Canary Seed (Phalaris canariensis L.) Peptides Prevent Obesity and Glucose Intolerance in Mice Fed a Western Diet
Abstract
1. Introduction
2. Results
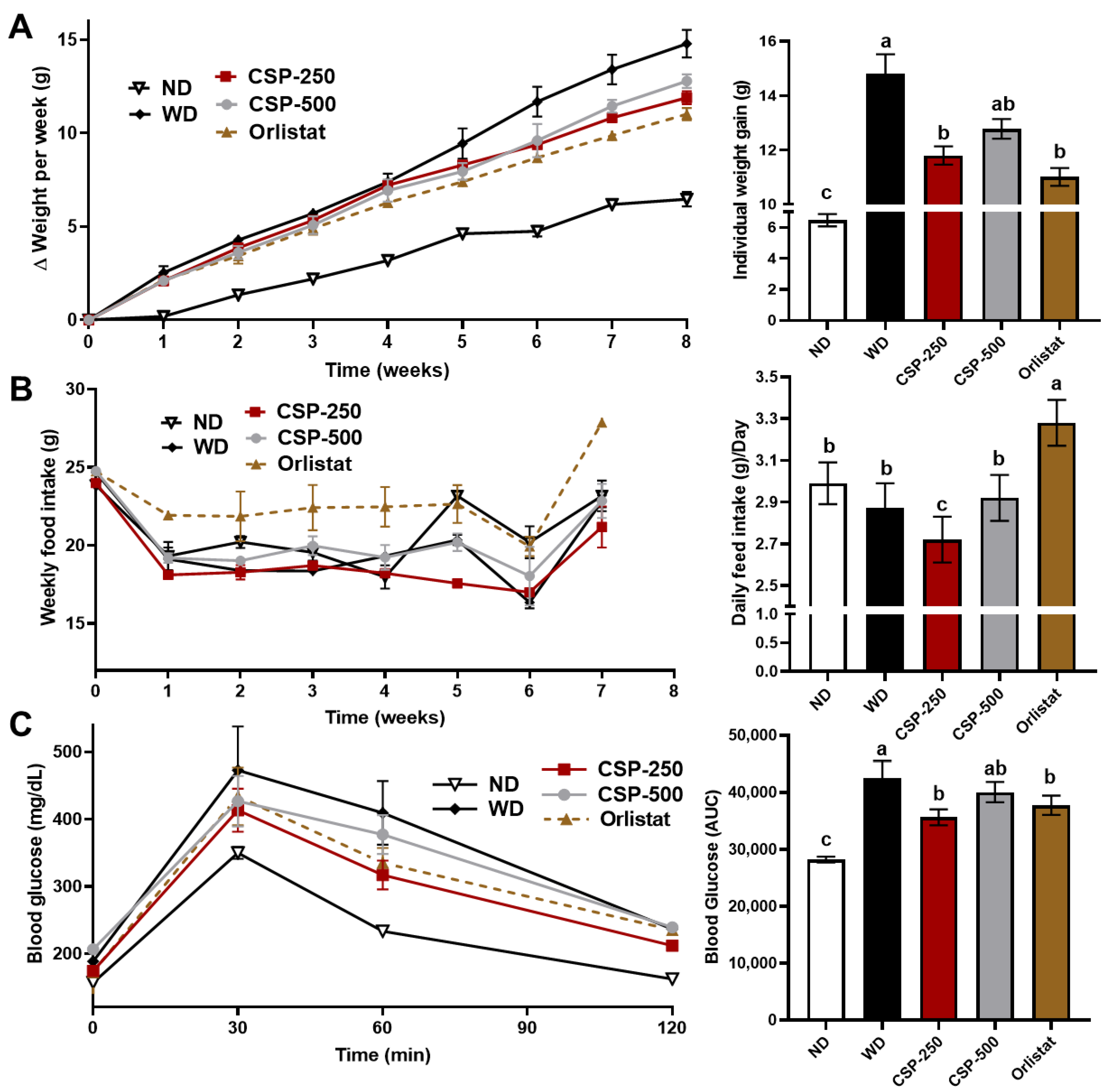
2.1. Effect of CSP on Body Weight, Feed Consumption, and Glucose Tolerance of Mice
2.2. Effect of CSP on Lipid Profiles, Organ Weight, and Fecal Proximal Composition
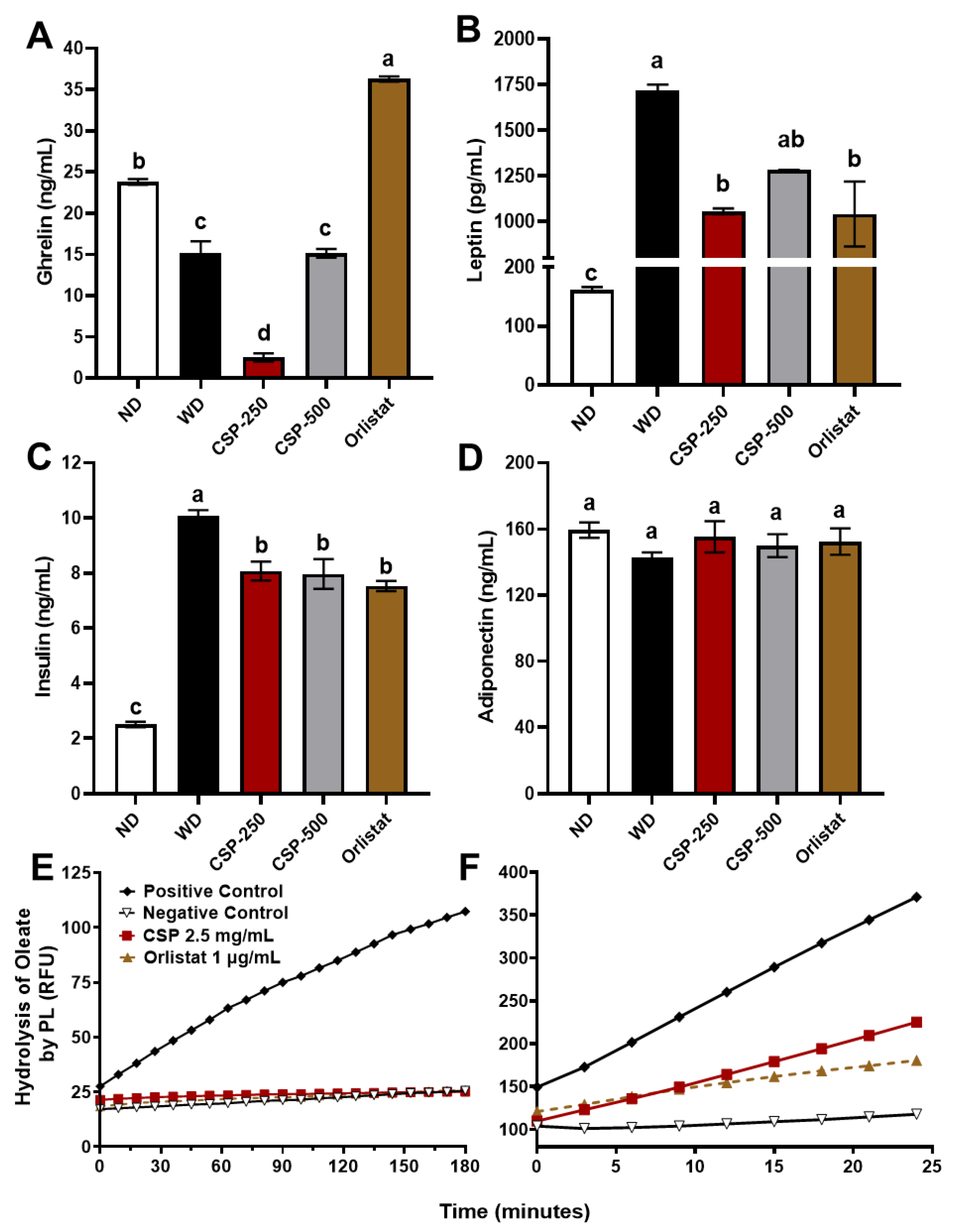
2.3. Modulatory Effect of CSP on Metabolic Hormones Levels and Pancreatic Lipase Activity
2.4. Effect of CSP on Liver Histopathology, Lipid Metabolism, and Gene Expression
3. Discussion
4. Materials and Methods
4.1. Preparation of Canary Seed Peptides (CSP)
4.2. Animals and Housing
4.3. Experimental Design, Diets, and Sample Collection
4.4. Intraperitoneal Glucose Tolerance Test (IPGTT)
4.5. Fecal Proximal Composition
4.6. Serum Biochemical Analysis
4.7. Measurement of Fasting Serum Levels of Metabolic Hormones
4.8. Pancreatic Lipase Inhibition
4.9. Histopathology
4.10. Hepatic Triglyceride Analysis
4.11. Gene Expression by Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Worldwide epidemic of obesity. In Obesity and Obstetrics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.; Pinto, D.C. Overview on the antihypertensive and anti-obesity effects of secondary metabolites from seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Kato, E. Bioactive compounds in plant materials for the prevention of diabetesand obesity. Biosci. Biotechnol. Biochem. 2019, 83, 975–985. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Daliri, E.B.-M.; Elahi, F.; Yeon, S.-J.; Tyagi, A.; Liu, S.; Madar, I.H.; Sultan, G.; Oh, D.-H. The role of bioactive peptides in diabetes and obesity. Foods 2021, 10, 2220. [Google Scholar] [CrossRef]
- Jang, E.; Moon, J.; Ko, J.; Ahn, C.; Lee, H.; Shin, J.; Park, C.; Kang, J. Novel black soy peptides with antiobesity effects: Activation of leptin-like signaling and AMP-activated protein kinase. Int. J. Obes. 2008, 32, 1161–1170. [Google Scholar] [CrossRef]
- Coronado-Cáceres, L.J.; Rabadán-Chávez, G.; Mojica, L.; Hernández-Ledesma, B.; Quevedo-Corona, L.; Lugo Cervantes, E. Cocoa Seed Proteins’(Theobroma cacao L.) Anti-Obesity Potential through Lipase Inhibition Using In Silico, In Vitro and In Vivo Models. Foods 2020, 9, 1359. [Google Scholar] [CrossRef]
- Mostafa, H.; Al-Ahbabi, N.; Adiamo, O.Q.; Mudgil, P.; Maqsood, S. Phoenix dactylifera L. seed protein hydrolysates as a potential source of peptides with antidiabetic and anti-hypercholesterolemic properties: An in vitro study. Food Biosci. 2022, 49, 101916. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hernandez, M.; Rabalski, I.; Hucl, P. Composition of hairless canary seed oil and starch-associated lipids and the relationship between starch pasting and thermal properties and its lipids. LWT 2020, 125, 109257. [Google Scholar] [CrossRef]
