Cloning and Characterization of Two Novel PR4 Genes from Picea asperata
Abstract
1. Introduction
2. Results
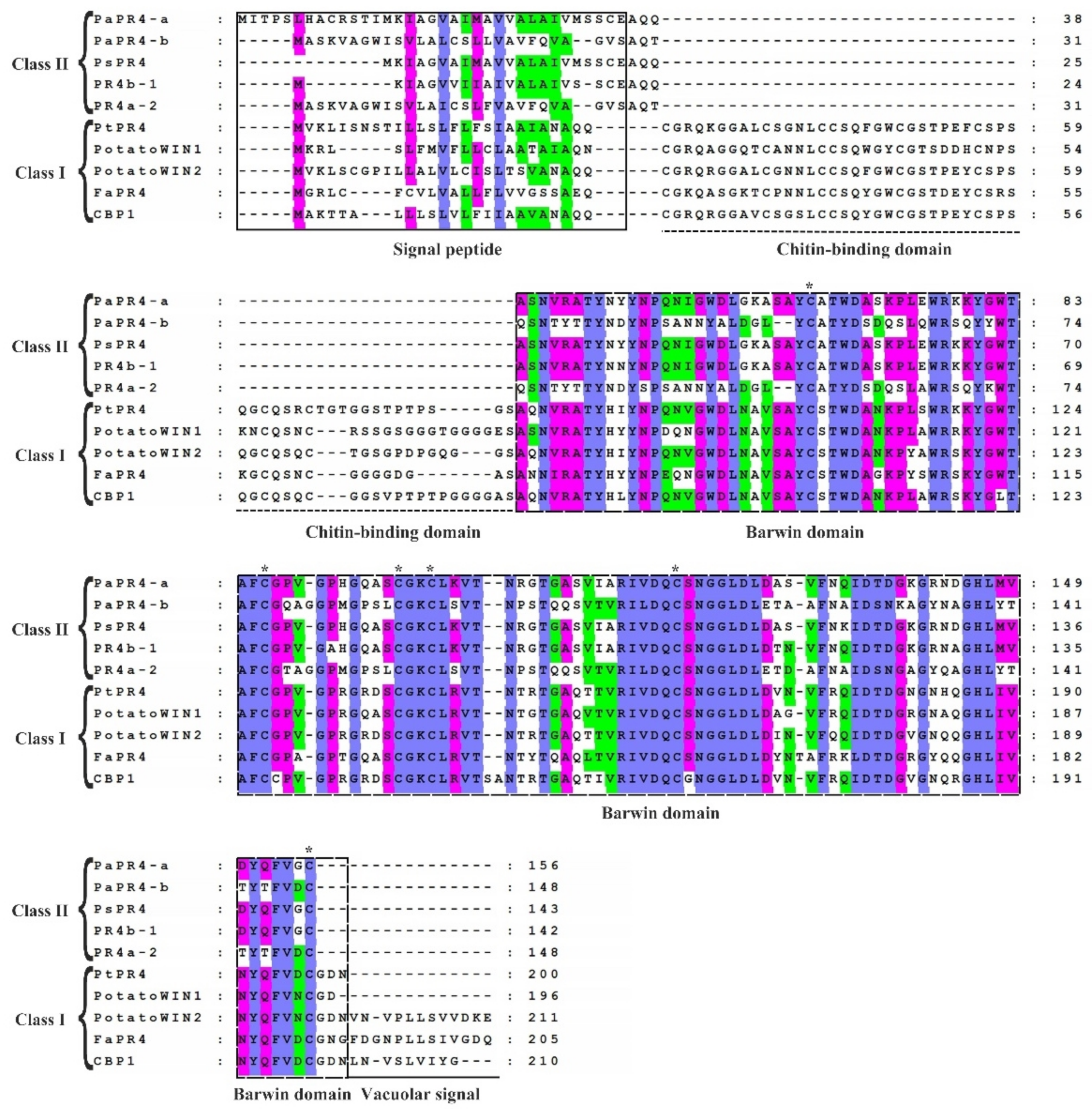
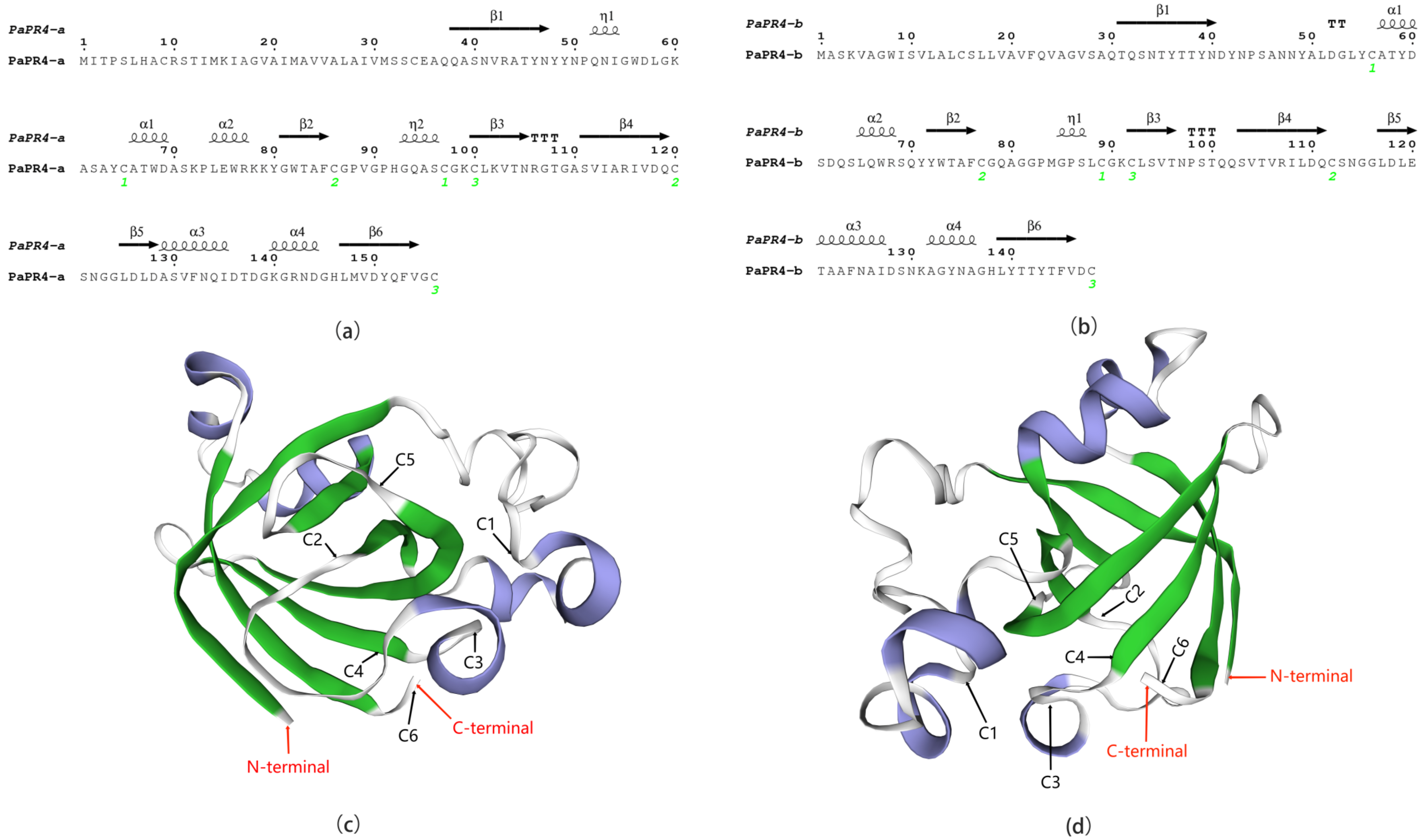
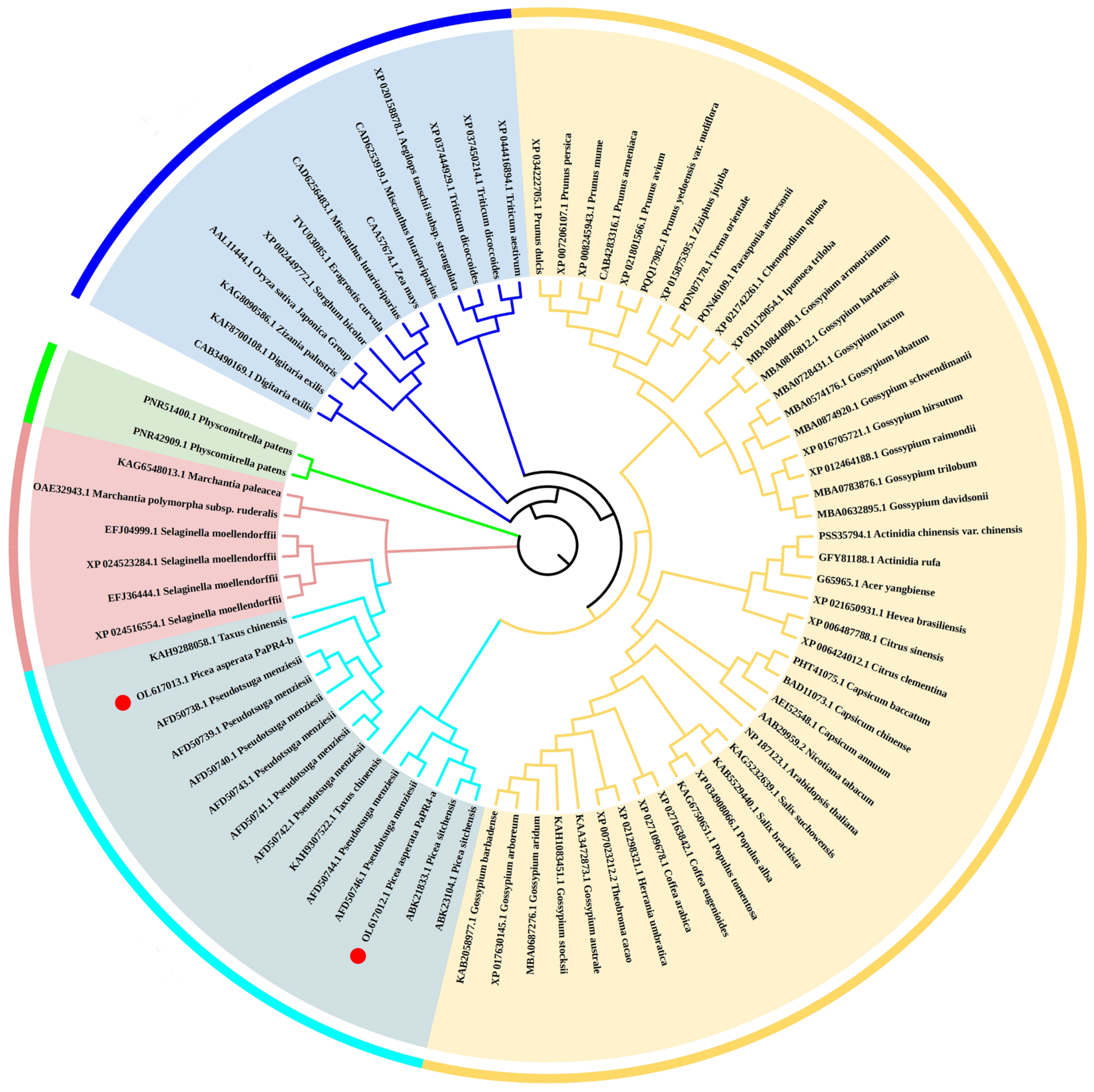
2.1. Cloning and Bioinformatics Analyses of PaPR4-a and PaPR4-b Genes
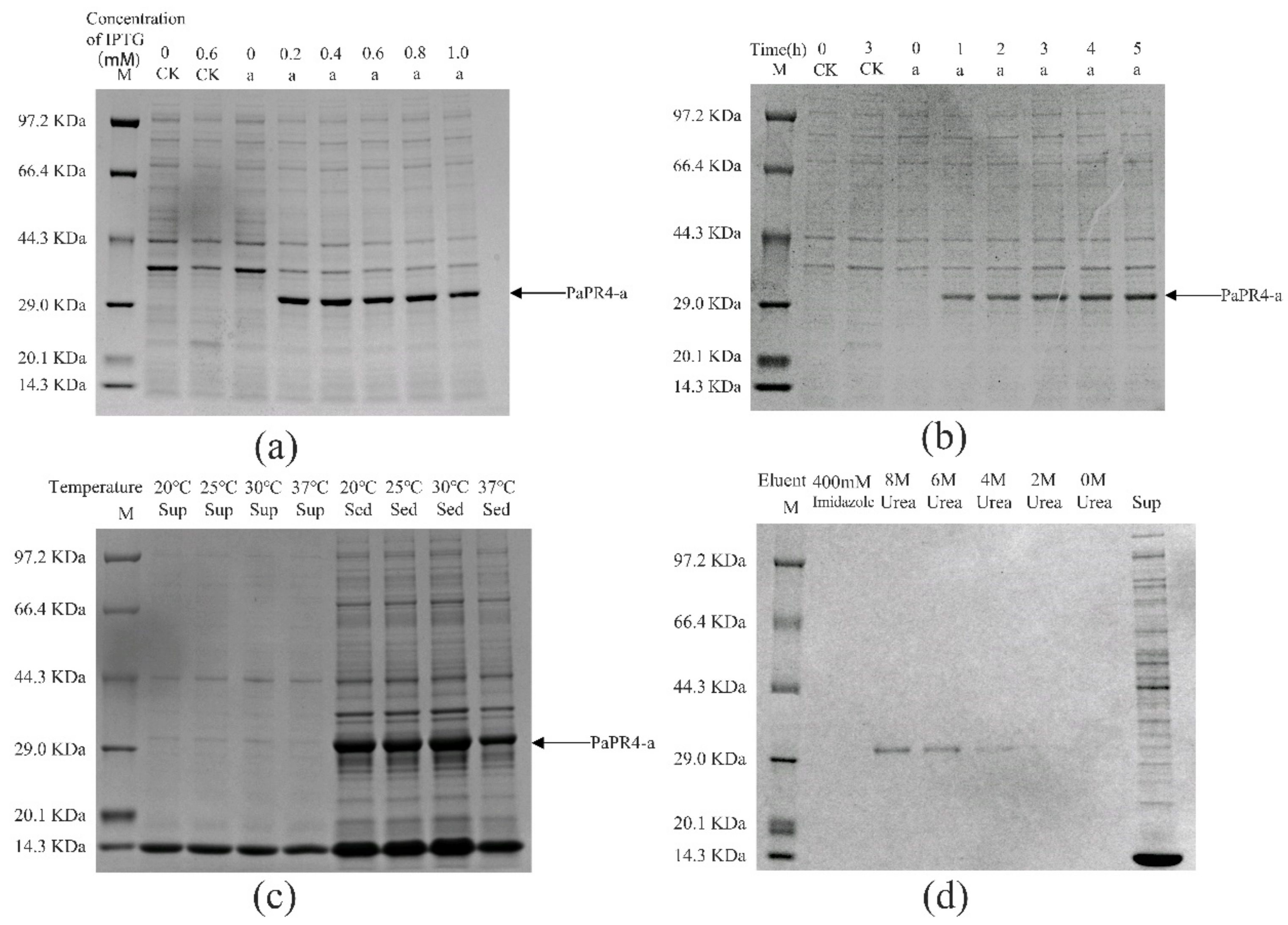
2.2. Prokaryotic Expression of PaPR4-a and PaPR4-b in E. coli
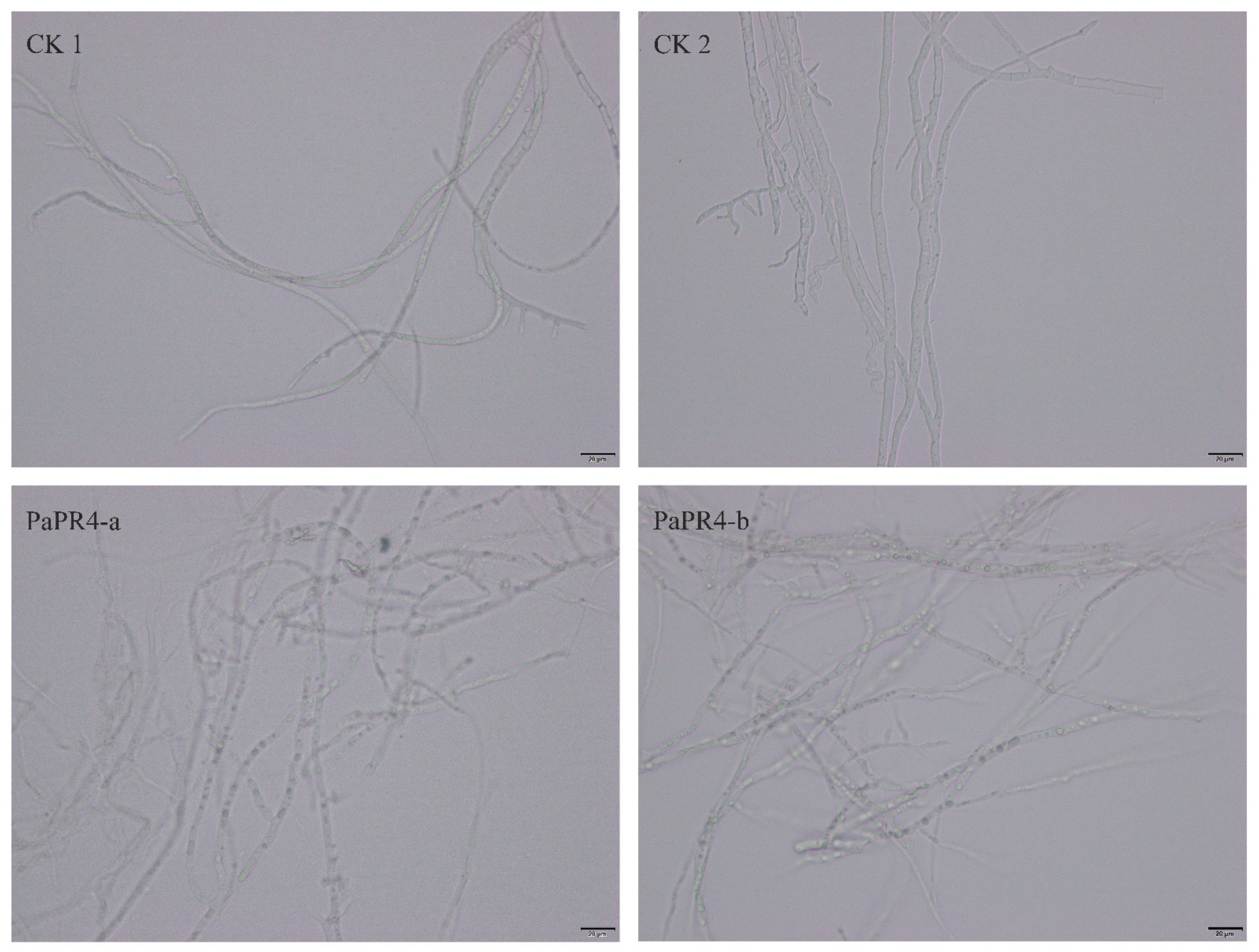
2.3. Antifungal Activity of Recombinant PaPR4-a and PaPR4-b Proteins
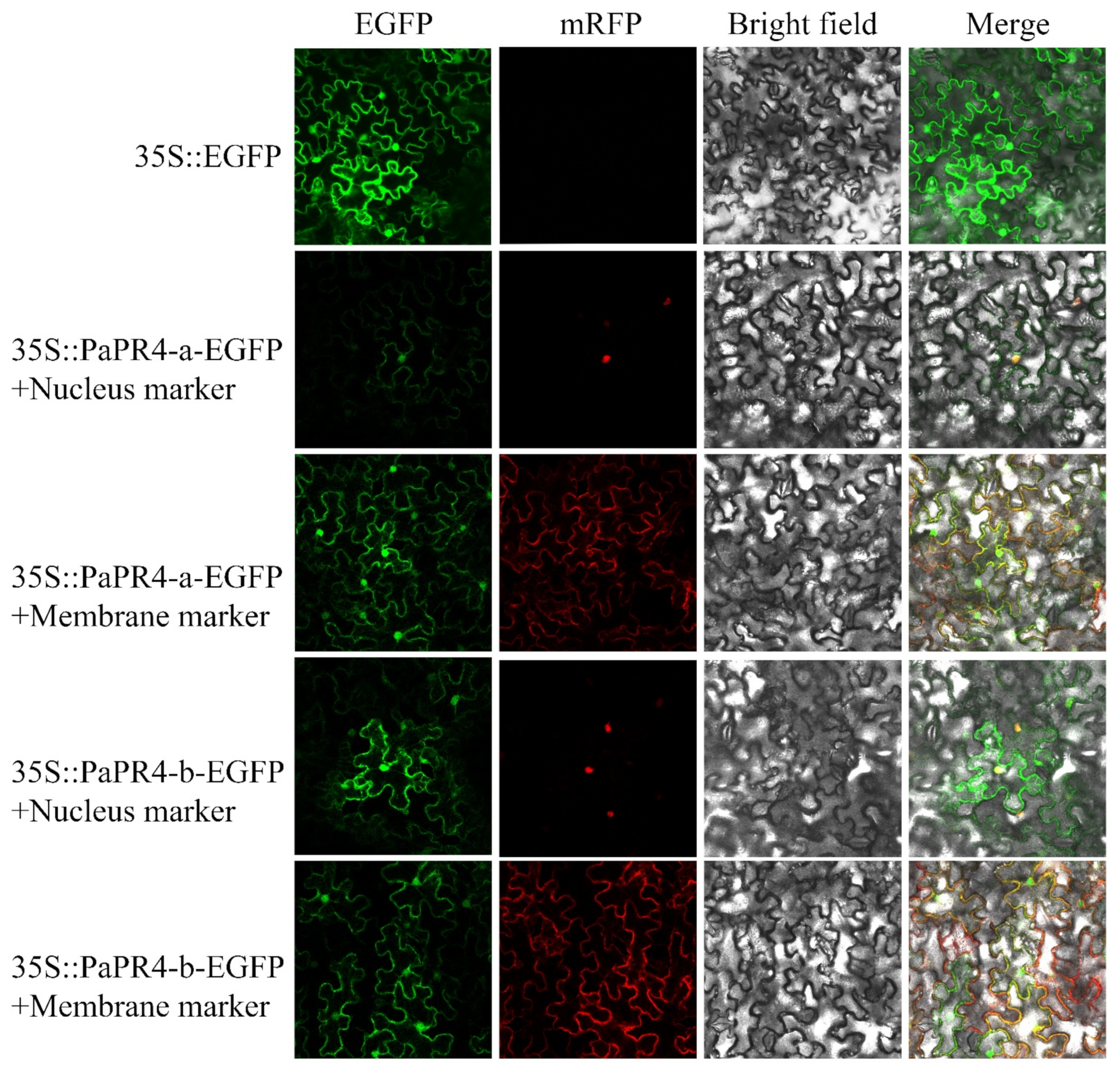
2.4. Subcellular Localization of PaPR4-a and PaPR4-b
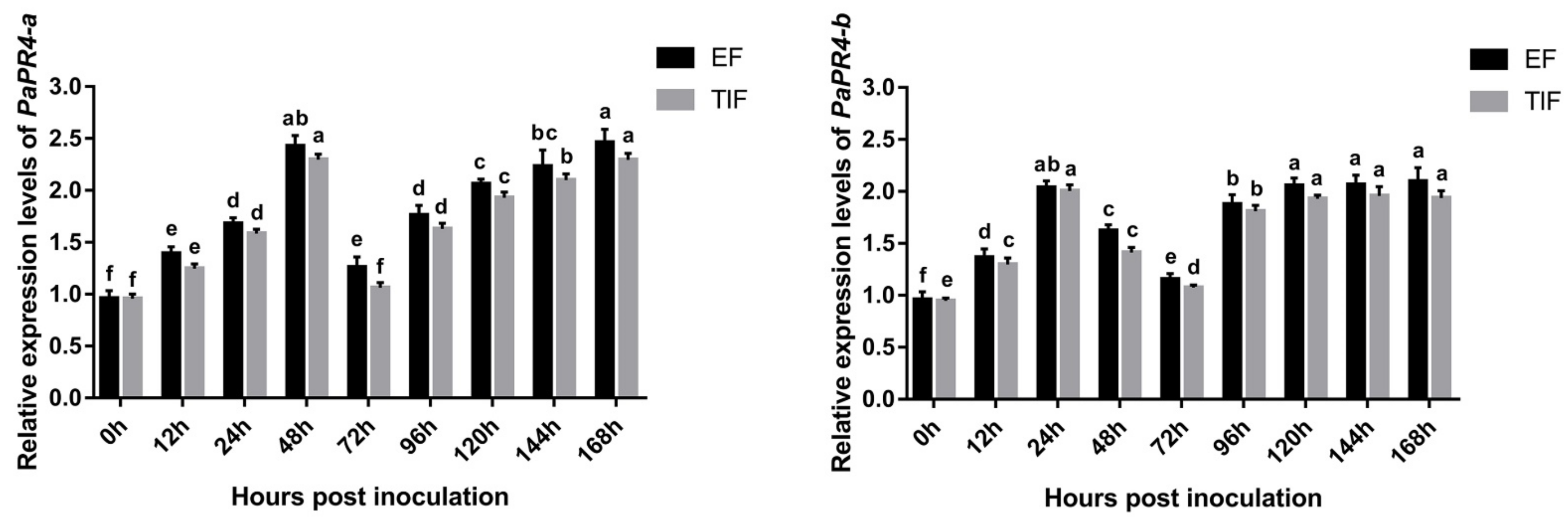
2.5. Quantitative Analysis of Gene Expression
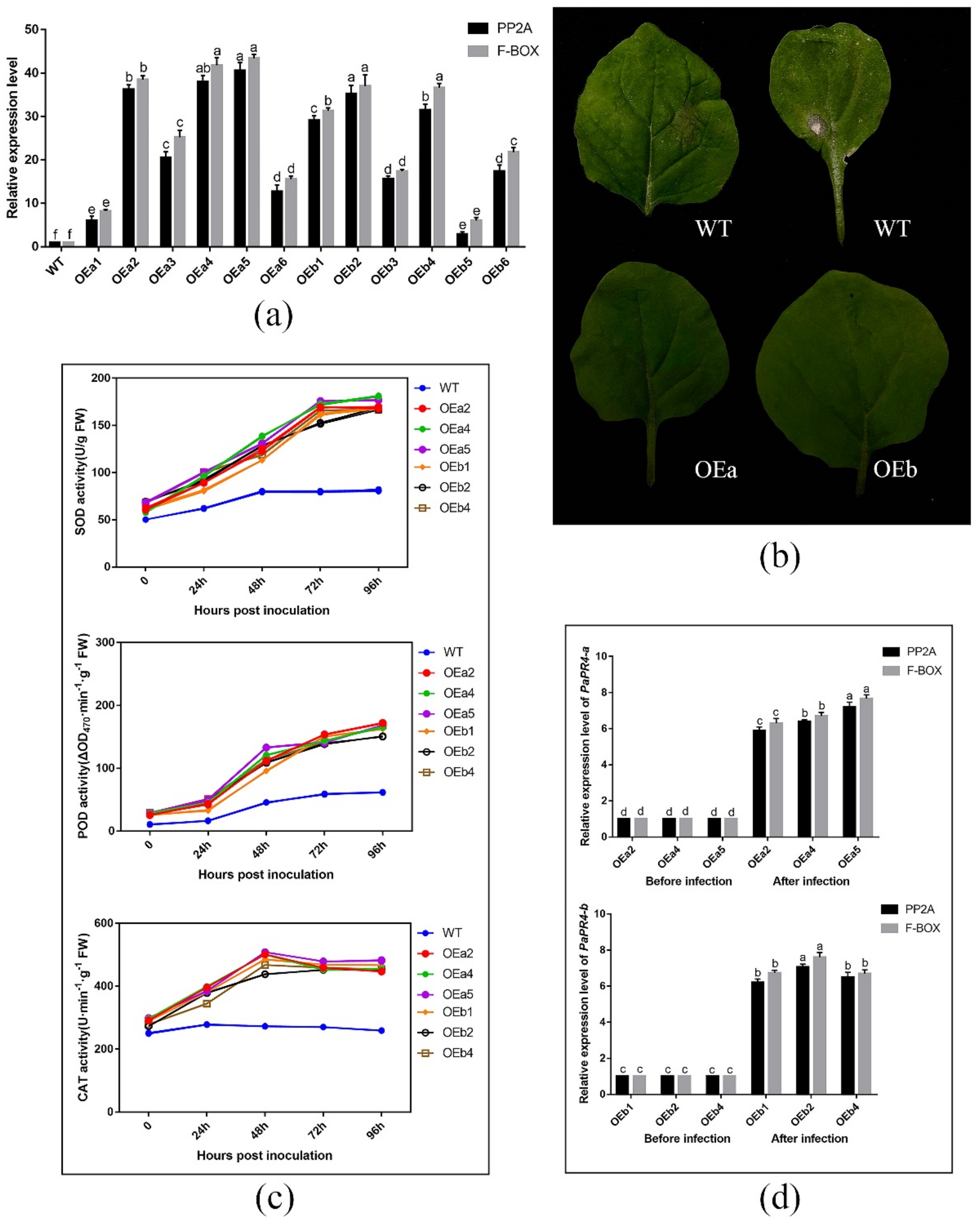
2.6. Functional Verification of PaPR4-a and PaPR4-b
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Plasmids
4.2. Total RNA Extraction and Reverse Transcription
4.3. Cloning of Full-Length PaPR4-a and PaPR4-b Genes
4.4. Bioinformatics Analyses of PaPR4-a and PaPR4-b
4.5. Expression of PaPR4-a and PaPR4-b in E. coli
4.5.1. Washing of Inclusion Bodies
4.5.2. Dissolution of Inclusion Bodies
4.5.3. Renaturation and Purification of PaPR4-a and PaPR4-b
4.6. Antifungal Activity of Recombinant PaPR4-a and PaPR4-b Proteins
4.7. Subcellular Localization of PaPR4-a and PaPR4-b
4.8. Pathogens and Inoculation Procedures
4.9. Functional Verification of PaPR4-a and PaPR4-b
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maimbo, M.M.; Ohnishi, K.K.; Hikichi, Y.Y.; Yoshioka, H.H.; Kiba, A.A. Induction of a Small Heat Shock Protein and Its Functional Roles in Nicotiana Plants in the Defense Response against Ralstonia solanacearum. Plant Physiol. 2007, 145, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Sajad Ali, B.A.G.A.; Javaid Akhter Bhat, A.T.S.T.; Prashant Yadav, S.R.A.G. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Felix, C.Z.A.G. Plants and animals: A different taste for microbes? Curr. Opin. Plant Biol. 2005, 8, 353–360. [Google Scholar] [CrossRef]
- Jones, J.L.D.J. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- Christensen, A.B.; Cho, B.H.; Næsby, M.; Gregersen, P.L.; Brandt, J.; Madriz-Ordeñana, K.; Collinge, D.B.; Thordal-Christensen, H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant Pathol. 2002, 3, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Nili Wang, B.X.L.X. Identification of a cluster of PR4-like genes involved in stress responses in rice. J. Plant Physiol. 2011, 168, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsen, S.; Poulsen, F.M. Three-dimensional structure in solution of barwin, a protein from barley seed. Biochemistry 1992, 31, 8783–8789. [Google Scholar] [CrossRef]
