Two Epitope Regions Revealed in the Complex of IL-17A and Anti-IL-17A VHH Domain
Abstract
:1. Introduction
2. Results
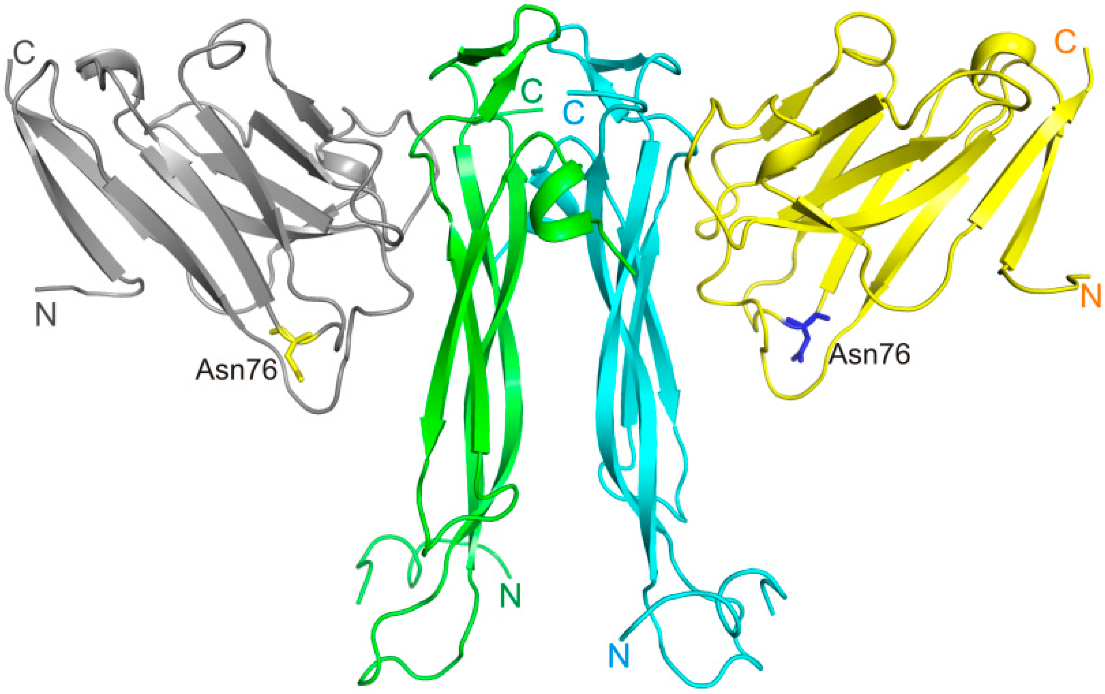
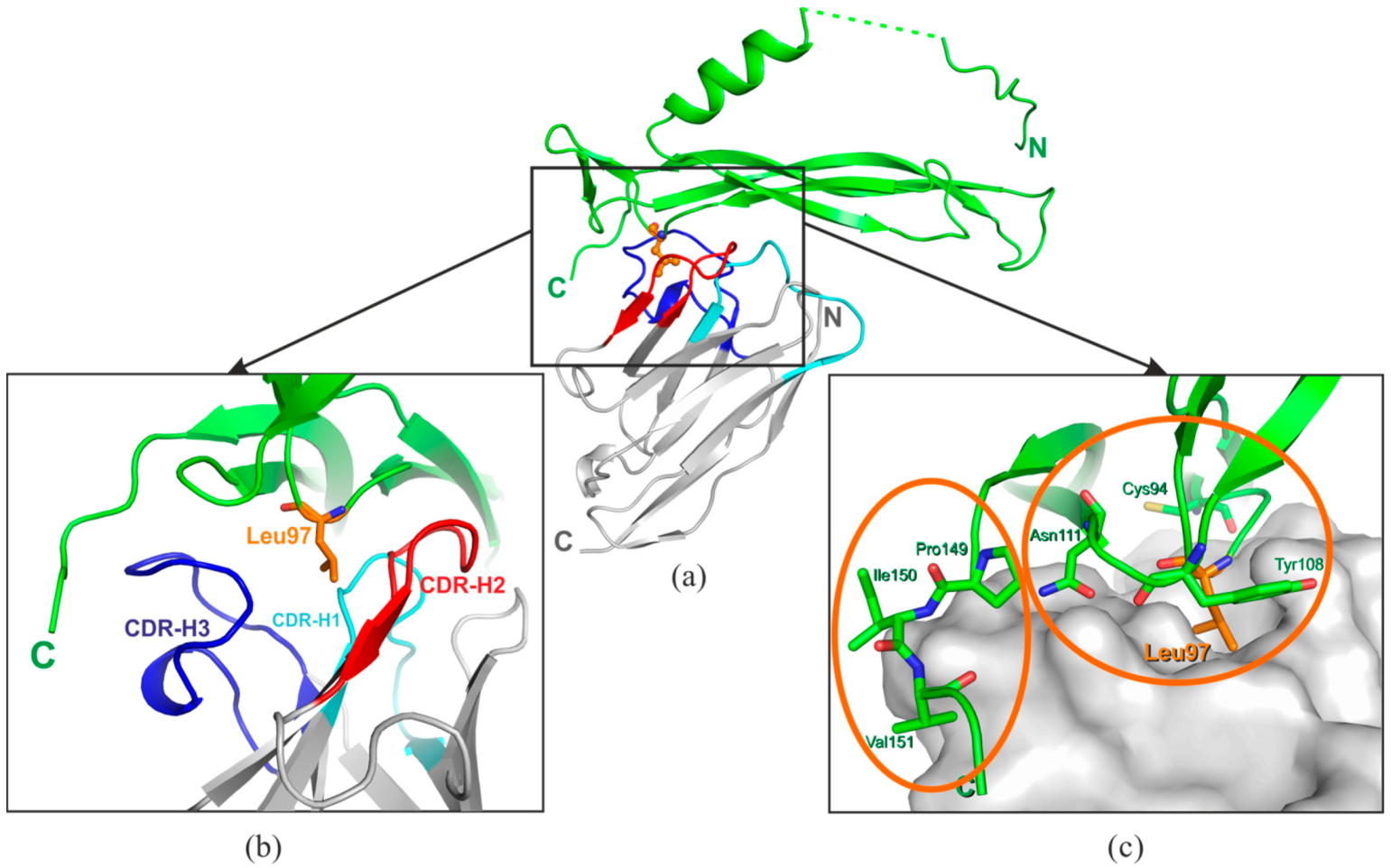
