The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans
Abstract
1. Introduction
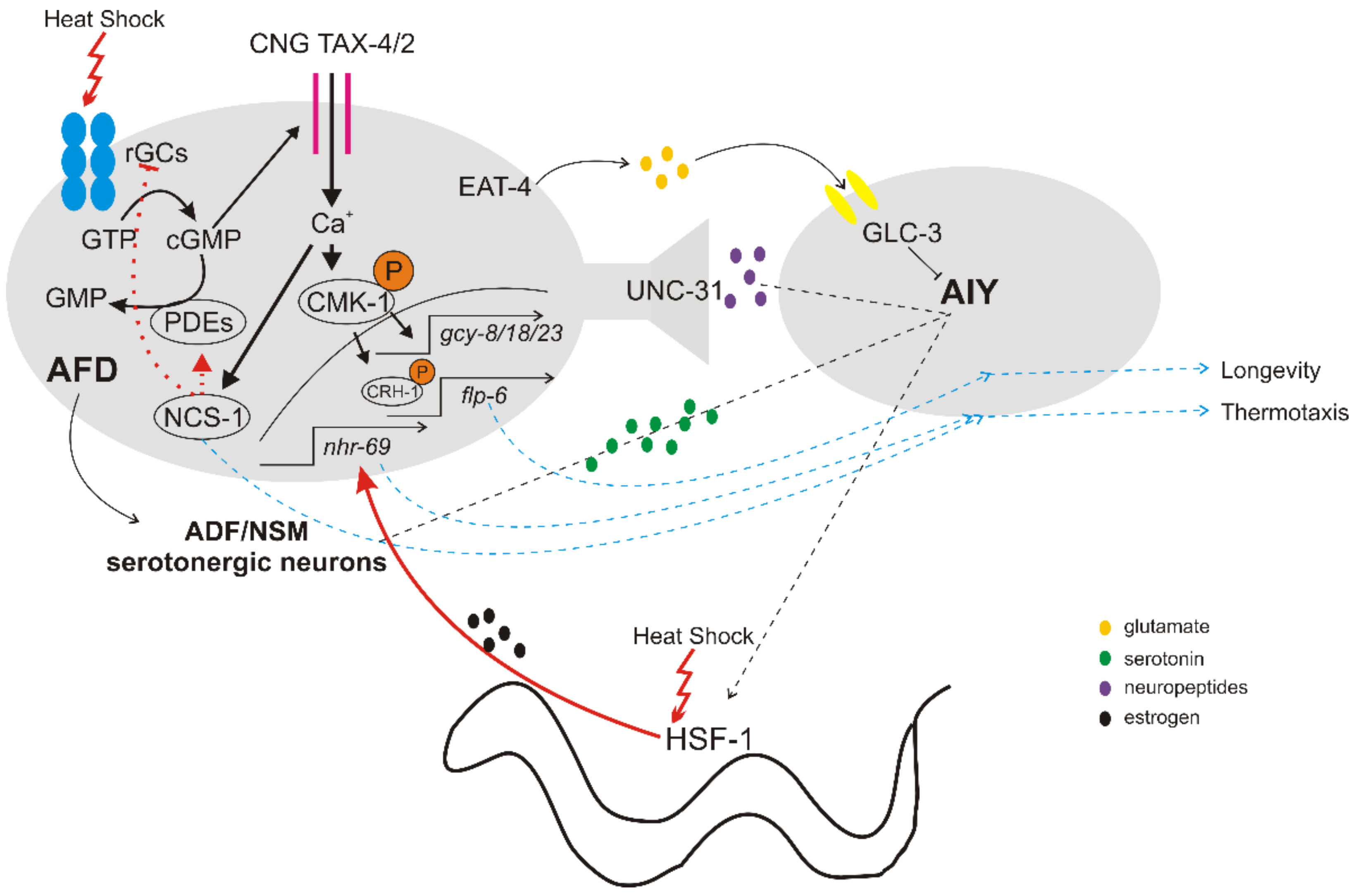
2. The Thermosensory Circuit
2.1. Detection of Temperature Changes
2.2. Downstream Signaling of Thermosensory Neurons
2.3. Cell Non-Autonomous Regulation of HSR
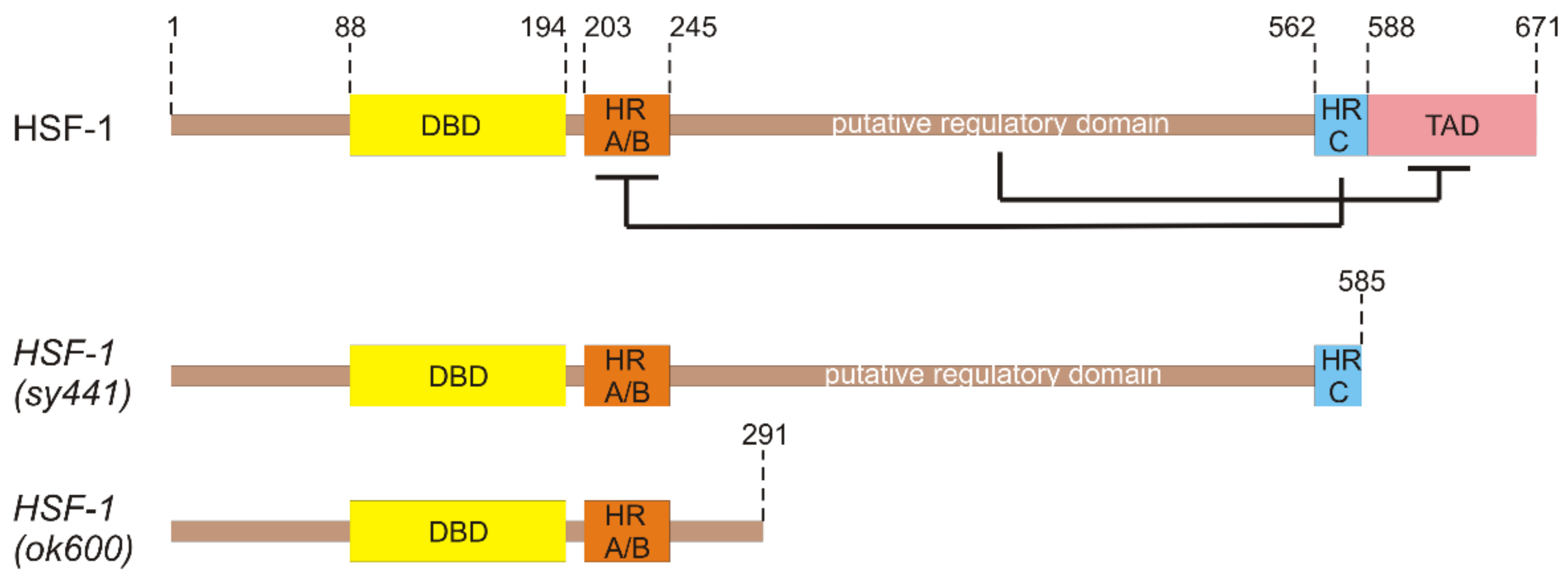
3. Functional Domains of HSF-1
4. HSF-1 Targets upon Heat Shock
4.1. Heat Shock Proteins, the Main Executive Body of HSR
4.2. Exoskeleton and Cytoskeleton Integrity Genes
5. The Post-Translational Fate of HSF-1
5.1. Phosphorylation
5.2. Sumoylation
5.3. Acetylation
6. Other Factors and Pathways That Aid Coping with Thermal Stress
6.1. The Insulin/IGF-1–Like Signaling (IIS)
6.2. HIF-1
6.3. Autophagy
6.4. Non-Coding RNAs
6.5. m5C Methylation
6.6. Mitochondrial Perturbation
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.E.; Artan, M.; Seo, K.; Lee, S.J. Regulation of lifespan by chemosensory and thermosensory systems: Findings in invertebrates and their implications in mammalian aging. Front. Genet. 2012, 3, 218. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, J.; Kim, Y.; Lee, S.V. Regulatory systems that mediate the effects of temperature on the lifespan of Caenorhabditis elegans. J. Neurogenet. 2020, 34, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Cornelius, T.; Morimoto, R.I. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science 2008, 320, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Vakkayil, K.L.; Hoppe, T. Temperature-Dependent Regulation of Proteostasis and Longevity. Front. Aging 2022, 3, 853588. [Google Scholar] [CrossRef]
- Ramot, D.; MacInnis, B.L.; Goodman, M.B. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat. Neurosci. 2008, 11, 908–915. [Google Scholar] [CrossRef]
- Brunquell, J.; Morris, S.; Lu, Y.; Cheng, F.; Westerheide, S.D. The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genom. 2016, 17, 559. [Google Scholar] [CrossRef]
- Higuchi-Sanabria, R.; Frankino, P.A.; Paul, J.W., 3rd; Tronnes, S.U.; Dillin, A. A Futile Battle? Protein Quality Control and the Stress of Aging. Dev. Cell 2018, 44, 139–163. [Google Scholar]
- Occhigrossi, L.; D’Eletto, M.; Barlev, N.; Rossin, F. The Multifaceted Role of HSF1 in Pathophysiology: Focus on Its Interplay with TG2. Int. J. Mol. Sci. 2021, 22, 6366. [Google Scholar] [CrossRef]
- Félix, M.A.; Duveau, F. Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol. 2012, 10, 59. [Google Scholar] [CrossRef]
- Harvey, S.C.; Viney, M.E. Thermal variation reveals natural variation between isolates of Caenorhabditis elegans. J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Clark, D.A.; Biron, D.; Mahadevan, L.; Samuel, A.D. Sensorimotor control during isothermal tracking in Caenorhabditis elegans. J. Exp. Biol. 2006, 209 Pt 23, 4652–4662. [Google Scholar] [CrossRef] [PubMed]
- Aoki, I.; Mori, I. Molecular biology of thermosensory transduction in C. elegans. Curr. Opin. Neurobiol. 2015, 34, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Glauser, D.A. Temperature sensing and context-dependent thermal behavior in nematodes. Curr. Opin. Neurobiol. 2022, 73, 102525. [Google Scholar] [CrossRef]
- Hedgecock, E.M.; Russell, R.L. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1975, 72, 4061–4065. [Google Scholar] [CrossRef]