- Neuhaus, J.M.U.B.; Sticher, L.; Meins, F.J.; Boller, T. A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proc. Natl. Acad. Sci. USA 1991, 88, 10362–10366. [Google Scholar] [CrossRef]
- Ponstein, A.S.; Bres-Vloemans, S.A.; Sela-Buurlage, M.B.; van den Elzen, P.J.M.; Melchers, L.S.; Cornelissen, B.J.C. A Nove1 Pathogen- and Wound-lnducible Tobacco (Nicotiana tabacum) Protein with Antifungal Activity. Physiol. Plant 1994, 104, 109–118. [Google Scholar] [CrossRef]
- Laura Bertini, S.P.M.P.; Caruso, C.C.A.C. Modular structure of HEL protein from Arabidopsis reveals new potential functions for PR-4 proteins. Biol. Chem. 2012, 393, 1533–1546. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cloning, H.J.L.M. Characterization of Pathogenesis Related Protein 4 Gene from Panax ginseng. Russ. J. Plant Physl. 2013, 61, 664–671. [Google Scholar] [CrossRef]
- Bertini, L.; Cascone, A.; Tucci, M.; D’Amore, R.; Di Berardino, I.; Buonocore, V.; Caporale, C.; Caruso, C. Molecular and functional analysis of new members of the wheat PR4 gene family. Biol. Chem. 2006, 387, 1101–1111. [Google Scholar] [CrossRef]
- Caporale, C.; Di Berardino, I.; Leonardi, L.; Bertini, L.; Cascone, A.; Buonocore, V.; Caruso, C. Wheat pathogenesis-related proteins of class 4 have ribonuclease activity. FEBS Lett. 2004, 575, 71–76. [Google Scholar] [CrossRef]
- Li, X.; Xia, B.; Jiang, Y.; Wu, Q.; Wang, C.; He, L.; Peng, F.; Wang, R. A new pathogenesis-related protein, LrPR4, from Lycoris radiata, and its antifungal activity against Magnaporthe grisea. Mol. Biol. Rep. 2010, 37, 995–1001. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Yang, C.; Han, S.; Yang, S.; Liu, G.; Zeng, Q.; Liu, Y. Molecular Identification and Antifungal Activity of a Defensin (PaDef) from Spruce. J. Plant Growth Regul. 2021, 41, 494–506. [Google Scholar] [CrossRef]
- Li, M.; Jiao, Y.; Wang, Y.; Zhang, N.; Wang, B.; Liu, R.; Yin, X.; Xu, Y.; Liu, G. CRISPR/Cas9-mediated VvPR4b editing decreases downy mildew resistance in grapevine (Vitis vinifera L.). Hortic. Res. 2020, 7, 149. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.H.; Zhang, Y.B.; Wang, Y.J.; Yang, G.; Yang, L.; Wang, R.Y.; Xie, Z.K. Isolation and Characterization of Two Distinct Class II PR4 Genes from the Oriental Lily Hybrid Sorbonne. Russ. J. Plant Physiol. 2017, 64, 707–717. [Google Scholar] [CrossRef]