- Jurado Rodríguez, K.Y. Desarrollo de un producto alimenticio galletas nutritivas basadas en alpiste para personas diabéticas, con el fin de optimizar su salud en la ciudad de Guayaquil. Universidad de Guayaquil. Bachelor’s Thesis, Facultad de Ciencias Administrativas, Universidad de Guayaquil, Guayaquil, Ecuador, 2015. [Google Scholar]
- Rodríguez, C.D.; Hernández, L.H.; Larios, R.M.; Ramos, C.C. Phalaris canariensis (alpiste) como alimento funcional. Una alternativa para el tratamiento de la hipertensión y reducción de índice de masa corporal en adultos mayores. Investig. Científica 2020, 14, 156–161. [Google Scholar]
- Valverde, M.E.; Orona-Tamayo, D.; Nieto-Rendón, B.; Paredes-López, O. Antioxidant and antihypertensive potential of protein fractions from flour and milk substitutes from canary seeds (Phalaris canariensis L.). Plant Foods Hum. Nutr. 2017, 72, 20–25. [Google Scholar] [CrossRef]
- Estrada-Salas, P.A.; Montero-Morán, G.M.; Martínez-Cuevas, P.P.; González, C.; Barba de la Rosa, A.P. Characterization of antidiabetic and antihypertensive properties of canary seed (Phalaris canariensis L.) peptides. J. Agric. Food Chem. 2014, 62, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Urbizo-Reyes, U.; Aguilar-Toalá, J.; Liceaga, A. Hairless canary seeds (Phalaris canariensis L.) as a potential source of antioxidant, antihypertensive, antidiabetic, and antiobesity biopeptides. Food Prod. Processing Nutr. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Kim, K.-H.; Anderson, J.M. Enzyme kinetics, molecular docking, and in silico characterization of canary seed (Phalaris canariensis L.) peptides with ACE and pancreatic lipase inhibitory activity. J. Funct. Foods 2022, 88, 104892. [Google Scholar] [CrossRef]
- Passos, C.S.; Carvalho, L.N.; Pontes Jr, R.B.; Campos, R.R.; Ikuta, O.; Boim, M.A. Blood pressure reducing effects of Phalaris canariensis in normotensive and spontaneously hypertensive rats. Can. J. Physiol. Pharmacol. 2012, 90, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.B.; Al Khalili, Y. Orlistat. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cavaliere, H.; Floriano, I.; Medeiros-Neto, G. Gastrointestinal side effects of orlistat may be prevented by concomitant prescription of natural fibers (psyllium mucilloid). Int. J. Obes. 2001, 25, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, Y.; Li, F.; Wu, T.; Shao, C.; Lin, Y.; Wang, W.; Feng, S.; Zhong, B. Effect of orlistat on liver fat content in patients with nonalcoholic fatty liver disease with obesity: Assessment using magnetic resonance imaging-derived proton density fat fraction. Ther. Adv. Gastroenterol. 