Methyl P-Coumarate Ameliorates the Inflammatory Response in Activated-Airway Epithelial Cells and Mice with Allergic Asthma
Abstract
1. Introduction
2. Results
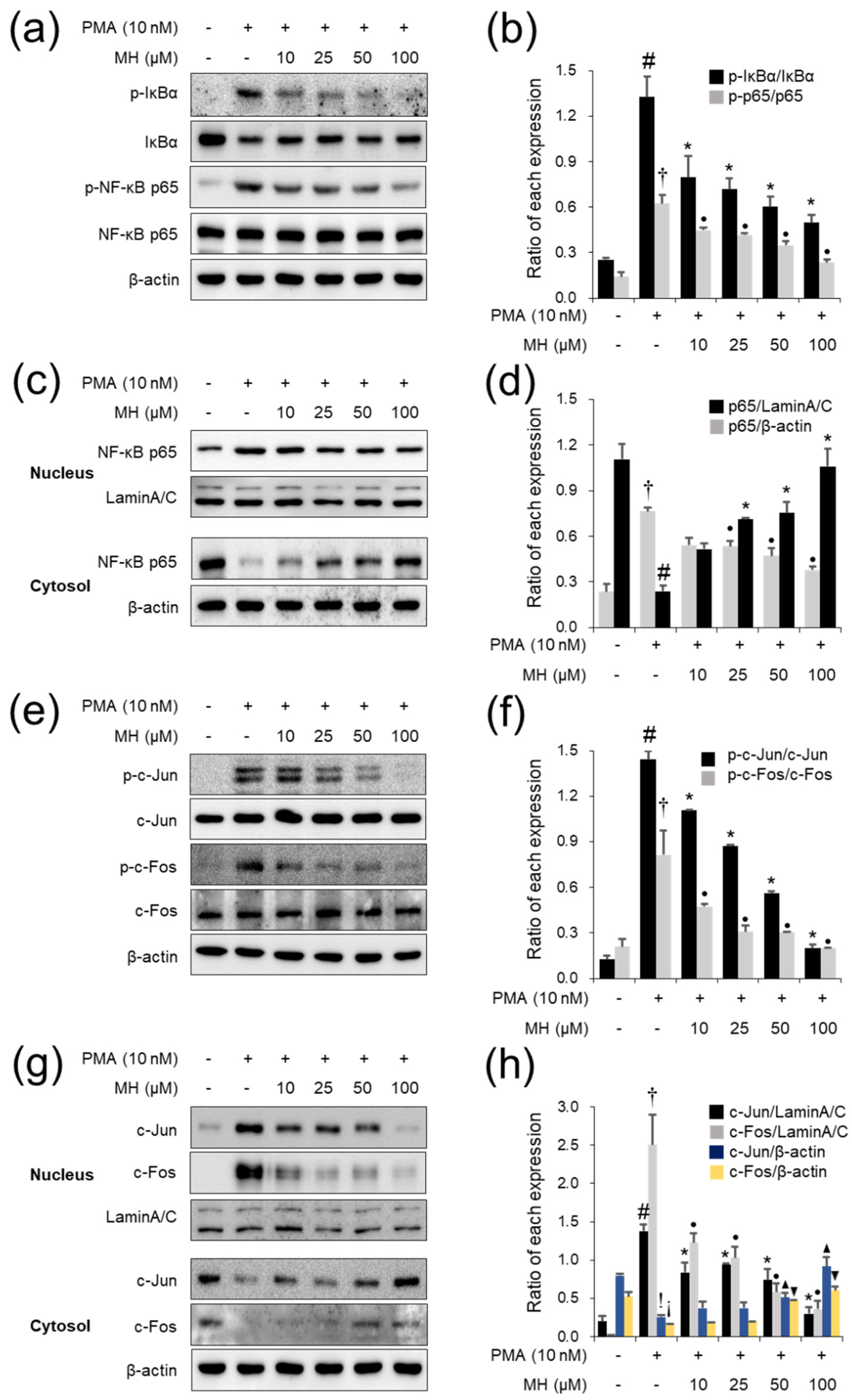
2.1. Suppressive Effects of MH against the PMA-Stimulated Inflammatory Response in A549 Cells
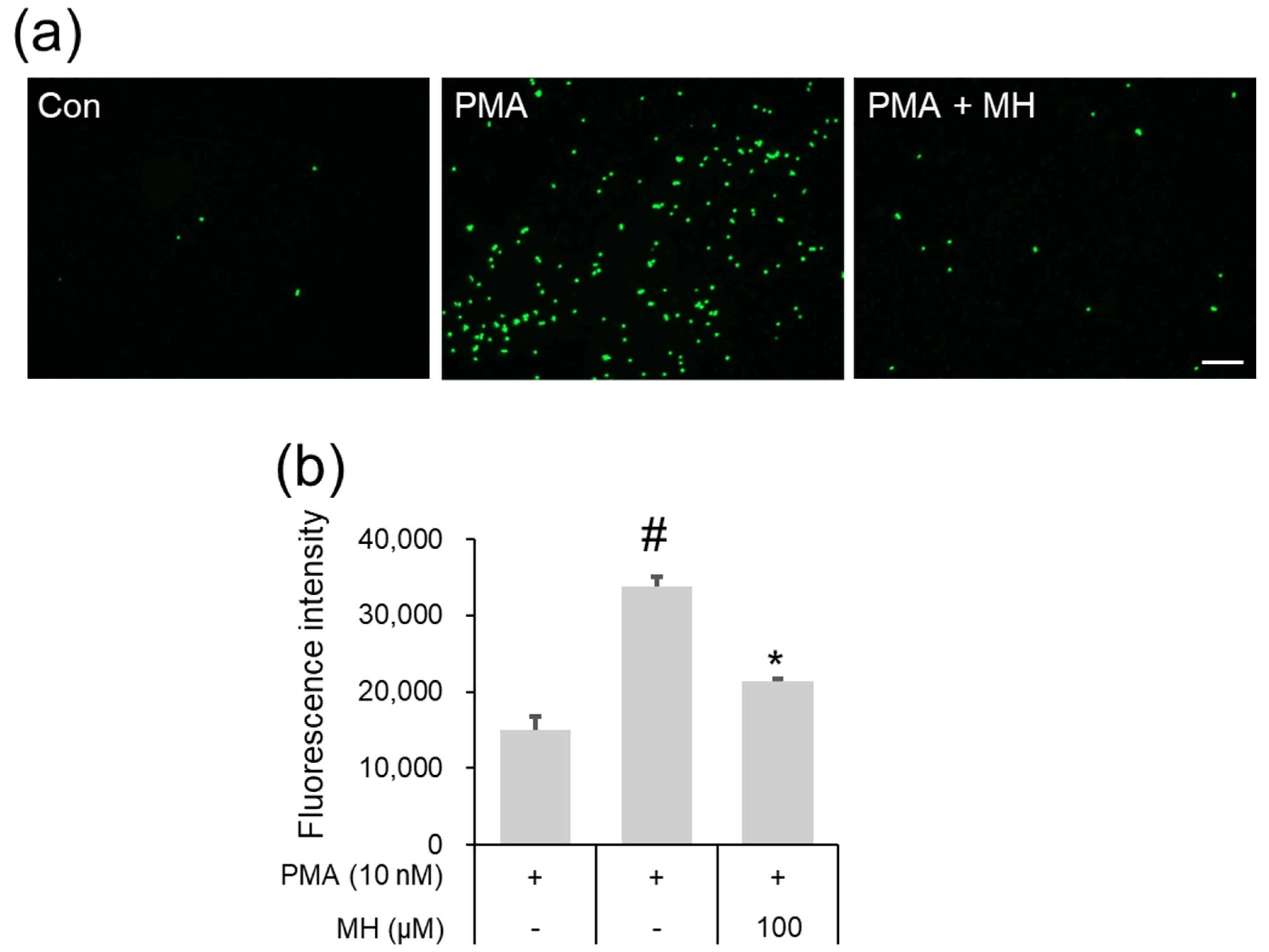
2.2. Suppressive Effects of MH on Adhesion of Eosinophil and Airway Epithelial Cells
2.3. Suppressive Effects of MH against the LPS-Induced Inflammatory Response in RAW264.7 Cells
2.4. Inhibitory Effects of MH on Recruitment of Immune Cells in Mice with AA
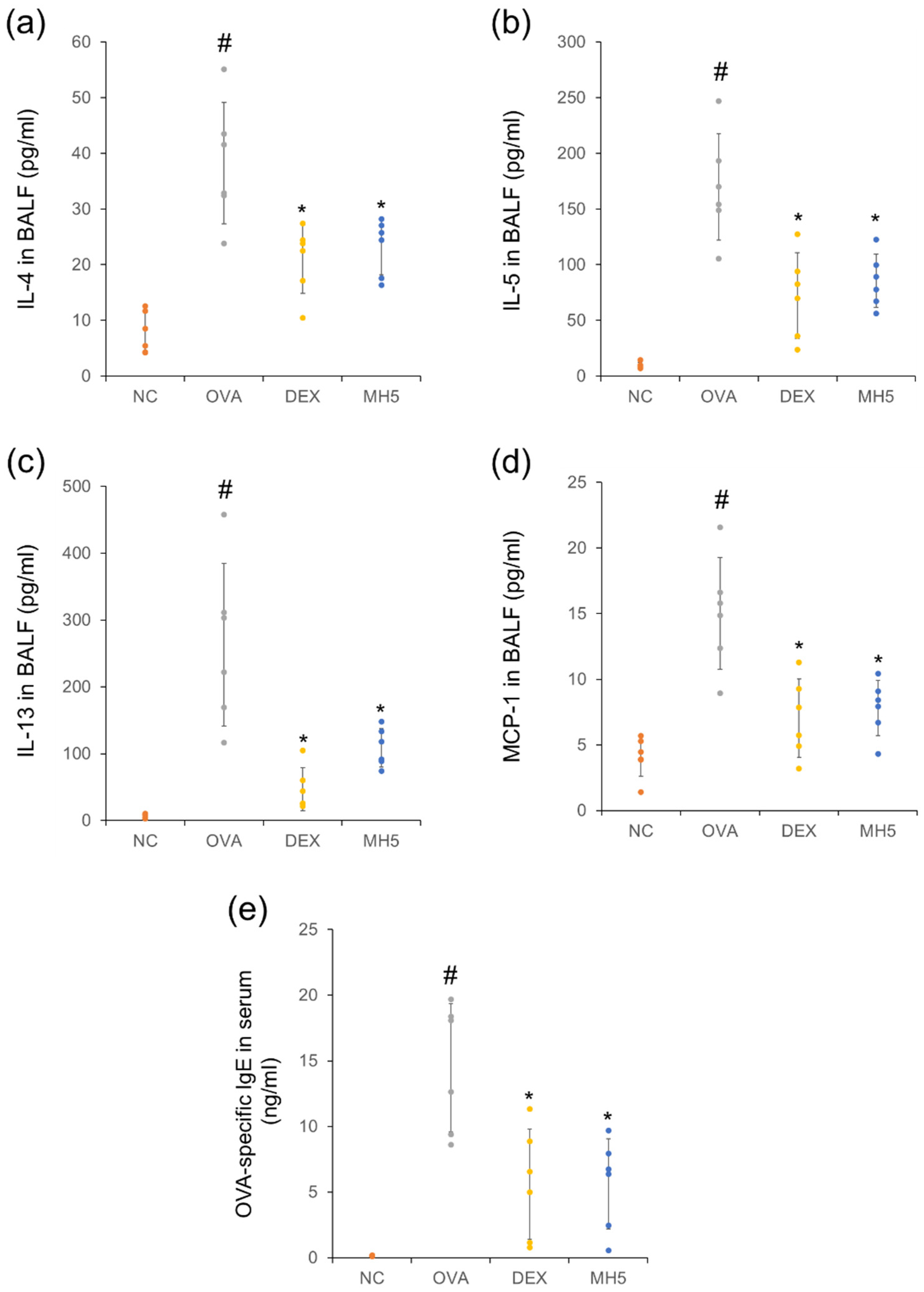
2.5. Suppressive Effects of MH on Secretion of Th2 Cytokines, MCP-1, and IgE in Mice with AA
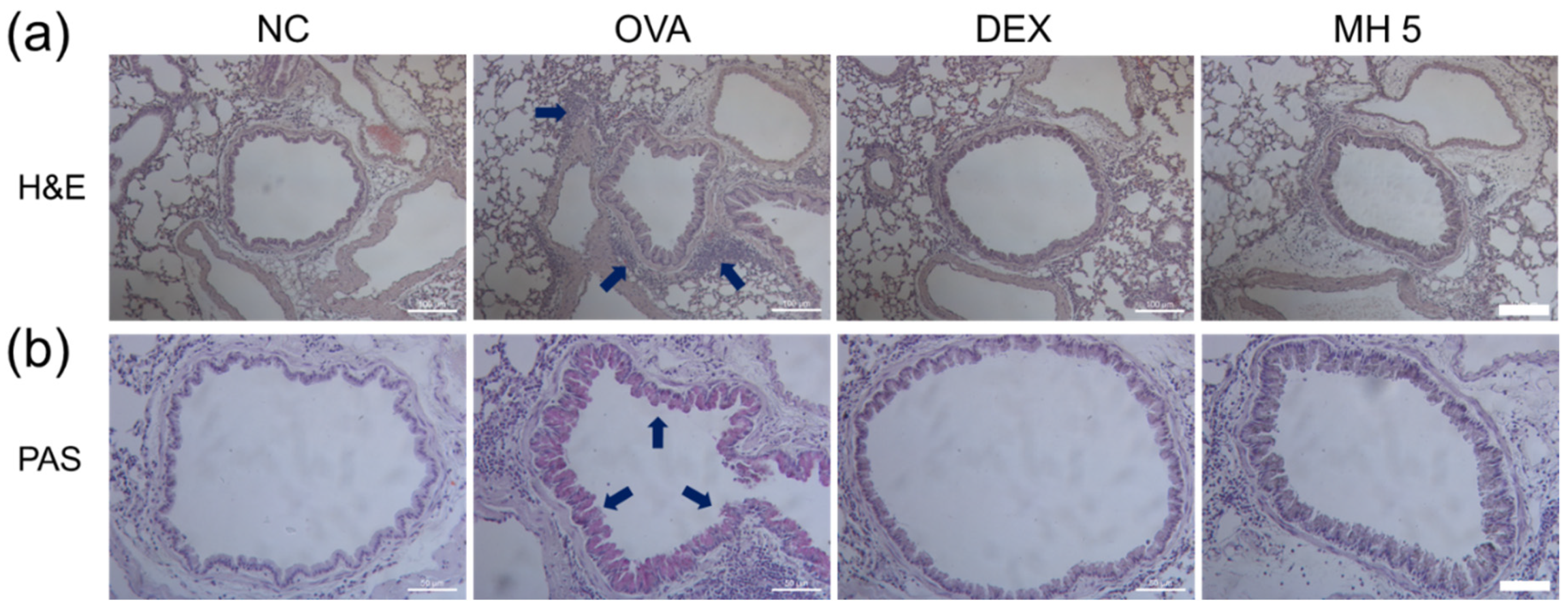
2.6. Suppressive Effects of MH on Immune Cell Influx and Mucus Secretion in Mice with AA
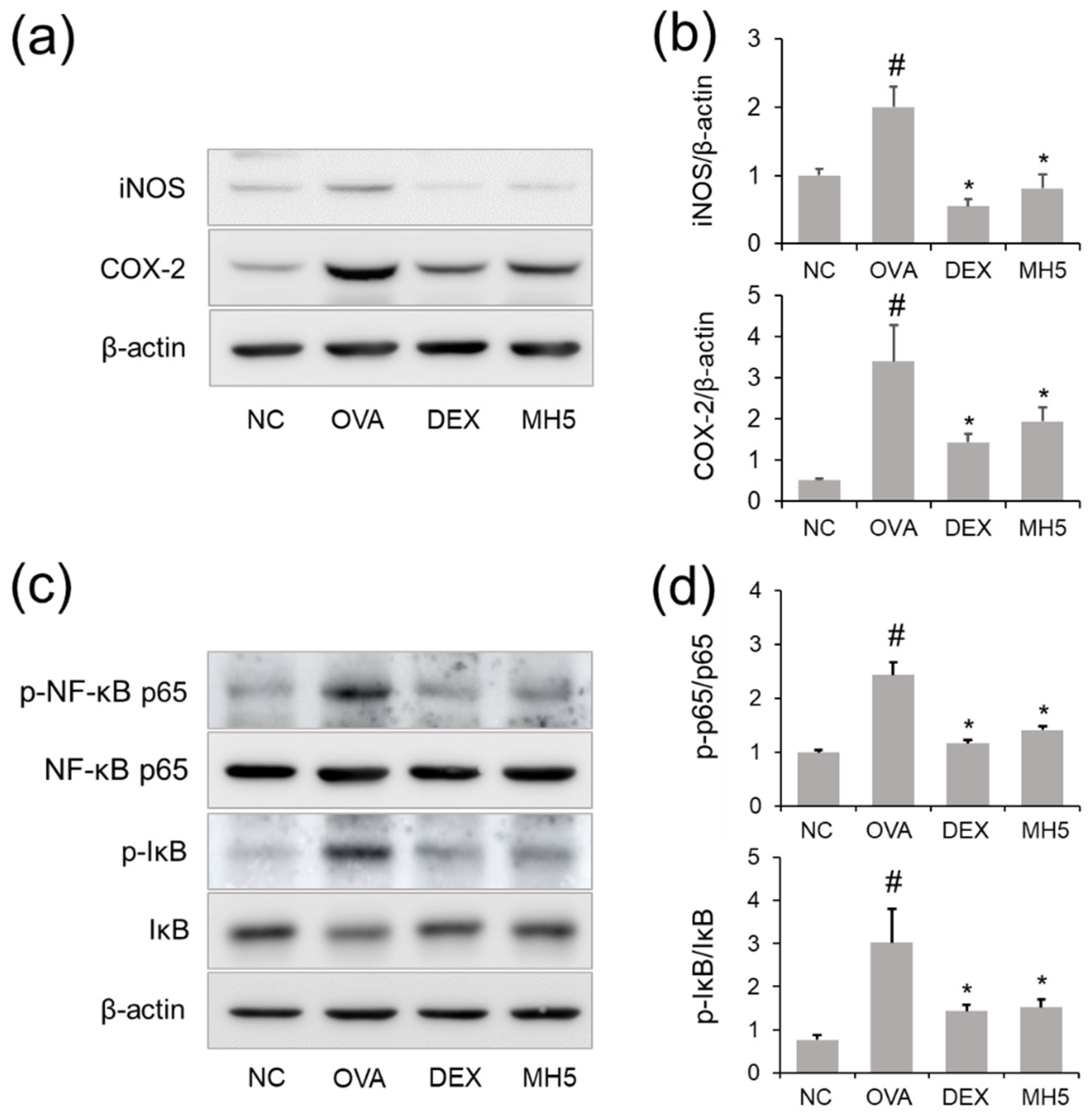
2.7. Suppressive Effects of MH on iNOS and COX-2 Expression in Mice with AA
2.8. Effects of MH on NF-κB Inactivation in Mice with AA
3. Discussion
4. Material and Methods
4.1. Reagents and Cell Culture
4.2. Nuclear and Cytoplasmic Extraction
4.3. Cell Adhesion Assays
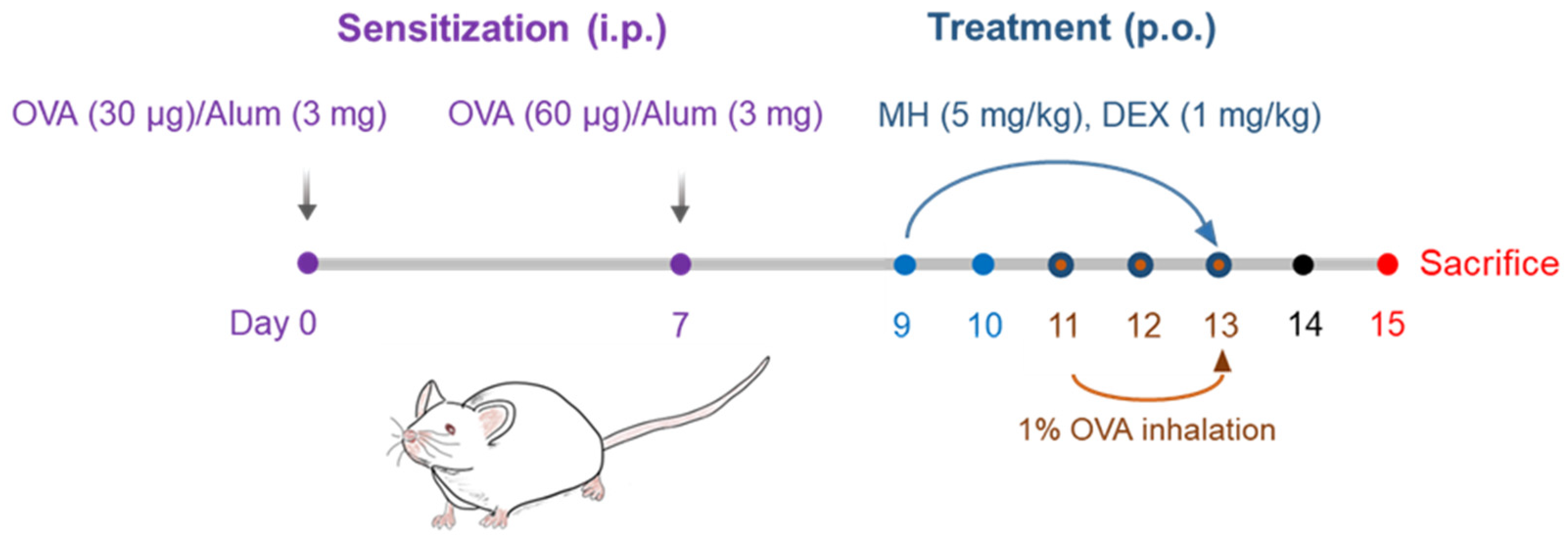
4.4. Establishment of the OVA-Induced AA mouse model
4.5. Immune Cell Count and ELISA
4.6. Western Blot Analysis
4.7. Histological Analysis
4.8. Ethics Statement
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bezzaoucha, A. Epidemiology of asthma in children and young adults in Algiers. Rev. Mal. Respir. 1992, 9, 417–423. [Google Scholar] [PubMed]
- Loftus, P.A.; Wise, S.K. Epidemiology of asthma. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 245–249. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.K.; Lee, J.W.; Xuezhen, X.; Harmalkar, D.S.; Song, J.G.; Park, J.W.; Hwang, D.; Min, J.H.; Kim, J.H.; Han, H.K.; et al. DK-1108 exerts anti-inflammatory activity against phorbol 12-myristate 13-acetate-induced inflammation and protective effect against OVA-induced allergic asthma. Biomed. Pharmacother. 2020, 132, 110950. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef]