- Esmail, S.M.; Aboulila, A.A.; El-Moneim, D.A. Variation in several pathogenesis—Related (PR) protein genes in wheat (Triticum aestivum) involved in defense against Puccinia striiformis f.sp. tritici. Physiol. Mol. Plant Pathol. 2020, 112, 101545. [Google Scholar] [CrossRef]
- Islam, M.A.; Sturrock, R.N.; Ekramoddoullah, A.K.M. Molecular cloning and gene transcription analyses of barwin-type PR-4 genes from Phellinus sulphurascens-infected Douglas-fir seedlings. For. Pathol. 2011, 42, 279–288. [Google Scholar] [CrossRef]
- Müller, M.M.; Valjakka, R.; Hantula, J. Genetic diversity of Lophodermium piceae in South Finland. For. Pathol. 2007, 37, 329–337. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Yang, S.; Zeng, Q.; He, Z.; Liu, Y. Cloning, Characterization and Expression of the Phenylalanine Ammonia-Lyase Gene (PaPAL) from Spruce Picea asperata. Forests 2019, 10, 613. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Jayaraj, J.; Muthukrishnan, S.; Liang, G.H.; Velazhahan, R. Jasmonic acid and salicylic acid induce accumulation of β-1,3-glucanase and thaumatin-like proteins in wheat and enhance resistance against Stagonospora nodorum. Biol. Plant. 2004, 48, 425–430. [Google Scholar] [CrossRef]
- Sabater-Jara, A.B.; Almagro, L.; Belchi-Navarro, S.; Barcelo, A.R.; Pedreno, M.A. Methyl jasmonate induces extracellular pathogenesis-related proteins in cell cultures of Capsicum chinense. Plant Signal. Behav. 2011, 6, 440–442. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Huang, Z.; Huang, L. Antioxidative response of metal-accumulator and non-accumulator plants under cadmium stress. Plant Soil 2008, 310, 137–149. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Mehdy, M.C. Active Oxygen Species in Plant Defense against Pathogens. Plant Physiol. 1994, 105, 467–472. [Google Scholar] [CrossRef]
- Kotchoni, S.O.; Gachomo, E.W. The reactive oxygen species network pathways:an essential prerequisite for perception of pathogen attack and the acquired disease resistance in plants. J. Biosci. 2006, 31, 389–404. [Google Scholar] [CrossRef]
- Munir, S.; Qureshi, M.; Shahzad, A. Antioxidant Response of Brassica Plants in Protection Against Alternaria Brassicicola. Pak. J. Bot. 2021, 53, 749–754. [Google Scholar] [CrossRef]
