The Influence of Freeze-Dried Alcohol-Water Extracts from Common Yarrow (Achillea millefolium L.) and German Chamomile (Matricaria chamomilla L.) on the Properties of Elastomer Vulcanizates
Abstract
:1. Introduction
2. Results
2.1. Characteristic of Extracts
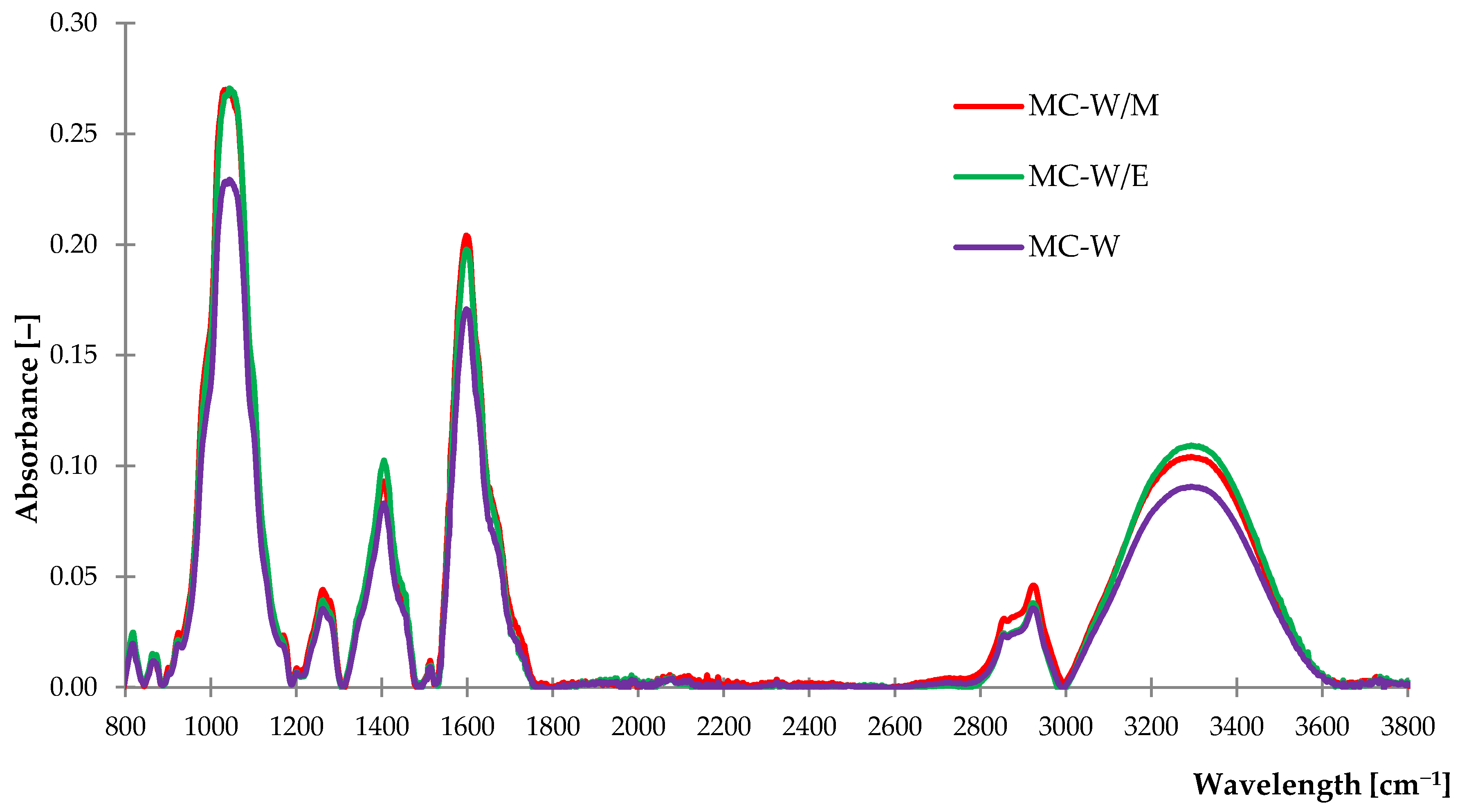
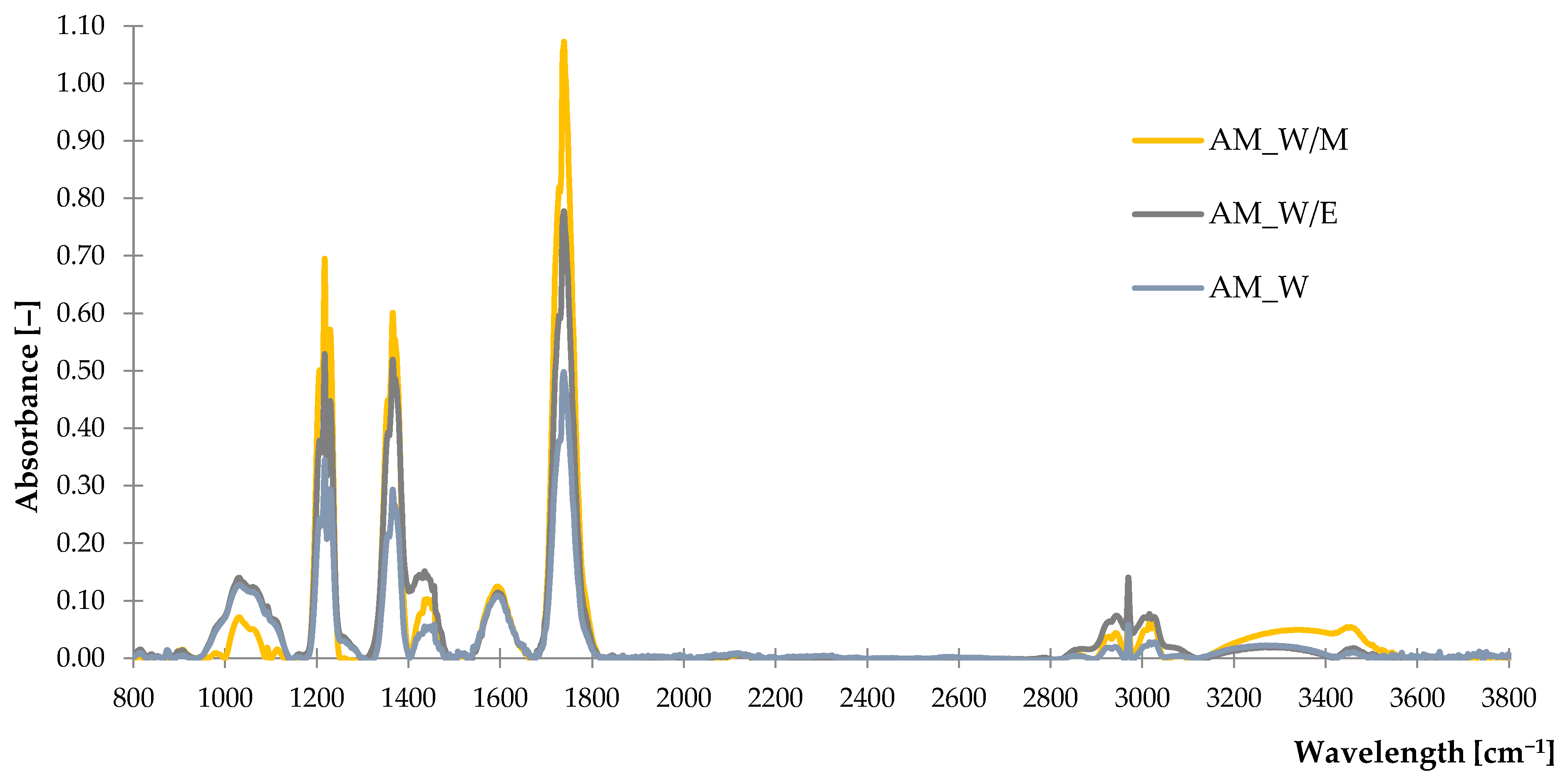
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
| Peak Assignments and Type of Vibration | Wave Number [cm−1] |
|---|---|
| v(O–H) phenols & alcohols, –C=Ow (overtone) and v(=C–Hvw) | 3600–3000 |
| vs (C–H) aliphatic and vas(–C–Hm, –CH3, –CH2) | 2970–2800 |
| v (C=O) | 1730–1690 |
| v(C=C) aryl, dvw(–CH2) and (–CH3) bending (scissoring) or vvw(–C–H) bending (rocking) | 1600–1500 |