- Mori, I.; Ohshima, Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 1995, 376, 344–348. [Google Scholar] [CrossRef]
- Goodman, M.B. Thermotaxis navigation behavior. In WormBook: The Online Review of C. elegans Biology; Wormbook: Pasadena, CA, USA, 2014; pp. 1–10. [Google Scholar]
- Ghosh, R.; Mohammadi, A.; Kruglyak, L.; Ryu, W.S. Multiparameter behavioral profiling reveals distinct thermal response regimes in Caenorhabditis elegans. BMC Biol. 2012, 10, 85. [Google Scholar] [CrossRef]
- Liu, S.; Schulze, E.; Baumeister, R. Temperature- and touch-sensitive neurons couple CNG and TRPV channel activities to control heat avoidance in Caenorhabditis elegans. PLoS ONE 2012, 7, e32360. [Google Scholar] [CrossRef]
- Goodman, M.B.; Sengupta, P. The extraordinary AFD thermosensor of C. elegans. Pflug. Arch. 2018, 470, 839–849. [Google Scholar] [CrossRef]
- Goodman, M.B.; Sengupta, P. How Caenorhabditis elegans Senses Mechanical Stress, Temperature, and Other Physical Stimuli. Genetics 2019, 212, 25–51. [Google Scholar] [CrossRef]
- Ward, S.; Thomson, N.; White, J.G.; Brenner, S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 1975, 160, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Beverly, M.; Anbil, S.; Sengupta, P. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J. Neurosci. 2011, 31, 11718–11727. [Google Scholar] [CrossRef] [PubMed]
- Biron, D.; Wasserman, S.; Thomas, J.H.; Samuel, A.D.; Sengupta, P. An olfactory neuron responds stochastically to temperature and modulates Caenorhabditis elegans thermotactic behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 11002–11007. [Google Scholar] [CrossRef] [PubMed]
- Kuhara, A.; Okumura, M.; Kimata, T.; Tanizawa, Y.; Takano, R.; Kimura, K.D.; Inada, H.; Matsumoto, K.; Mori, I. Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 2008, 320, 803–807. [Google Scholar] [CrossRef]
- Yu, S.; Avery, L.; Baude, E.; Garbers, D.L. Guanylyl cyclase expression in specific sensory neurons: A new family of chemosensory receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 3384–3387. [Google Scholar] [CrossRef]
- Ortiz, C.O.; Etchberger, J.F.; Posy, S.L.; Frøkjaer-Jensen, C.; Lockery, S.; Honig, B.; Hobert, O. Searching for neuronal left/right asymmetry: Genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics 2006, 173, 131–149. [Google Scholar] [CrossRef]
- Inada, H.; Ito, H.; Satterlee, J.; Sengupta, P.; Matsumoto, K.; Mori, I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 2006, 172, 2239–2252. [Google Scholar] [CrossRef]
- Wasserman, S.M.; Beverly, M.; Bell, H.W.; Sengupta, P. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr. Biol. 2011, 21, 353–362. [Google Scholar] [CrossRef]
- Komatsu, H.; Mori, I.; Rhee, J.S.; Akaike, N.; Ohshima, Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 1996, 17, 707–718. [Google Scholar] [CrossRef]
- Coburn, C.M.; Bargmann, C.I. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 1996, 17, 695–706. [Google Scholar] [CrossRef]
- Clark, D.A.; Biron, D.; Sengupta, P.; Samuel, A.D. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci. 2006, 26, 7444–7451. [Google Scholar] [CrossRef] [PubMed]