- Liu, G.; Su, X.; Guan, L.; Hu, F. Comparison of Defensive Enzyme Activities in the Leaves of Seven Oriental Lily Hybrids after Inoculation with Botrytis elliptica. J. Am. Soc. Hortic. Sci. 2019, 144, 55–62. [Google Scholar] [CrossRef]
- Rahman, M.U.; Hanif, M.; Shah, K.; Ahmad, B.; Wang, X. In vitro evaluation of berries of various Vitis genotypes for disease resistance to Botrytis cinerea. Vitis 2019, 58, 123–130. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.L.; Zhu, Y.; Yang, X.M.; Zhang, K.N.; Xiao, Z.Y.; Wang, H.; Zhao, J.H.; Zhang, L.L.; Li, G.B.; et al. Osa-miR398b boosts H2 O2 production and rice blast disease-resistance via multiple superoxide dismutases. N. Phytol. 2019, 222, 1507–1522. [Google Scholar] [CrossRef]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Nasiri, J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L.). Eur. J. Plant Pathol. 2017, 147, 279–294. [Google Scholar] [CrossRef]
- Kumar, N.; Ebel, R.C.; Roberts, P.D. Superoxide dismutase activity in kumquat leaves infected with Xanthomonas axonopodis pv. citri. J. Hortic. Sci. Biotechnol. 2011, 86, 62–68. [Google Scholar] [CrossRef]
- Kunos, V.; Cséplő, M.; Seress, D.; Eser, A.; Kende, Z.; Uhrin, A.; Bányai, J.; Bakonyi, J.; Pál, M.; Mészáros, K. The Stimulation of Superoxide Dismutase Enzyme Activity and Its Relation with the Pyrenophora teres f. teres Infection in Different Barley Genotypes. Sustainability 2022, 14, 2597. [Google Scholar] [CrossRef]
- Hamidi, S.R.; Safdari, Y.; Arabi, M.S. Test bacterial inclusion body for activity prior to start denaturing and refolding processes to obtain active eukaryotic proteins. Protein Expr. Purif. 2019, 154, 147–151. [Google Scholar] [CrossRef]
- Goulding, C.W.; Perry, L.J. Protein production in Escherichia coli for structural studies by X-ray crystallography. J. Struct. Biol. 2003, 142, 133–143. [Google Scholar] [CrossRef]
- Oneda, H.; Inouye, K. Refolding and Recovery of Recombinant Human Matrix Metalloproteinase 7 (Matrilysin) from Inclusion Bodies Expressed by Escherichia coli. J. Biochem. 1999, 126, 905–911. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Zhu, H.; Zhu, T. Cloning and Expression of the Chitinase Encoded by ChiKJ406136 from Streptomyces Sampsonii (Millard & Burr) Waksman KJ40 and Its Antifungal Effect. Forests 2018, 9, 699. [Google Scholar] [CrossRef]
- Bassard, J.; Richert, L.; Geerinck, J.; Renault, H.; Duval, F.; Ullmann, P.; Schmitt, M.; Meyer, E.; Mutterer, J.; Boerjan, W.; et al. Protein—Protein and Protein—Membrane Associations in the Lignin Pathway. Plant Cell 2012, 24, 4465–4482. [Google Scholar] [CrossRef] [PubMed]
