Choosing the Probe for Single-Molecule Fluorescence Microscopy
Abstract
1. Introduction
2. Comparisons of Fluorophores for Single-Molecule Applications in the Literature
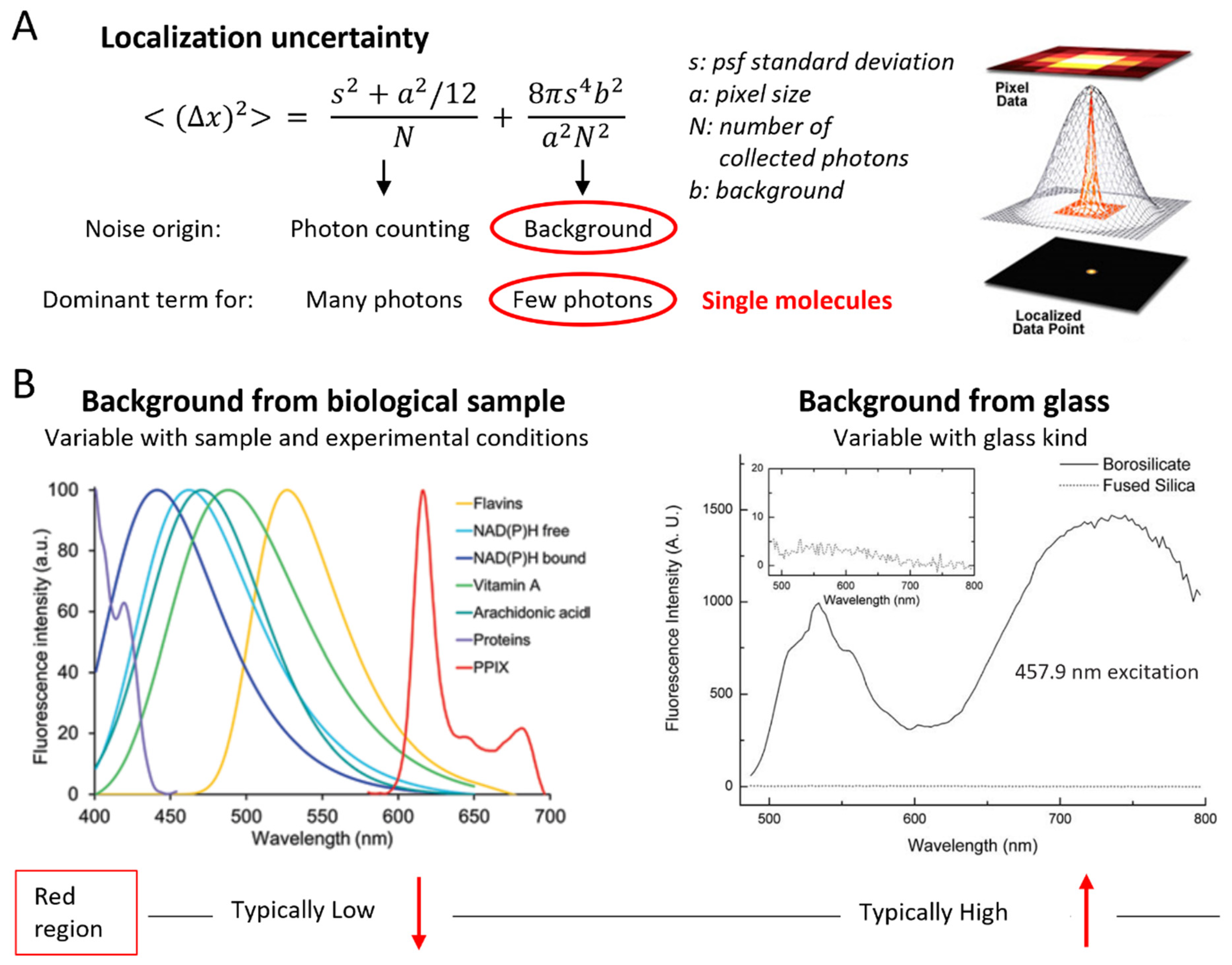
3. Single-Molecule Signal-to-Noise Ratio
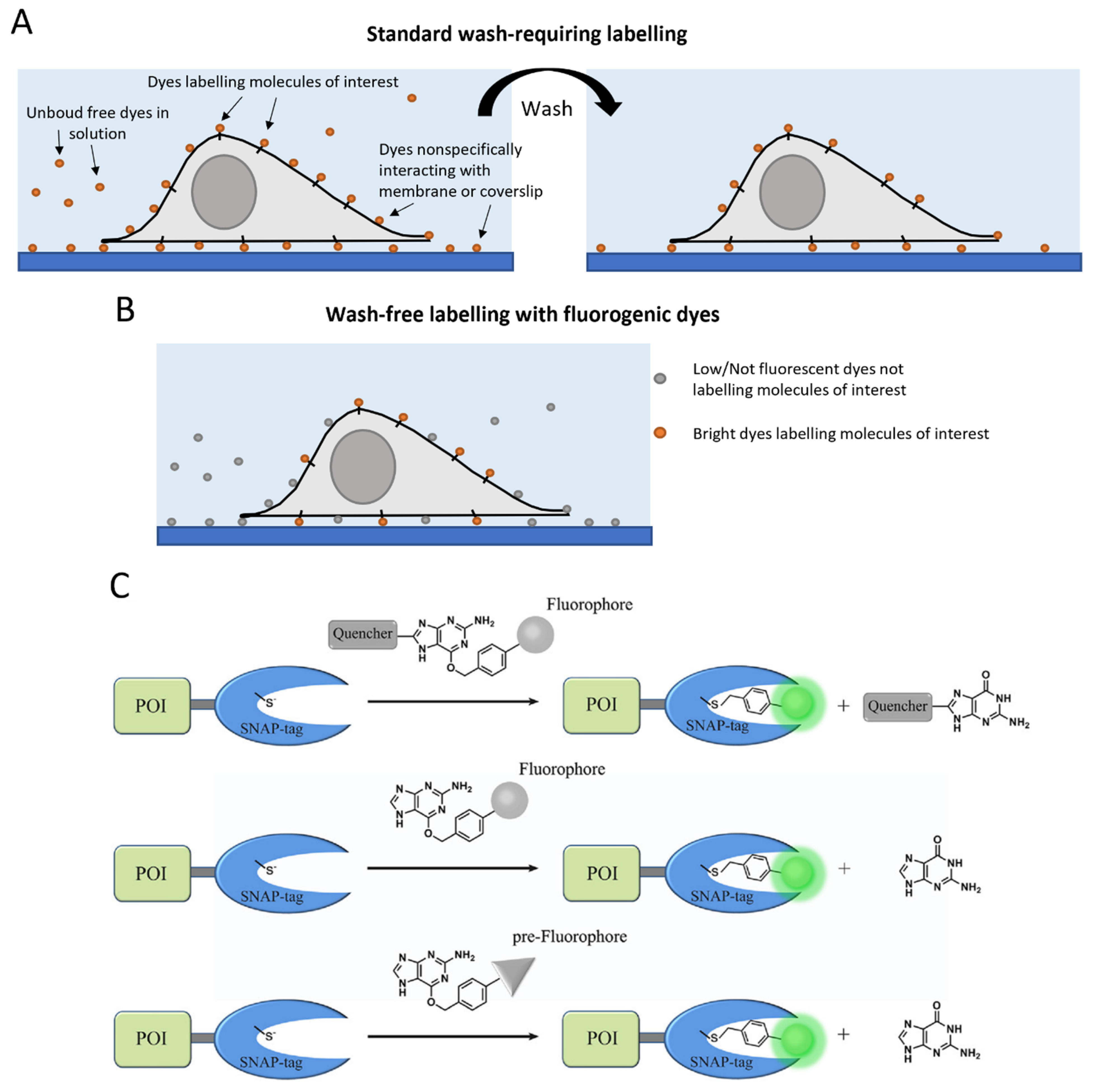
4. Aspecific Interactions
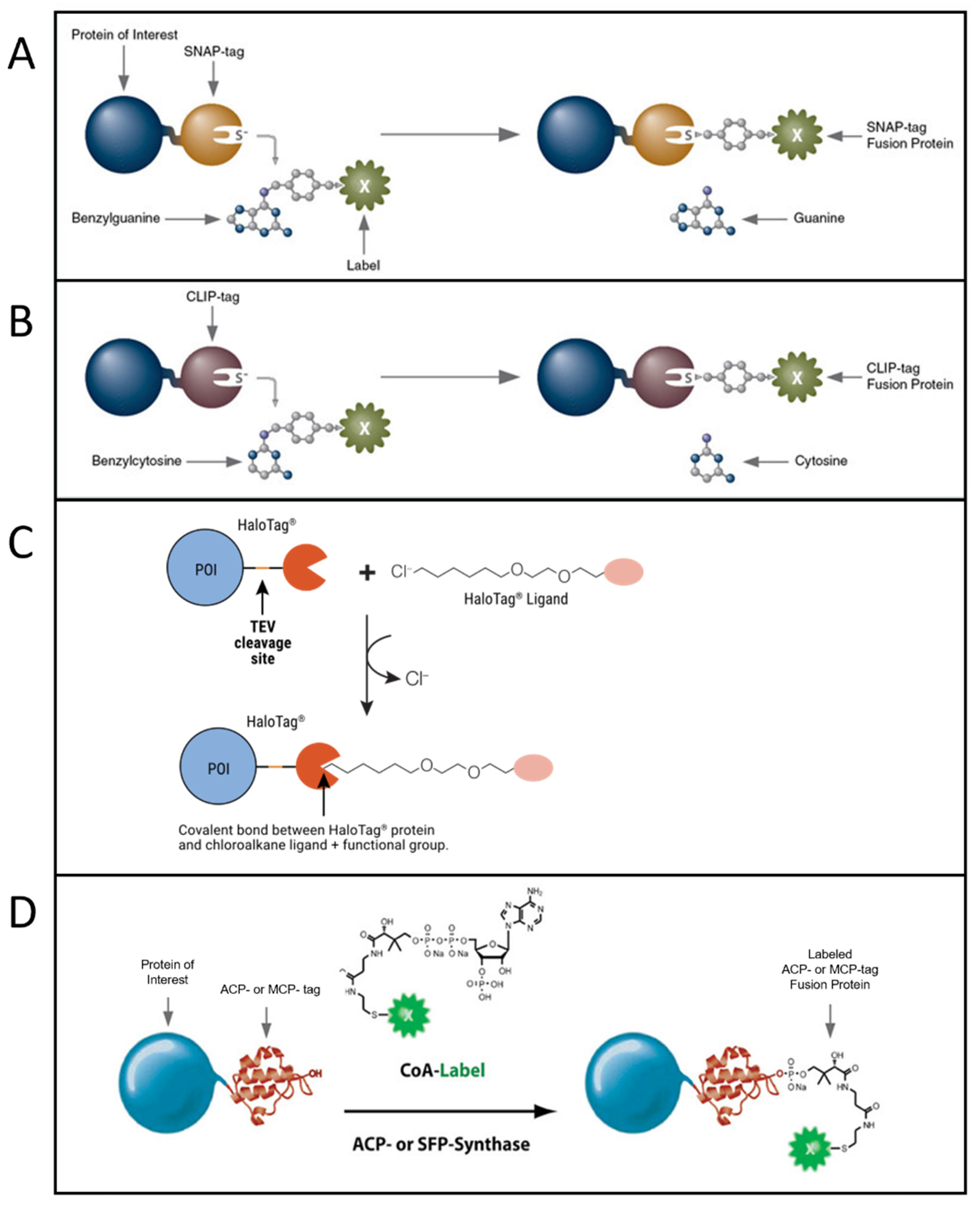
5. Fluorescent Probes for Single Molecule Microscopy
5.1. Single-Molecule Techniques
5.2. Fluorescent Proteins
5.3. Organic Dyes
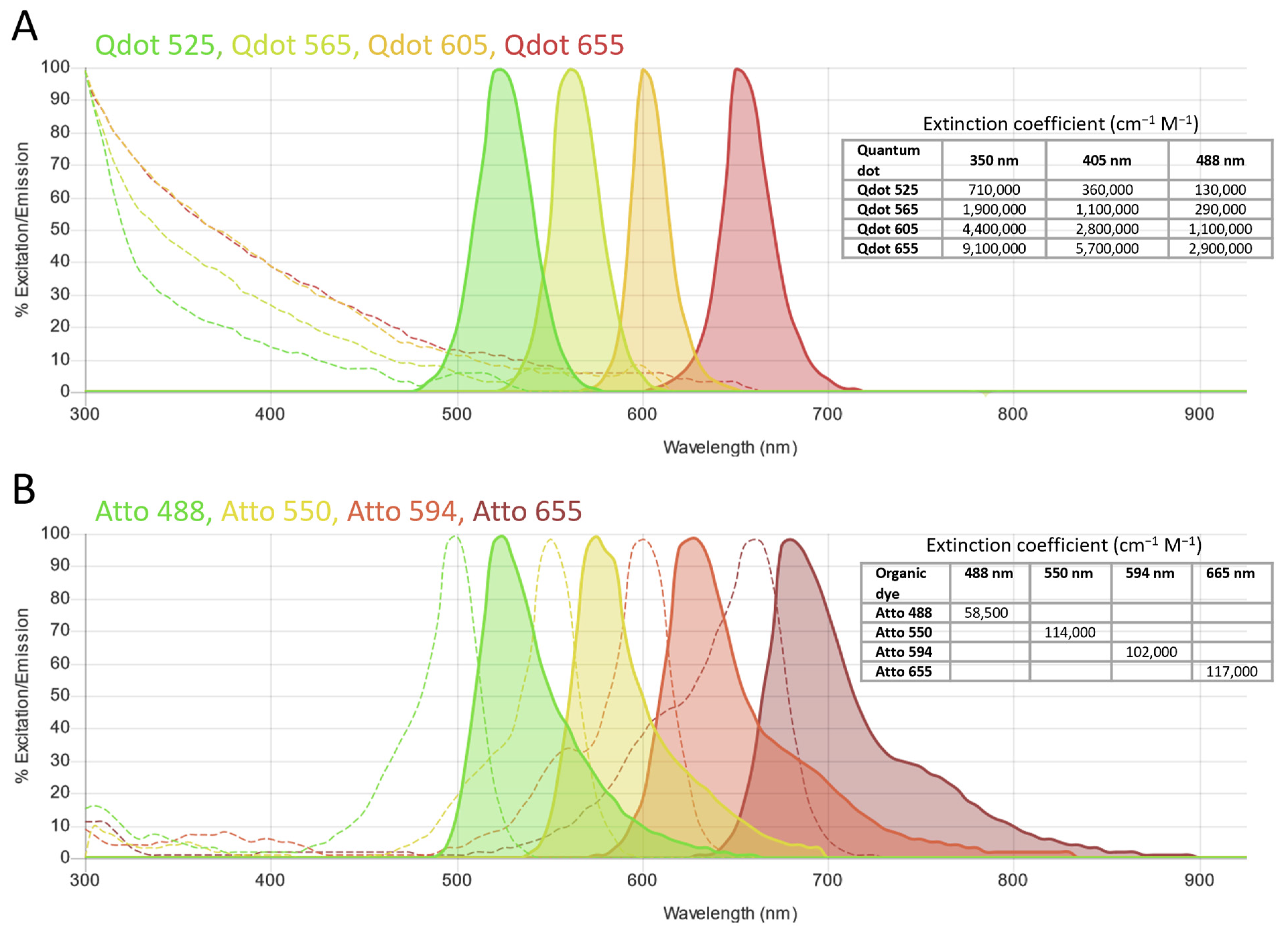
5.4. Quantum Dots and Other Nanoparticles
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hickey, S.M.; Ung, B.; Bader, C.; Brooks, R.; Lazniewska, J.; Johnson, I.R.D.; Sorvina, A.; Logan, J.; Martini, C.; Moore, C.R.; et al. Fluorescence microscopy—An outline of hardware, biological handling, and fluorophore considerations. Cells 2022, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.W.; Conchello, J.A. Fluorescence microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef]
- Liu, Z.; Lavis, L.D.; Betzig, E. Imaging Live-Cell Dynamics and Structure at the Single-Molecule Level. Mol. Cell 2015, 58, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Shashkova, S.; Leake, M.C. Single-molecule fluorescence microscopy review: Shedding new light on old problems. Biosci. Rep. 2017, 37, BSR20170031. [Google Scholar] [CrossRef] [PubMed]
- Axmann, M.; Madl, J.; Schütz, G.J. Single-Molecule Microscopy in the Life Sciences. In Fluorescence Microscopy; Wiley: Hoboken, NJ, USA, 2013; pp. 293–343. [Google Scholar]
- Munsky, B.; Fox, Z.; Neuert, G. Integrating single-molecule experiments and discrete stochastic models to understand heterogeneous gene transcription dynamics. Methods 2015, 85, 12–21. [Google Scholar] [CrossRef]
- Janissen, R.; Eslami-Mossallam, B.; Artsimovitch, I.; Depken, M.; Dekker, N.H. High-throughput single-molecule experiments reveal heterogeneity, state switching, and three interconnected pause states in transcription. Cell Rep. 2022, 39, 110749. [Google Scholar] [CrossRef]
- Mashanov, G.I.; Nenasheva, T.A.; Mashanova, A.; Lape, R.; Birdsall, N.J.M.; Sivilotti, L.; Molloy, J.E. Heterogeneity of cell membrane structure studied by single molecule tracking. Faraday Discuss. 2021, 232, 358–374. [Google Scholar] [CrossRef]
- Semrau, S.; Schmidt, T. Membrane heterogeneity—From lipid domains to curvature effects. Soft Matter 2009, 5, 3174–3186. [Google Scholar] [CrossRef]
- Marchetti, L.; Callegari, A.; Luin, S.; Signore, G.; Viegi, A.; Beltram, F.; Cattaneo, A. Ligand signature in the membrane dynamics of single TrkA receptor molecules. J. Cell Sci. 2013, 126, 4445–4456. [Google Scholar] [CrossRef]
- Tian, H.; Furstenberg, A.; Huber, T. Labeling and single-molecule methods to monitor G proteincoupled receptor dynamics. Chem. Rev. 2017, 117, 186–245. [Google Scholar] [CrossRef]
- Hoze, N.; Nair, D.; Hosy, E.; Sieben, C.; Manley, S.; Herrmann, A.; Sibarita, J.B.; Choquet, D.; Holcmana, D. Heterogeneity of AMPA receptor trafficking and molecular interactions revealed by superresolution analysis of live cell imaging. Proc. Natl. Acad. Sci. USA 2012, 109, 17052–17057. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; McHale, K.; Louis, J.M.; Eaton, W.A. Single-molecule fluorescence experiments determine protein folding transition path times. Science 2012, 335, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Deniz, A.A.; Laurence, T.A.; Beligere, G.S.; Dahan, M.; Martin, A.B.; Chemla, D.S.; Dawson, P.E.; Schultz, P.G.; Weiss, S. Single-molecule protein folding: Diffusion fluorescence resonance energy transfer studies of the denaturation of chymotrypsin inhibitor 2. Proc. Natl. Acad. Sci. USA 2000, 97, 5179–5184. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chen, K.; Yan, R.; Li, W.; Xu, K. Single-molecule displacement mapping unveils nanoscale heterogeneities in intracellular diffusivity. Nat. Methods 2020, 17, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Zhang, Z.L.; Sun, E.Z.; Peng, J.; Xie, M.; Tian, Z.Q.; Lin, Y.; Pang, D.W. Visualizing the endocytic and exocytic processes of wheat germ agglutinin by quantum dot-based single-particle tracking. Biomaterials 2011, 32, 7616–7624. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.; Clarke, D. Single Molecule Fluorescence Detection and Tracking in Mammalian Cells: The State-of-the-Art and Future Perspectives. Int. J. Mol. Sci. 2012, 13, 14742–14765. [Google Scholar] [CrossRef]
- Miller, H.; Zhou, Z.; Shepherd, J.; Wollman, A.J.M.; Leake, M.C. Single-molecule techniques in biophysics: A review of the progress in methods and applications. Rep. Prog. Phys. 2018, 81, 024601. [Google Scholar] [CrossRef]
- Kapanidis, A.N.; Weiss, S. Fluorescent probes and bioconjugation chemistries for single-molecule fluorescence analysis of biomolecules. J. Chem. Phys. 2002, 117, 10953–10964. [Google Scholar] [CrossRef]