| sv(C=C) aromatic | 1440 |
| v(O–H) bending | 1390–1310 |
| v(–C–H, –CH3) | 1372–1337 |
| v(–C–C(=O))–O | 1250–1160 |
| v(–C–O), v(–C–H), v(–O–H) | 1056 |
| vm,vw(–C–O), | 1044/1023 |
| v(C–O), (C–C) | 1020–1030 |
| v(O–C–O) v(C–C–C) | 1090–1020 900 |
2.1.2. Near-Infrared Spectroscopy (NIR)
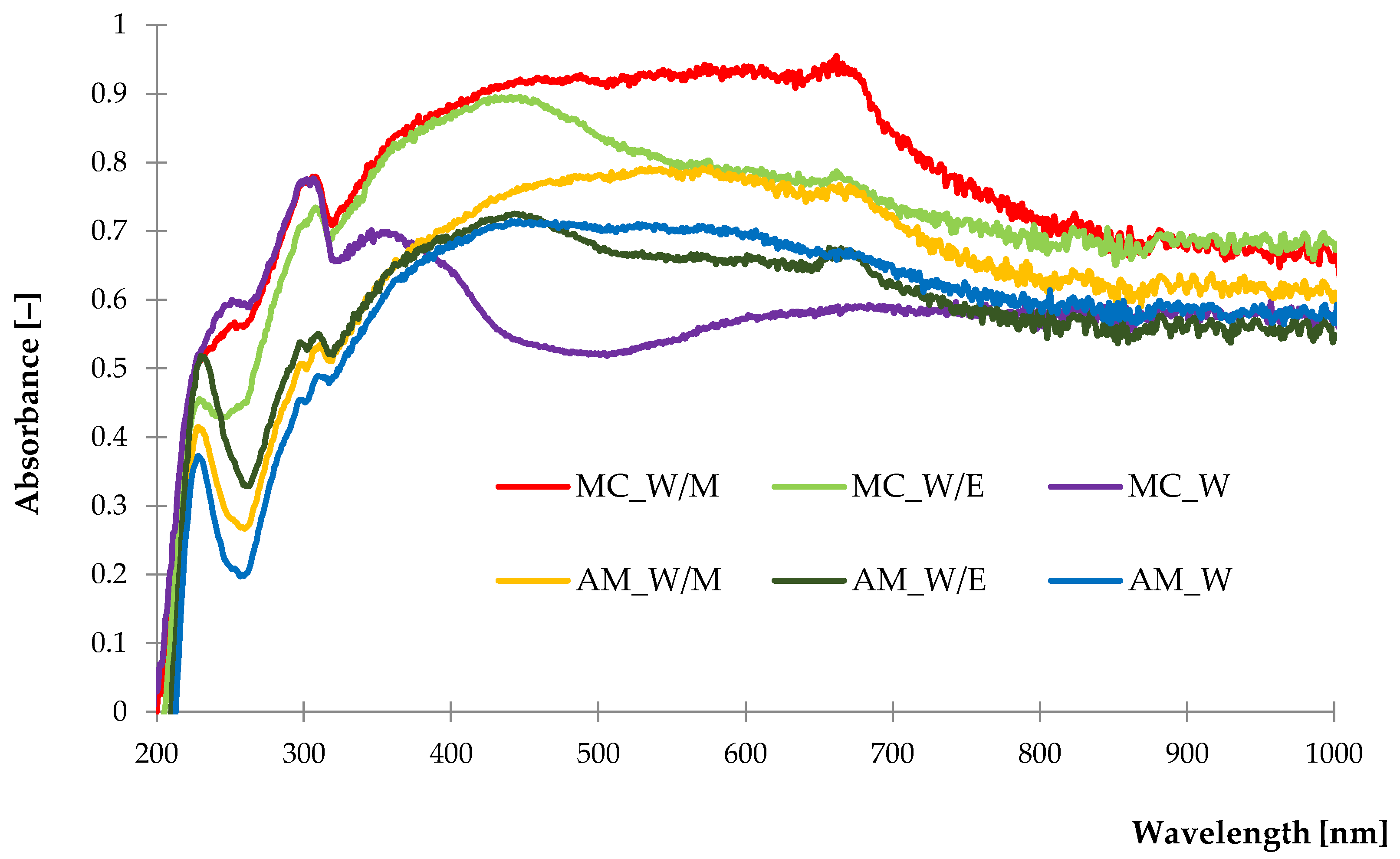
2.1.3. UV-Vis Diffuse Reflectance Spectroscopy
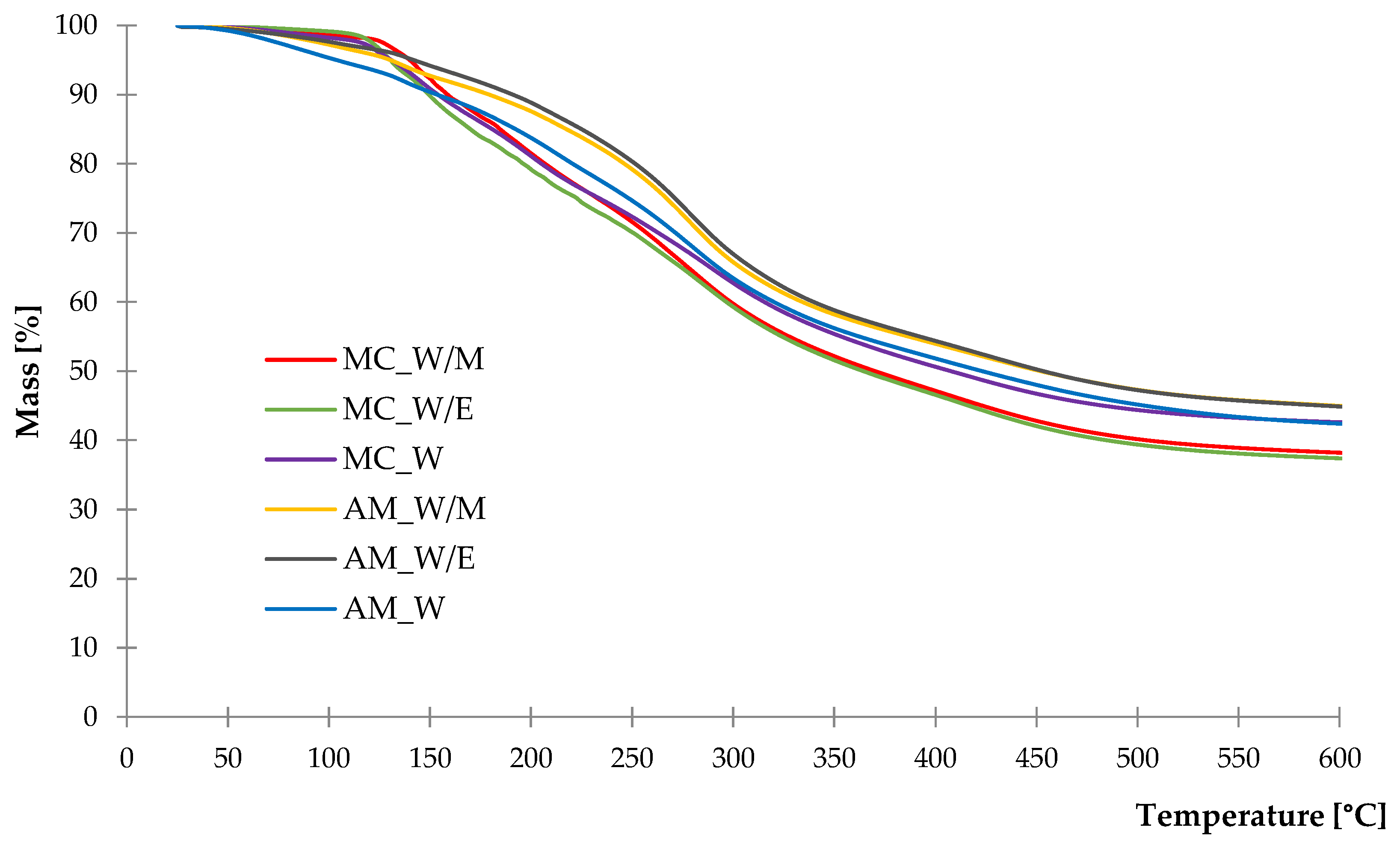
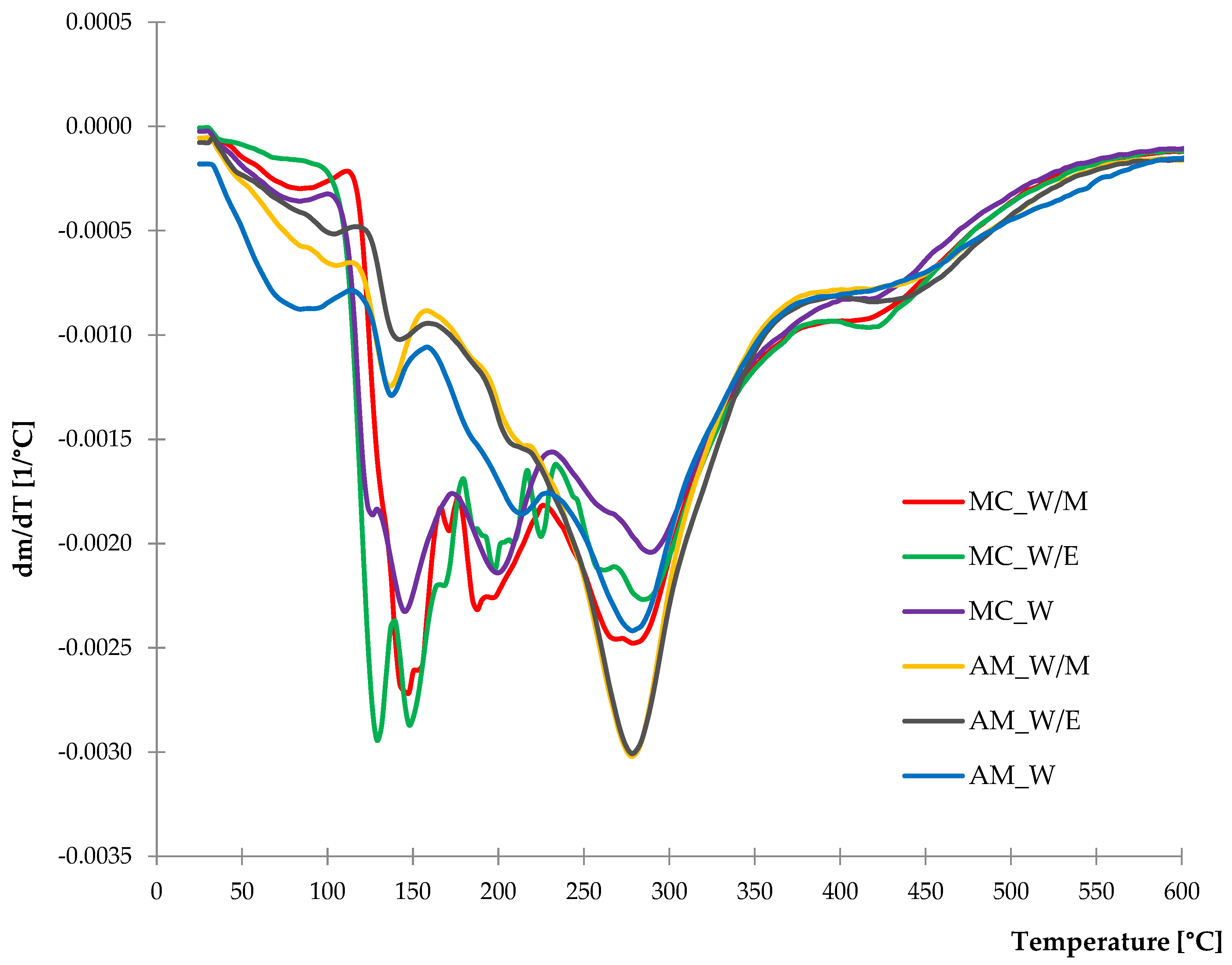
2.1.4. Thermal Stability
2.1.5. Total Phenolic Content (TPC) and Antioxidant Activity
2.2. Characteristic of Vulcanizates
2.2.1. Rheometric Properties
2.2.2. Cross-Linking Density
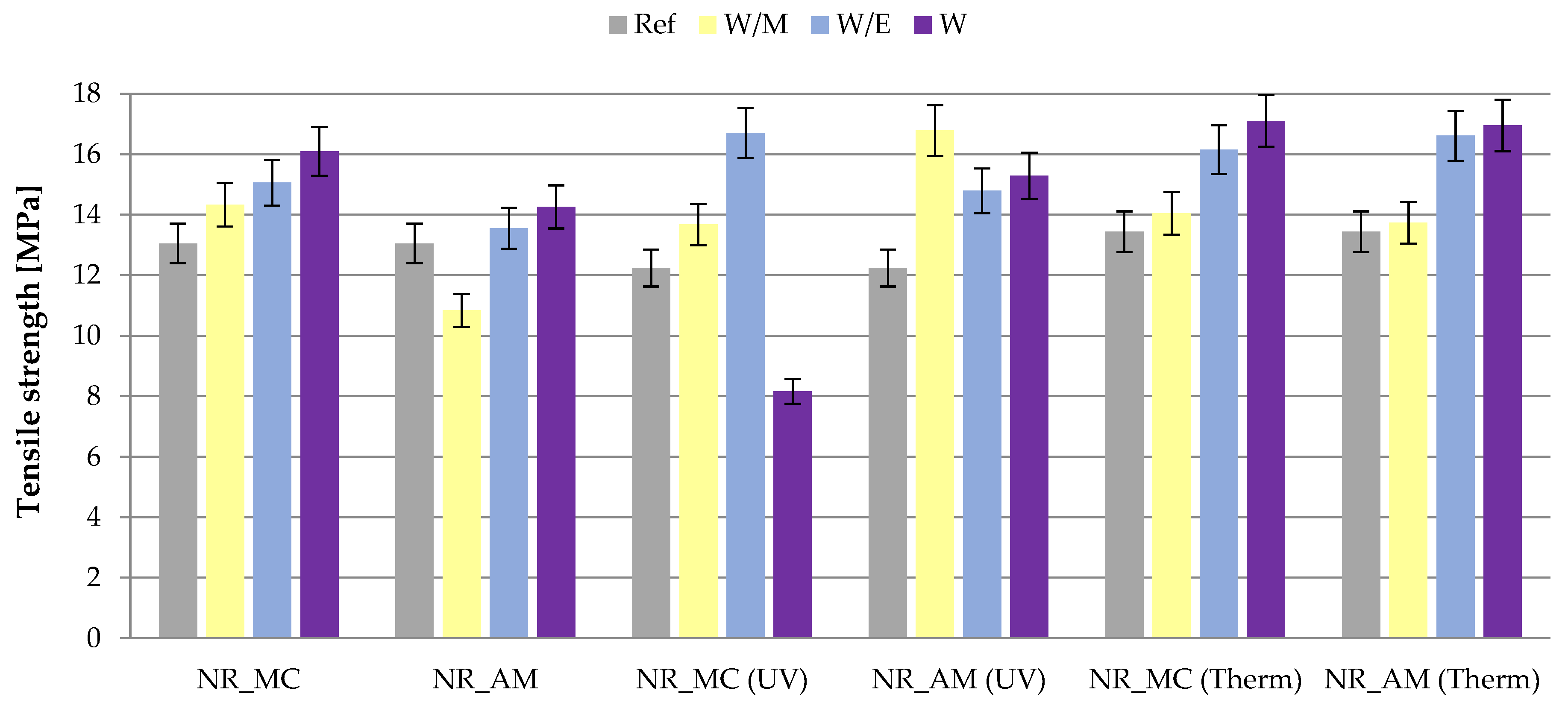
