety Ningmai 9 and its derivatives. Mol. Plant Breed. 2017, 15, 1053–1063. [Google Scholar]
- Meng, L.Z.; Liu, H.W.; Yang, L.; Mai, C.Y.; Yu, L.Q.; Li, H.J.; Zhang, H.J.; Zhou, Y. Effects of the Vrn-D1b allele associated with facultative growth habit on agronomic traits in common wheat. Euphytica 2016, 211, 113–122. [Google Scholar] [CrossRef]
- Dixon, P.M.; Moore, K.J.; Santen, E.V. The analysis of combined experiments. In Applied Statistics in Agricultura, Biolgica, and Environmental Siences; Glaz, B., Yeater, K.M., Eds.; American Society of Agronomy, Soil Science Society of America, Crop Science Society of America: Madison, WI, USA, 2018. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Huang, Y.; Xue, X.; Yu, S.; Li, T.; Liu, H.; Yang, L.; Zhou, Y.; Li, H.; Zhang, H. Effects of Fhb1, Fhb2 and Fhb5 on Fusarium Head Blight Resistance and the Development of Promising Lines in Winter Wheat. Int. J. Mol. Sci. 2022, 23, 15047. https://doi.org/10.3390/ijms232315047
Dai X, Huang Y, Xue X, Yu S, Li T, Liu H, Yang L, Zhou Y, Li H, Zhang H. Effects of Fhb1, Fhb2 and Fhb5 on Fusarium Head Blight Resistance and the Development of Promising Lines in Winter Wheat. International Journal of Molecular Sciences. 2022; 23(23):15047. https://doi.org/10.3390/ijms232315047
Chicago/Turabian StyleDai, Xuran, Yiwen Huang, Xinhui Xue, Shuo Yu, Teng Li, Hongwei Liu, Li Yang, Yang Zhou, Hongjie Li, and Hongjun Zhang. 2022. "Effects of Fhb1, Fhb2 and Fhb5 on Fusarium Head Blight Resistance and the Development of Promising Lines in Winter Wheat" International Journal of Molecular Sciences 23, no. 23: 15047. https://doi.org/10.3390/ijms232315047
APA StyleDai, X., Huang, Y., Xue, X., Yu, S., Li, T., Liu, H., Yang, L., Zhou, Y., Li, H., & Zhang, H. (2022). Effects of Fhb1, Fhb2 and Fhb5 on Fusarium Head Blight Resistance and the Development of Promising Lines in Winter Wheat. International Journal of Molecular Sciences, 23(23), 15047. https://doi.org/10.3390/ijms232315047