- Dominic, E.A.; Deb, K.C. Enhancement of soluble protein expression through the use of fusion tags. Curr. Opin. Biotechnol. 2006, 17, 353–358. [Google Scholar] [CrossRef]
- Naomi, E.C. Turning protein crystallisation from an art into a science. Curr. Opin. Struct. Biol. 2004, 14, 577–583. [Google Scholar] [CrossRef]
- Hu, X.; Reddy, A.S.N. Cloning and expression of a PR5-like protein from Arabidopsis: Inhibition of fungal growth by bacterially expressed protein. Plant Mol. Biol. 1997, 34, 949–959. [Google Scholar] [CrossRef]
- Li, D.; Jian, G.; Zhang, Y.; Ai, T. Bacterial expression of a Trichosanthes kirilowii defensin (TDEF1) and its antifungal activity on Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2007, 71, 146–151. [Google Scholar] [CrossRef]
- Hammarström, M.; Hellgren, N.; van den Berg, S.; Berglund, H.; Härd, T. Rapid screening for improved solubility of small human proteins produced as fusion proteins in Escherichia coli. Protein Sci. 2002, 11, 313–321. [Google Scholar] [CrossRef]
- Kataeva, I.; Chang, J.; Xu, H.; Luan, C.; Zhou, J.; Uversky, V.N.; Lin, D.; Horanyi, P.; Liu, Z.J.; Ljungdahl, L.G.; et al. Improving solubility of Shewanella oneidensis MR-1 and Clostridium thermocellum JW-20 proteins expressed into Esherichia coli. J. Proteome Res. 2005, 4, 1942–1951. [Google Scholar] [CrossRef]
- Ohno, R.; Takumi, S.; Nakamura, C. Expression of a cold-responsive Lt-Cor gene and development of freezing tolerance during cold acclimation in wheat (Triticum aestivum L.). J. Exp. Bot. 2001, 52, 2367–2374. [Google Scholar] [CrossRef]
- Ohno, R.; Takumi, S. Extracellular trafficking of a wheat cold-responsive protein, WLT10. J. Plant Physiol. 2015, 174, 71–74. [Google Scholar] [CrossRef]
- Zaynab, M.; Peng, J.; Sharif, Y.; Al-Yahyai, R.; Jamil, A.; Hussain, A.; Khan, K.A.; Alotaibi, S.S.; Li, S. Expression profiling of pathogenesis-related Protein-1 (PR-1) genes from Solanum tuberosum reveals its critical role in phytophthora infestans infection. Microb. Pathog. 2021, 161, 105290. [Google Scholar] [CrossRef]
- Hou, M.; Xu, W.; Bai, H.; Liu, Y.; Li, L.; Liu, L.; Liu, B.; Liu, G. Characteristic expression of rice pathogenesis-related proteins in rice leaves during interactions with Xanthomonas oryzae pv. oryzae. Plant Cell Rep. 2011, 31, 895–904. [Google Scholar] [CrossRef]