2019, 12, 1756284819879047. [Google Scholar] [CrossRef] [PubMed]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of pancreatic lipase: State of the art and clinical perspectives. EXCLI J. 2014, 13, 897. [Google Scholar] [PubMed]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Tinline-Goodfellow, C.T.; West, D.W.; Malowany, J.M.; Gillen, J.B.; Moore, D.R. An acute reduction in habitual protein intake attenuates post exercise anabolism and may bias oxidation-derived protein requirements in resistance trained men. Front. Nutr. 2020, 7, 55. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Aragon, A.A. How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. J. Int. Soc. Sports Nutr. 2018, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, T.; Bumbie, G.Z.; Hu, H.; Chen, Q.; Lu, C.; Tang, Z. Effects of dietary crude protein levels on fecal crude protein, amino acids flow amount, fecal and ileal microbial amino acids composition and amino acid digestibility in growing pigs. Animals 2020, 10, 2092. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F.; Gonçalves, G.A.; Inácio, F.D.; Koehnlein, E.A.; De Souza, C.G.M.; Bracht, A.; Peralta, R.M. Inhibition of pancreatic lipase and triacylglycerol intestinal absorption by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Nutrients 2015, 7, 5601–5614. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tonge, P.J. Drug–target residence time: Critical information for lead optimization. Curr. Opin. Chem. Biol. 2010, 14, 467–474. [Google Scholar] [CrossRef]
- Feltrin, K.L. The Role of the Free Fatty Acid, Lauric Acid, in Appetite Regulation and Its Potential as an Appetite-Suppressant. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2008. [Google Scholar]
- Feltrin, K.L.; Patterson, M.; Ghatei, M.A.; Bloom, S.R.; Meyer, J.H.; Horowitz, M.; Feinle-Bisset, C. Effect of fatty acid chain length on suppression of ghrelin and stimulation of PYY, GLP-2 and PP secretion in healthy men. Peptides 2006, 27, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Lindqvist, A.; Krabisch, L.; Rehfeld, J.F.; Erlanson-Albertsson, C. Appetite suppression through delayed fat digestion. Physiol. Behav. 2006, 89, 563–568. [Google Scholar] [CrossRef]