- Gooding, J.J.; Gaus, K. Single-Molecule Sensors: Challenges and Opportunities for Quantitative Analysis. Angew. Chem. Int. Ed. 2016, 55, 11354–11366. [Google Scholar] [CrossRef]
- Michalet, X.; Colyer, R.A.; Scalia, G.; Ingargiola, A.; Lin, R.; Millaud, J.E.; Weiss, S.; Siegmund, O.H.W.; Tremsin, A.S.; Vallerga, J.V.; et al. Development of new photon-counting detectors for single-molecule fluorescence microscopy. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120035. [Google Scholar] [CrossRef]
- Leake, M.C. Analytical tools for single-molecule fluorescence imaging in cellulo. Phys. Chem. Chem. Phys. 2014, 16, 12635–12647. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.; Zhang, L.; Granick, S. Methods to track single-molecule trajectories. Langmuir 2006, 22, 5266–5272. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.E.; Larson, D.R.; Webb, W.W. Precise nanometer localization analysis for individual fluorescent probes. Biophys. J. 2002, 82, 2775–2783. [Google Scholar] [CrossRef]
- Vandenberk, N.; Barth, A.; Borrenberghs, D.; Hofkens, J.; Hendrix, J. Evaluation of Blue and Far-Red Dye Pairs in Single-Molecule Förster Resonance Energy Transfer Experiments. J. Phys. Chem. B 2018, 122, 4249–4266. [Google Scholar] [CrossRef]
- Zhang, Z.; Yomo, D.; Gradinaru, C. Choosing the right fluorophore for single-molecule fluorescence studies in a lipid environment. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Zanetti-Domingues, L.C.; Tynan, C.J.; Rolfe, D.J.; Clarke, D.T.; Martin-Fernandez, M. Hydrophobic Fluorescent Probes Introduce Artifacts into Single Molecule Tracking Experiments Due to Non-Specific Binding. PLoS ONE 2013, 8, e74200. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, R.; Convertino, D.; Calvello, M.; Ceccarelli, L.; Bonsignore, F.; Ravelli, C.; Cattaneo, A.; Martini, C.; Luin, S.; Mitola, S.; et al. Fluorolabeling of the PPTase-Related Chemical Tags: Comparative Study of Different Membrane Receptors and Different Fluorophores in the Labeling Reactions. Front. Mol. Biosci. 2020, 7, 195. [Google Scholar] [CrossRef]
- Bosch, P.J.; Corrêa, I.R.; Sonntag, M.H.; Ibach, J.; Brunsveld, L.; Kanger, J.S.; Subramaniam, V. Evaluation of fluorophores to label SNAP-Tag fused proteins for multicolor single-molecule tracking microscopy in live cells. Biophys. J. 2014, 107, 803–814. [Google Scholar] [CrossRef]
- Tsunoyama, T.A.; Watanabe, Y.; Goto, J.; Naito, K.; Kasai, R.S.; Suzuki, K.G.N.; Fujiwara, T.K.; Kusumi, A. Super-long single-molecule tracking reveals dynamic-anchorage-induced integrin function. Nat. Chem. Biol. 2018, 14, 497–506. [Google Scholar] [CrossRef]
- Lehmann, M.; Lichtner, G.; Klenz, H.; Schmoranzer, J. Novel organic dyes for multicolor localization-based super-resolution microscopy. J. Biophotonics 2016, 9, 161–170. [Google Scholar] [CrossRef]
- Dempsey, G.T.; Vaughan, J.C.; Chen, K.H.; Bates, M.; Zhuang, X. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 2011, 8, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Bittel, A.M.; Nickerson, A.; Saldivar, I.S.; Dolman, N.J.; Nan, X.; Gibbs, S.L. Methodology for quantitative characterization of fluorophore photoswitching to predict superresolution microscopy image quality. Sci. Rep. 2016, 6, 29687. [Google Scholar] [CrossRef] [PubMed]
- Kügel, W.; Muschielok, A.; Michaelis, J. Bayesian-inference-based fluorescence correlation spectroscopy and single-molecule burst analysis reveal the influence of dye selection on DNA hairpin dynamics. ChemPhysChem 2012, 13, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Bonsignore, F.; Gobbo, F.; Amodeo, R.; Calvello, M.; Jacob, A.; Signore, G.; Schirripa Spagnolo, C.; Porciani, D.; Mainardi, M.; et al. Fast-diffusing p75NTR monomers support apoptosis and growth cone collapse by neurotrophin ligands. Proc. Natl. Acad. Sci. USA 2019, 116, 21563–21572. [Google Scholar] [CrossRef]
- Jahnke, K.; Grubmüller, H.; Igaev, M.; Göpfrich, K. Choice of fluorophore affects dynamic DNA nanostructures. Nucleic Acids Res. 2021, 49, 4186–4195. [Google Scholar] [CrossRef] [PubMed]
- Hayashi-Takanaka, Y.; Stasevich, T.J.; Kurumizaka, H.; Nozaki, N.; Kimura, H. Evaluation of chemical fluorescent dyes as a protein conjugation partner for live cell imaging. PLoS ONE 2014, 9, e106271. [Google Scholar] [CrossRef]
- Marchetti, L.; De Nadai, T.; Bonsignore, F.; Calvello, M.; Signore, G.; Viegi, A.; Beltram, F.; Luin, S.; Cattaneo, A. Site-Specific Labeling of Neurotrophins and Their Receptors via Short and Versatile Peptide Tags. PLoS ONE 2014, 9, e113708. [Google Scholar] [CrossRef] [PubMed]
- Spectra Viewer|Chroma Technology Corp. Available online: https://www.chroma.com/spectra-viewer (accessed on 13 October 2022).