- Shi, X.; Tian, Z.; Liu, J.; van der Vossen, E.A.; Xie, C. A potato pathogenesis-related protein gene, StPRp27, contributes to race-nonspecific resistance against Phytophthora infestans. Mol. Biol. Rep. 2012, 39, 1909–1916. [Google Scholar] [CrossRef]
- Lu, H.-C.; Lin, J.H.; Chua, A.C.N.; Chung, T.; Tsai, I.-C.; Tzen, J.T.C.; Chou, W. Cloning and expression of pathogenesis-related protein 4 from jelly fig (Ficus awkeotsang Makino) achenes associated with ribonuclease, chitinase and anti-fungal activities. Plant Physiol. Biochem. 2012, 56, 1–13. [Google Scholar] [CrossRef]
- Pereira, M.S.; de Andrade, S.E.; Matos, L.E.; Oliveira, D.S.A.; Silva, A.B.; Santos, L.L.L.; Peres, G.K.; Da, S.G.A.; Pirovani, C.P.; Micheli, F. The pathogenesis-related protein PR-4b from Theobroma cacao presents RNase activity, Ca2+ and Mg2+ dependent-DNase activity and antifungal action on Moniliophthora perniciosa. BMC Plant Biol. 2014, 14, 161. [Google Scholar] [CrossRef][Green Version]
- Guevara-Morato, M.A.; de Lacoba, M.G.; Garcia-Luque, I.; Serra, M.T. Characterization of a pathogenesis-related protein 4 (PR-4) induced in Capsicum chinense L3 plants with dual RNase and DNase activities. J. Exp. Bot. 2010, 61, 3259–3271. [Google Scholar] [CrossRef]
- Mittler, R.; Lam, E. Characterization of nuclease activities and DNA fragmentation induced upon hypersensitive response cell death and mechanical stress. Plant Mol. Biol. 1997, 34, 209–221. [Google Scholar] [CrossRef]
- Caruso, C.; Caporale, C.; Chilosi, G.; Vacca, F.; Bertini, L.; Magro, P.; Poerio, E.; Buonocore, V. Structural and antifungal properties of a pathogenesis-related protein from wheat kernel. J. Protein Chem. 1996, 15, 35–44. [Google Scholar] [CrossRef]
- Zhu, T.; Song, F.; Zheng, Z. Molecular Characterization of the Rice Pathogenesis-related Protein, OsPR-4b, and Its Antifungal Activity Against Rhizoctonia solani. J. Phytopathol. 2006, 154, 378–384. [Google Scholar] [CrossRef]
- Nandi, A.K. Application of Antimicrobial Proteins and Peptides in Developing Disease-Resistant Plants. In Plant Pathogen Resistance Biotechnology; Collinge, D.B., Ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2016; pp. 51–70. [Google Scholar]
- Anam Moosa, A.F.; Shahbaz, T.S.; Sajid, A.K. Transgenic expression of antifungal pathogenesis- related proteins against phytopathogenic fungi—15 years of success. Isr. J. Plant Sci. 2017, 65, 38–54. [Google Scholar] [CrossRef]