- Sharma, N.; Akkoyunlu, M.; Rabin, R.L. Macrophages-common culprit in obesity and asthma. Allergy 2018, 73, 1196–1205. [Google Scholar] [CrossRef]
- Ishioka, S. Role of cytokines in pathophysiology of asthma. Nihon Naika Gakkai Zasshi 1996, 85, 184–188. [Google Scholar] [PubMed]
- Truyen, E.; Coteur, L.; Dilissen, E.; Overbergh, L.; Dupont, L.J.; Ceuppens, J.L.; Bullens, D.M. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax 2006, 61, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Chun, W.; Lee, H.J.; Min, J.H.; Kim, S.M.; Seo, J.Y.; Ahn, K.S.; Oh, S.R. The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells 2021, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, K.; Ogawa, T.; Shimizu, S.; Shimizu, T. Epidermal growth factor receptor inhibitor AG1478 inhibits mucus hypersecretion in airway epithelium. Am. J. Rhinol. Allergy 2016, 30, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Dunican, E.M.; Elicker, B.M.; Gierada, D.S.; Nagle, S.K.; Schiebler, M.L.; Newell, J.D.; Raymond, W.W.; Lachowicz-Scroggins, M.E.; Di Maio, S.; Hoffman, E.A.; et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J. Clin. Investig. 2018, 128, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Martins, M.A.; Tiberio, I.F. Nitric oxide in asthma physiopathology. ISRN Allergy 2011, 2011, 832560. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Ryu, H.W.; Kwon, O.K.; Hwang, D.; Kim, M.G.; Min, J.H.; Zhang, Z.; Kim, S.Y.; Paik, J.H.; Oh, S.R.; et al. Callicarpa japonica Thunb. ameliorates allergic airway inflammation by suppressing NF-kappaB activation and upregulating HO-1 expression. J. Ethnopharmacol. 2021, 267, 113523. [Google Scholar] [CrossRef] [PubMed]
- Daham, K.; James, A.; Balgoma, D.; Kupczyk, M.; Billing, B.; Lindeberg, A.; Henriksson, E.; FitzGerald, G.A.; Wheelock, C.E.; Dahlen, S.E.; et al. Effects of selective COX-2 inhibition on allergen-induced bronchoconstriction and airway inflammation in asthma. J. Allergy Clin. Immunol. 2014, 134, 306–313. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, S.M.; Min, J.H.; Kim, M.G.; Kwon, O.K.; Hwang, D.; Oh, J.H.; Park, M.W.; Chun, W.; Lee, H.J.; et al. 3,4,5-Trihydroxycinnamic acid exerts anti-asthmatic effects in vitro and in vivo. Int. Immunopharmacol. 2020, 88, 107002. [Google Scholar] [CrossRef]