- Takeishi, A.; Yu, Y.V.; Hapiak, V.M.; Bell, H.W.; O’Leary, T.; Sengupta, P. Receptor-type Guanylyl Cyclases Confer Thermosensory Responses in C. elegans. Neuron 2016, 90, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; O’Halloran, D.; Goodman, M.B. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. J. Gen. Physiol. 2013, 142, 437–449. [Google Scholar] [CrossRef]
- Yu, Y.V.; Bell, H.W.; Glauser, D.; Van Hooser, S.D.; Goodman, M.B.; Sengupta, P. CaMKI-dependent regulation of sensory gene expression mediates experience-dependent plasticity in the operating range of a thermosensory neuron. Neuron 2014, 84, 919–926. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nakano, S.; Amano, M.; Tsuboi, D.; Nishioka, T.; Ikeda, S.; Yokoyama, G.; Kaibuchi, K.; Mori, I. Single-Cell Memory Regulates a Neural Circuit for Sensory Behavior. Cell Rep. 2016, 14, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Sugi, T.; Nonomura, M.; Mori, I. Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis. EMBO Rep. 2011, 12, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Glauser, D.A.; Chen, W.C.; Agin, R.; Macinnis, B.L.; Hellman, A.B.; Garrity, P.A.; Tan, M.W.; Goodman, M.B. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics 2011, 188, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Saro, G.; Lia, A.S.; Thapliyal, S.; Marques, F.; Busch, K.E.; Glauser, D.A. Specific Ion Channels Control Sensory Gain, Sensitivity, and Kinetics in a Tonic Thermonociceptor. Cell Rep. 2020, 30, 397–408.e4. [Google Scholar] [CrossRef]
- Schild, L.C.; Glauser, D.A. Dual Color Neural Activation and Behavior Control with Chrimson and CoChR in Caenorhabditis elegans. Genetics 2015, 200, 1029–1034. [Google Scholar] [CrossRef]
- Ippolito, D.; Thapliyal, S.; Glauser, D.A. Ca(2+)/CaM binding to CaMKI promotes IMA-3 importin binding and nuclear translocation in sensory neurons to control behavioral adaptation. Elife 2021, 10, e71443. [Google Scholar] [CrossRef]
- Narayan, A.; Laurent, G.; Sternberg, P.W. Transfer characteristics of a thermosensory synapse in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2011, 108, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Kuhara, A.; Nakamura, F.; Okochi, Y.; Mori, I. Bidirectional regulation of thermotaxis by glutamate transmissions in Caenorhabditis elegans. EMBO J. 2011, 30, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Horoszok, L.; Raymond, V.; Sattelle, D.B.; Wolstenholme, A.J. GLC-3: A novel fipronil and BIDN-sensitive, but picrotoxinin-insensitive, L-glutamate-gated chloride channel subunit from Caenorhabditis elegans. Br. J. Pharmacol. 2001, 132, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Saro, G.; Lia, A.S.; Poole, R.J.; Falquet, L.; Glauser, D.A. Identification of avoidance genes through neural pathway-specific forward optogenetics. PLoS Genet. 2019, 15, e1008509. [Google Scholar] [CrossRef] [PubMed]
- Byrne Rodgers, J.; Ryu, W.S. Targeted thermal stimulation and high-content phenotyping reveal that the C. elegans escape response integrates current behavioral state and past experience. PLoS ONE 2020, 15, e0229399. [Google Scholar] [CrossRef]
- Marques, F.; Falquet, L.; Vandewyer, E.; Beets, I.; Glauser, D.A. Signaling via the FLP-14/FRPR-19 neuropeptide pathway sustains nociceptive response to repeated noxious stimuli in C. elegans. PLoS Genet. 2021, 17, e1009880. [Google Scholar] [CrossRef]