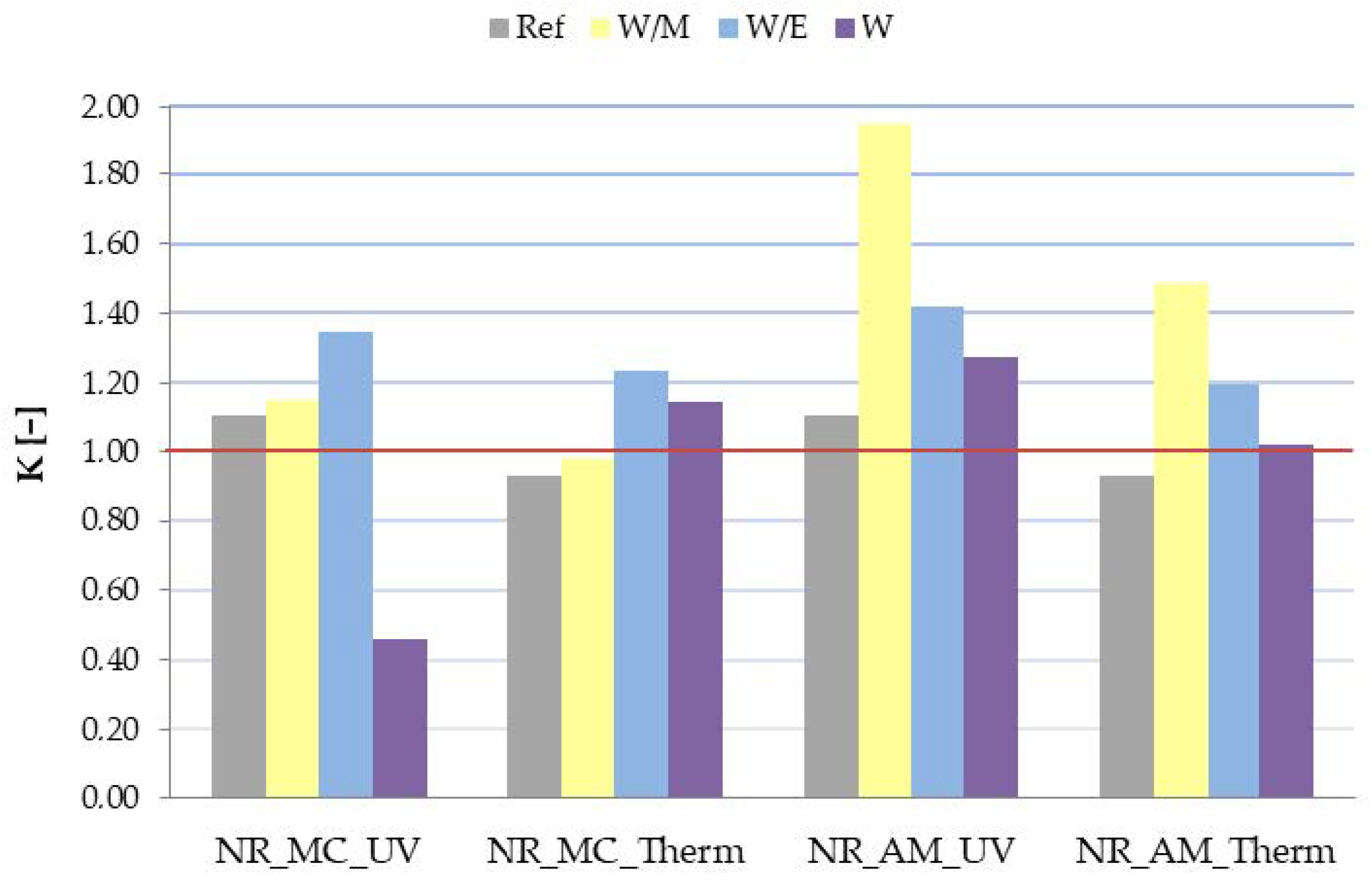
2.2.3. Mechanical Properties
2.2.4. Color Stability
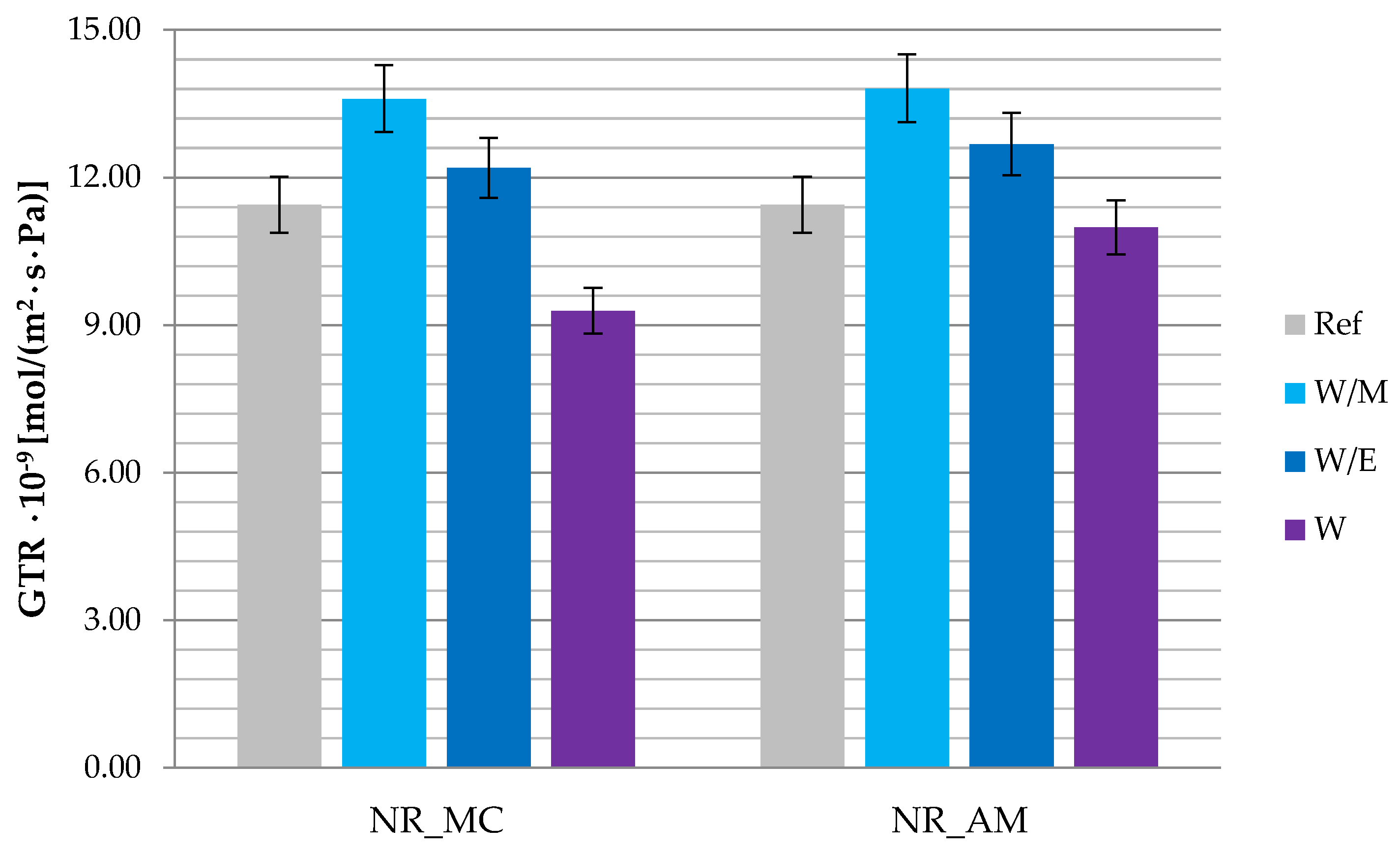
2.2.5. Barrier Properties
3. Materials and Methods
3.1. Materials
- Elastomer matrix: natural rubber RSS I (NR) provided by Torimex Chemicals Sp. z.o.o. (Konstantynów Łódzki, Poland);
- Cross-linking system: sulphur (Siarkopol, Tarnobrzeg, Poland), stearin (POCH, Gliwice, Poland), 2-mercaptobenzothiazole (MBT) (Sigma-Aldrich, Poznań, Poland), micro-sized zinc oxide (ZnO) (Huta Będzin, Poland);
- Dried plants: Matricaria chamomilla L. and Achillea millefolium L. provided by Ziołowy Zakątek (Koryciny, Poland);
- Both plants used for this study was purchased from certificated food company. Leaves of plants were harvested in the area of Podlaskie Voivodeship (Poland) before the flowering. Dried leaves were packed into paper bags. Products were ground in food chopper to obtain powders.
3.2. Preparation of Freeze-Dried Extracts
- MC-W/M—freeze-dried extract of M. chamomilla L. from water-methanol solvent;
- MC-W/E—freeze-dried extract of M. chamomilla L. from water-ethanol solvent;
- MC-W—freeze-dried extract of M. chamomilla L. from water solvent;
- AM-W/M—freeze-dried extract of A. millefolium L. from water-methanol solvent;
- AM-W/E—freeze-dried extract of A. millefolium L. from water-ethanol solvent;
- AM-W—freeze-dried extract of A. millefolium L. from water solvent.