- Lee, J.W.; Ryu, H.W.; Kim, D.Y.; Kwon, O.K.; Jang, H.J.; Kwon, H.J.; Kim, S.Y.; Lee, S.U.; Kim, S.M.; Oh, E.S.; et al. Biflavonoid-rich fraction from Daphne pseudomezereum var. koreana Hamaya exerts anti-inflammatory effect in an experimental animal model of allergic asthma. J. Ethnopharmacol. 2021, 265, 113386. [Google Scholar] [CrossRef]
- Xu, T.; Ge, X.; Lu, C.; Dai, W.; Chen, H.; Xiao, Z.; Wu, L.; Liang, G.; Ying, S.; Zhang, Y.; et al. Baicalein attenuates OVA-induced allergic airway inflammation through the inhibition of the NF-kappaB signaling pathway. Aging 2019, 11, 9310–9327. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Singh, D.K.; Dash, D.; Singh, R. Intranasal curcumin regulates chronic asthma in mice by modulating NF-kB activation and MAPK signaling. Phytomedicine 2018, 51, 29–38. [Google Scholar] [CrossRef]
- Patil, R.H.; Kumar, M.N.; Kumar, K.K.M.; Nagesh, R.; Kavya, K.; Babu, R.L.; Ramesh, G.T.; Sharma, S.C. Dexamethasone inhibits inflammatory response via down regulation of AP-1 transcription factor in human lung epithelial cells. Gene 2018, 645, 85–94. [Google Scholar] [CrossRef]
- Lee, J.W.; Min, J.H.; Kim, M.G.; Kim, S.M.; Kwon, O.K.; Oh, T.K.; Lee, J.K.; Kim, T.Y.; Lee, S.W.; Choi, S.; et al. Pistacia weinmannifolia root exerts a protective role in ovalbumininduced lung inflammation in a mouse allergic asthma model. Int. J. Mol. Med. 2019, 44, 2171–2180. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.M.; Min, J.H.; Kwon, O.K.; Park, M.H.; Park, J.W.; Ahn, H.I.; Hwang, J.Y.; Oh, S.R.; Lee, J.W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef] [PubMed]
- Maruthamuthu, V.; Henry, L.J.K.; Ramar, M.K.; Kandasamy, R. Myxopyrum serratulum ameliorates airway inflammation in LPS-stimulated RAW 264.7 macrophages and OVA-induced murine model of allergic asthma. J. Ethnopharmacol. 2020, 255, 112369. [Google Scholar] [CrossRef] [PubMed]