- Lee, S.J.; Kenyon, C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 2009, 19, 715–722. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, H.J.; Tseng, W.C.; Hsu, J.M.; Huang, T.T.; Chen, C.H.; Pan, C.L. A C. elegans Thermosensory Circuit Regulates Longevity through crh-1/CREB-Dependent flp-6 Neuropeptide Signaling. Dev. Cell 2016, 39, 209–223. [Google Scholar] [CrossRef]
- Sugi, T.; Nishida, Y.; Mori, I. Regulation of behavioral plasticity by systemic temperature signaling in Caenorhabditis elegans. Nat. Neurosci. 2011, 14, 984–992. [Google Scholar] [CrossRef]
- Lindquist, S.; Craig, E.A. The heat-shock proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Stringham, E.G.; Candido, E.P. Targeted single-cell induction of gene products in Caenorhabditis elegans: A new tool for developmental studies. J. Exp. Zool. 1993, 266, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Plagens, R.N.; Mossiah, I.; Kim Guisbert, K.S.; Guisbert, E. Chronic temperature stress inhibits reproduction and disrupts endocytosis via chaperone titration in Caenorhabditis elegans. BMC Biol. 2021, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Prahlad, V.; Morimoto, R.I. Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 14204–14209. [Google Scholar] [CrossRef] [PubMed]
- Tatum, M.C.; Ooi, F.K.; Chikka, M.R.; Chauve, L.; Martinez-Velazquez, L.A.; Steinbusch, H.W.M.; Morimoto, R.I.; Prahlad, V. Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase. Curr. Biol. 2015, 25, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10 (Suppl. S10–17). [Google Scholar] [CrossRef]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef]
- Teixeira-Castro, A.; Ailion, M.; Jalles, A.; Brignull, H.R.; Vilaça, J.L.; Dias, N.; Rodrigues, P.; Oliveira, J.F.; Neves-Carvalho, A.; Morimoto, R.I.; et al. Neuron-specific proteotoxicity of mutant ataxin-3 in C. elegans: Rescue by the DAF-16 and HSF-1 pathways. Hum. Mol. Genet. 2011, 20, 2996–3009. [Google Scholar] [CrossRef]
- Van Oosten-Hawle, P.; Porter, R.S.; Morimoto, R.I. Regulation of organismal proteostasis by transcellular chaperone signaling. Cell 2013, 153, 1366–1378. [Google Scholar] [CrossRef]
- Douglas, P.M.; Baird, N.A.; Simic, M.S.; Uhlein, S.; McCormick, M.A.; Wolff, S.C.; Kennedy, B.K.; Dillin, A. Heterotypic Signals from Neural HSF-1 Separate Thermotolerance from Longevity. Cell Rep. 2015, 12, 1196–1204. [Google Scholar] [CrossRef]
- Chauve, L.; Hodge, F.; Murdoch, S.; Masoudzadeh, F.; Mann, H.J.; Lopez-Clavijo, A.F.; Okkenhaug, H.; West, G.; Sousa, B.C.; Segonds-Pichon, A.; et al. Neuronal HSF-1 coordinates the propagation of fat desaturation across tissues to enable adaptation to high temperatures in C. elegans. PLoS Biol. 2021, 19, e3001431. [Google Scholar] [CrossRef] [PubMed]
- Maman, M.; Carvalhal Marques, F.; Volovik, Y.; Dubnikov, T.; Bejerano-Sagie, M.; Cohen, E. A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans. J. Neurosci. 2013, 33, 6102–6111. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, C.; Ching, T.T.; Nishimura, M.; Davis, A.E.; Gelino, S.; Catan, H.H.; Yu, X.; Chu, C.C.; Ong, B.; Panowski, S.H.; et al. Integrin-linked kinase modulates longevity and thermotolerance in C. elegans through neuronal control of HSF-1. Aging Cell 2014, 13, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Gracida, X.; Dion, M.F.; Harris, G.; Zhang, Y.; Calarco, J.A. An Elongin-Cullin-SOCS Box Complex Regulates Stress-Induced Serotonergic Neuromodulation. Cell Rep. 2017, 21, 3089–3101. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Min, S.; Prahlad, V. Gene bookmarking by the heat shock transcription factor programs the insulin-like signaling pathway. Mol. Cell 2021, 81, 4843–4860. [Google Scholar] [CrossRef]
