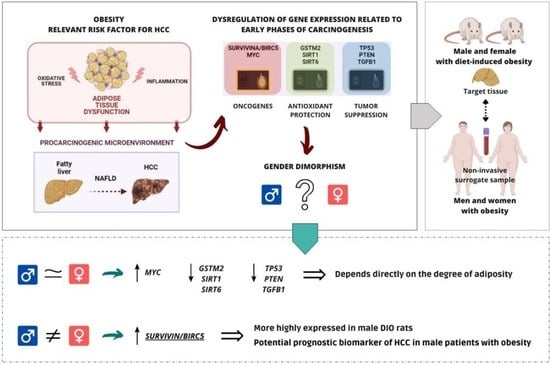
Gender Dimorphism in Hepatic Carcinogenesis-Related Gene Expression Associated with Obesity as a Low-Grade Chronic Inflammatory Disease
Abstract
:1. Introduction
2. Results
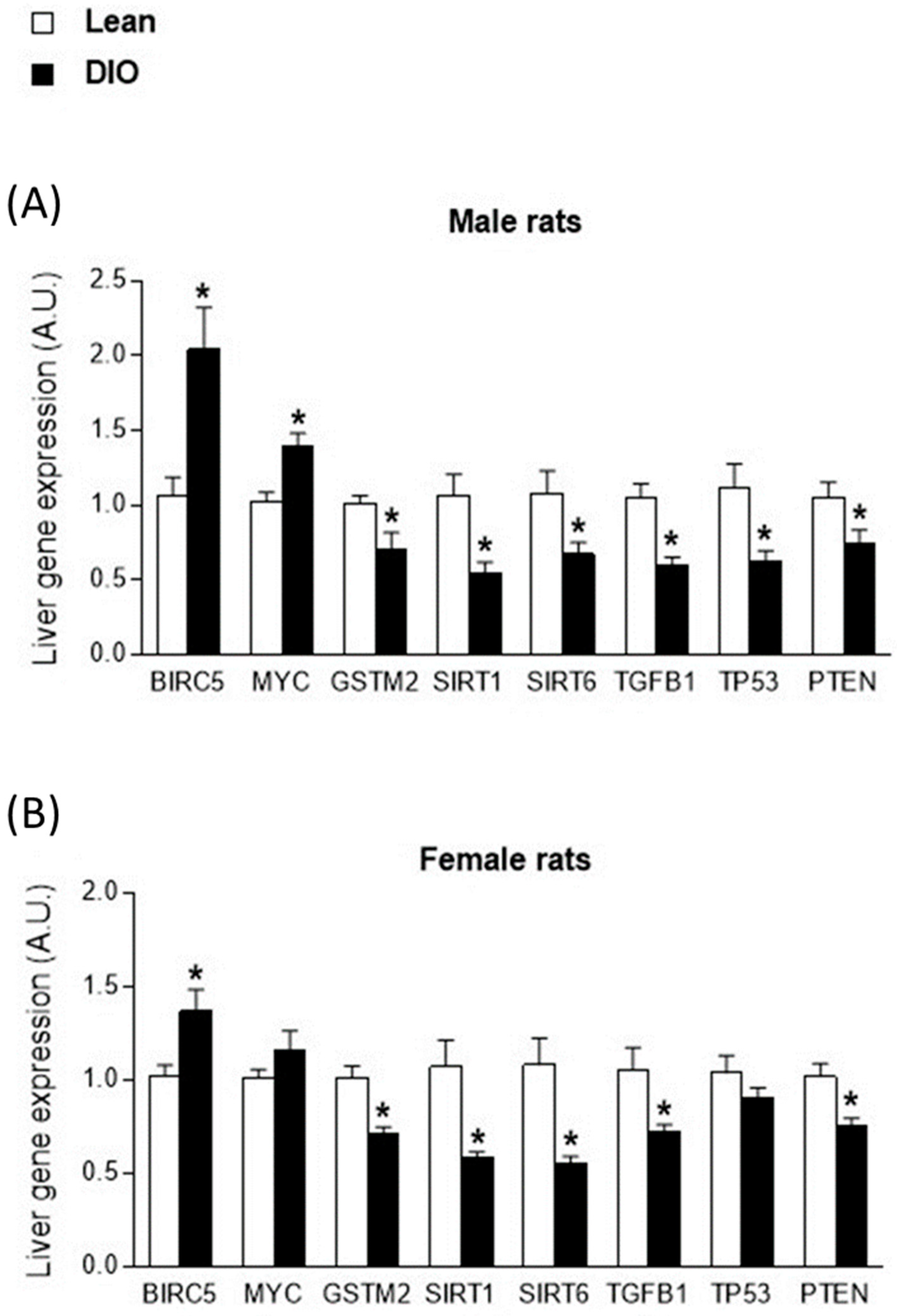
2.1. Sex-Biased Carcinogenesis-Related Gene Expression in Livers from Obese Rats
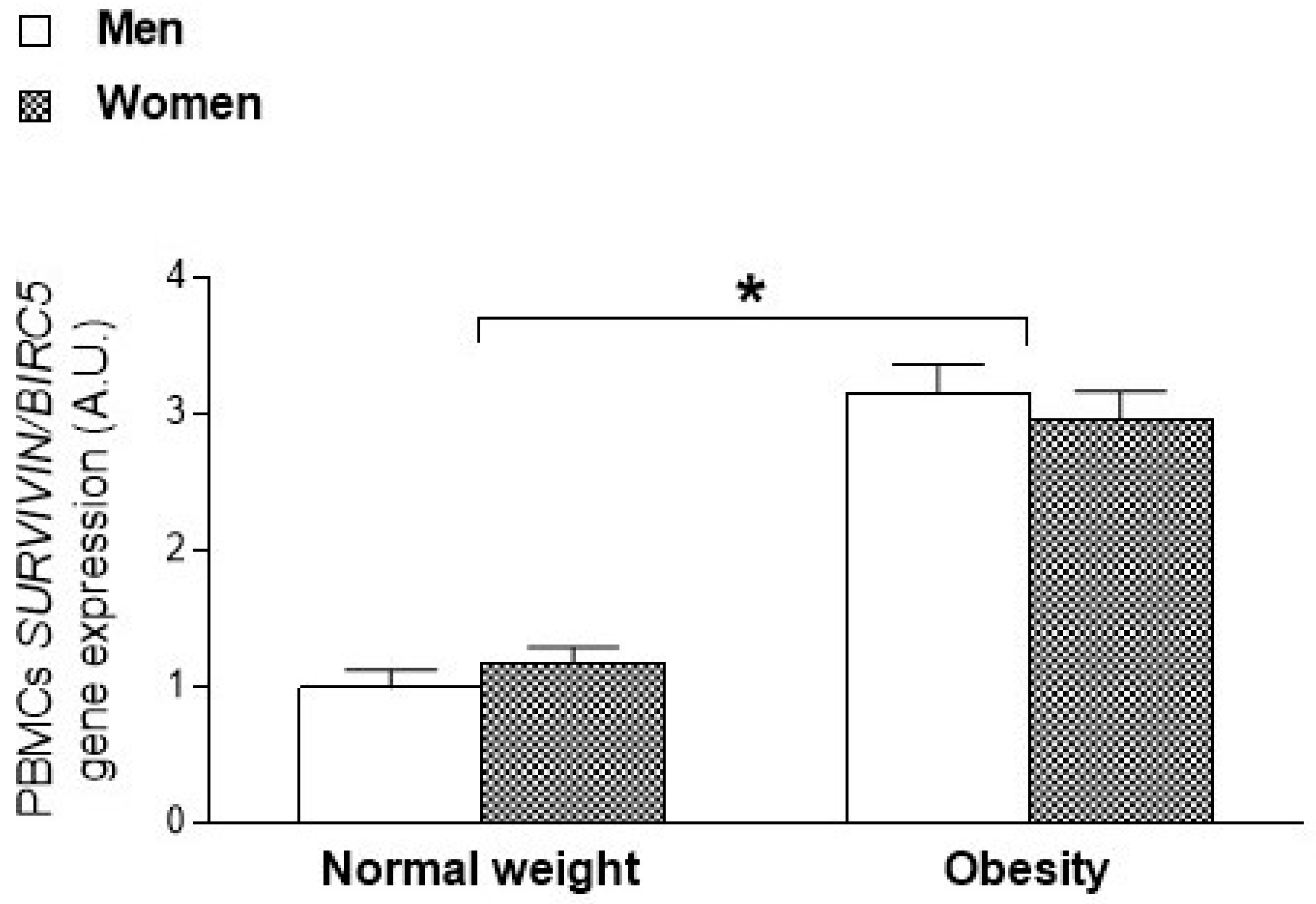
2.2. Sexual Dimorphism in SURVIVIN/BIRC5 Oncogene Expression According to the Degree of Adiposity in Animals and Humans
3. Discussion
4. Methods and Materials
4.1. Animals with Diet-Induced Obesity (DIO)
4.2. Patients with Obesity and Normal Weight Individuals
4.3. Gene Expression Assessment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinn, J.L.; Snyder, M. Sexual Dimorphism in Mammalian Gene Expression. Trends Genet. 2005, 21, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Gemma, C.; Rakyan, V.K.; Holland, M.L. Sexually Dimorphic Gene Expression Emerges with Embryonic Genome Activation and Is Dynamic throughout Development. BMC Genom. 2015, 16, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, A.S.; Shapiro, B.H. Sex-Dependent Expression of Seven Housekeeping Genes in Rat Liver. J. Gastroenterol. Hepatol. 2006, 21, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, L.; Körner, C.; Ott, F.; Volke, D.; Cokan, K.B.; Juvan, P.; Brosch, M.; Hofmann, U.; Hoffmann, R.; Rozman, D.; et al. Sex-Dependent Dynamics of Metabolism in Primary Mouse Hepatocytes. Arch. Toxicol. 2021, 95, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Clodfelter, K.H.; Holloway, M.G.; Hodor, P.; Park, S.-H.; Ray, W.J.; Waxman, D.J. Sex-Dependent Liver Gene Expression Is Extensive and Largely Dependent upon Signal Transducer and Activator of Transcription 5b (STAT5b): STAT5b-Dependent Activation of Male Genes and Repression of Female Genes Revealed by Microarray Analysis. Mol. Endocrinol. 2006, 20, 1333–1351. [Google Scholar] [CrossRef] [Green Version]
- Kwekel, J.C.; Desai, V.G.; Moland, C.L.; Branham, W.S.; Fuscoe, J.C. Age and Sex Dependent Changes in Liver Gene Expression during the Life Cycle of the Rat. BMC Genom. 2010, 11, 675. [Google Scholar] [CrossRef] [Green Version]