- Zeghbib, W.; Boudjouan, F.; Vasconcelos, V.; Lopes, G. Phenolic Compounds’ Occurrence in Opuntia Species and Their Role in the Inflammatory Process: A Review. Molecules 2022, 27, 4763. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Kwon, H.J.; Seo, C.S.; Choi, S.J.; Shin, N.R.; Kim, S.H.; Kim, Y.H.; Kim, J.C.; Kim, M.S.; Shin, I.S. 4-Hydroxycinnamic acid suppresses airway inflammation and mucus hypersecretion in allergic asthma induced by ovalbumin challenge. Phytother. Res. 2020, 34, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Kim, M.G.; Kim, S.M.; Park, J.W.; Chun, W.; Lee, H.J.; Oh, S.R.; Ahn, K.S.; Lee, J.W. 3,4,5-Trihydroxycinnamic acid exerts a protective effect on pulmonary inflammation in an experimental animal model of COPD. Int. Immunopharmacol. 2020, 85, 106656. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Nihei, K.-I.; Kubo, I. Intracellular oxidation of methyl p-coumarate is involved in anti-melanogenic and cytotoxic activities against melanoma cells. Phytochem. Lett. 2022, 50, 89–94. [Google Scholar] [CrossRef]
- Hooperb, S.N.J.; Jurgens, T.; Chandler, R.F.; Stevens, M.F. Methyl p-coumarate: A cytotoxic constituent from Comptonia peregrina. Phytochemistry 1984, 23, 2096–2097. [Google Scholar] [CrossRef]
- Quek, A.; Kassim, N.K.; Lim, P.C.; Tan, D.C.; Mohammad Latif, M.A.; Ismail, A.; Shaari, K.; Awang, K. alpha-Amylase and dipeptidyl peptidase-4 (DPP-4) inhibitory effects of Melicope latifolia bark extracts and identification of bioactive constituents using in vitro and in silico approaches. Pharm. Biol. 2021, 59, 964–973. [Google Scholar] [CrossRef]
- Zhang, W.M.; Wang, W.; Zhang, J.J.; Wang, Z.R.; Wang, Y.; Hao, W.J.; Huang, W.Y. Antibacterial Constituents of Hainan Morinda citrifolia (Noni) Leaves. J. Food Sci. 2016, 81, M1192–M1196. [Google Scholar] [CrossRef]
- Yuan, S.; Li, W.; Li, Q.; Wang, L.; Cao, J.; Jiang, W. Defense Responses, Induced by p-Coumaric Acid and Methyl p-Coumarate, of Jujube (Ziziphus jujuba Mill.) Fruit against Black Spot Rot Caused by Alternaria alternata. J. Agric. Food Chem. 2019, 67, 2801–2810. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Guo, L.; Chen, Y. Determination of three compounds in Aloe vera by capillary electrophoresis. Biomed. Chromatogr. 2004, 18, 112–116. [Google Scholar] [CrossRef]