- Spectra Viewer|Tocris Bioscience. Available online: https://www.tocris.com/resources/spectral-viewer (accessed on 13 October 2022).
- Fluorescence SpectraViewer. Available online: https://www.thermofisher.com/order/fluorescence-spectraviewer#!/ (accessed on 13 October 2022).
- Fluorescence Spectrum Viewer|AAT Bioquest. Available online: https://www.aatbio.com/fluorescence-excitation-emission-spectrum-graph-viewer (accessed on 13 October 2022).
- Spectra Analyzer. Available online: https://www.biolegend.com/en-us/spectra-analyzer (accessed on 13 October 2022).
- Spectra Viewer. Available online: https://public.brain.mpg.de/shiny/apps/SpectraViewer/ (accessed on 13 October 2022).
- FPbase Fluorescence Spectra Viewer. Available online: https://www.fpbase.org/spectra/ (accessed on 13 October 2022).
- FluoroFinder. Available online: https://app.fluorofinder.com/ffsv/svs/78847dc03520013b647f0211f09e3eab (accessed on 23 October 2022).
- Grimm, J.B.; Brown, T.A.; English, B.P.; Lionnet, T.; Lavis, L.D. Synthesis of Janelia Fluor HaloTag and SNAP-Tag Ligands and Their Use in Cellular Imaging Experiments BT—Super-Resolution Microscopy: Methods and Protocols. Methods Mol. Biol. 2017, 1663, 179–188. [Google Scholar]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef]
- Jaqaman, K.; Loerke, D.; Mettlen, M.; Kuwata, H.; Grinstein, S.; Schmid, S.L.; Danuser, G. Robust single-particle tracking in live-cell time-lapse sequences. Nat. Methods 2008, 5, 695–702. [Google Scholar] [CrossRef]
- Sakon, J.J.; Weninger, K.R. Detecting the conformation of individual proteins in live cells. Nat. Methods 2010, 7, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Reyhani, A. Evaluating single molecule detection methods for microarrays with high dynamic range for quantitative single cell analysis. Sci. Rep. 2017, 7, 17957. [Google Scholar] [CrossRef] [PubMed]
- Aubin, J.E. Autofluorescence of viable cultured mammalian cells. J. Histochem. Cytochem. 1979, 27, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.C.; Meyer, R.A.; Zaruba, M.E.; McKhann, G.M. Cellular autofluorescence—Is it due to flavins? J. Histochem. Cytochem. 1979, 27, 44–48. [Google Scholar] [CrossRef]
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 2005, 11, 227–256. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, H.; Zheng, D.; Cao, Q.; Wang, Y.; Harris, D.; Wang, Y. Sudan black B reduces autofluorescence in murine renal tissue. Arch. Pathol. Lab. Med. 2011, 135, 1335–1342. [Google Scholar] [CrossRef]
- Andersson, H.; Baechi, T.; Hoechl, M.; Richter, C. Autofluorescence of living cells. J. Microsc. 1998, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sustarsic, M.; Kapanidis, A.N. Taking the ruler to the jungle: Single-molecule FRET for understanding biomolecular structure and dynamics in live cells. Curr. Opin. Struct. Biol. 2015, 34, 52–59. [Google Scholar] [CrossRef]
- König, I.; Zarrine-Afsar, A.; Aznauryan, M.; Soranno, A.; Wunderlich, B.; Dingfelder, F.; Stüber, J.C.; Plückthun, A.; Nettels, D.; Schuler, B. Single-molecule spectroscopy of protein conformational dynamics in live eukaryotic cells. Nat. Methods 2015, 12, 773–779. [Google Scholar] [CrossRef]
- Palmer, G.M.; Keely, P.J.; Breslin, T.M.; Ramanujam, N. Autofluorescence Spectroscopy of Normal and Malignant Human Breast Cell Lines. Photochem. Photobiol. 2003, 78, 462–469. [Google Scholar] [CrossRef]
- Croce, A.C.; Spano, A.; Locatelli, D.; Barni, S.; Sciola, L.; Bottiroli, G. Dependence of fibroblast autofluorescence properties on normal and transformed conditions. Role of the metabolic activity. Photochem. Photobiol. 1999, 69, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Pitts, J.D.; Sloboda, R.D.; Dragnev, K.H.; Dmitrovsky, E.; Mycek, M.-A. Autofluorescence characteristics of immortalized and carcinogen-transformed human bronchial epithelial cells. J. Biomed. Opt. 2001, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog. Retin. Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef] [PubMed]
- de Castro, J.V.; Gonçalves, C.S.; Martins, E.P.; Miranda-Lorenzo, I.; Cerqueira, M.T.; Longatto-Filho, A.; Pinto, A.A.; Reis, R.L.; Sousa, N.; Heeschen, C.; et al. Intracellular autofluorescence as a new biomarker for cancer stem cells in glioblastoma. Cancers 2021, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Huang, J.S.; Wang, Y.Y.; Chen, K.C.; Wong, T.Y.; Chen, Y.C.; Wu, C.W.; Chan, L.P.; Lin, Y.C.; Kao, Y.H.; et al. Novel quantitative analysis of autofluorescence images for oral cancer screening. Oral Oncol. 2017, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, K.; Aebisher, D.; Międzybrodzka, A.; Cieślar, G.; Kawczyk-Krupka, A. Methods for bladder cancer diagnosis—The role of autofluorescence and photodynamic diagnosis. Photodiagnosis Photodyn. Ther. 2019, 27, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, G.; Croce, A.C.; Locatelli, D.; Nano, R.; Giombelli, E.; Messina, A.; Benericetti, E. Brain Tissue Autofluorescence: An Aid for Intraoperative Delineation of Tumor Resection Margins. Cancer Detect. Prev. 1998, 22, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, G.; Croce, A.C.; Locatelli, D.; Marchesini, R.; Pignoli, E.; Tomatis, S.; Cuzzoni, C.; Di Palma, S.; Dalfante, M.; Spinelli, P. Natural fluorescence of normal and neoplastic human colon: A comprehensive “ex vivo” study. Lasers Surg. Med. 1995, 16, 48–60. [Google Scholar] [CrossRef]
- Chacko, J.V.; Eliceiri, K.W. Autofluorescence lifetime imaging of cellular metabolism: Sensitivity toward cell density, pH, intracellular, and intercellular heterogeneity. Cytom. Part A 2019, 95, 56–69. [Google Scholar] [CrossRef]
- Awasthi, K.; Moriya, D.; Nakabayashi, T.; Li, L.; Ohta, N. Sensitive detection of intracellular environment of normal and cancer cells by autofluorescence lifetime imaging. J. Photochem. Photobiol. B Biol. 2016, 165, 256–265. [Google Scholar] [CrossRef]
- Heaster, T.M.; Walsh, A.J.; Zhao, Y.; Hiebert, S.W.; Skala, M.C. Autofluorescence imaging identifies tumor cell-cycle status on a single-cell level. J. Biophotonics 2018, 11, e201600276. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.R.; Ross, S.T.; Davidson, M.W. Single molecule localization microscopy for superresolution. J. Opt. 2013, 15, 094001. [Google Scholar] [CrossRef]
- Croce, A.C.; Bottiroli, G. Autofluorescence spectroscopy for monitoring Metabolism in Animal cells and tissues. In Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2017; pp. 15–43. [Google Scholar]
- Wazawa, T.; Ueda, M. Total internal reflection fluorescence microscopy in single molecule nanobioscience. Adv. Biochem. Eng. Biotechnol. 2005, 95, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Gambin, Y.; Vandelinder, V.; Ferreon, A.C.M.; Lemke, E.A.; Groisman, A.; Deniz, A.A. Visualizing a one-way protein encounter complex by ultrafast single-molecule mixing. Nat. Methods 2011, 8, 239–241. [Google Scholar] [CrossRef]
- Friedman, L.J.; Chung, J.; Gelles, J. Viewing dynamic assembly of molecular complexes by multi-wavelength single-molecule fluorescence. Biophys. J. 2006, 91, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Staughton, T.J.; McGillicuddy, C.J.; Weinberg, P.D. Techniques for reducing the interfering effects of autofluorescence in fluorescence microscopy: Improved detection of sulphorhodamine B-labelled albumin in arterial tissue. J. Microsc. 2001, 201, 70–76. [Google Scholar] [CrossRef]
- Hess, S.T.; Gould, T.J.; Gunewardene, M.; Bewersdorf, J.; Mason, M.D. Ultrahigh resolution imaging of biomolecules by fluorescence photoactivation localization microscopy. Methods Mol. Biol. 2009, 544, 483–522. [Google Scholar] [CrossRef]
- Razali, W.A.W.; Sreenivasan, V.K.A.; Bradac, C.; Connor, M.; Goldys, E.M.; Zvyagin, A.V. Wide-field time-gated photoluminescence microscopy for fast ultrahigh-sensitivity imaging of photoluminescent probes. J. Biophotonics 2016, 9, 848–858. [Google Scholar] [CrossRef]
- Davis, L.M.; Parker, W.C.; Ball, D.A.; Williams, J.G.; Bashford, G.R.; Sheaff, P.; Eckles, R.D.; Lamb, D.T.; Middendorf, L.R. Imaging of single-chromophore molecules in aqueous solution near a fused-silica interface. Multiphot. Microsc. Biomed. Sci. 2001, 4262, 301. [Google Scholar] [CrossRef]
- Maes, C.F.; Yan, C. Laser-induced fluorescence in fused silica and other optical materials. In Fluorescence Detection IV; Menzel, E.R., Katzir, A., Eds.; SPIE: Bellingham, WA, USA, 1996; pp. 93–102. [Google Scholar]
- Yan, Y.-J.; Wang, B.-W.; Yang, C.-M.; Wu, C.-Y.; Ou-Yang, M. Autofluorescence Detection Method for Dental Plaque Bacteria Detection and Classification: Example of Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Streptococcus mutans. Dent. J. 2021, 9, 74. [Google Scholar] [CrossRef]
- Rabouw, F.T.; Cogan, N.M.B.; Berends, A.C.; van der Stam, W.; Vanmaekelbergh, D.; Koenderink, A.F.; Krauss, T.D.; Donega, C.D.M. Non-blinking single-photon emitters in silica. Sci. Rep. 2016, 6, 21187. [Google Scholar] [CrossRef] [PubMed]
- Pinkel, D.; Segraves, R.; Sudar, D.; Clark, S.; Poole, I.; Kowbel, D.; Collins, C.; Kuo, W.L.; Chen, C.; Zhai, Y.; et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nat. Genet. 1998, 20, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Shilova, O.N.; Shilov, E.S.; Deyev, S.M. The effect of trypan blue treatment on autofluorescence of fixed cells. Cytom. Part A 2017, 91, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Zanetti-Domingues, L.C.; Martin-Fernandez, M.L.; Needham, S.R.; Rolfe, D.J.; Clarke, D.T. A Systematic Investigation of Differential Effects of Cell Culture Substrates on the Extent of Artifacts in Single-Molecule Tracking. PLoS ONE 2012, 7, e45655. [Google Scholar] [CrossRef] [PubMed]
- Quinn, A.; Mantz, H.; Jacobs, K.; Bellion, M.; Santen, L. Protein adsorption kinetics in different surface potentials. EPL 2008, 81, 56003. [Google Scholar] [CrossRef]