- Albertsson, P.-Å.; Köhnke, R.; Emek, S.C.; Mei, J.; Rehfeld, J.F.; Åkerlund, H.-E.; Erlanson-Albertsson, C. Chloroplast membranes retard fat digestion and induce satiety: Effect of biological membranes on pancreatic lipase/co-lipase. Biochem. J. 2007, 401, 727–733. [Google Scholar] [CrossRef]
- Ojeda, L.; Da Ruiz, Y.; Martínez, F.; Odreman, R.; Torri, J.; Villegas, R.; Ybarra, L.P.; Machado, N.N. Effects of the seed of phalaris canariensis and the change of diet on serum lipids in rats. Emir. J. Food Agric. 2021, 33, 287–292. [Google Scholar] [CrossRef]
- Almaraz, L.M.; Ramírez, J.L.E.; Santiago, J. Effects profiles of complete aqueous extract and hexane and aqueous fractions of Phalaris canariensis L. seeds on fructose-induced metabolic syndrome in rats. Int. J. Herb. Med. 2017, 5, 39–46. [Google Scholar]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and prevention of hepatic steatosis. Gastroenterol. Hepatol. 2015, 11, 167. [Google Scholar]
- Richard, J.; Lingvay, I. Hepatic steatosis and Type 2 diabetes: Current and future treatment considerations. Expert Rev. Cardiovasc. Ther. 2011, 9, 321–328. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Teratani, T.; Tomita, K.; Furuhashi, H.; Sugihara, N.; Higashiyama, M.; Nishikawa, M.; Irie, R.; Takajo, T.; Wada, A.; Horiuchi, K. Lipoprotein lipase up-regulation in hepatic stellate cells exacerbates liver fibrosis in nonalcoholic steatohepatitis in mice. Hepatol. Commun. 2019, 3, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, A.L.; Shulman, G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology 2014, 59, 713–723. [Google Scholar] [CrossRef]
- Pérez-Echarri, N.; Pérez-Matute, P.; Marcos-Gómez, B.; Marti, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Down-regulation in muscle and liver lipogenic genes: EPA ethyl ester treatment in lean and overweight (high-fat-fed) rats. J. Nutr. Biochem. 2009, 20, 705–714. [Google Scholar] [CrossRef]
- Chow, J.D.; Lawrence, R.T.; Healy, M.E.; Dominy, J.E.; Liao, J.A.; Breen, D.S.; Byrne, F.L.; Kenwood, B.M.; Lackner, C.; Okutsu, S. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol. Metab. 2014, 3, 419–431. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M.; Madrigales Ahuatzi, D.; Horcacitas, M.d.C.; Garcia Baez, E.; Cruz Victoria, T.; Mota-Flores, J.M. Ameliorative effect of hexane extract of Phalaris canariensis on high fat diet-induced obese and streptozotocin-induced diabetic mice. Evid.-Based Complementary Altern. Med. 2014, 2014, 145901. [Google Scholar] [CrossRef]