3.3. Preparation of Rubber Mixtures and Vulcanizates
3.4. Freeze-Dried Extracts Characterization Methods
- DPPH
- ABTS
3.5. Vulcanizates Characterization Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedek, B.; Kopp, B. Achillea millefolium L. s.l. revisited: Recent findings confirm the traditional use. Wien. Med. Wochenschr. 2007, 157, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Daniel, P.S.; Lourenço, E.L.B.; Da Cruz, R.M.S.; De Souza Gonçalves, C.H.; Das Almas, L.R.M.; Hoscheid, J.; Da Silva, C.; Jacomassi, E.; Junior, L.B.; Alberton, O. Composition and antimicrobial activity of essential oil of yarrow (Achillea millefolium L.). Aust. J. Crop Sci. 2020, 14, 545–550. [Google Scholar] [CrossRef]
- Lemmens-Gruber, R.; Marchart, E.; Rawnduzi, P.; Engel, N.; Benedek, B.; Kopp, B. Investigation of the Spasmolytic Activity of the Flavonoid Fraction of Achillea millefolium s.l. on Isolated Guinea-pig Ilea. Arzneimittelforschung 2006, 56, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedek, B.; Geisz, N.; Jäger, W.; Thalhammer, T.; Kopp, B. Choleretic effects of yarrow (Achillea millefolium s.l.) in the isolated perfused rat liver. Phytomedicine 2006, 13, 702–706. [Google Scholar] [CrossRef]
- Candan, F.; Unlu, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sökmen, A.; Akpulat, H. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J. Ethnopharmacol. 2003, 87, 215–220. [Google Scholar] [CrossRef]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef]
- Yaeesh, S.; Jamal, Q.; Khan, A.-U.; Gilani, A.H. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother. Res. 2006, 20, 546–551. [Google Scholar] [CrossRef]
- Nemeth, E. Biological Activities of Yarrow Species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151–3167. [Google Scholar] [CrossRef]
- Glasl, S.; Rothwangl-Wiltschnigg, K. Yarrow (Achillea millefolium L. s. l.): Pharmaceutical quality of commercial samples. Pharmazie 2008, 63, 23–26. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU J. Pharm. Sci. 2011, 19, 173–186. [Google Scholar]
- Benedek, B.; Gjoncaj, N.; Saukel, J.; Kopp, B. Distribution of Phenolic Compounds in Middleeuropean Taxa of the Achillea millefolium L. Aggregate. Chem. Biodivers. 2007, 4, 849–857. [Google Scholar] [CrossRef]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research1. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical composition of the essential oils and extracts of Achillea species and their biological activities: A review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, T.; Geetha, R.V.; Roy, A.; Aravind Kumar, S. Yarrow (Achillea millefolium Linn.) a herbal medicinal plant with broad therapeutic use—A review. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 136–141. [Google Scholar]
- Taheri, J.B.; Azimi, S.; Rafieian, N.; Zanjani, H.A. Herbs in dentistry. Int. Dent. J. 2011, 61, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyev, A.M.; Bagirova, M.; Yaman, S.; Koc, R.C.; Abamor, E.S.; Ates, S.C.; Baydar, S.Y.; Elcicek, S.; Oztel, O.N. Development of New Antiherpetic Drugs Based on Plant Compounds. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Elsevier: Amsterdam, The Netherlands, 2013; pp. 245–259. [Google Scholar]
- Hameed, I.H.; Mohammed, G.J.; Kamal, S.A. A Review: Uses and Pharmacological Activity of Matricaria Chamomilla. Indian J. Public Health Res. Dev. 2018, 9, 200. [Google Scholar] [CrossRef]
- Gosztola, B.; Sárosi, S.; Németh, E. Variability of the Essential Oil Content and Composition of Chamomile (Matricaria recutita L.) affected by Weather Conditions. Nat. Prod. Commun. 2010, 5, 1934578X1000500325. [Google Scholar] [CrossRef] [Green Version]
- Haghi, G.; Hatami, A.; Safaei, A.; Mehran, M. Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res. Pharm. Sci. 2015, 9, 31–37. [Google Scholar]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). J. Essent. Oil Bear. Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Kapalka, G.M. Nutritional and Herbal Therapies for Children and Adolescents; Academic Press: Cambridge, MA, USA, 2010; pp. 13–46. [Google Scholar] [CrossRef]
- Kazemi, M. Chemical Composition and Antimicrobial Activity of Essential Oil of Matricaria recutita. Int. J. Food Prop. 2014, 18, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Sebai, H.; Jabri, M.-A.; Souli, A.; Rtibi, K.; Selmi, S.; Tebourbi, O.; El-Benna, J.; Sakly, M. Antidiarrheal and antioxidant activities of chamomile (Matricaria recutita L.) decoction extract in rats. J. Ethnopharmacol. 2014, 152, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R. The essential oil of Chamomilla recutita (L.) Rausch. Cultivated and wild growing in Poland. Ann. Univ. Mariae Curie-Sklodowska 2011, 24, 199–206. [Google Scholar]
- Shebbo, S.; El Joumaa, M.; Kawach, R.; Borjac, J. Hepatoprotective effect of Matricaria chamomilla aqueous extract against 1,2-Dimethylhydrazine-induced carcinogenic hepatic damage in mice. Heliyon 2020, 6, e04082. [Google Scholar] [CrossRef]
- Jarrahi, M.; Vafaei, A.A.; Taherian, A.A.; Miladi, H.; Pour, A.R. Evaluation of topical Matricaria chamomilla extract activity on linear incisional wound healing in albino rats. Nat. Prod. Res. 2010, 24, 697–702. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, J.; Shankar, E. Chamomile: A herbal medicine of the past with a bright future (Review). Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghalian, K.; Abdoshah, S.; Khalighi-Sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.). Plant Physiol. Biochem. 2011, 49, 201–207. [Google Scholar] [CrossRef] [PubMed]
- El Mihyaoui, A.; da Silva, J.C.G.E.; Charfi, S.; Castillo, M.E.C.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Turk, B.; Baričevič, D.; Batič, F. Essential oil content, chamazulene content and antioxidative properties of Achillea millefolium agg. extracts from Slovenia. Acta Agric. Slov. 2021, 117, 1–10. [Google Scholar] [CrossRef]