- Breault-Turcot, J.; Chaurand, P.; Masson, J.F. Unravelling nonspecific adsorption of complex protein mixture on surfaces with SPR and MS. Anal. Chem. 2014, 86, 9612–9619. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-specific adsorption reduction methods in biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef]
- Stüber, J.C.; Richter, C.P.; Bellón, J.S.; Schwill, M.; König, I.; Schuler, B.; Piehler, J.; Plückthun, A. Apoptosis-inducing anti-HER2 agents operate through oligomerization-induced receptor immobilization. Commun. Biol. 2021, 4, 762. [Google Scholar] [CrossRef] [PubMed]
- Shelby, S.A.; Holowka, D.; Baird, B.; Veatch, S.L. Distinct stages of stimulated fcεri receptor clustering and immobilization are identified through superresolution imaging. Biophys. J. 2013, 105, 2343–2354. [Google Scholar] [CrossRef]
- Qin, M.; Hou, S.; Wang, L.K.; Feng, X.Z.; Wang, R.; Yang, Y.L.; Wang, C.; Yu, L.; Shao, B.; Qiao, M.Q. Two methods for glass surface modification and their application in protein immobilization. Colloids Surf. B Biointerfaces 2007, 60, 243–249. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Review of the research on anti-protein fouling coatings materials. Prog. Org. Coatings 2020, 147. [Google Scholar] [CrossRef]
- Luk, Y.Y.; Kato, M.; Mrksich, M. Self-assembled monolayers of alkanethiolates presenting mannitol groups are inert to protein adsorption and cell attachment. Langmuir 2000, 16, 9604–9608. [Google Scholar] [CrossRef]
- Hua, B.; Han, K.Y.; Zhou, R.; Kim, H.; Shi, X.; Abeysirigunawardena, S.C.; Jain, A.; Singh, D.; Aggarwal, V.; Woodson, S.A.; et al. An improved surface passivation method for single-molecule studies. Nat. Methods 2014, 11, 1233–1236. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Y.; Qin, M.; Cao, Y.; Wang, W. A simple procedure to improve the surface passivation for single molecule fluorescence studies. Phys. Biol. 2015, 12, 045006. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Fiedler, J.; Engelhard, E.; Ameringer, T.; Tugulu, S.; Klok, H.A.; Brenner, R.E.; Moeller, M. A novel star PEG-derived surface coating for specific cell adhesion. J. Biomed. Mater. Res.—Part A 2005, 74, 607–617. [Google Scholar] [CrossRef]
- Revyakin, A.; Zhang, Z.; Coleman, R.A.; Li, Y.; Inouye, C.; Lucas, J.K.; Park, S.R.; Chu, S.; Tjian, R. Transcription initiation by human RNA polymerase II visualized at single-molecule resolution. Genes Dev. 2012, 26, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Ogle, R.C.; Robey, F.A.; Sasaki, M.; Martin, G.R.; Yamada, Y.; Kleinman, H.K. A Pentapeptide from the Laminin B1 Chain Mediates Cell Adhesion and Binds the 67 000 Laminin Receptor. Biochemistry 1987, 26, 6896–6900. [Google Scholar] [CrossRef]
- Taite, L.J.; Yang, P.; Jun, H.W.; West, J.L. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2008, 84, 108–116. [Google Scholar] [CrossRef]
- Schlapak, R.; Armitage, D.; Saucedo-Zeni, N.; Chrzanowski, W.; Hohage, M.; Caruana, D.; Howorka, S. Selective protein and DNA adsorption on PLL-PEG films modulated by ionic strength. Soft Matter 2009, 5, 613–621. [Google Scholar] [CrossRef]
- Tessmar, J.K.; Göpferich, A.M. Customized PEG-derived copolymers for tissue-engineering applications. Macromol. Biosci. 2007, 7, 23–39. [Google Scholar] [CrossRef]
- Hughes, L.D.; Rawle, R.J.; Boxer, S.G. Choose Your Label Wisely: Water-Soluble Fluorophores Often Interact with Lipid Bilayers. PLoS ONE 2014, 9, e87649. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, J.; Kühn, S.; Tarnawski, M.; Gotthard, G.; Tünnermann, J.; Tänzer, T.; Karpenko, J.; Mertes, N.; Xue, L.; Uhrig, U.; et al. Kinetic and Structural Characterization of the Self-Labeling Protein Tags HaloTag7, SNAP-tag, and CLIP-tag. Biochemistry 2021, 60, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Garcia-Parajo, M.F. A review of progress in single particle tracking: From methods to biophysical insights. Reports Prog. Phys. 2015, 78, 12. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, D.M.; Sengupta, P.; Lippincott-Schwartz, J.; Verkhusha, V.V. Photocontrollable fluorescent proteins for superresolution imaging. Annu. Rev. Biophys. 2014, 43, 303–329. [Google Scholar] [CrossRef]
- Ha, T. Single-molecule fluorescence resonance energy transfer. Methods 2001, 25, 78–86. [Google Scholar] [CrossRef]
- Roy, R.; Hohng, S.; Ha, T. A practical guide to single-molecule FRET. Nat. Methods 2008, 5, 507–516. [Google Scholar] [CrossRef]
- Clausen, M.P.; Lagerholm, B.C. The Probe Rules in Single Particle Tracking. Curr. Protein Pept. Sci. 2011, 12, 699–713. [Google Scholar] [CrossRef]
- Lelek, M.; Gyparaki, M.T.; Beliu, G.; Schueder, F.; Griffié, J.; Manley, S.; Jungmann, R.; Sauer, M.; Lakadamyali, M.; Zimmer, C. Single-molecule localization microscopy. Nat. Rev. Methods Prim. 2021, 1, 39. [Google Scholar] [CrossRef]
- Pujals, S.; Albertazzi, L. Super-resolution Microscopy for Nanomedicine Research. ACS Nano 2019, 13, 9707–9712. [Google Scholar] [CrossRef]
- Kikuchi, K.; Adair, L.D.; Lin, J.; New, E.J.; Kaur, A. Photochemical Mechanisms of Fluorophores Employed in Single-Molecule Localization Microscopy. Angew. Chem. Int. Ed. 2022, e202204745. [Google Scholar] [CrossRef]
- Shimomura, O.; Johnson, F.H.; Saiga, Y. Extraction, Purification and Properties of Aequorin, a Bioluminescent Protein from the Luminous Hydromedusan, Aequorea. J. Cell. Comp. Physiol. 1962, 59, 223–239. [Google Scholar] [CrossRef]
- Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 1998, 67, 509–544. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Patterson, G.H.; Davidson, M.W. Advances in fluorescent protein technology. J. Cell Sci. 2007, 120, 4247–4260. [Google Scholar] [CrossRef]
- Kremers, G.J.; Gilbert, S.G.; Cranfill, P.J.; Davidson, M.W.; Piston, D.W. Fluorescent proteins at a glance. J. Cell Sci. 2011, 124, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Snapp, E.L. Fluorescent proteins: A cell biologist’s user guide. Trends Cell Biol. 2009, 19, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.M.; Fossati, M.; Francolini, M.; Snapp, E.L. Assessing the Tendency of Fluorescent Proteins to Oligomerize Under Physiologic Conditions. Traffic 2012, 13, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, D.A.; Violin, J.D.; Newton, A.C.; Tsien, R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 2002, 296, 913–916. [Google Scholar] [CrossRef]
- Costantini, L.M.; Baloban, M.; Markwardt, M.L.; Rizzo, M.; Guo, F.; Verkhusha, V.V.; Snapp, E.L. A palette of fluorescent proteins optimized for diverse cellular environments. Nat. Commun. 2015, 6, 7670. [Google Scholar] [CrossRef]
- Marchetti, L.; Comelli, L.; D’Innocenzo, B.; Puzzi, L.; Luin, S.; Arosio, D.; Calvello, M.; Mendoza-Maldonado, R.; Peverali, F.; Trovato, F.; et al. Homeotic proteins participate in the function of human-DNA replication origins. Nucleic Acids Res. 2010, 38, 8105–8119. [Google Scholar] [CrossRef]
- Miyawaki, A. Development of probes for cellular functions using fluorescent proteins and fluorescence resonance energy transfer. Annu. Rev. Biochem. 2011, 80, 357–373. [Google Scholar] [CrossRef]
- Hochreiter, B.; Garcia, A.P.; Schmid, J.A. Fluorescent proteins as genetically encoded FRET biosensors in life sciences. Sensors 2015, 15, 26281–26314. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.J.; St-Pierre, F.; Gong, Y.; Marshall, J.D.; Cranfill, P.J.; Baird, M.A.; McKeown, M.R.; Wiedenmann, J.; Davidson, M.W.; Schnitzer, M.J.; et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 2012, 9, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Bajar, B.T.; Wang, E.S.; Zhang, S.; Lin, M.Z.; Chu, J. A guide to fluorescent protein FRET pairs. Sensors 2016, 16, 1488. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.A.; Springer, G.H.; Granada, B.; Piston, D.W. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 2004, 22, 445–449. [Google Scholar] [CrossRef]
- Piston, D.W.; Kremers, G.J. Fluorescent protein FRET: The good, the bad and the ugly. Trends Biochem. Sci. 2007, 32, 407–414. [Google Scholar] [CrossRef]
- Albertazzi, L.; Arosio, D.; Marchetti, L.; Ricci, F.; Beltram, F. Quantitative FRET analysis with the E0GFP-mCherry fluorescent protein pair. Photochem. Photobiol. 2009, 85, 287–297. [Google Scholar] [CrossRef]
- Mo, G.C.H.; Posner, C.; Rodriguez, E.A.; Sun, T.; Zhang, J. A rationally enhanced red fluorescent protein expands the utility of FRET biosensors. Nat. Commun. 2020, 11, 1848. [Google Scholar] [CrossRef]
- Bindels, D.S.; Haarbosch, L.; Van Weeren, L.; Postma, M.; Wiese, K.E.; Mastop, M.; Aumonier, S.; Gotthard, G.; Royant, A.; Hink, M.A.; et al. MScarlet: A bright monomeric red fluorescent protein for cellular imaging. Nat. Methods 2016, 14, 53–56. [Google Scholar] [CrossRef]
- Bajar, B.T.; Wang, E.S.; Lam, A.J.; Kim, B.B.; Jacobs, C.L.; Howe, E.S.; Davidson, M.W.; Lin, M.Z.; Chu, J. Improving brightness and photostability of green and red fluorescent proteins for live cell imaging and FRET reporting. Sci. Rep. 2016, 6, 20889. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef]
- Shroff, H.; Galbraith, C.G.; Galbraith, J.A.; Betzig, E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat. Methods 2008, 5, 417–423. [Google Scholar] [CrossRef]
- Biteen, J.S.; Thompson, M.A.; Tselentis, N.K.; Bowman, G.R.; Shapiro, L.; Moerner, W.E. Super-resolution imaging in live Caulobacter crescentus cells using photoswitchable EYFP. Nat. Methods 2008, 5, 947–949. [Google Scholar] [CrossRef]
- De Zitter, E.; Thédié, D.; Mönkemöller, V.; Hugelier, S.; Beaudouin, J.; Adam, V.; Byrdin, M.; Van Meervelt, L.; Dedecker, P.; Bourgeois, D. Mechanistic investigation of mEos4b reveals a strategy to reduce track interruptions in sptPALM. Nat. Methods 2019, 16, 707–710. [Google Scholar] [CrossRef]
- Henriques, R.; Griffiths, C.; Rego, E.H.; Mhlanga, M.M. PALM and STORM: Unlocking live-cell super-resolution. Biopolymers 2011, 95, 322–331. [Google Scholar] [CrossRef]