- Achouri, A.; L’Hocine, L.; Martineau-Côté, D.; Sirois, S.; Pitre, M.; Mason, E.; Abdel-Aal, E.M.; Hucl, P. Scale up fractionation of components from novel glabrous brown and yellow canary seeds (Phalaris canariensis L.) and techno-functional properties of the resulting protein isolates. Food Res. Int. 2020, 137, 109751. [Google Scholar] [CrossRef] [PubMed]
- Urbizo-Reyes, U.; Kim, K.-H.; Reddivari, L.; Anderson, J.M.; Liceaga, A.M. Oxidative Stress Protection by Canary Seed (Phalaris canariensis L.) Peptides in Caco-2 Cells and Caenorhabditis elegans. Nutrients 2022, 14, 2415. [Google Scholar] [CrossRef] [PubMed]
- Small, L.; Ehrlich, A.; Iversen, J.; Ashcroft, S.P.; Trošt, K.; Moritz, T.; Hartmann, B.; Holst, J.J.; Treebak, J.T.; Zierath, J.R. Comparative analysis of oral and intraperitoneal glucose tolerance tests in mice. Mol. Metab. 2022, 57, 101440. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Lowery, R.G. A high-throughput method for measuring drug residence time using the transcreener ADP assay. SLAS DISCOVERY: Adv. Life Sci. RD 2017, 22, 915–922. [Google Scholar] [CrossRef]
- Adhyatmika, A.; Beljaars, L.; Putri, K.S.; Habibie, H.; Boorsma, C.E.; Reker-Smit, C.; Luangmonkong, T.; Guney, B.; Haak, A.; Mangnus, K.A. Osteoprotegerin is more than a possible serum marker in liver fibrosis: A study into its function in human and murine liver. Pharmaceutics 2020, 12, 471. [Google Scholar] [CrossRef]
- Smith, A.; Yu, X.; Yin, L. Diazinon exposure activated transcriptional factors CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) and induced adipogenesis in 3T3-L1 preadipocytes. Pestic. Biochem. Physiol. 2018, 150, 48–58. [Google Scholar] [CrossRef]
- Siersbæk, M.; Varticovski, L.; Yang, S.; Baek, S.; Nielsen, R.; Mandrup, S.; Hager, G.L.; Chung, J.H.; Grøntved, L. High fat diet-induced changes of mouse hepatic transcription and enhancer activity can be reversed by subsequent weight loss. Sci. Rep. 2017, 7, 40220. [Google Scholar] [CrossRef]
- Pfohl, M.; DaSilva, N.A.; Marques, E.; Agudelo, J.; Liu, C.; Goedken, M.; Slitt, A.L.; Seeram, N.P.; Ma, H. Hepatoprotective and anti-inflammatory effects of a standardized pomegranate (Punica granatum) fruit extract in high fat diet-induced obese C57BL/6 mice. Int. J. Food Sci. Nutr. 2021, 72, 499–510. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]


| Treatment Group | Fecal Proximal Composition (% in Dry Weight) | Organ and Tissue Weights | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude Protein | Crude Fat | Crude Fiber | Nitrogen Free Extract | Ash | Kidney (mg) | Spleen (mg) | Liver (%, w/bwt) | Epididymal Fat (%, w/bwt) | |
| Normal diet | 17.64 ± 0.04 ab | 2.23 ± 0.30 b | 33.08 ± 0.93 a | 47.48 ± 0.93 b | 20.27 ± 0.63 a | 371.64 ± 4.36 a | 86.27 ± 3.50 a | 4.03 ± 0.06 b | 3.07 ± 0.27 d |