- Waxman, D.J.; Holloway, M.G. Sex Differences in the Expression of Hepatic Drug Metabolizing Enzymes. Mol. Pharmacol. 2009, 76, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Schiffrin, M.; Winkler, C.; Quignodon, L.; Naldi, A.; Trötzmüller, M.; Köfeler, H.; Henry, H.; Parini, P.; Desvergne, B.; Gilardi, F. Sex Dimorphism of Nonalcoholic Fatty Liver Disease (NAFLD) in Pparg-Null Mice. Int. J. Mol. Sci. 2021, 22, 9969. [Google Scholar] [CrossRef]
- Kurt, Z.; Barrere-Cain, R.; LaGuardia, J.; Mehrabian, M.; Pan, C.; Hui, S.T.; Norheim, F.; Zhou, Z.; Hasin, Y.; Lusis, A.J.; et al. Tissue-Specific Pathways and Networks Underlying Sexual Dimorphism in Non-Alcoholic Fatty Liver Disease. Biol. Sex Differ. 2018, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Ruggieri, A.; Barbati, C.; Malorni, W. Cellular and Molecular Mechanisms Involved in Hepatocellular Carcinoma Gender Disparity. Int. J. Cancer 2010, 127, 499–504. [Google Scholar] [CrossRef]
- Hanna, D.; Sugamori, K.S.; Bott, D.; Grant, D.M. The Impact of Sex on Hepatotoxic, Inflammatory and Proliferative Responses in Mouse Models of Liver Carcinogenesis. Toxicology 2020, 442, 152546. [Google Scholar] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [PubMed]
- Wu, E.M.; Wong, L.L.; Hernandez, B.Y.; Ji, J.-F.; Jia, W.; Kwee, S.A.; Kalathil, S. Gender Differences in Hepatocellular Cancer: Disparities in Nonalcoholic Fatty Liver Disease/Steatohepatitis and Liver Transplantation. Hepatoma Res. 2018, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.-R.; Feldstein, A.F.; Zein, N.N. The Incidence and Risk Factors of Hepatocellular Carcinoma in Patients with Nonalcoholic Steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, A.; Jia, S.; Huang, J. Recent Advances in the Molecular Mechanism of Sex Disparity in Hepatocellular Carcinoma. Oncol. Lett. 2019, 17, 4222–4228. [Google Scholar] [CrossRef] [Green Version]
- Sayaf, K.; Gabbia, D.; Russo, F.P.; De Martin, S. The Role of Sex in Acute and Chronic Liver Damage. Int. J. Mol. Sci. 2022, 23, 10654. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Wang, C.; Huang, Y.-Z.; Zhang, L.-L. Nonalcoholic Fatty Liver Disease Shows Significant Sex Dimorphism. World J. Clin. Cases 2022, 10, 1457–1472. [Google Scholar] [CrossRef]
- Zoller, H.; Tilg, H. Nonalcoholic Fatty Liver Disease and Hepatocellular Carcinoma. Metabolism 2016, 65, 1151–1160. [Google Scholar] [CrossRef]