- Das, S.; Ooi, F.K.; Cruz Corchado, J.; Fuller, L.C.; Weiner, J.A.; Prahlad, V. Serotonin signaling by maternal neurons upon stress ensures progeny survival. Elife 2020, 9, e55246. [Google Scholar] [CrossRef]
- Edwards, S.L.; Erdenebat, P.; Morphis, A.C.; Kumar, L.; Wang, L.; Chamera, T.; Georgescu, C.; Wren, J.D.; Li, J. Insulin/IGF-1 signaling and heat stress differentially regulate HSF1 activities in germline development. Cell Rep 2021, 36, 109623. [Google Scholar] [CrossRef]
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127 Pt 2, 261–266. [Google Scholar] [CrossRef]
- Hajdu-Cronin, Y.M.; Chen, W.J.; Sternberg, P.W. The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics 2004, 168, 1937–1949. [Google Scholar] [CrossRef]
- Anckar, J.; Sistonen, L. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv. Exp. Med. Biol. 2007, 594, 78–88. [Google Scholar]
- Green, M.; Schuetz, T.J.; Sullivan, E.K.; Kingston, R.E. A heat shock-responsive domain of human HSF1 that regulates transcription activation domain function. Mol. Cell Biol. 1995, 15, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chauve, L.; Phelps, G.; Brielmann, R.M.; Morimoto, R.I. E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response. Genes Dev. 2016, 30, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.A.; Douglas, P.M.; Simic, M.S.; Grant, A.R.; Moresco, J.J.; Wolff, S.C.; Yates, J.R., 3rd; Manning, G.; Dillin, A. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science 2014, 346, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.F.; Morimoto, R.I. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell 2004, 15, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.L.; Plagens, R.N.; Kim Guisbert, K.S.; Guisbert, E. Standardized Methods for Measuring Induction of the Heat Shock Response in Caenorhabditis elegans. J. Vis. Exp. 2020, 161, e61030. [Google Scholar] [CrossRef]
- Chiang, W.C.; Ching, T.T.; Lee, H.C.; Mousigian, C.; Hsu, A.L. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell 2012, 148, 322–334. [Google Scholar] [CrossRef]
- Vihervaara, A.; Duarte, F.M.; Lis, J.T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet. 2018, 19, 385–397. [Google Scholar] [CrossRef]
- Trinklein, N.D.; Murray, J.I.; Hartman, S.J.; Botstein, D.; Myers, R.M. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol. Biol. Cell 2004, 15, 1254–1261. [Google Scholar] [CrossRef]
- Garrigues, J.M.; Tsu, B.V.; Daugherty, M.D.; Pasquinelli, A.E. Diversification of the Caenorhabditis heat shock response by Helitron transposable elements. Elife 2019, 8, e51139. [Google Scholar] [CrossRef] [PubMed]
- Guisbert, E.; Czyz, D.M.; Richter, K.; McMullen, P.D.; Morimoto, R.I. Identification of a tissue-selective heat shock response regulatory network. PLoS Genet. 2013, 9, e1003466. [Google Scholar] [CrossRef] [PubMed]