- Niu, L.; Yu, J. Investigating intracellular dynamics of FtsZ cytoskeleton with photoactivation single-molecule tracking. Biophys. J. 2008, 95, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Suárez, M.; Ting, A.Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 929–943. [Google Scholar] [CrossRef]
- Wang, S.; Moffitt, J.R.; Dempsey, G.T.; Xie, X.S.; Zhuang, X. Characterization and development of photoactivatable fluorescent proteins for single-molecule-based superresolution imaging. Proc. Natl. Acad. Sci. USA 2014, 111, 8452–8457. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Zhang, R.; Yuan, J. Characterization of Photophysical Properties of Photoactivatable Fluorescent Proteins for Super-Resolution Microscopy. J. Phys. Chem. B 2020, 124, 1892–1897. [Google Scholar] [CrossRef]
- Luin, S.; Voliani, V.; Lanza, G.; Bizzarri, R.; Amat, P.; Tozzini, V.; Serresi, M.; Beltram, F. Raman Study of Chromophore States in Photochromic Fluorescent Proteins. J. Am. Chem. Soc. 2009, 131, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Vaughan, J.C. Switchable Fluorophores for Single-Molecule Localization Microscopy. Chem. Rev. 2018, 118, 9412–9454. [Google Scholar] [CrossRef]
- Abbruzzetti, S.; Bizzarri, R.; Luin, S.; Nifosì, R.; Storti, B.; Viappiani, C.; Beltram, F. Photoswitching of E222Q GFP mutants: “Concerted” mechanism of chromophore isomerization and protonation. Photochem. Photobiol. Sci. 2010, 9, 1307–1319. [Google Scholar] [CrossRef]
- Bizzarri, R.; Serresi, M.; Cardarelli, F.; Abbruzzetti, S.; Campanini, B.; Viappiani, C.; Beltram, F. Single amino acid replacement makes Aequorea victoria fluorescent proteins reversibly photoswitchable. J. Am. Chem. Soc. 2010, 132, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Z.; He, K.; Wu, J.; Li, N.; Zhao, R.; Yuan, J.; Xiao, H.; Zhang, Y.; Fang, X. Quantitative Characterization of the Membrane Dynamics of Newly Delivered TGF-β Receptors by Single-Molecule Imaging. Anal. Chem. 2018, 90, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Douglass, A.D.; Vale, R.D. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 2005, 121, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Li, N.; Zhao, L.; Sun, Y.; Ruan, H.; Zhang, M.; Yuan, J.; Fang, X. Super-resolution imaging and tracking of TGF-β receptor II on living cells. Sci. Bull. 2016, 61, 632–638. [Google Scholar] [CrossRef][Green Version]
- Manley, S.; Gillette, J.M.; Patterson, G.H.; Shroff, H.; Hess, H.F.; Betzig, E.; Lippincott-Schwartz, J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 2008, 5, 155–157. [Google Scholar] [CrossRef]
- Lee, Y.; Phelps, C.; Huang, T.; Mostofian, B.; Wu, L.; Zhang, Y.; Tao, K.; Chang, Y.H.; Stork, P.J.S.; Gray, J.W.; et al. High-throughput single-particle tracking reveals 1 nested membrane domains that dictate krasg12d 2 diffusion and trafficking. Elife 2019, 8, e46393. [Google Scholar] [CrossRef]
- Manley, S.; Gillette, J.M.; Lippincott-Schwartz, J. Single-Particle Tracking Photoactivated Localization Microscopy for Mapping Single-Molecule Dynamics. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2010; pp. 109–120. [Google Scholar]
- Shcherbakova, D.M.; Baloban, M.; Verkhusha, V.V. Near-infrared fluorescent proteins engineered from bacterial phytochromes. Curr. Opin. Chem. Biol. 2015, 27, 52–63. [Google Scholar] [CrossRef]
- Khan, F.I.; Hassan, F.; Anwer, R.; Juan, F.; Lai, D. Comparative Analysis of Bacteriophytochrome Agp2 and Its Engineered Photoactivatable NIR Fluorescent Proteins PAiRFP1 and PAiRFP2. Biomolecules 2020, 10, 1286. [Google Scholar] [CrossRef]
- Shcherbakova, D.M.; Subach, O.M.; Verkhusha, V.V. Red Fluorescent Proteins: Advanced Imaging Applications and Future Design. Angew. Chem. Int. Ed. 2012, 51, 10724–10738. [Google Scholar] [CrossRef]
- Fu, Y.; Finney, N.S. Small-molecule fluorescent probes and their design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef]
- Ni, M.; Zhuo, S.; So, P.T.C.; Yu, H. Fluorescent probes for nanoscopy: Four categories and multiple possibilities. J. Biophotonics 2017, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Li, N.; Fang, X. Single-molecule fluorescence imaging in living cells. Annu. Rev. Phys. Chem. 2013, 64, 459–480. [Google Scholar] [CrossRef]
- Banaz, N.; Mäkelä, J.; Uphoff, S. Choosing the right label for single-molecule tracking in live bacteria: Side-by-side comparison of photoactivatable fluorescent protein and Halo tag dyes. J. Phys. D. Appl. Phys. 2019, 52, 064002. [Google Scholar] [CrossRef] [PubMed]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An introduction to performing immunofluorescence staining. In Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2019; pp. 299–311. [Google Scholar]
- Oddone, A.; Vilanova, I.V.; Tam, J.; Lakadamyali, M. Super-resolution imaging with stochastic single-molecule localization: Concepts, technical developments, and biological applications. Microsc. Res. Tech. 2014, 77, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Wieser, S.; Moertelmaier, M.; Fuertbauer, E.; Stockinger, H.; Schütz, G.J. (Un)confined diffusion of CD59 in the plasma membrane determined by high-resolution single molecule microscopy. Biophys. J. 2007, 92, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Jaqaman, K.; Kuwata, H.; Touret, N.; Collins, R.; Trimble, W.S.; Danuser, G.; Grinstein, S. Cytoskeletal Control of CD36 Diffusion Promotes Its Receptor and Signaling Function. Cell 2011, 146, 593–606. [Google Scholar] [CrossRef]
- Wombacher, R.; Cornish, V.W. Chemical tags: Applications in live cell fluorescence imaging. J. Biophotonics 2011, 4, 391–402. [Google Scholar] [CrossRef]
- Schneider, A.F.L.; Hackenberger, C.P.R. Fluorescent labelling in living cells. Curr. Opin. Biotechnol. 2017, 48, 61–68. [Google Scholar] [CrossRef]
- Freidel, C.; Kaloyanova, S.; Peneva, K. Chemical tags for site-specific fluorescent labeling of biomolecules. Amino Acids 2016, 48, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Keppler, A.; Gendreizig, S.; Gronemeyer, T.; Pick, H.; Vogel, H.; Johnsson, K. A general method for the covalent labeling of fusion proteins with small molecules In Vivo. Nat. Biotechnol. 2003, 21, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Juillerat, A.; Heinis, C.; Corrêa, I.R.; Kindermann, M.; Beaufils, F.; Johnsson, K. An Engineered Protein Tag for Multiprotein Labeling in Living Cells. Chem. Biol. 2008, 15, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Los, G.V.; Encell, L.P.; McDougall, M.G.; Hartzell, D.D.; Karassina, N.; Zimprich, C.; Wood, M.G.; Learish, R.; Ohana, R.F.; Urh, M.; et al. HaloTag: A Novel Protein Labeling Technology for Cell Imaging and Protein Analysis. ACS Chem. Biol. 2008, 3, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Encell, L.P. Development of a Dehalogenase-Based Protein Fusion Tag Capable of Rapid, Selective and Covalent Attachment to Customizable Ligands. Curr. Chem. Genom. 2012, 6, 55–71. [Google Scholar] [CrossRef]
- England, C.G.; Luo, H.; Cai, W. HaloTag Technology: A Versatile Platform for Biomedical Applications. Bioconjug. Chem. 2015, 26, 975–986. [Google Scholar] [CrossRef]
- Yin, J.; Straight, P.D.; McLoughlin, S.M.; Zhou, Z.; Lin, A.J.; Golan, D.E.; Kelleher, N.L.; Kolter, R.; Walsh, C.T. Genetically encoded short peptide tag for versatile protein labeling by Sfp phosphopantetheinyl transferase. Proc. Natl. Acad. Sci. USA 2005, 102, 15815–15820. [Google Scholar] [CrossRef]
- George, N.; Pick, H.; Vogel, H.; Johnsson, N.; Johnsson, K. Specific labeling of cell surface proteins with chemically diverse compounds. J. Am. Chem. Soc. 2004, 126, 8896–8897. [Google Scholar] [CrossRef]
- Cole, N.B. Site-specific protein labeling with SNAP-tags. Curr. Protoc. Protein Sci. 2013, 2013, 30.1.1–30.1.16. [Google Scholar] [CrossRef]
- Maurel, D.; Comps-Agrar, L.; Brock, C.; Rives, M.-L.; Bourrier, E.; Ayoub, M.A.; Bazin, H.; Tinel, N.; Durroux, T.; Prézeau, L.; et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: Application to GPCR oligomerization. Nat. Methods 2008, 5, 561–567. [Google Scholar] [CrossRef]
- Calebiro, D.; Rieken, F.; Wagner, J.; Sungkaworn, T.; Zabel, U.; Borzi, A.; Cocucci, E.; Zurn, A.; Lohse, M.J. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. USA 2013, 110, 743–748. [Google Scholar] [CrossRef]
- Ibach, J.; Radon, Y.; Gelléri, M.; Sonntag, M.H.; Brunsveld, L.; Bastiaens, P.I.H.; Verveer, P.J. Single Particle Tracking Reveals that EGFR Signaling Activity Is Amplified in Clathrin-Coated Pits. PLoS ONE 2015, 10, e0143162. [Google Scholar] [CrossRef]
- Asher, W.B.; Geggier, P.; Holsey, M.D.; Gilmore, G.T.; Pati, A.K.; Meszaros, J.; Terry, D.S.; Mathiasen, S.; Kaliszewski, M.J.; McCauley, M.D.; et al. Single-molecule FRET imaging of GPCR dimers in living cells. Nat. Methods 2021, 18, 397–405. [Google Scholar] [CrossRef]
- Klein, T.; Löschberger, A.; Proppert, S.; Wolter, S.; Van De Linde, S.; Sauer, M. Live-cell dSTORM with SNAP-tag fusion proteins. Nat. Methods 2011, 8, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Sungkaworn, T.; Jobin, M.-L.; Burnecki, K.; Weron, A.; Lohse, M.J.; Calebiro, D. Single-molecule imaging reveals receptor–G protein interactions at cell surface hot spots. Nature 2017, 550, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Vafabakhsh, R.; Levitz, J.; Isacoff, E.Y. Conformational dynamics of a class C G-protein-coupled receptor. Nature 2015, 524, 497–501. [Google Scholar] [CrossRef]
- HaloTag® Technology: Novel New Applications. Available online: https://ita.promega.com/resources/pubhub/2019/halotag-technology-novel-new-applications/ (accessed on 20 October 2022).
- HaloTag® Technology: Focus on Imaging Protocol. Available online: https://ita.promega.com/resources/protocols/technical-manuals/0/halotag-technology-focus-on-imaging-protocol/ (accessed on 16 October 2022).