- Kutlu, O.; Kaleli, H.N.; Ozer, E. Molecular Pathogenesis of Nonalcoholic Steatohepatitis- (NASH-) Related Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2018, 2018, 8543763. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism 2019, 92, 82–97. [Google Scholar] [CrossRef]
- Peak, J.G.; Peak, M.J.; Foote, C.S. Observations on the Photosensitized Breakage of DNA by 2-Thiouracil and 334-Nm Ultraviolet Radiation. Photochem. Photobiol. 1986, 44, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, S.; Scamporrino, A.; Filippello, A.; Di Pino, A.; Scicali, R.; Malaguarnera, R.; Purrello, F.; Piro, S. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int. J. Mol. Sci. 2021, 22, 11905. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Divella, R.; Mazzocca, A.; Daniele, A.; Sabbà, C.; Paradiso, A. Obesity, Nonalcoholic Fatty Liver Disease and Adipocytokines Network in Promotion of Cancer. Int. J. Biol. Sci. 2019, 15, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Yang, M. Current Options and Future Directions for NAFLD and NASH Treatment. Int. J. Mol. Sci. 2021, 22, 7571. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Carreira, M.C.; Amil, M.; Mosteiro, C.S.; Garcia-Caballero, T.; Fernandez-Quintela, A.; Portillo, M.P.; Casanueva, F.F.; Crujeiras, A.B. An Energy Restriction-Based Weight Loss Intervention Is Able to Reverse the Effects of Obesity on the Expression of Liver Tumor-Promoting Genes. FASEB J. 2020, 34, 2312–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crujeiras, A.B.; Cabia, B.; Carreira, M.C.; Amil, M.; Cueva, J.; Andrade, S.; Seoane, L.M.; Pardo, M.; Sueiro, A.; Baltar, J.; et al. Secreted Factors Derived from Obese Visceral Adipose Tissue Regulate the Expression of Breast Malignant Transformation Genes. Int. J. Obes. 2016, 40, 514–523. [Google Scholar] [CrossRef]
- Carreira, M.C.; Izquierdo, A.G.; Amil, M.; Casanueva, F.F.; Crujeiras, A.B. Oxidative Stress Induced by Excess of Adiposity Is Related to a Downregulation of Hepatic SIRT6 Expression in Obese Individuals. Oxid. Med. Cell. Longev. 2018, 2018, 6256052. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Carreira, M.C.; Rodriguez-Carnero, G.; Fernandez-Quintela, A.; Sueiro, A.M.; Martinez-Olmos, M.A.; Guzman, G.; De Luis, D.; Pinhel, M.A.S.; Nicoletti, C.F.; et al. Weight Loss Normalizes Enhanced Expression of the Oncogene Survivin in Visceral Adipose Tissue and Blood Leukocytes from Individuals with Obesity. Int. J. Obes. 2021, 45, 206–216. [Google Scholar] [CrossRef]