- Morton, E.A.; Lamitina, T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 2013, 12, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Morimoto, R.I. Stress and the cell nucleus: Dynamics of gene expression and structural reorganization. Gene Expr. 1999, 7, 261–270. [Google Scholar] [PubMed]
- Melnick, M.; Gonzales, P.; Cabral, J.; Allen, M.A.; Dowell, R.D.; Link, C.D. Heat shock in C. elegans induces downstream of gene transcription and accumulation of double-stranded RNA. PLoS ONE 2019, 14, e0206715. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef]
- Shalgi, R.; Hurt, J.A.; Krykbaeva, I.; Taipale, M.; Lindquist, S.; Burge, C.B. Widespread regulation of translation by elongation pausing in heat shock. Mol. Cell 2013, 49, 439–452. [Google Scholar] [CrossRef]
- Dutta, N.; Garcia, G.; Higuchi-Sanabria, R. Hijacking Cellular Stress Responses to Promote Lifespan. Front. Aging 2022, 3, 860404. [Google Scholar] [CrossRef]
- Jee, H. Size dependent classification of heat shock proteins: A mini-review. J. Exerc. Rehabil. 2016, 12, 255–259. [Google Scholar] [CrossRef]
- Snutch, T.P.; Baillie, D.L. Alterations in the pattern of gene expression following heat shock in the nematode Caenorhabditis elegans. Can. J. Biochem. Cell Biol. 1983, 61, 480–487. [Google Scholar] [CrossRef]
- McColl, G.; Rogers, A.N.; Alavez, S.; Hubbard, A.E.; Melov, S.; Link, C.D.; Bush, A.I.; Kapahi, P.; Lithgow, G.J. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab. 2010, 12, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Higuchi-Sanabria, R.; Paul, J.W., 3rd; Durieux, J.; Benitez, C.; Frankino, P.A.; Tronnes, S.U.; Garcia, G.; Daniele, J.R.; Monshietehadi, S.; Dillin, A. Spatial regulation of the actin cytoskeleton by HSF-1 during aging. Mol. Biol. Cell 2018, 29, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Sóti, C.; Csermely, P. Molecular chaperones and the aging process. Biogerontology 2000, 1, 225–233. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Sun, F.; Zhang, J.; Feng, J.; Liu, H.; Rajendran, K.V.; Sun, L.; Zhang, Y.; Jiang, Y.; et al. RNA-Seq reveals expression signatures of genes involved in oxygen transport, protein synthesis, folding, and degradation in response to heat stress in catfish. Physiol. Genom. 2013, 45, 462–476. [Google Scholar] [CrossRef]
- Dams, S.D.; de Liefde-van Beest, M.; Nuijs, A.M.; Oomens, C.W.; Baaijens, F.P. Heat shocks enhance procollagen type I and III expression in fibroblasts in ex vivo human skin. Skin Res. Technol. 2011, 17, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Nagata, K. Hsp47 as a collagen-specific molecular chaperone. Methods Enzymol. 2011, 499, 167–182. [Google Scholar] [PubMed]
- Gomez-Pastor, R.; Burchfiel, E.T.; Neef, D.W.; Jaeger, A.M.; Cabiscol, E.; McKinstry, S.U.; Doss, A.; Aballay, A.; Lo, D.C.; Akimov, S.S.; et al. Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington’s disease. Nat. Commun. 2017, 8, 14405. [Google Scholar] [CrossRef]
- Batista-Nascimento, L.; Neef, D.W.; Liu, P.C.; Rodrigues-Pousada, C.; Thiele, D.J. Deciphering human heat shock transcription factor 1 regulation via post-translational modification in yeast. PLoS ONE 2011, 6, e15976. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Loew, C.; Körner, R.; Pinkert, S.; Theis, M.; Hayer-Hartl, M.; Buchholz, F.; Hartl, F.U. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell 2014, 156, 975–985. [Google Scholar] [CrossRef]