- Howyzeh, M.S.; Aslani, S.; Pooraskari, O. Essential Oil Profile of an Iranian Yarrow (Achillea millefolium). J. Essent. Oil Bear. Plants 2019, 22, 295–300. [Google Scholar] [CrossRef]
- Baskran, R.N.R.; Lakshmanan, R. Assessment of effect of chamomile oil on dental anxiety for patients undergoing extraction—A randomized controlled trial. Drug Invent. Today 2019, 11, 1875–1879. [Google Scholar]
- Ribeiro, S.O.; Fontaine, V.; Mathieu, V.; Zhiri, A.; Baudoux, D.; Stévigny, C.; Souard, F. Antibacterial and Cytotoxic Activities of Ten Commercially Available Essential Oils. Antibiotics 2020, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Syakri, S.; Ismail, I.; Amal, N.M.; Masjidi, N.A.; Tahir, K.A. Characterization and Anti-aging Tests of Peel-Off Gel Masks Made from Ethanolic Extract of Yarrow (Achillea millefolium). Open Access Maced. J. Med. Sci. 2021, 9, 1591–1595. [Google Scholar] [CrossRef]
- Demirtas, A.; Musa, S.A.A.; Pekcan, M.; Demirbas, Y.S.; Piskin, I.; Emre, B.; Toprak, N.; Ozturk, H. Yoğurt Otu (Galium Aparine) ve Civan Perçemi (Achillea millefolium) Ekstraktlarının In Vitro Yarı-Sürekli Kültür Sisteminde (RUSITEC) Rumen Mikrobiyal Fermentasyonu Üzerine Etkileri. Kafkas Univ. Veter-Fak. Derg. 2020, 26. [Google Scholar] [CrossRef]
- Mouhid, L.; de Cedrón, M.G.; Quijada-Freire, A.; Fernández-Marcos, P.J.; Reglero, G.; Fornari, T.; de Molina, A.R. Yarrow Supercritical Extract Ameliorates the Metabolic Stress in a Model of Obesity Induced by High-Fat Diet. Nutrients 2019, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agatonovic-Kustrin, S.; Ortakand, D.B.; Morton, D.W.; Yusof, A.P. Rapid evaluation and comparison of natural products and antioxidant activity in calendula, feverfew, and German chamomile extracts. J. Chromatogr. A 2015, 1385, 103–110. [Google Scholar] [CrossRef]
- Fukunaga, E.; Hirao, Y.; Ogata-Ikeda, I.; Nishimura, Y.; Seo, H.; Oyama, Y. Bisabololoxide A, One of the Constituents in German Chamomile Extract, Attenuates Cell Death Induced by Calcium Overload. Phytother. Res. 2013, 28, 685–691. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakano, Y.; Inayama, K.; Sakai, A.; Kamiya, T. Dietary intake of the flower extracts of German Chamomile (Matricaria recutita L.) inhibited compound 48/80-induced itch-scratch responses in mice. Phytomedicine 2003, 10, 657–664. [Google Scholar] [CrossRef]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential Application of Peppermint (Mentha piperita L.), German Chamomile (Matricaria chamomilla L.) and Yarrow (Achillea millefolium L.) as Active Fillers in Natural Rubber Biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Rybiński, P.; Strzelec, K. Modified Nanoclays/Straw Fillers as Functional Additives of Natural Rubber Biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef]
- Jamroży, M.; Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Gajda, P.; Tyliszczak, B. The Impact of the Matricaria chamomilla L. Extract, Starch Solution and the Photoinitiator on Physiochemical Properties of Acrylic Hydrogels. Materials 2022, 15, 2837. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Efenberger–Szmechtyk, M.; Nowak, A.; Strzelec, K. Anti-Oxidative Activity of Alcohol-Water Extracts from Field Horsetail (Equisteum arvense) in Elastomer Vulcanizates Subjected to Accelerated Aging Processes. Materials 2020, 13, 4903. [Google Scholar] [CrossRef] [PubMed]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Efenberger-Szmechtyk, M.; Strzelec, K. Antioxidant and Anti–Aging Activity of Freeze–Dried Alcohol–Water Extracts from Common Nettle (Urtica dioica L.) and Peppermint (Mentha piperita L.) in Elastomer Vulcanizates. Polymers 2022, 14, 1460. [Google Scholar] [CrossRef] [PubMed]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef]
- Taraj, K.; Malollari, I.; Andoni, A.; Ciko, L.; Lazo, P.; Ylli, F.; Osmeni, A.; Como, A. Eco-extraction of albanian chamomile essential oils by liquid CO2 at different temperatures and characterisation by FTIR spectroscopy. J. Environ. Prot. Ecol. 2017, 18, 117–124. [Google Scholar]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011, 5, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Abdelhameed, R.M.; Abdel-Gawad, H.; Taha, M.; Hegazi, B. Separation of bioactive chamazulene from chamomile extract using metal-organic framework. J. Pharm. Biomed. Anal. 2017, 146, 126–134. [Google Scholar] [CrossRef]
- Pljevljakusic, D.; Ristic, M.; Šavikin, K. Screening of yarrow (Achillea millefolium Agg.) populations in Serbia for yield components and essential oil composition. Lek. Sirovine 2017, 37, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Elmastaş, M.; Dermirtas, I.; Isildak, O.; Aboul-Enein, H.Y. Antioxidant Activity of S-Carvone Isolated from Spearmint (Mentha Spicata L. Fam Lamiaceae). J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1465–1475. [Google Scholar] [CrossRef]
- Sadowska, U.; Matwijczuk, A.; Niemczynowicz, A.; Dróżdż, T.; Żabiński, A. Spectroscopic Examination and Chemometric Analysis of Essential Oils Obtained from Peppermint Herb (Mentha piperita L.) and Caraway Fruit (Carum carvi L.) Subjected to Pulsed Electric Fields. Processes 2019, 7, 466. [Google Scholar] [CrossRef] [Green Version]
- Afrin, T.; Karobi, S.N.; Rahman, M.M.; Mollah, M.Y.A.; Susan, A.B.H. Water Structure Modification by Sugars and Its Consequence on Micellization Behavior of Cetyltrimethylammonium Bromide in Aqueous Solution. J. Solut. Chem. 2013, 42, 1488–1499. [Google Scholar] [CrossRef]