- Boccardo, N.A.; Segretin, M.E.; Hernandez, I.; Mirkin, F.G.; Chacón, O.; Lopez, Y.; Borrás-Hidalgo, O.; Bravo-Almonacid, F.F. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci. Rep. 2019, 9, 2791. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.; Dechert, C.; Király, L.; Fodor, J.; Michel, K.; Kogel, K.; Hückelhoven, R. Functional assessment of the pathogenesis-related protein PR-1b in barley. Plant Sci. 2003, 165, 1275–1280. [Google Scholar] [CrossRef]
- Fagoaga, C.; Rodrigo, I.; Conejero, V.; Hinarejos, C.; Tuset, J.J.; Arnau, J.; Pina, J.A.; Navarro, L.; Pena, L. Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Mol. Breed. 2001, 7, 175–185. [Google Scholar] [CrossRef]
- Alexander, D.; Goodman, R.M.; Gut-Rella, M.; Glascock, C.; Weymann, K.; Friedrich, L.; Maddoxi, D.; Ahl-Goy, P.; Luntz, T.; Ward, E.; et al. Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein la. Proc. Natl. Acad. Sci. USA 1993, 90, 7327–7331. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Velazhahan, R.; Oliva, N.; Ona, I.; Mew, T.; Khush, G.S.; Muthukrishnan, S.; Datta, S.K. Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor. Appl. Genet. 1999, 98, 1138–1145. [Google Scholar] [CrossRef]
- Cui, J.; Luan, Y.; Jiang, N.; Bao, H.; Meng, J. Comparative transcriptome analysis between resistant and susceptible tomato allows the identification of lncRNA16397 conferring resistance toPhytophthora infestans by co-expressing glutaredoxin. Plant J. 2017, 89, 577–589. [Google Scholar] [CrossRef]
- Cui, J.; Xu, P.; Meng, J.; Li, J.; Jiang, N.; Luan, Y. Transcriptome signatures of tomato leaf induced by Phytophthora infestans and functional identification of transcription factor SpWRKY3. Theor. Appl. Genet. 2018, 131, 787–800. [Google Scholar] [CrossRef]
- Wu, J.; Yang, R.; Yang, Z.; Yao, S.; Zhao, S.; Wang, Y.; Li, P.; Song, X.; Jin, L.; Zhou, T.; et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat. Plants 2017, 3, 16203. [Google Scholar] [CrossRef]
- Kroj, T.; Rudd, J.J.; Nürnberger, T.; Gäbler, Y.; Lee, J.; Scheel, D. Mitogen-activated Protein Kinases Play an Essential Role in Oxidative Burst-independent Expression of Pathogenesis-related Genes in Parsley. J. Biol. Chem. 2003, 278, 2256–2264. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, Y.J.; Lee, S.C.; Hong, J.K.; Hwang, B.K. Hydrogen Peroxide Generation by the Pepper Extracellular Peroxidase CaPO2 Activates Local and Systemic Cell Death and Defense Response to Bacterial Pathogens. Plant Physiol. 2007, 145, 890–904. [Google Scholar] [CrossRef]