- Cabia, B.; Andrade, S.; Carreira, M.C.; Casanueva, F.F.; Crujeiras, A.B. A Role for Novel Adipose Tissue-Secreted Factors in Obesity-Related Carcinogenesis. Obes. Rev. 2016, 17, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A Unique Target for Tumor Therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, C.V. C-Myc Target Genes Involved in Cell Growth, Apoptosis, and Metabolism. Mol. Cell. Biol. 1999, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.G.; Lee, Y.H.; Park, H.S.; Ryoo, K.; Kang, K.W.; Park, J.; Eom, S.J.; Kim, M.J.; Chang, T.S.; Choi, S.Y.; et al. Glutathione S-Transferase Mu Modulates the Stress-Activated Signals by Suppressing Apoptosis Signal-Regulating Kinase 1. J. Biol. Chem. 2001, 276, 12749–12755. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Wang, J.; Xiang, H.; Lu, Y.; Wu, T. Role and Clinical Significance of TGF-β1 and TGF-βR1 in Malignant Tumors (Review). Int. J. Mol. Med. 2021, 47, 431–432. [Google Scholar] [CrossRef]
- Brady, C.A.; Jiang, D.; Mello, S.S.; Johnson, T.M.; Jarvis, L.A.; Kozak, M.M.; Kenzelmann Broz, D.; Basak, S.; Park, E.J.; McLaughlin, M.E.; et al. Distinct P53 Transcriptional Programs Dictate Acute DNA-Damage Responses and Tumor Suppression. Cell 2011, 145, 571–583. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yu, W.; Xiao, H.; Lin, K. BIRC5 Is a Prognostic Biomarker Associated with Tumor Immune Cell Infiltration. Sci. Rep. 2021, 11, 390. [Google Scholar] [CrossRef]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibino, S.; Kawazoe, T.; Kasahara, H.; Itoh, S.; Ishimoto, T.; Sakata-Yanagimoto, M.; Taniguchi, K. Inflammation-Induced Tumorigenesis and Metastasis. Int. J. Mol. Sci. 2021, 22, 5421. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, Y.; Sarkar, D. Association of Adipose Tissue and Adipokines with Development of Obesity-Induced Liver Cancer. Int. J. Mol. Sci. 2021, 22, 2163. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Obesity as a Cause of Hepatocellular Carcinoma. Ann. Hepatol. 2015, 14, 299–303. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Henry, L. Epidemiology of Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. JHEP Rep. Innov. Hepatol. 2021, 3, 100305. [Google Scholar] [CrossRef]