- Kubo, I.; Nihei, K.; Tsujimoto, K. Methyl p-coumarate, a melanin formation inhibitor in B16 mouse melanoma cells. Bioorg. Med. Chem. 2004, 12, 5349–5354. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.A.; Lee, J.W.; Shin, S.Y.; Kwon, J.H.; Lee, H.J.; Kim, S.S.; Kwon, Y.S.; Chun, W. Methyl p-Hydroxycinnamate Suppresses Lipopolysaccharide-Induced Inflammatory Responses through Akt Phosphorylation in RAW264.7 Cells. Biomol. Ther. 2014, 22, 10–16. [Google Scholar] [CrossRef]
- Kim, S.M.; Min, J.H.; Kim, J.H.; Choi, J.; Park, J.M.; Lee, J.; Goo, S.H.; Oh, J.H.; Kim, S.H.; Chun, W.; et al. Methyl phydroxycinnamate exerts antiinflammatory effects in mouse models of lipopolysaccharideinduced ARDS. Mol. Med. Rep. 2022, 25, 37. [Google Scholar] [CrossRef]
- Burke-Gaffney, A.; Hellewell, P.G. A CD18/ICAM-1-dependent pathway mediates eosinophil adhesion to human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 408–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Poynter, M.E.; Irvin, C.G. Interleukin-6 as a biomarker for asthma: Hype or is there something else? Eur. Respir. J. 2016, 48, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Ludviksdottir, D.; Janson, C.; Nettelbladt, O.; Bjornsson, E.; Roomans, G.M.; Boman, G.; Seveus, L.; Venge, P. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. BHR Group. Am. J. Respir. Crit. Care Med. 2000, 162, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, H.; Ohno, I.; Honma, M.; Tanno, Y.; Yamauchi, K.; Tamura, G.; Shirato, K. IL-5, IL-8 and GM-CSF immunostaining of sputum cells in bronchial asthma and chronic bronchitis. Clin. Exp. Allergy 1995, 25, 720–728. [Google Scholar] [CrossRef]
- Lee, Y.G.; Jeong, J.J.; Nyenhuis, S.; Berdyshev, E.; Chung, S.; Ranjan, R.; Karpurapu, M.; Deng, J.; Qian, F.; Kelly, E.A.; et al. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2015, 52, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wang, A.; He, Q.; Yang, Q.; Liu, L.; Zhang, G.; Huang, Y.; Ding, X.; Yu, H.; Hu, S. Phenotypic switch in lung interstitial macrophage polarization in an ovalbumin-induced mouse model of asthma. Exp. Ther. Med. 2017, 14, 1284–1292. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Q.; Wang, Y.; Song, X.; Zhang, Y. Blockade of CCL2/CCR2 signaling pathway prevents inflammatory monocyte recruitment and attenuates OVA-Induced allergic asthma in mice. Immunol. Lett. 2019, 214, 30–36. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef]