- Schulz, H.; Engelhardt, U.H.; Wegent, A.; Drews, H.-H.; Lapczynski, S. Application of Near-Infrared Reflectance Spectroscopy to the Simultaneous Prediction of Alkaloids and Phenolic Substances in Green Tea Leaves. J. Agric. Food Chem. 1999, 47, 5064–5067. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Determination of phenolic compounds of grape skins during ripening by NIR spectroscopy. LWT 2011, 44, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Valdivieso, D.; Font, R.; Blanco-Díaz, M.T.; Moreno-Rojas, J.M.; Gómez, P.; Alonso-Moraga, Á.; Del Río-Celestino, M. Application of near-infrared reflectance spectroscopy for predicting carotenoid content in summer squash fruit. Comput. Electron. Agric. 2014, 108, 71–79. [Google Scholar] [CrossRef]
- Davey, M.W.; Saeys, W.; Hof, E.; Ramon, H.; Swennen, R.L.; Keulemans, J. Application of Visible and Near-Infrared Reflectance Spectroscopy (Vis/NIRS) to Determine Carotenoid Contents in Banana (Musa spp.) Fruit Pulp. J. Agric. Food Chem. 2009, 57, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Brenna, O.V.; Berardo, N. Application of Near-Infrared Reflectance Spectroscopy (NIRS) to the Evaluation of Carotenoids Content in Maize. J. Agric. Food Chem. 2004, 52, 5577–5582. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Huck, C.W. Breakthrough Potential in Near-Infrared Spectroscopy: Spectra Simulation. A Review of Recent Developments. Front. Chem. 2019, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, Y.; Morisawa, Y. Principles and Characteristics of NIR Spectroscopy; Springer: Singapore, 2020; pp. 11–35. [Google Scholar] [CrossRef]
- Ali, M.; Emsley, A.; Herman, H.; Heywood, R. Spectroscopic studies of the ageing of cellulosic paper. Polymer 2001, 42, 2893–2900. [Google Scholar] [CrossRef]
- Metrohm, A.G. NIR Spectroscopy: A Guide to Near-Infrared Spectroscopic Analysis of Industrial Manufacturing Processes; Monograph: Herisau, Switzerland, 2013. [Google Scholar]
- Kowalski, R.; Kowalska, G. Phenolic acid contents in fruits of aubergine (Solanum melongena L.). Polish J. Food Nutr. Sci. 2005, 14, 55. [Google Scholar]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Remote Sensing of Environment Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Zaghdoudi, K.; Ngomo, O.; Vanderesse, R.; Arnoux, P.; Myrzakhmetov, B.; Frochot, C.; Guiavarc’H, Y. Extraction, Identification and Photo-Physical Characterization of Persimmon (Diospyros kaki L.) Carotenoids. Foods 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.M.B.; Kobelnik, M.; Regasini, L.O.; Dutra, L.A.; Bolzani, V.D.S.; Ribeiro, C.A. Synthesis and evaluation of the thermal behavior of flavonoids. J. Therm. Anal. 2016, 127, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; Macêdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Da Costa, E.M.; Filho, J.M.B.; do Nascimento, T.G.; Macêdo, R.O. Thermal characterization of the quercetin and rutin flavonoids. Thermochim. Acta 2002, 392–393, 79–84. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of microparticles of carotenoids from natural and synthetic sources for applications in food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Corbu, A.R.; Rotaru, A.; Nour, V. Edible vegetable oils enriched with carotenoids extracted from by-products of sea buckthorn (Hippophae rhamnoides ssp. sinensis): The investigation of some characteristic properties, oxidative stability and the effect on thermal behaviour. J. Therm. Anal. 2019, 142, 735–747. [Google Scholar] [CrossRef]
- Nour, V.; Corbu, A.R.; Rotaru, P.; Karageorgou, I.; Lalas, S. Effect of carotenoids, extracted from dry tomato waste, on the stability and characteristics of various vegetable oils. Grasas Aceites 2018, 69, 238. [Google Scholar] [CrossRef] [Green Version]
- Guz, L.; Adaszek, Ł.; Wawrzykowski, J.; Ziętek, J.; Winiarczyk, S. In vitro antioxidant and antibabesial activities of the extracts of Achillea millefolium. Pol. J. Vet. Sci. 2019, 22, 369–376. [Google Scholar] [CrossRef]
- Catani, M.V.; Rinaldi, F.; Tullio, V.; Gasperi, V.; Savini, I. Comparative Analysis of Phenolic Composition of Six Commercially Available Chamomile (Matricaria chamomilla L.) Extracts: Potential Biological Implications. Int. J. Mol. Sci. 2021, 22, 10601. [Google Scholar] [CrossRef]
- Sotiropoulou, N.S.; Megremi, S.F.; Tarantilis, P. Evaluation of Antioxidant Activity, Toxicity, and Phenolic Profile of Aqueous Extracts of Chamomile (Matricaria chamomilla L.) and Sage (Salvia officinalis L.) Prepared at Different Temperatures. Appl. Sci. 2020, 10, 2270. [Google Scholar] [CrossRef] [Green Version]
- Toplan, G.G.; Taşkın, T.; İşcan, G.; Göger, F.; Kürkçüoğlu, M.; Civaş, A.; Ecevit-Genç, G.; Mat, A.; Başer, K.H.C. Comparative Studies on Essential Oil and Phenolic Content with In Vitro Antioxidant, Anticholinesterase, Antimicrobial Activities of Achillea biebersteinii Afan. and A. millefolium subsp. millefolium Afan. L. Growing in Eastern Turkey. Molecules 2022, 27, 1956. [Google Scholar] [CrossRef] [PubMed]
- Turker, A.U.; Yildirim, A.B.; Tas, I.; Ozkan, E.; Turker, H. Evaluation of some traditional medicinal plants: Phytochemical profile, antibacterial and antioxidant potentials. Romanian Biotechnol. Lett. 2021, 26, 2499–2510. [Google Scholar] [CrossRef]
- Khimi, S.R.; Pickering, K.L. A new method to predict optimum cure time of rubber compound using dynamic mechanical analysis. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Marković, G.; Marinović-Cincović, M.; Valentova, H.; Ilavsky, M.; Radovanović, B.; Budinski-Simendić, J. Curing Characteristics and Dynamic Mechanical Behaviour of Reinforced Acrylonitrile-Butadiene/Chlorosulfonated Polyethylene Rubber Blends. Mater. Sci. Forum 2005, 494, 475–480. [Google Scholar]
- Ondrušová, D.; Labaj, I.; Pajtášová, M.; Vršková, J. Preparation and properties of new elastomeric systems containing alternative fillers. MATEC Web Conf. 2019, 254, 07003. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G. Degradation Studies Realized on Natural Rubber and Plasticized Potato Starch Based Eco-Composites Obtained by Peroxide Cross-Linking. Int. J. Mol. Sci. 2018, 19, 2862. [Google Scholar] [CrossRef]