- Brunquell, J.; Raynes, R.; Bowers, P.; Morris, S.; Snyder, A.; Lugano, D.; Deonarine, A.; Westerheide, S.D. CCAR-1 is a negative regulator of the heat-shock response in Caenorhabditis elegans. Aging Cell 2018, 17, e12813. [Google Scholar] [CrossRef]
- Tonkiss, J.; Calderwood, S.K. Regulation of heat shock gene transcription in neuronal cells. Int. J. Hyperth. 2005, 21, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Guettouche, T.; Boellmann, F.; Lane, W.S.; Voellmy, R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Laskovs, M.; Williams, R.I.; Mahadevan, A.; Labbadia, J. A Mitochondrial Stress-Specific Form of HSF1 Protects against Age-Related Proteostasis Collapse. Dev. Cell 2020, 54, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, R.; Denison, C.; Bening-Abu-Shach, U.; Chisholm, A.D.; Gygi, S.P.; Broday, L. SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev. Cell 2009, 17, 724–735. [Google Scholar] [CrossRef]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M., Jr.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef]
- Rivard, R.S.; Morris, J.M.; Youngman, M.J. The PP2A/4/6 subfamily of phosphoprotein phosphatases regulates DAF-16 and confers resistance to environmental stress in postreproductive adult C. elegans. PLoS ONE 2020, 15, e0229812. [Google Scholar] [CrossRef]
- Biglou, S.G.; Bendena, W.G.; Chin-Sang, I. An overview of the insulin signaling pathway in model organisms Drosophila melanogaster and Caenorhabditis elegans. Peptides 2021, 145, 170640. [Google Scholar] [CrossRef]
- Oh, S.W.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2005, 102, 4494–4499. [Google Scholar] [CrossRef]
- Tepper, R.G.; Ashraf, J.; Kaletsky, R.; Kleemann, G.; Murphy, C.T.; Bussemaker, H.J. PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 2013, 154, 676–690. [Google Scholar] [CrossRef]
- Shen, C.; Nettleton, D.; Jiang, M.; Kim, S.K.; Powell-Coffman, J.A. Roles of the HIF-1 hypoxia-inducible factor during hypoxia response in Caenorhabditis elegans. J. Biol. Chem. 2005, 280, 20580–20588. [Google Scholar] [CrossRef]
- Treinin, M.; Shliar, J.; Jiang, H.; Powell-Coffman, J.A.; Bromberg, Z.; Horowitz, M. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol. Genom. 2003, 14, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Carranza, A.D.V.; Saragusti, A.; Chiabrando, G.A.; Carrari, F.; Asis, R. Effects of chlorogenic acid on thermal stress tolerance in C. elegans via HIF-1, HSF-1 and autophagy. Phytomedicine 2020, 66, 153132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Leboutet, R.; Largeau, C.; Zentout, S.; Lefebvre, C.; Delahodde, A.; Culetto, E.; Legouis, R. Autophagy facilitates mitochondrial rebuilding after acute heat stress via a DRP-1-dependent process. J. Cell Biol. 2021, 220, e201909139. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Nakai, A. The heat shock factor family and adaptation to proteotoxic stress. FEBS J. 2010, 277, 4112–4125. [Google Scholar] [PubMed]
- Kumsta, C.; Hansen, M. Hormetic heat shock and HSF-1 overexpression improve C. elegans survival and proteostasis by inducing autophagy. Autophagy 2017, 13, 1076–1077. [Google Scholar] [CrossRef]