- Sohn, W.; Lee, H.W.; Lee, S.; Lim, J.H.; Lee, M.W.; Park, C.H.; Yoon, S.K. Obesity and the Risk of Primary Liver Cancer: A Systematic Review and Meta-Analysis. Clin. Mol. Hepatol. 2021, 27, 157–174. [Google Scholar] [CrossRef]
- Hassoun, Z.; Gores, G.J. Treatment of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2003, 1, 10–18. [Google Scholar] [CrossRef]
- Befeler, A.S.; Di Bisceglie, A.M. Hepatocellular Carcinoma: Diagnosis and Treatment. Gastroenterology 2002, 122, 1609–1619. [Google Scholar] [CrossRef] [Green Version]
- Lonardo, A.; Suzuki, A. Sexual Dimorphism of NAFLD in Adults. Focus on Clinical Aspects and Implications for Practice and Translational Research. J. Clin. Med. 2020, 9, 1278. [Google Scholar] [CrossRef]
- Fäldt Beding, A.; Larsson, P.; Helou, K.; Einbeigi, Z.; Parris, T.Z. Pan-cancer analysis identifies BIRC5 as a prognostic biomarker. BMC Cancer 2022, 22, 322. [Google Scholar] [CrossRef]
- Zaffaroni, N.; Pennati, M.; Daidone, M.G. Survivin as a Target for New Anticancer Interventions. J. Cell. Mol. Med. 2005, 9, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Validating Survivin as a Cancer Therapeutic Target. Nat. Rev. Cancer 2003, 3, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Shiraki, K.; Sugimoto, K.; Yamanaka, T.; Fujikawa, K.; Ito, M.; Takase, K.; Moriyama, M.; Kawano, H.; Hayashida, M.; et al. Survivin Promotes Cell Proliferation in Human Hepatocellular Carcinoma. Hepatology 2000, 31, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Du, W.; Fan, D. Survivin: The Promising Target in Hepatocellular Carcinoma Gene Therapy. Cancer Biol. Ther. 2008, 7, 555–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udoh, U.-A.S.; Banerjee, M.; Rajan, P.K.; Sanabria, J.D.; Smith, G.; Schade, M.; Sanabria, J.A.; Nakafuku, Y.; Sodhi, K.; Pierre, S.V.; et al. Tumor-Suppressor Role of the A1-Na/K-ATPase Signalosome in NASH Related Hepatocellular Carcinoma. Int. J. Mol. Sci. 2022, 23, 7359. [Google Scholar] [CrossRef]