- Datta, S.; Stocek, R.; Naskar, K. Influence of Ultraviolet Radiation on Mechanical Properties of a Photoinitiator Compounded High Vinyl Styrene–Butadiene–Styrene Block Copolymer. Polymers 2021, 13, 1287. [Google Scholar] [CrossRef]
- Komethi, M.; Othman, N.; Ismail, H.; Sasidharan, S. Comparative study on natural antioxidant as an aging retardant for natural rubber vulcanizates. J. Appl. Polym. Sci. 2011, 124, 1490–1500. [Google Scholar] [CrossRef]
- Perrin, D.; Léger, R.; Otazaghine, B.; Ienny, P. Hyperelastic behavior of modified sepiolite/SEBS thermoplastic elastomers. J. Mater. Sci. 2017, 52, 7591–7604. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 1–29. [Google Scholar]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A.; Kucharska, A.Z.; Fecka, I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Rybiński, P.; Maniukiewicz, W.; Zaborski, M. Aluminum-Magnesium Hydroxycarbonate/Azo Dye Hybrids as Novel Multifunctional Colorants for Elastomer Composites. Polymers 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, M.; Sowińska, A.; Kucharska, J. Organic Zinc Salts as Pro-Ecological Activators for Sulfur Vulcanization of Styrene–Butadiene Rubber. Polymers 2019, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Marzec, A.; Szadkowski, B.; Rogowski, J.; Rybiński, P.; Maniukiewicz, W. Novel eco-friendly hybrid pigment with improved stability as a multifunctional additive for elastomer composites with reduced flammability and pH sensing properties. Dye. Pigment. 2020, 186, 108965. [Google Scholar] [CrossRef]

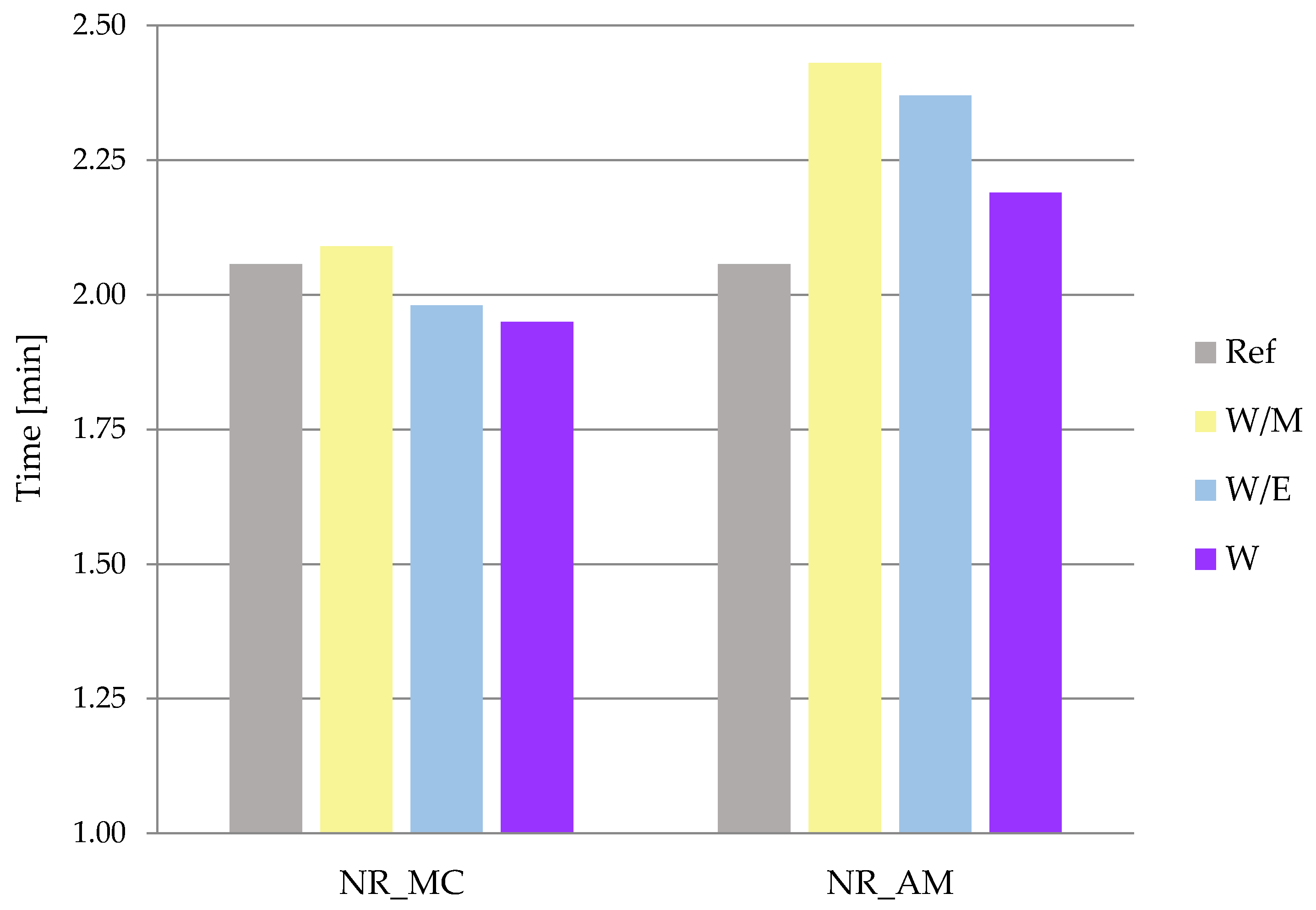

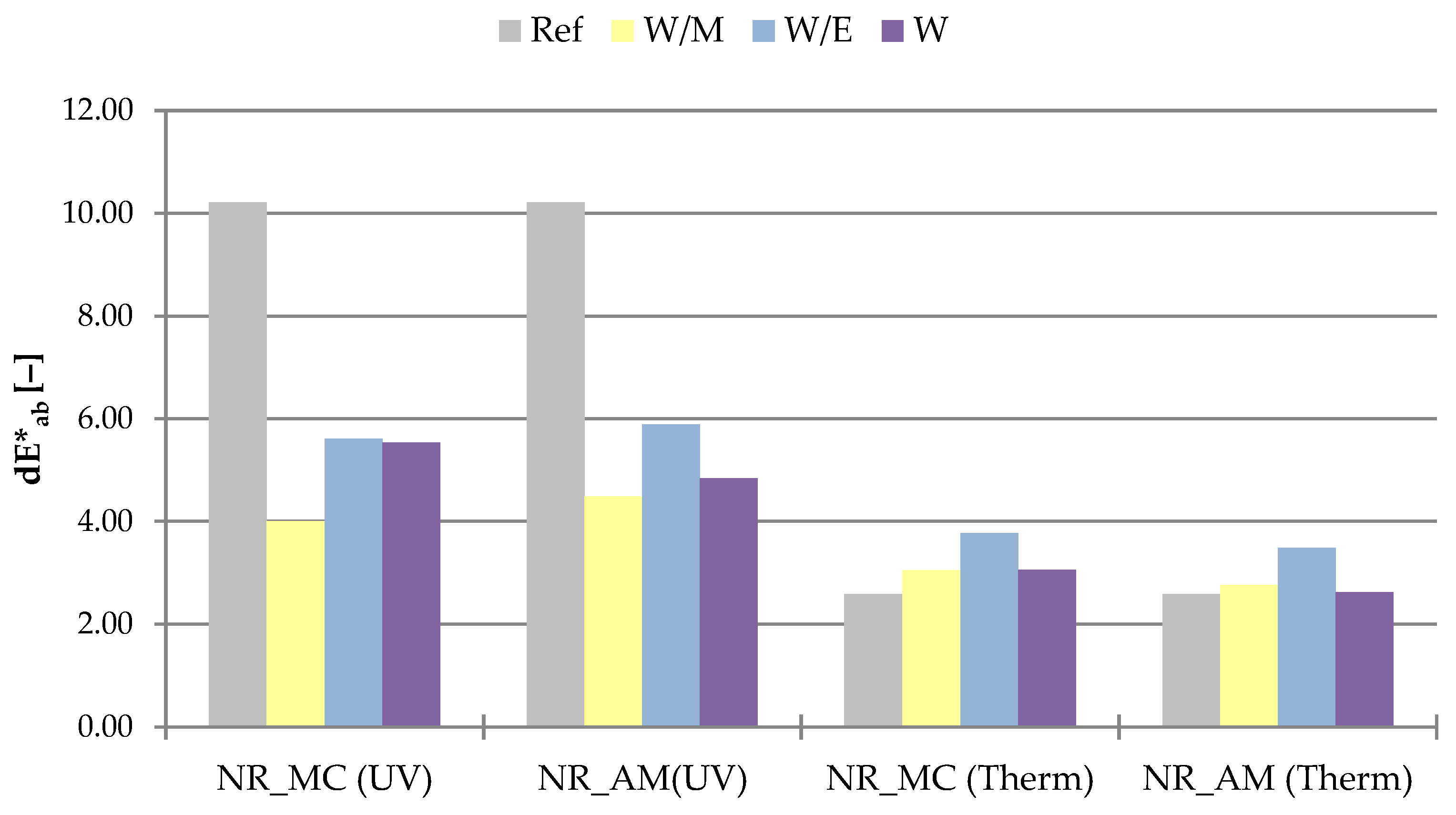
| Peak Assignments and Type of Vibration | Wavelength [cm−1] |
|---|---|
| s(–CHn) | 4034–4040 |
| s(–C–H) | 4375–4408 |
| s(–O–H) | 4531–4890 |
| s(–O–H) | 5093–5233 |
| s(–C–H) first overtone | 5551–5928 |
| s(–O–H) | 6670–7068 |
| s(–C–H) second overtone | 8093–8550 |
| Sample | Δm25–120 °C (%) | Δm120–170 °C (%) | Δm170–230 °C (%) | Δm230–310 °C (%) | Δm310–600 °C (%) | R600 (%) |
|---|---|---|---|---|---|---|
| MC_W/M | 1.93 | 10.04 | 12.54 | 17.70 | 19.58 | 38.21 |
| MC_W/E | 2.40 | 12.37 | 11.76 | 16.16 | 19.91 | 37.40 |
| MC_W | 3.12 | 9.78 | 11.55 | 14.69 | 18.26 | 42.60 |
| AM_W/M | 4.15 | 4.83 | 8.06 | 19.25 | 18.74 | 44.97 |
| AM_W/E | 3.38 | 4.23 | 8.27 | 19.35 | 19.89 | 44.88 |
| AM_W | 6.36 | 5.31 | 10.03 | 16.72 | 19.18 | 42.40 |
| Sample | TPC (µgGAE/mL) | Antioxidant Activity (mgTE/mL) | |
|---|---|---|---|
| ABTS | DPPH | ||
| MC_W/M | 14,207.8 ± 39.2 b | 11.82 ± 0.11 a | 23.57 ± 0.29 b |
| MC_W/E | 8327.6 ± 177.7 c | 10.88 ± 0.14 b | 24.21 ± 0.22 a |
| MC_W | 1038.2 ± 30.0 d | 1.68 ± 0.03 d | 2.39 ± 0.01 d |
| AM_W/M | 15,881.8 ± 606.1 a | 11.49 ± 0.02 a | 23.28 ± 0.27 b |
| AM_W/E | 7961.8 ± 282.2 c | 7.75 ± 0.20 c | 15.76 ± 0.90 c |
| AM_W | 276.8 ± 19.6 e | 0.48 ± 0.03 e | 1.16 ± 0.14 e |
| Sample | γe × 10−5 (mol/cm3) | ||
|---|---|---|---|
| Unaged | UV | Therm | |
| Ref | 1.63 ± 0.02 | 1.84 ± 0.03 | 1.82 ± 0.03 |
| NR_MC_W/M | 1.93 ± 0.03 | 1.92 ± 0.04 | 1.94 ± 0.02 |
| NR_MC_W/E | 1.60 ± 0.03 | 1.65 ± 0.03 | 1.75 ± 0.03 |
| NR_MC_W | 1.52 ± 0.04 | 1.62 ± 0.02 | 1.41 ± 0.01 |
| NR_AM_W/M | 1.87 ± 0.03 | 1.88 ± 0.01 | 1.92 ± 0.04 |
| NR_AM_W/E | 1.46 ± 0.04 | 1.54 ± 0.03 | 1.51 ± 0.02 |
| NR_AM_W | 1.31 ± 0.02 | 1.50 ± 0.03 | 1.40 ± 0.04 |
| Sample | Eb (%) | EbUV (%) | EbTherm (%) |
|---|---|---|---|
| Ref | 663.43 ± 1.59 | 586.59 ± 4.80 | 584.68 ± 6.24 |
| NR_MC_W/M | 617.75 ± 3.32 | 782.24 ± 2.25 | 626.25 ± 5.50 |
| NR_MC_W/E | 644.19 ± 4.25 | 784.10 ± 3.23 | 743.91 ± 4.57 |
| NR_MC_W | 646.01 ± 5.37 | 756.13 ± 2.55 | 697.56 ± 3.49 |
| NR_AM_W/M | 584.85 ± 4.88 | 760.09 ± 5.13 | 754.37 ± 4.12 |
| NR_AM_W/E | 627.80 ± 6.01 | 788.24 ± 3.99 | 570.92 ± 5.20 |
| NR_AM_W | 650.20 ± 4.37 | 772.37 ± 2.47 | 558.08 ± 3.56 |
| Sample Name | Extract | NR | Stearin | ZnO | MBT | Sulfur |
|---|---|---|---|---|---|---|
| (phr 1) | ||||||
| Reference Sample (Ref) | 0 | 100 | 1 | 5 | 2 | 2 |
| NR-MC-W/M 2 | 5.0 | 100 | 1 | 5 | 2 | 2 |
| NR-MC-W/E 3 | ||||||
| NR-MC-W 4 | ||||||
| NR-AM-W/M 5 | ||||||
| NR-AM-W/E 6 | ||||||
| NR-AM-W 7 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksieiev, A.; Masłowski, M.; Efenberger-Szmechtyk, M.; Strzelec, K. The Influence of Freeze-Dried Alcohol-Water Extracts from Common Yarrow (Achillea millefolium L.) and German Chamomile (Matricaria chamomilla L.) on the Properties of Elastomer Vulcanizates. Int. J. Mol. Sci. 2022, 23, 15048. https://doi.org/10.3390/ijms232315048
Aleksieiev A, Masłowski M, Efenberger-Szmechtyk M, Strzelec K. The Influence of Freeze-Dried Alcohol-Water Extracts from Common Yarrow (Achillea millefolium L.) and German Chamomile (Matricaria chamomilla L.) on the Properties of Elastomer Vulcanizates. International Journal of Molecular Sciences. 2022; 23(23):15048. https://doi.org/10.3390/ijms232315048
Chicago/Turabian StyleAleksieiev, Andrii, Marcin Masłowski, Magdalena Efenberger-Szmechtyk, and Krzysztof Strzelec. 2022. "The Influence of Freeze-Dried Alcohol-Water Extracts from Common Yarrow (Achillea millefolium L.) and German Chamomile (Matricaria chamomilla L.) on the Properties of Elastomer Vulcanizates" International Journal of Molecular Sciences 23, no. 23: 15048. https://doi.org/10.3390/ijms232315048
APA StyleAleksieiev, A., Masłowski, M., Efenberger-Szmechtyk, M., & Strzelec, K. (2022). The Influence of Freeze-Dried Alcohol-Water Extracts from Common Yarrow (Achillea millefolium L.) and German Chamomile (Matricaria chamomilla L.) on the Properties of Elastomer Vulcanizates. International Journal of Molecular Sciences, 23(23), 15048. https://doi.org/10.3390/ijms232315048