- Hong, Y.; Cui, J.; Liu, Z.; Luan, Y. SpWRKY6 acts as a positive regulator during tomato resistance to Phytophthora infestans infection. Biochem. Bioph. Res. Co. 2018, 506, 787–792. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, D.; Liu, Y.; Luo, C.; Zhou, Y.; Zhang, L. Overexpression of a NF-YB3 transcription factor from Picea wilsonii confers tolerance to salinity and drought stress in transformed Arabidopsis thaliana. Plant Physiol. Biochem. 2015, 94, 153–164. [Google Scholar] [CrossRef]
- Kolosova, N.; Breuil, C.; Bohlmann, J. Cloning and characterization of chitinases from interior spruce and lodgepole pine. Phytochemistry 2014, 101, 32–39. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Asiegbu, F.O.; Yang, C.; Han, S.; Yang, S.; Zeng, Q.; Liu, Y. Molecular Identification and Antifungal Properties of Four Thaumatin-like Proteins in Spruce (Picea likiangensis). Forests 2021, 12, 1268. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Oh, S.; Park, J.M.; Joung, Y.H.; Lee, S.; Chung, E.; Kim, S.; Yu, S.H.; Choi, D. A plant EPF-type zinc-finger protein, CaPIF1, involved in defence against pathogens. Mol. Plant Pathol. 2005, 6, 269–285. [Google Scholar] [CrossRef]
- Liu, D.; Shi, L.; Han, C.; Yu, J.; Li, D.; Zhang, Y. Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE 2012, 7, e46451. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Liu, L.; Li, C.; Yang, C.; Li, S.; Han, S.; Lin, T.; Liu, Y. Cloning and Characterization of Two Novel PR4 Genes from Picea asperata. Int. J. Mol. Sci. 2022, 23, 14906. https://doi.org/10.3390/ijms232314906
Zhao W, Liu L, Li C, Yang C, Li S, Han S, Lin T, Liu Y. Cloning and Characterization of Two Novel PR4 Genes from Picea asperata. International Journal of Molecular Sciences. 2022; 23(23):14906. https://doi.org/10.3390/ijms232314906
Chicago/Turabian StyleZhao, Weidong, Lijuan Liu, Chengsong Li, Chunlin Yang, Shujiang Li, Shan Han, Tiantian Lin, and Yinggao Liu. 2022. "Cloning and Characterization of Two Novel PR4 Genes from Picea asperata" International Journal of Molecular Sciences 23, no. 23: 14906. https://doi.org/10.3390/ijms232314906
APA StyleZhao, W., Liu, L., Li, C., Yang, C., Li, S., Han, S., Lin, T., & Liu, Y. (2022). Cloning and Characterization of Two Novel PR4 Genes from Picea asperata. International Journal of Molecular Sciences, 23(23), 14906. https://doi.org/10.3390/ijms232314906