- Ip, W.K.; Wong, C.K.; Lam, C.W. Interleukin (IL)-4 and IL-13 up-regulate monocyte chemoattractant protein-1 expression in human bronchial epithelial cells: Involvement of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase 1/2 and Janus kinase-2 but not c-Jun NH2-terminal kinase 1/2 signalling pathways. Clin. Exp. Immunol. 2006, 145, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Leon, B.; Ballesteros-Tato, A. Modulating Th2 Cell Immunity for the Treatment of Asthma. Front. Immunol. 2021, 12, 637948. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Canonica, G.W.; Matucci, A.; Paolini, R.; Triggiani, M.; Paggiaro, P. Targeted therapy in severe asthma today: Focus on immunoglobulin E. Drug Des. Devel Ther. 2017, 11, 1979–1987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chipps, B.E.; Marshik, P.L. Targeted IgE Therapy for Patients With Moderate to Severe Asthma. Biotechnol. Healthc. 2004, 1, 56–61. [Google Scholar] [PubMed]
- Rael, E.L.; Lockey, R.F. Interleukin-13 signaling and its role in asthma. World Allergy Organ. J. 2011, 4, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Ferrando, M.; Varricchi, G.; Passalacqua, G.; Canonica, G.W. A Critical Evaluation of Anti-IL-13 and Anti-IL-4 Strategies in Severe Asthma. Int. Arch. Allergy Immunol. 2016, 170, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Heffler, E.; Crimi, C.; Maglio, A.; Vatrella, A.; Pelaia, G.; Canonica, G.W. Interleukins 4 and 13 in Asthma: Key Pathophysiologic Cytokines and Druggable Molecular Targets. Front. Pharmacol. 2022, 13, 851940. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lee, J.W.; Kim, M.O.; Lee, R.W.; Kang, M.J.; Kim, S.M.; Min, J.H.; Oh, E.S.; Song, Y.N.; Jung, S.; et al. Daphnodorin C isolated from the stems of Daphne kiusiana Miquel attenuates airway inflammation in a mouse model of chronic obstructive pulmonary disease. Phytomedicine 2022, 96, 153848. [Google Scholar] [CrossRef]