- Mazza, D.; Ganguly, S.; McNally, J.G. Monitoring dynamic binding of chromatin proteins In Vivo by single-molecule tracking. Methods Mol. Biol. 2013, 1042, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Huseyin, M.K.; Klose, R.J. Live-cell single particle tracking of PRC1 reveals a highly dynamic system with low target site occupancy. Nat. Commun. 2021, 12, 887. [Google Scholar] [CrossRef]
- Hipp, L.; Beer, J.; Kuchler, O.; Reisser, M.; Sinske, D.; Michaelis, J.; Gebhardt, J.C.M.; Knöll, B. Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proc. Natl. Acad. Sci. USA 2019, 116, 880–889. [Google Scholar] [CrossRef]
- Yin, J.; Liu, F.; Li, X.; Walsh, C.T. Labeling Proteins with Small Molecules by Site-Specific Posttranslational Modification. J. Am. Chem. Soc. 2004, 126, 7754–7755. [Google Scholar] [CrossRef]
- Yin, J.; Lin, A.J.; Buckett, P.D.; Wessling-Resnick, M.; Golan, D.E.; Walsh, C.T. Single-cell FRET imaging of transferrin receptor trafficking dynamics by Sfp-catalyzed, site-specific protein labeling. Chem. Biol. 2005, 12, 999–1006. [Google Scholar] [CrossRef]
- Zhou, Z.; Cironi, P.; Lin, A.J.; Xu, Y.; Hrvatin, S.; Golan, D.E.; Silver, P.A.; Walsh, C.T.; Yin, J. Genetically Encoded Short Peptide Tags for Orthogonal Protein Labeling by Sfp and AcpS Phosphopantetheinyl Transferases. ACS Chem. Biol. 2007, 2, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lin, A.J.; Golan, D.E.; Walsh, C.T. Site-specific protein labeling by Sfp phosphopantetheinyl transferase. Nat. Protoc. 2006, 1, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, F.; Bonsignore, F.; Amodeo, R.; Cattaneo, A.; Marchetti, L. Site-specific direct labeling of neurotrophins and their receptors: From biochemistry to advanced imaging applications. In Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2018; pp. 295–314. [Google Scholar]
- Callegari, A.; Luin, S.; Marchetti, L.; Duci, A.; Cattaneo, A.; Beltram, F. Single particle tracking of acyl carrier protein (ACP)-tagged TrkA receptors in PC12nnr5 cells. J. Neurosci. Methods 2012, 204, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Kasai, R.S.; Ito, S.V.; Awane, R.M.; Fujiwara, T.K.; Kusumi, A. The Class-A GPCR Dopamine D2 Receptor Forms Transient Dimers Stabilized by Agonists: Detection by Single-Molecule Tracking. Cell Biochem. Biophys. 2018, 76, 29–37. [Google Scholar] [CrossRef] [PubMed]
- De Nadai, T.; Marchetti, L.; Di Rienzo, C.; Calvello, M.; Signore, G.; Di Matteo, P.; Gobbo, F.; Turturro, S.; Meucci, S.; Viegi, A.; et al. Precursor and mature NGF live tracking: One versus many at a time in the axons. Sci. Rep. 2016, 6, 20272. [Google Scholar] [CrossRef]
- Convertino, D.; Fabbri, F.; Mishra, N.; Mainardi, M.; Cappello, V.; Testa, G.; Capsoni, S.; Albertazzi, L.; Luin, S.; Marchetti, L.; et al. Graphene promotes axon elongation through local stall of nerve growth factor signaling endosomes. Nano Lett. 2020, 20, 3633–3641. [Google Scholar] [CrossRef]
- Munro, J.B.; Nath, A.; Färber, M.; Datta, S.A.K.; Rein, A.; Rhoades, E.; Mothes, W. A Conformational Transition Observed in Single HIV-1 Gag Molecules during In Vitro Assembly of Virus-Like Particles. J. Virol. 2014, 88, 3577–3585. [Google Scholar] [CrossRef]
- Suzuki, K.G.N.; Kasai, R.S.; Hirosawa, K.M.; Nemoto, Y.L.; Ishibashi, M.; Miwa, Y.; Fujiwara, T.K.; Kusumi, A. Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 2012, 8, 774–783. [Google Scholar] [CrossRef]
- Malkusch, N.; Dörfler, T.; Nagy, J.; Eilert, T.; Michaelis, J. smFRET experiments of the RNA polymerase II transcription initiation complex. Methods 2017, 120, 115–124. [Google Scholar] [CrossRef]
- Niekamp, S.; Sung, J.; Huynh, W.; Bhabha, G.; Vale, R.D.; Stuurman, N. Nanometer-accuracy distance measurements between fluorophores at the single-molecule level. Proc. Natl. Acad. Sci. USA 2019, 116, 4275–4284. [Google Scholar] [CrossRef]
- Niekamp, S.; Stuurman, N.; Zhang, N.; Vale, R.D. Three-color single-molecule imaging reveals conformational dynamics of dynein undergoing motility. Proc. Natl. Acad. Sci. USA 2021, 118, e2101391118. [Google Scholar] [CrossRef] [PubMed]
- Benke, A.; Olivier, N.; Gunzenhäuser, J.; Manley, S. Multicolor single molecule tracking of stochastically active synthetic dyes. Nano Lett. 2012, 12, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, R.S.; Baguley, S.W.; Richens, J.H.; Wissner, R.F.; Xi, Z.; Allgeyer, E.S.; Zhong, S.; Thompson, A.D.; Lowe, N.; Butler, R.; et al. Labeling Strategies Matter for Super-Resolution Microscopy: A Comparison between HaloTags and SNAP-tags. Cell Chem. Biol. 2019, 26, 584–592.e6. [Google Scholar] [CrossRef] [PubMed]
- Presman, D.M.; Ball, D.A.; Paakinaho, V.; Grimm, J.B.; Lavis, L.D.; Karpova, T.S.; Hager, G.L. Quantifying transcription factor binding dynamics at the single-molecule level in live cells. Methods 2017, 123, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S.; Mely, Y. Fluorescent environment-sensitive dyes as reporters of biomolecular interactions. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 35–58. [Google Scholar]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef]
- Hoelzel, C.A.; Zhang, X. Visualizing and Manipulating Biological Processes by Using HaloTag and SNAP-Tag Technologies. ChemBioChem 2020, 21, 1935–1946. [Google Scholar] [CrossRef]
- Kozma, E.; Kele, P. Fluorogenic probes for super-resolution microscopy. Org. Biomol. Chem. 2019, 17, 215–233. [Google Scholar] [CrossRef]
- Chyan, W.; Raines, R.T. Enzyme-Activated Fluorogenic Probes for Live-Cell and In Vivo Imaging. ACS Chem. Biol. 2018, 13, 1810–1823. [Google Scholar] [CrossRef]
- Li, C.; Tebo, A.G.; Gautier, A. Fluorogenic labeling strategies for biological imaging. Int. J. Mol. Sci. 2017, 18, 1473. [Google Scholar] [CrossRef]
- Bachollet, S.P.J.T.; Addi, C.; Pietrancosta, N.; Mallet, J.M.; Dumat, B. Fluorogenic Protein Probes with Red and Near-Infrared Emission for Genetically Targeted Imaging**. Chem.—A Eur. J. 2020, 26, 14467–14473. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, K.; Dunham, N.P.; Liu, H.; Fares, M.; Boal, A.K.; Li, X.; Zhang, X. The Cation−π Interaction Enables a Halo-Tag Fluorogenic Probe for Fast No-Wash Live Cell Imaging and Gel-Free Protein Quantification. Biochemistry 2017, 56, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Bachollet, S.P.J.T.; Shpinov, Y.; Broch, F.; Benaissa, H.; Gautier, A.; Pietrancosta, N.; Mallet, J.M.; Dumat, B. An expanded palette of fluorogenic HaloTag probes with enhanced contrast for targeted cellular imaging. Org. Biomol. Chem. 2022, 20, 3619–3628. [Google Scholar] [CrossRef]
- Leng, S.; Qiao, Q.L.; Gao, Y.; Miao, L.; Deng, W.G.; Xu, Z.C. SNAP-tag fluorogenic probes for wash free protein labeling. Chin. Chem. Lett. 2017, 28, 1911–1915. [Google Scholar] [CrossRef]
- Prifti, E.; Reymond, L.; Umebayashi, M.; Hovius, R.; Riezman, H.; Johnsson, K. A fluorogenic probe for snap-tagged plasma membrane proteins based on the solvatochromic molecule nile red. ACS Chem. Biol. 2014, 9, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-K.; Hsieh, P.-Y.; Zhuang, Y.-D.; Hsia, C.-Y.; Huang, C.-L.; Lai, H.-P.; Lin, H.-S.; Chen, I.-C.; Hsu, H.-Y.; Tan, K.-T. A rapid SNAP-tag fluorogenic probe based on an environment-sensitive fluorophore for no-wash live cell imaging. ACS Chem. Biol. 2014, 9, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Fares, M.; Grainger, L.S.; Wolstenholme, C.H.; Hou, A.; Liu, Y.; Zhang, X. A SNAP-tag fluorogenic probe mimicking the chromophore of the red fluorescent protein Kaede. Org. Biomol. Chem. 2019, 17, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Liu, W.; Chen, J.; Zhou, W.; Yin, W.; Miao, L.; Cui, J.; Xu, Z. A naphthalimide-derived fluorogenic probe for SNAP-tag with a fast record labeling rate. Dye. Pigment. 2017, 147, 327–333. [Google Scholar] [CrossRef]
- Wang, L.; Tran, M.; D’Este, E.; Roberti, J.; Koch, B.; Xue, L.; Johnsson, K. A general strategy to develop cell permeable and fluorogenic probes for multicolour nanoscopy. Nat. Chem. 2020, 12, 165–172. [Google Scholar] [CrossRef]
- Joo, C.; Ha, T. Single-molecule FRET with total internal reflection microscopy. Cold Spring Harb. Protoc. 2012, 7, 1223–1237. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, C.; Lee, N.K. Real-time submillisecond single-molecule FRET dynamics of freely diffusing molecules with liposome tethering. Nat. Commun. 2015, 6, 6992. [Google Scholar] [CrossRef]
- Mukherjee, S.K.; Knop, J.-M.; Oliva, R.; Möbitz, S.; Winter, R. Untangling the interaction of α-synuclein with DNA i-motifs and hairpins by volume-sensitive single-molecule FRET spectroscopy. RSC Chem. Biol. 2021, 2, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Brunger, A.T.; Strop, P.; Vrljic, M.; Chu, S.; Weninger, K.R. Three-dimensional molecular modeling with single molecule FRET. J. Struct. Biol. 2011, 173, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sielaff, H.; Börsch, M.; Grüber, G. Conformational dynamics of the rotary subunit F in the A3B3DF complex of Methanosarcina mazei Gö1 A-ATP synthase monitored by single-molecule FRET. FEBS Lett. 2017, 591, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Förster-Radius R0 of Selected ATTO-Dye Pairs. Available online: https://www.atto-tec.com/fileadmin/user_upload/Katalog_Flyer_Support/R_0_-Tabelle_2018_web.pdf (accessed on 17 October 2022).
- R0 Values for Some Alexa Fluor Dyes—Table 1.6|Thermo Fisher Scientific—IT. Available online: https://www.thermofisher.com/it/en/home/references/molecular-probes-the-handbook/tables/r0-values-for-some-alexa-fluor-dyes.html (accessed on 17 October 2022).
- Hohng, S.; Joo, C.; Ha, T. Single-molecule three-color FRET. Biophys. J. 2004, 87, 1328–1337. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Hohng, S. Single-Molecule Three-Color FRET with Both Negligible Spectral Overlap and Long Observation Time. PLoS ONE 2010, 5, e12270. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Ragunathan, K.; Joo, C.; Ha, T.; Hohng, S. Single-molecule four-color FRET. Angew. Chem.—Int. Ed. 2010, 49, 9922–9925. [Google Scholar] [CrossRef]
- Ratzke, C.; Hellenkamp, B.; Hugel, T. Four-colour FRET reveals directionality in the Hsp90 multicomponent machinery. Nat. Commun. 2014, 5, 4192. [Google Scholar] [CrossRef]
- Kim, H.; Abeysirigunawarden, S.C.; Chen, K.; Mayerle, M.; Ragunathan, K.; Luthey-Schulten, Z.; Ha, T.; Woodson, S.A. Protein-guided RNA dynamics during early ribosome assembly. Nature 2014, 506, 334–338. [Google Scholar] [CrossRef]
- Ernst, S.; Düser, M.G.; Zarrabi, N.; Börsch, M. Three-color Förster resonance energy transfer within single FOF1-ATP synthases: Monitoring elastic deformations of the rotary double motor in real time. J. Biomed. Opt. 2012, 17, 011004. [Google Scholar] [CrossRef]
- Milles, S.; Koehler, C.; Gambin, Y.; Deniz, A.A.; Lemke, E.A. Intramolecular three-colour single pair FRET of intrinsically disordered proteins with increased dynamic range. Mol. Biosyst. 2012, 8, 2531–2534. [Google Scholar] [CrossRef]
- Jung, J.; Han, K.Y.; Koh, H.R.; Lee, J.; Choi, Y.M.; Kim, C.; Kim, S.K. Effect of single-base mutation on activity and folding of 10-23 deoxyribozyme studied by three-color single-molecule ALEX FRET. J. Phys. Chem. B 2012, 116, 3007–3012. [Google Scholar] [CrossRef] [PubMed]
- Stein, I.H.; Steinhauer, C.; Tinnefeld, P. Single-molecule four-color FRET visualizes energy-transfer paths on DNA origami. J. Am. Chem. Soc. 2011, 133, 4193–4195. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Photobleaching of organic fluorophores: Quantitative characterization, mechanisms, protection. Methods Appl. Fluoresc. 2020, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Gong, W.; Li, W.; Sharma, A.; Shim, I.; Zhang, W.; Das, P.; Pan, W.; Liu, L.; Yang, Z.; et al. Organic fluorescent probes for stochastic optical reconstruction microscopy (STORM): Recent highlights and future possibilities. Coord. Chem. Rev. 2019, 380, 17–34. [Google Scholar] [CrossRef]
- Vogelsang, J.; Cordes, T.; Forthmann, C.; Steinhauer, C.; Tinnefeid, P. Controlling the fluorescence of ordinary oxazine dyes for single-molecule switching and superresolution microscopy. Proc. Natl. Acad. Sci. USA 2009, 106, 8107–8112. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, H.; Liu, Y. Optimized Stochastic Optical Reconstruction Microscopy for Imaging Chromatin Structure in Pathological Tissue. Curr. Protoc. Cytom. 2020, 94, e78. [Google Scholar] [CrossRef]
- Olivier, N.; Keller, D.; Gönczy, P.; Manley, S. Resolution Doubling in 3D-STORM Imaging through Improved Buffers. PLoS ONE 2013, 8, e69004. [Google Scholar] [CrossRef]
- Vaughan, J.C.; Dempsey, G.T.; Sun, E.; Zhuang, X. Phosphine quenching of cyanine dyes as a versatile tool for fluorescence microscopy. J. Am. Chem. Soc. 2013, 135, 1197–1200. [Google Scholar] [CrossRef]
- Olivier, N.; Keller, D.; Rajan, V.S.; Gönczy, P.; Manley, S. Simple buffers for 3D STORM microscopy. Biomed. Opt. Express 2013, 4, 885. [Google Scholar] [CrossRef]
- Sample Preparation|Abbelight. Available online: https://www.abbelight.com/solutions/abbelight-research/ (accessed on 14 November 2022).