- Civelek, M.; Mehrkens, J.F.; Carstens, N.M.; Fitzenberger, E.; Wenzel, U. Inhibition of mitophagy decreases survival of Caenorhabditis elegans by increasing protein aggregation. Mol. Cell Biochem. 2019, 452, 123–131. [Google Scholar] [CrossRef]
- Nehammer, C.; Podolska, A.; Mackowiak, S.D.; Kagias, K.; Pocock, R. Specific microRNAs regulate heat stress responses in Caenorhabditis elegans. Sci. Rep. 2015, 5, 8866. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Brunquell, J.; Snyder, A.; Cheng, F.; Westerheide, S.D. HSF-1 is a regulator of miRNA expression in Caenorhabditis elegans. PLoS ONE 2017, 12, e0183445. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, W.P.; Pagliuso, D.C.; Garrigues, J.M.; Chen, J.S.; Aalto, A.P.; Pasquinelli, A.E. Remodeling of the Caenorhabditis elegans non-coding RNA transcriptome by heat shock. Nucleic Acids Res. 2019, 47, 9829–9841. [Google Scholar] [CrossRef] [PubMed]
- Pagliuso, D.C.; Bodas, D.M.; Pasquinelli, A.E. Recovery from heat shock requires the microRNA pathway in Caenorhabditis elegans. PLoS Genet. 2021, 17, e1009734. [Google Scholar] [CrossRef] [PubMed]
- Tuorto, F.; Herbst, F.; Alerasool, N.; Bender, S.; Popp, O.; Federico, G.; Reitter, S.; Liebers, R.; Stoecklin, G.; Gröne, H.J.; et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. EMBO J. 2015, 34, 2350–2362. [Google Scholar] [CrossRef]
- Navarro, I.C.; Tuorto, F.; Jordan, D.; Legrand, C.; Price, J.; Braukmann, F.; Hendrick, A.G.; Akay, A.; Kotter, A.; Helm, M.; et al. Translational adaptation to heat stress is mediated by RNA 5-methylcytosine in Caenorhabditis elegans. EMBO J. 2021, 40, e105496. [Google Scholar] [CrossRef]
- Labbadia, J.; Brielmann, R.M.; Neto, M.F.; Lin, Y.F.; Haynes, C.M.; Morimoto, R.I. Mitochondrial Stress Restores the Heat Shock Response and Prevents Proteostasis Collapse during Aging. Cell Rep. 2017, 21, 1481–1494. [Google Scholar] [CrossRef]
- Taouktsi, E.; Kyriakou, E.; Smyrniotis, S.; Borbolis, F.; Bondi, L.; Avgeris, S.; Trigazis, E.; Rigas, S.; Voutsinas, G.E.; Syntichaki, P. Organismal and Cellular Stress Responses upon Disruption of Mitochondrial Lonp1 Protease. Cells 2022, 11, 1363. [Google Scholar] [CrossRef]
- Gibellini, L.; De Gaetano, A.; Mandrioli, M.; Van Tongeren, E.; Bortolotti, C.A.; Cossarizza, A.; Pinti, M. The biology of Lonp1: More than a mitochondrial protease. Int. Rev. Cell Mol. Biol. 2020, 354, 1–61. [Google Scholar]
- Voos, W.; Pollecker, K. The Mitochondrial Lon Protease: Novel Functions off the Beaten Track? Biomolecules 2020, 10, 253. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakou, E.; Taouktsi, E.; Syntichaki, P. The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 23, 14907. https://doi.org/10.3390/ijms232314907
Kyriakou E, Taouktsi E, Syntichaki P. The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans. International Journal of Molecular Sciences. 2022; 23(23):14907. https://doi.org/10.3390/ijms232314907
Chicago/Turabian StyleKyriakou, Eleni, Eirini Taouktsi, and Popi Syntichaki. 2022. "The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans" International Journal of Molecular Sciences 23, no. 23: 14907. https://doi.org/10.3390/ijms232314907
APA StyleKyriakou, E., Taouktsi, E., & Syntichaki, P. (2022). The Thermal Stress Coping Network of the Nematode Caenorhabditis elegans. International Journal of Molecular Sciences, 23(23), 14907. https://doi.org/10.3390/ijms232314907