- Kim, S.M.; Ryu, H.W.; Kwon, O.K.; Min, J.H.; Park, J.M.; Kim, D.Y.; Oh, S.R.; Lee, S.J.; Ahn, K.S.; Lee, J.W. Protective Effect of Paulownia tomentosa Fruits in an Experimental Animal Model of Acute Lung Injury. Microbiol. Biotechnol. Lett. 2022, 50, 310–318. [Google Scholar] [CrossRef]
- Qian, J.; Ma, X.; Xun, Y.; Pan, L. Protective effect of forsythiaside A on OVA-induced asthma in mice. Eur. J. Pharmacol. 2017, 812, 250–255. [Google Scholar] [CrossRef]
- Li, J.; Kartha, S.; Iasvovskaia, S.; Tan, A.; Bhat, R.K.; Manaligod, J.M.; Page, K.; Brasier, A.R.; Hershenson, M.B. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L690–L699. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Lee, A.Y.; Song, J.H.; Yang, S.; Park, I.; Lim, J.O.; Jung, T.Y.; Ko, J.W.; Kim, J.C.; Lim, K.S.; et al. Scrophularia buergeriana attenuates allergic inflammation by reducing NF-kappaB activation. Phytomedicine 2020, 67, 153159. [Google Scholar] [CrossRef]
- Lu, X.; Xu, C.; Yang, R.; Zhang, G. Ganoderic Acid A Alleviates OVA-Induced Asthma in Mice. Inflammation 2021, 44, 1908–1915. [Google Scholar] [CrossRef]
- Oh, S.W.; Cha, J.Y.; Jung, J.E.; Chang, B.C.; Kwon, H.J.; Lee, B.R.; Kim, D.Y. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-kappaB inhibition. J. Ethnopharmacol. 2011, 136, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cai, S.; Nie, J.; Li, Y.; Shi, G.; Hao, J.; Fu, W.; Tan, H.; Chen, S.; Li, B.; et al. The natural compound nujiangexanthone A suppresses mast cell activation and allergic asthma. Biochem. Pharmacol. 2016, 100, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Ryu, H.W.; Ahn, H.I.; Min, J.H.; Kim, S.M.; Kim, M.G.; Kwon, O.K.; Hwang, D.; Kim, S.Y.; Choi, S.; et al. The Anti-Inflammatory Effect of Trichilia martiana C. DC. in the Lipopolysaccharide-Stimulated Inflammatory Response in Macrophages and Airway Epithelial Cells and in LPS-Challenged Mice. J. Microbiol. Biotechnol. 