| Western diet | 15.29 ± 0.05 c | 2.58 ± 0.01 b | 28.9 ± 1.21 a | 47.56 ± 1.21 b | 21.85 ± 0.16 a | 390.76 ± 0.76 a | 85.25 ± 0.50 a | 5.42 ± 0.48 a | 6.68 ± 0.20 a |
| CSP-250 | 16.14 ± 0.01 bc | 3.03 ± 0.01 b | 30.41 ± 1.06 a | 48.02 ± 1.06 b | 21.04 ± 0.41 a | 386.50 ± 10.25 a | 80.4 ± 0.93 a | 4.70 ± 0.01 ab | 5.97 ± 0.12 bc |
| CSP-500 | 18.83 ± 0.91 a | 3.02 ± 0.39 b | 31.58 ± 0.80 a | 48.74 ± 0.80 b | 20.34 ± 0.63 a | 369.08 ± 12.83 a | 84.60 ± 3.70 a | 4.61 ± 0.25 ab | 6.28 ± 0.45 ab |
| Orlistat | 12.62 ± 0.15 d | 38.67 ± 0.16 a | 19.9 ± 0.32 a | 93.42 ± 0.32 a | 13.1 ± 0.19 b | 402.08 ± 7.43 a | 81.50 ± 0.50 a | 4.46 ± 0.29 ab | 5.41 ± 0.32 c |
| Macro-Nutrient | Normal Diet | Western Diet |
|---|---|---|
| TD.05230 | TD.88137 | |
| Protein (%) | 17.3 | 17.3 |
| Carbohydrate (%) | 63.5 | 48.5 |
| Fat (%) | 5.2 | 21.2 |
| Energy (kcal/g) | 3.7 | 4.5 |
| Ingredient | Formula (g/kg) | |
| Casein | 195 | 195 |
| DL-methionine | 3 | 3 |
| Sucrose | 341 | 341 |
| Corn Starch | 212 | 150 |
| Maltodextrin | 100 | N/A |
| Anhydrous Milkfat | 37 | 210 |
| Soybean oil | 13 | N/A |
| cholesterol | N/A | 1.5 |
| cellulose | 50 | 50 |
| Mineral mix, (AIN-76) | 35 | 35 |
| Calcium Carbonate | 4 | 4 |
| Vitamin Mix (Teklab-40060) | 10 | 10 |
| Ethoxyquien-antioxidant | 0.01 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Li, S.; Kim, K.-H.; Cox, A.D.; Anderson, J.M. Canary Seed (Phalaris canariensis L.) Peptides Prevent Obesity and Glucose Intolerance in Mice Fed a Western Diet. Int. J. Mol. Sci. 2022, 23, 14927. https://doi.org/10.3390/ijms232314927
Urbizo-Reyes U, Liceaga AM, Reddivari L, Li S, Kim K-H, Cox AD, Anderson JM. Canary Seed (Phalaris canariensis L.) Peptides Prevent Obesity and Glucose Intolerance in Mice Fed a Western Diet. International Journal of Molecular Sciences. 2022; 23(23):14927. https://doi.org/10.3390/ijms232314927
Chicago/Turabian StyleUrbizo-Reyes, Uriel, Andrea M. Liceaga, Lavanya Reddivari, Shiyu Li, Kee-Hong Kim, Abigail D. Cox, and Joseph M. Anderson. 2022. "Canary Seed (Phalaris canariensis L.) Peptides Prevent Obesity and Glucose Intolerance in Mice Fed a Western Diet" International Journal of Molecular Sciences 23, no. 23: 14927. https://doi.org/10.3390/ijms232314927
APA StyleUrbizo-Reyes, U., Liceaga, A. M., Reddivari, L., Li, S., Kim, K.-H., Cox, A. D., & Anderson, J. M. (2022). Canary Seed (Phalaris canariensis L.) Peptides Prevent Obesity and Glucose Intolerance in Mice Fed a Western Diet. International Journal of Molecular Sciences, 23(23), 14927. https://doi.org/10.3390/ijms232314927