- Su, C. Survivin in Survival of Hepatocellular Carcinoma. Cancer Lett. 2016, 379, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Fields, A.C.; Cotsonis, G.; Sexton, D.; Santoianni, R.; Cohen, C. Survivin Expression in Hepatocellular Carcinoma: Correlation with Proliferation, Prognostic Parameters, and Outcome. Mod. Pathol. 2004, 17, 1378–1385. [Google Scholar] [CrossRef] [Green Version]
- Mamori, S.; Asakura, T.; Ohkawa, K.; Tajiri, H. Survivin Expression in Early Hepatocellular Carcinoma and Post-Treatment with Anti-Cancer Drug under Hypoxic Culture Condition. World J. Gastroenterol. 2007, 13, 5306–5311. [Google Scholar] [CrossRef] [Green Version]
- Katzke, V.A.; Kaaks, R.; Kühn, T. Lifestyle and Cancer Risk. Cancer J. 2015, 21, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Besaratinia, A.; Caceres, A.; Tommasi, S. DNA Hydroxymethylation in Smoking-Associated Cancers. Int. J. Mol. Sci. 2022, 23, 2657. [Google Scholar] [CrossRef]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary Fat and Cancer—Which Is Good, Which Is Bad, and the Body of Evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef] [PubMed]
| Lean | Obese | |||
|---|---|---|---|---|
| Sex | Male | Female | Male | Female |
| n | 10 | 10 | 10 | 10 |
| Age (weeks) | 8 | 6 | 8 | 6 |
| Body weight (g) | 562.3 ± 15.2 | 226.4 ± 10.8 # | 663.5 ± 76.4 * | 245.8 ± 10.3 *,# |
| Fat mass (g) | 52.4 ± 15.6 | 16.0 ± 4.26 # | 146.8 ± 59.7 * | 30.4 ± 5.32 *,# |
| Fat mass (%) | 9.30 ± 2.67 | 7.02 ± 1.71 # | 21.6 ± 6.50 * | 12.4 ± 2.12 *,# |
| Fat-free mass (g) | 420.0 ± 16.1 | 173.3 ± 10.1 # | 424.7 ± 27.6 | 170.6 ± 8.64 # |
| Fat-free mass (%) | 74.7 ± 2.34 | 76.6 ± 4.08 | 64.5 ± 6.23 * | 69.4 ± 2.73 *,# |
| Lean | Obese | ANOVA p-Value | |||||
|---|---|---|---|---|---|---|---|
| GENE | Male | Female | Male | Female | Group | Sex | Group * Sex |
| SURVIVIN/BIRC5 | 1.06 ± 0.39 | 1.02 ± 0.19 | 2.04 ± 0.89 | 1.37 ± 0.37 | <0.001 * | 0.041 * | 0.068 |
| MYC | 1.02 ± 0.20 | 1.01 ± 0.15 | 1.40 ± 0.24 | 1.16 ± 0.33 | 0.003 * | 0.144 | 0.183 |
| GSTM2 | 1.01 ± 0.16 | 1.02 ± 0.18 | 0.70 ± 0.34 | 0.71 ± 0.11 | <0.001 * | 0.931 | 0.981 |
| SIRT1 | 1.06 ± 0.46 | 1.07 ± 0.44 | 0.51 ± 0.24 | 0.59 ± 0.09 | <0.001 * | 0.807 | 0.899 |
| SIRT6 | 1.08 ± 0.46 | 1.08 ± 0.45 | 0.67 ± 0.25 | 0.55 ± 0.12 | <0.001 * | 0.604 | 0.604 |