- Uno, S.N.; Kamiya, M.; Yoshihara, T.; Sugawara, K.; Okabe, K.; Tarhan, M.C.; Fujita, H.; Funatsu, T.; Okada, Y.; Tobita, S.; et al. A spontaneously blinking fluorophore based on intramolecular spirocyclization for live-cell super-resolution imaging. Nat. Chem. 2014, 6, 681–689. [Google Scholar] [CrossRef]
- Chu, L.A.; Lu, C.H.; Yang, S.M.; Liu, Y.T.; Feng, K.L.; Tsai, Y.C.; Chang, W.K.; Wang, W.C.; Chang, S.W.; Chen, P.; et al. Rapid single-wavelength lightsheet localization microscopy for clarified tissue. Nat. Commun. 2019, 10, 4762. [Google Scholar] [CrossRef] [PubMed]
- Uno, S.N.; Kamiya, M.; Morozumi, A.; Urano, Y. A green-light-emitting, spontaneously blinking fluorophore based on intramolecular spirocyclization for dual-colour super-resolution imaging. Chem. Commun. 2017, 54, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Morozumi, A.; Kamiya, M.; Uno, S.N.; Umezawa, K.; Kojima, R.; Yoshihara, T.; Tobita, S.; Urano, Y. Spontaneously Blinking Fluorophores Based on Nucleophilic Addition/Dissociation of Intracellular Glutathione for Live-Cell Super-resolution Imaging. J. Am. Chem. Soc. 2020, 142, 9625–9633. [Google Scholar] [CrossRef]
- Tyson, J.; Hu, K.; Zheng, S.; Kidd, P.; Dadina, N.; Chu, L.; Toomre, D.; Bewersdorf, J.; Schepartz, A. Extremely Bright, Near-IR Emitting Spontaneously Blinking Fluorophores Enable Ratiometric Multicolor Nanoscopy in Live Cells. ACS Cent. Sci. 2021, 7, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Chi, W.; Qiao, Q.; Wang, C.; Zheng, J.; Zhou, W.; Xu, N.; Wu, X.; Jiang, X.; Tan, D.; Xu, Z.; et al. Descriptor Δ G C-O Enables the Quantitative Design of Spontaneously Blinking Rhodamines for Live-Cell Super-Resolution Imaging. Angew. Chem. 2020, 132, 20390–20398. [Google Scholar] [CrossRef]
- Tachibana, R.; Kamiya, M.; Morozumi, A.; Miyazaki, Y.; Fujioka, H.; Nanjo, A.; Kojima, R.; Komatsu, T.; Ueno, T.; Hanaoka, K.; et al. Design of spontaneously blinking fluorophores for live-cell super-resolution imaging based on quantum-chemical calculations. Chem. Commun. 2020, 56, 13173–13176. [Google Scholar] [CrossRef]
- Zheng, Q.; Ayala, A.X.; Chung, I.; Weigel, A.V.; Ranjan, A.; Falco, N.; Grimm, J.B.; Tkachuk, A.N.; Wu, C.; Lippincott-Schwartz, J.; et al. Rational Design of Fluorogenic and Spontaneously Blinking Labels for Super-Resolution Imaging. ACS Cent. Sci. 2019, 5, 1602–1613. [Google Scholar] [CrossRef]
- Werther, P.; Yserentant, K.; Braun, F.; Kaltwasser, N.; Popp, C.; Baalmann, M.; Herten, D.; Wombacher, R. Live-Cell Localization Microscopy with a Fluorogenic and Self-Blinking Tetrazine Probe. Angew. Chem. Int. Ed. 2020, 59, 804–810. [Google Scholar] [CrossRef]
- Sharonov, A.; Hochstrasser, R.M. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. USA 2006, 103, 18911–18916. [Google Scholar] [CrossRef]
- Tas, R.P.; Albertazzi, L.; Voets, I.K. Small Peptide-Protein Interaction Pair for Genetically Encoded, Fixation Compatible Peptide-PAINT. Nano Lett. 2021, 21, 9509–9516. [Google Scholar] [CrossRef]
- van Wee, R.; Filius, M.; Joo, C. Completing the canvas: Advances and challenges for DNA-PAINT super-resolution imaging. Trends Biochem. Sci. 2021, 46, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Nieves, D.J.; Gaus, K.; Baker, M.A.B. DNA-based super-resolution microscopy: DNA-PAINT. Genes 2018, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Schnitzbauer, J.; Strauss, M.T.; Schlichthaerle, T.; Schueder, F.; Jungmann, R. Super-resolution microscopy with DNA-PAINT. Nat. Protoc. 2017, 12, 1198–1228. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Bannai, H.; Lévi, S.; Schweizer, C.; Dahan, M.; Triller, A. Imaging the lateral diffusion of membrane molecules with quantum dots. Nat. Protoc. 2007, 1, 2628–2634. [Google Scholar] [CrossRef]
- Howarth, M.; Takao, K.; Hayashi, Y.; Ting, A.Y. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA 2005, 102, 7583–7588. [Google Scholar] [CrossRef]
- Tada, H.; Higuchi, H.; Wanatabe, T.M.; Ohuchi, N. In Vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007, 67, 1138–1144. [Google Scholar] [CrossRef]
- Varela, J.A.; Dupuis, J.P.; Etchepare, L.; Espana, A.; Cognet, L.; Groc, L. Targeting neurotransmitter receptors with nanoparticles In Vivo allows single-molecule tracking in acute brain slices. Nat. Commun. 2016, 7, 10947. [Google Scholar] [CrossRef]
- Amodeo, R.; Nifosì, R.; Giacomelli, C.; Ravelli, C.; La Rosa, L.; Callegari, A.; Trincavelli, M.L.; Mitola, S.; Luin, S.; Marchetti, L. Molecular insight on the altered membrane trafficking of TrkA kinase dead mutants. Biochim. Biophys. Acta—Mol. Cell Res. 2020, 1867, 118614. [Google Scholar] [CrossRef]
- Low-Nam, S.T.; Lidke, K.A.; Cutler, P.J.; Roovers, R.C.; Van Bergen En Henegouwen, P.M.P.; Wilson, B.S.; Lidke, D.S. ErbB1 dimerization is promoted by domain co-confinement and stabilized by ligand binding. Nat. Struct. Mol. Biol. 2011, 18, 1244–1249. [Google Scholar] [CrossRef]
- Bannai, H. Molecular membrane dynamics: Insights into synaptic function and neuropathological disease. Neurosci. Res. 2018, 129, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Bonasio, R.; Carman, C.V.; Kim, E.; Sage, P.T.; Love, K.R.; Mempel, T.R.; Springer, T.A.; Von Andrian, U.H. Specific and covalent labeling of a membrane protein with organic fluorochromes and quantum dots. Proc. Natl. Acad. Sci. USA 2007, 104, 14753–14758. [Google Scholar] [CrossRef] [PubMed]
- Sunbul, M.; Yen, M.; Zou, Y.; Yin, J. Enzyme catalyzed site-specific protein labeling and cell imaging with quantum dots. Chem. Commun. 2008, 45, 5927–5929. [Google Scholar] [CrossRef] [PubMed]
- So, M.-K.; Yao, H.; Rao, J. HaloTag protein-mediated specific labeling of living cells with quantum dots. Biochem. Biophys. Res. Commun. 2008, 374, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Opazo, P.; Labrecque, S.; Tigaret, C.M.; Frouin, A.; Wiseman, P.W.; De Koninck, P.; Choquet, D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 2010, 67, 239–252. [Google Scholar] [CrossRef]
- Gómez-Varela, D.; Kohl, T.; Schmidt, M.; Rubio, M.E.; Kawabe, H.; Nehring, R.B.; Schäfer, S.; Stühmer, W.; Pardo, L.A. Characterization of Eag1 Channel Lateral Mobility in Rat Hippocampal Cultures by Single-Particle-Tracking with Quantum Dots. PLoS ONE 2010, 5, e8858. [Google Scholar] [CrossRef]
- Won, S.; Kim, H.D.; Kim, J.Y.; Lee, B.C.; Chang, S.; Park, C.S. Movements of individual BKCa channels in live cell membrane monitored by site-specific labeling using quantum dots. Biophys. J. 2010, 99, 2853–2862. [Google Scholar] [CrossRef]
- Chang, J.C.; Rosenthal, S.J. Visualization of lipid raft membrane compartmentalization in living RN46A neuronal cells using single quantum dot tracking. ACS Chem. Neurosci. 2012, 3, 737–743. [Google Scholar] [CrossRef][Green Version]
- Murcia, M.J.; Minner, D.E.; Mustata, G.M.; Ritchie, K.; Naumann, C.A. Design of quantum dot-conjugated lipids for long-term, high-speed tracking experiments on cell surfaces. J. Am. Chem. Soc. 2008, 130, 15054–15062. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Mattoussi, H.; Medintz, I.L. Delivering quantum dots into cells: Strategies, progress and remaining issues. Anal. Bioanal. Chem. 2009, 393, 1091–1105. [Google Scholar] [CrossRef]
- Muro, E.; Fragola, A.; Pons, T.; Lequeux, N.; Ioannou, A.; Skourides, P.; Dubertret, B. Comparing intracellular stability and targeting of sulfobetaine quantum dots with other surface chemistries in live cells. Small 2012, 8, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Courty, S.; Luccardini, C.; Bellaiche, Y.; Cappello, G.; Dahan, M. Tracking individual kinesin motors in living cells using single quantum-dot imaging. Nano Lett. 2006, 6, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kambara, T.; Gonda, K.; Higuchi, H. Intracellular imaging of targeted proteins labeled with quantum dots. Exp. Cell Res. 2008, 314, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Efros, A.L.; Nesbitt, D.J. Origin and control of blinking in quantum dots. Nat. Nanotechnol. 2016, 11, 661–671. [Google Scholar] [CrossRef]
- Mahler, B.; Spinicelli, P.; Buil, S.; Quelin, X.; Hermier, J.P.; Dubertret, B. Towards non-blinking colloidal quantum dots. Nat. Mater. 2008, 7, 659–664. [Google Scholar] [CrossRef]
- Lane, L.A.; Smith, A.M.; Lian, T.; Nie, S. Compact and blinking-suppressed quantum dots for single-particle tracking in live cells. J. Phys. Chem. B 2014, 118, 14140–14147. [Google Scholar] [CrossRef]
- Sergé, A.; Bertaux, N.; Rigneault, H.; Marguet, D. Dynamic multiple-target tracing to probe spatiotemporal cartography of cell membranes. Nat. Methods 2008, 5, 687–694. [Google Scholar] [CrossRef]
- Arnspang, E.C.; Brewer, J.R.; Lagerholm, B.C. Multi-Color Single Particle Tracking with Quantum Dots. PLoS ONE 2012, 7, e48521. [Google Scholar] [CrossRef]
- You, C.; Marquez-Lago, T.T.; Richter, C.P.; Wilmes, S.; Moraga, I.; Garcia, K.C.; Leier, A.; Piehler, J. Receptor dimer stabilization by hierarchical plasma membrane microcompartments regulates cytokine signaling. Sci. Adv. 2016, 2, e1600452. [Google Scholar] [CrossRef]
- Ito, Y.; Sakata-Sogawa, K.; Tokunaga, M. Multi-color single-molecule tracking and subtrajectory analysis for quantification of spatiotemporal dynamics and kinetics upon T cell activation. Sci. Rep. 2017, 7, 6994. [Google Scholar] [CrossRef]
- Clausen, M.P.; Arnspang, E.C.; Ballou, B.; Bear, J.E.; Lagerholm, B.C. Simultaneous Multi-Species Tracking in Live Cells with Quantum Dot Conjugates. PLoS ONE 2014, 9, e97671. [Google Scholar] [CrossRef] [PubMed]
- Extinction Coefficients of Qdot Nanocrystal Streptavidin Conjugates at Ultraviolet and Visible Wavelengths—Table 6.10|Thermo Fisher Scientific—IT. Available online: https://www.thermofisher.com/it/en/home/references/molecular-probes-the-handbook/tables/extinction-coefficients-of-qdot-streptavidin-conjugates-at-common-wavelengths.html (accessed on 23 October 2022).