2020, 30, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Lee, J.W.; Lee, J.K.; Song, Y.N.; Oh, E.S.; Ro, H.; Yoon, D.; Jeong, Y.H.; Park, J.Y.; Hong, S.T.; et al. Black Ginseng Extract Suppresses Airway Inflammation Induced by Cigarette Smoke and Lipopolysaccharides In Vivo. Antioxidants 2022, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, M.O.; Song, Y.N.; Min, J.H.; Kim, S.M.; Kang, M.J.; Oh, E.S.; Lee, R.W.; Jung, S.; Ro, H.; et al. Compound K ameliorates airway inflammation and mucus secretion through the regulation of PKC signaling in vitro and in vivo. J. Ginseng Res. 2022, 46, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Park, H.A.; Kwon, O.K.; Ryu, H.W.; Min, J.H.; Park, M.W.; Park, M.H.; Paik, J.H.; Choi, S.; Paryanto, I.; Yuniato, P.; et al. Physalis peruviana L. inhibits ovalbumininduced airway inflammation by attenuating the activation of NFkappaB and inflammatory molecules. Int. J. Mol. Med. 2019, 43, 1830–1838. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-W.; Choi, J.; Lee, J.; Park, J.-M.; Kim, S.-M.; Min, J.-H.; Seo, D.-Y.; Goo, S.-H.; Kim, J.-H.; Kwon, O.-K.; et al. Methyl P-Coumarate Ameliorates the Inflammatory Response in Activated-Airway Epithelial Cells and Mice with Allergic Asthma. Int. J. Mol. Sci. 2022, 23, 14909. https://doi.org/10.3390/ijms232314909
Park J-W, Choi J, Lee J, Park J-M, Kim S-M, Min J-H, Seo D-Y, Goo S-H, Kim J-H, Kwon O-K, et al. Methyl P-Coumarate Ameliorates the Inflammatory Response in Activated-Airway Epithelial Cells and Mice with Allergic Asthma. International Journal of Molecular Sciences. 2022; 23(23):14909. https://doi.org/10.3390/ijms232314909
Chicago/Turabian StylePark, Ji-Won, Jinseon Choi, Juhyun Lee, Jin-Mi Park, Seong-Man Kim, Jae-Hong Min, Da-Yun Seo, Soo-Hyeon Goo, Ju-Hee Kim, Ok-Kyoung Kwon, and et al. 2022. "Methyl P-Coumarate Ameliorates the Inflammatory Response in Activated-Airway Epithelial Cells and Mice with Allergic Asthma" International Journal of Molecular Sciences 23, no. 23: 14909. https://doi.org/10.3390/ijms232314909
APA StylePark, J.-W., Choi, J., Lee, J., Park, J.-M., Kim, S.-M., Min, J.-H., Seo, D.-Y., Goo, S.-H., Kim, J.-H., Kwon, O.-K., Lee, K., Ahn, K.-S., Oh, S.-R., & Lee, J.-W. (2022). Methyl P-Coumarate Ameliorates the Inflammatory Response in Activated-Airway Epithelial Cells and Mice with Allergic Asthma. International Journal of Molecular Sciences, 23(23), 14909. https://doi.org/10.3390/ijms232314909