| TGFB1 | 1.05 ± 0.30 | 1.06 ± 0.38 | 0.60 ± 0.17 | 0.72 ± 0.12 | <0.001 * | 0.424 | 0.503 |
| TP53 | 1.11 ± 0.50 | 1.04 ± 0.30 | 0.62 ± 0.23 | 0.91 ± 0.16 | 0.004 * | 0.323 | 0.085 |
| PTEN | 1.05 ± 0.33 | 1.02 ± 0.22 | 0.74 ± 0.30 | 0.75 ± 0.14 | 0.001 * | 0.911 | 0.797 |
| Normal Weight | Obesity | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| n | 10 | 19 | 10 | 12 |
| Age (years) | 40.2 ± 8.82 | 33.5 ± 9.00 | 42.50 ± 10.8 | 35.7 ± 10.8 |
| Height (m) | 1.73 ± 0.06 | 1.65 ± 0.07 | 1.74 ± 0.05 | 1.64 ± 0.06 |
| Body weight (kg) | 67.2 ± 7.81 | 57.6 ± 4.07 | 114.2 ± 20.2 * | 113.2 ± 21.6 * |
| BMI (kg/m2) | 23.5 ± 2.33 | 21.4 ± 1.72 | 38.0 ± 6.90 * | 41.9 ± 7.70 * |
| WC (cm) | 86.2 ± 7.66 | 76.9 ± 7.89 | 120.8 ± 19.6 * | 122.8 ± 15.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo, A.G.; Carreira, M.C.; Rodriguez-Carnero, G.; Perez-Lois, R.; Seoane, L.M.; Casanueva, F.F.; Crujeiras, A.B. Gender Dimorphism in Hepatic Carcinogenesis-Related Gene Expression Associated with Obesity as a Low-Grade Chronic Inflammatory Disease. Int. J. Mol. Sci. 2022, 23, 15002. https://doi.org/10.3390/ijms232315002
Izquierdo AG, Carreira MC, Rodriguez-Carnero G, Perez-Lois R, Seoane LM, Casanueva FF, Crujeiras AB. Gender Dimorphism in Hepatic Carcinogenesis-Related Gene Expression Associated with Obesity as a Low-Grade Chronic Inflammatory Disease. International Journal of Molecular Sciences. 2022; 23(23):15002. https://doi.org/10.3390/ijms232315002
Chicago/Turabian StyleIzquierdo, Andrea G., Marcos C. Carreira, Gemma Rodriguez-Carnero, Raquel Perez-Lois, Luisa M. Seoane, Felipe F. Casanueva, and Ana B. Crujeiras. 2022. "Gender Dimorphism in Hepatic Carcinogenesis-Related Gene Expression Associated with Obesity as a Low-Grade Chronic Inflammatory Disease" International Journal of Molecular Sciences 23, no. 23: 15002. https://doi.org/10.3390/ijms232315002
APA StyleIzquierdo, A. G., Carreira, M. C., Rodriguez-Carnero, G., Perez-Lois, R., Seoane, L. M., Casanueva, F. F., & Crujeiras, A. B. (2022). Gender Dimorphism in Hepatic Carcinogenesis-Related Gene Expression Associated with Obesity as a Low-Grade Chronic Inflammatory Disease. International Journal of Molecular Sciences, 23(23), 15002. https://doi.org/10.3390/ijms232315002