- ATTO-TEC GmbH—Data-Table. Available online: https://www.atto-tec.com/support/datatable/ (accessed on 23 October 2022).
- Abraham, L.; Lu, H.Y.; Falcão, R.C.; Scurll, J.; Jou, T.; Irwin, B.; Tafteh, R.; Gold, M.R.; Coombs, D. Limitations of Qdot labelling compared to directly-conjugated probes for single particle tracking of B cell receptor mobility. Sci. Rep. 2017, 7, 11379. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wu, C.; Chen, L.; Ramirez, A.; Bearer, E.L.; Li, W.P.; Mobley, W.C.; Chu, S. One at a time, live tracking of NGF axonal transport using quantum dots. Proc. Natl. Acad. Sci. USA 2007, 104, 13666–13671. [Google Scholar] [CrossRef]
- Groc, L.; Lafourcade, M.; Heine, M.; Renner, M.; Racine, V.; Sibarita, J.B.; Lounis, B.; Choquet, D.; Cognet, L. Surface trafficking of neurotransmitter receptor: Comparison between single-molecule/quantum dot strategies. J. Neurosci. 2007, 27, 12433–12437. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Nagai, T. Extensive use of FRET in biological imaging. J. Electron Microsc. 2013, 62, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. FRET as a biomolecular research tool—Understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.C.; Algar, W.R.; Medintz, I.L.; Hildebrandt, N. Quantum dots for Förster Resonance Energy Transfer (FRET). TrAC—Trends Anal. Chem. 2020, 125, 115819. [Google Scholar] [CrossRef]
- Snee, P.T. Semiconductor quantum dot FRET: Untangling energy transfer mechanisms in bioanalytical assays. TrAC—Trends Anal. Chem. 2020, 123, 115750. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Hildebrandt, N. Recent developments in lanthanide-to-quantum dot FRET using time-gated fluorescence detection and photon upconversion. TrAC—Trends Anal. Chem. 2016, 84, 60–71. [Google Scholar] [CrossRef]
- Goryacheva, O.A.; Beloglazova, N.V.; Vostrikova, A.M.; Pozharov, M.V.; Sobolev, A.M.; Goryacheva, I.Y. Lanthanide-to-quantum dot Förster resonance energy transfer (FRET): Application for immunoassay. Talanta 2017, 164, 377–385. [Google Scholar] [CrossRef]
- Hohng, S.; Ha, T. Single-molecule quantum-dot fluorescence resonance energy transfer. ChemPhysChem 2005, 6, 956–960. [Google Scholar] [CrossRef]
- Sugawa, M.; Nishikawa, S.; Iwane, A.H.; Yanagida, T.; Biju, V. Single-Molecule FRET imaging for enzymatic reactions at high ligand concentrations. Small 2010, 6, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Piper, J.D.; Abell, C.; Klenerman, D.; Kang, D.J.; Ying, L. Fluorescence resonance energy transfer between a quantum dot donor and a dye acceptor attached to DNA. Chem. Commun. 2005, 38, 4807–4809. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Nakayama, K.; Itoh, K.; Itoh, T.; Ishikawa, M.; Biju, V. Reversible dimerization of EGFR revealed by single-molecule fluorescence imaging using quantum dots. Chem.—A Eur. J. 2010, 16, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor-acceptor combinations. Angew. Chem.—Int. Ed. 2006, 45, 4562–4589. [Google Scholar] [CrossRef]
- Heyes, C.D. Quantum Dots in Single Molecule Spectroscopy; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Medintz, I.L.; Clapp, A.R.; Mattoussi, H.; Goldman, E.R.; Fisher, B.; Mauro, J.M. Self-assembled nanoscale biosensors based on quantum dot FRET donors. Nat. Mater. 2003, 2, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhanghao, K.; Wang, H.; Liu, Y.; Wang, F.; Zhang, X.; Shi, K.; Gao, J.; Jin, D.; Xi, P. Versatile Application of Fluorescent Quantum Dot Labels in Super-resolution Fluorescence Microscopy. ACS Photonics 2016, 3, 1611–1618. [Google Scholar] [CrossRef]
- Hoyer, P.; Staudt, T.; Engelhardt, J.; Hell, S.W. Quantum dot blueing and blinking enables fluorescence nanoscopy. Nano Lett. 2011, 11, 245–250. [Google Scholar] [CrossRef]
- Xu, J.; Tehrani, K.F.; Kner, P. Multicolor 3D super-resolution imaging by quantum dot stochastic optical reconstruction microscopy. ACS Nano 2015, 9, 2917–2925. [Google Scholar] [CrossRef]
- Wang, Y.; Fruhwirth, G.; Cai, E.; Ng, T.; Selvin, P.R. 3D super-resolution imaging with blinking quantum dots. Nano Lett. 2013, 13, 5233–5241. [Google Scholar] [CrossRef]
- Jin, D.; Xi, P.; Wang, B.; Zhang, L.; Enderlein, J.; Van Oijen, A.M. Nanoparticles for super-resolution microscopy and single-molecule tracking. Nat. Methods 2018, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, H.; Kang, M.; Baday, M.; Lee, S.H. High spatial and temporal resolution using upconversion nanoparticles and femtosecond pulsed laser in single particle tracking. Curr. Appl. Phys. 2022, 44, 40–45. [Google Scholar] [CrossRef]


| Considered System | Application | Analyzed Properties | Best Dyes | Ref. |
|---|---|---|---|---|
| Freely diffusing double-stranded DNA molecules | smFRET | Photostability, brightness, fluorescence lifetime and FRET performance | Alexa 488 and Atto 647N | [25] |
| Dyes on synthetic lipid membranes | Single-molecule studies | Aspecific interactions | Alexa 488, Atto 488 | [26] |
| EGFR labeled via anti-EGRF affibody in live cells | SMT | Aspecific interactions | Alexa 488, CF640R | [27] |
| TrkA receptor labeled via ACP-derived tag in live cells | SMT | Aspecific interactions, mean fluorescence intensity and photostability | Abberior STAR 635p | [28] |
| EGFR labeled via SNAP tag in live cells | SMT | Aspecific interactions and photostability | Dy 549, CF 640 | [29] |
| Integrins labeled via ACP-tag in live cells | SMT | Photostability | SeTau 647 | [30] |
| Immunolabeled microtubules in fixed cells | STORM | Image quality | CF 555, CF 568, CF 647, Dy 547, Alexa 647 | [31] |
| Immunolabeled microtubules in fixed cells | 3-color STORM | Image quality | CF 568, CF 647, CF 680 | [31] |
| Labeled antibodies adsorbed to coverslip; immunolabeled microtubules and clathrin-coated pits | STORM | Photon numbers, duty cycles, survival fractions and switching cycles | Alexa 647, Dy 654, Cy5 | [32] |
| Labeled antibodies adsorbed to coverslip; immunolabeled microtubules and clathrin-coated pits | 4-color STORM | Photon numbers, duty cycles, survival fractions and switching cycles | Atto 488, Cy3B, Alexa 647 and DyLight 750 | [32] |
| Immunolabeled microtubules in fixed cells | SMLM | Image quality | Alexa 647, Cy5, Atto 488 | [33] |
| Considered System | Measurement | Worst Dyes, to Be Excluded from Single-Molecule Imaging | Best Dyes for Low Aspecific Interactions | Ref. |
|---|---|---|---|---|
| Labeling with anti-EGFR affibody-dye on cells grown on PEG-BSA nanogel surfaces | Diffusion coefficient in single-particle tracking | AL 546, AT 647N, AL 555, Cy3, CF 568, AT 565 | AL 488, CF 640R, TMR | [27] |
| Interactions of dyes with lipid vesicles and supported lipid bilayers (diverse kinds of lipids) | Partition coefficient into the lipid membrane via fluorescence correlation spectroscopy and visualization of adsorbed dyes in TIRF | AT 647N, Cy5, AT 594 | AT 488, AL 488, AT 532, Fluorescein | [26] |
| Labeling of receptors tagged with ACP-derived tags in cells | Incubation of not transfected cells with CoA dyes | AT 550, AL 568, AT 633 | AL 488, AT 488, Abb. 488, Abb. 635p, AL 647 | [28] |
| Labeling of SNAP-tagged proteins in cells | Incubation of non-transfected cells with BG-dyes | AT 550, AT 565, AT 620, AT 633, AT 647N, Dy 630, Dy 651, Abb. 635 | AL 647, AT 532, Dy 634, Cf 633, Dy 649, Dy 648, Dy 549, Cf 640 | [29] |
| Dye interaction with EggPC unilamellar vesicles | Dialysis on vesicle–dye mixtures | BODIPY-TMR M, AT 550 M, Cy 3 SE, AL 633 M, AT 647 M, sulforhodamine B, Texas Red M | AL 488 SE, AT 488 SE, AL 532 SE, AT 532 SE, AL 555 M, AL 568 HY, AL 647 SE, AL 647 M, Chromeo 488 SE, OG 488 SE, OG 514 SE | [102] |
| Labeling with anti-EGFR affibody-dye | Diffusion coefficients in cells | At 647 N, AL 546 | AL 488 | [85] |
| FPs | ODs | Qdots | |
|---|---|---|---|
| Brightness | 103–104 M−1 cm−1 | 104–105 M−1 cm−1 | 105–105 M−1 cm−1 |
| Photostability | Low | Medium | High |
| Size | 4 nm | <1 nm | 15 nm (up to 50 nm with functionalization) |
| Conjugation | Genetically encoded: specific, stoichiometry-controlled, easy also intracellularly. | Chemical coupling. Several protocols available for specific, efficient and stoichiometry-controlled labeling. Drawback: aspecific adsorption (resolved by fluorogenic dyes). | Chemical coupling typically requiring functionalization with large molecules. Challenges: intracellular delivery, control of stoichiometry and efficiency, steric hindrance. |
| Sm-FRET | Use of CFP-YFP couple and derivatives; research for improvement (bright red FPs) to overcome limitations. | Several available D-A couples, even multicolor FRET achieved. | Limited applications, used as donors with OD acceptors. |
| SMT | Limited applications due to poor SNR and photostability. | Many applications, not invasive. Drawbacks: short tracks, difficult multicolor. Typically: ~10 s of observation at 30–50 Hz with localization precision of 20–30 nm [30,35]. | Longer tracks, easier multicolor. Drawbacks: possible perturbations on the labeled molecule, blinking. Typically: up to minutes of observation at 50–200 Hz with localization precision of about 10 nm [10,104]. |
| SMLM | PALM with cell-friendly media. Localization precision: 10–50 nm [105]. | STORM (using a photoswitching buffer); PAINT, DNA-PAINT. Localization precision: 20–30 nm [31,32]. | Limited applications, specialized methods under development. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirripa Spagnolo, C.; Luin, S. Choosing the Probe for Single-Molecule Fluorescence Microscopy. Int. J. Mol. Sci. 2022, 23, 14949. https://doi.org/10.3390/ijms232314949
Schirripa Spagnolo C, Luin S. Choosing the Probe for Single-Molecule Fluorescence Microscopy. International Journal of Molecular Sciences. 2022; 23(23):14949. https://doi.org/10.3390/ijms232314949
Chicago/Turabian StyleSchirripa Spagnolo, Chiara, and Stefano Luin. 2022. "Choosing the Probe for Single-Molecule Fluorescence Microscopy" International Journal of Molecular Sciences 23, no. 23: 14949. https://doi.org/10.3390/ijms232314949
APA StyleSchirripa Spagnolo, C., & Luin, S. (2022). Choosing the Probe for Single-Molecule Fluorescence Microscopy. International Journal of Molecular Sciences, 23(23), 14949. https://doi.org/10.3390/ijms232314949