2.1. Structure of the VHH Domain in Complex with IL-17A
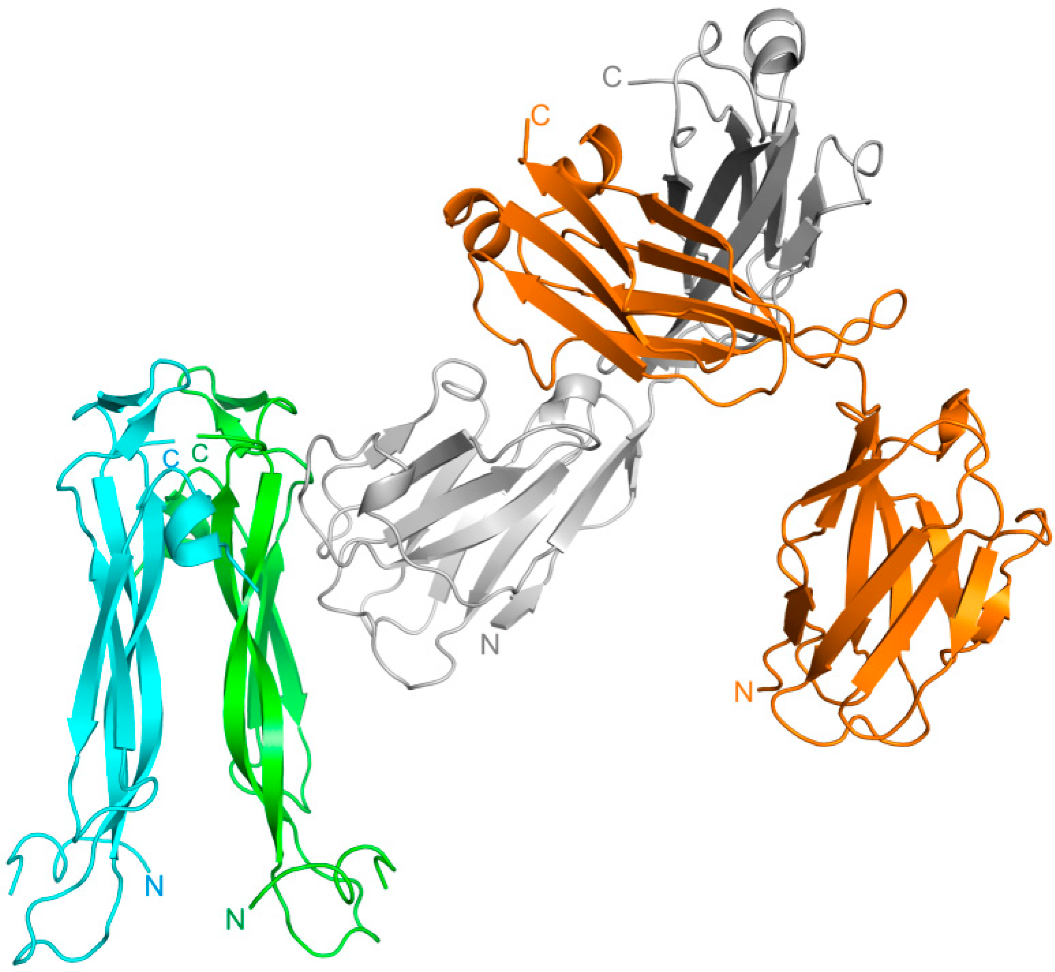
2.2. Modeling of the IL-17A/Netakimab Fab Fragment Complex
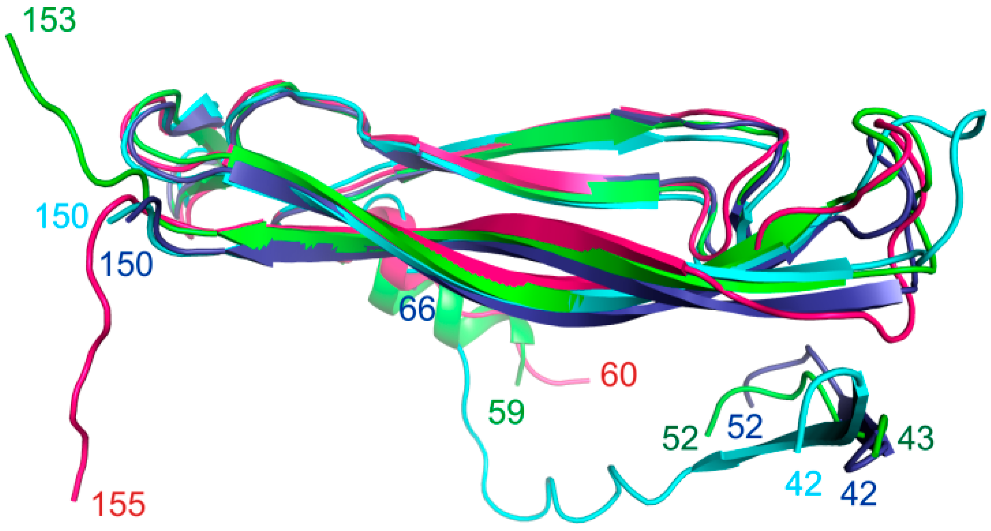
2.3. The Structure of Anti-IL-17A-N Mutant Form
3. Discussion
3.1. Interactions of the VHH Domain with IL-17A
3.2. Crystal Contacts between of the VHH Domain and IL-17A Are of No Functional Significance
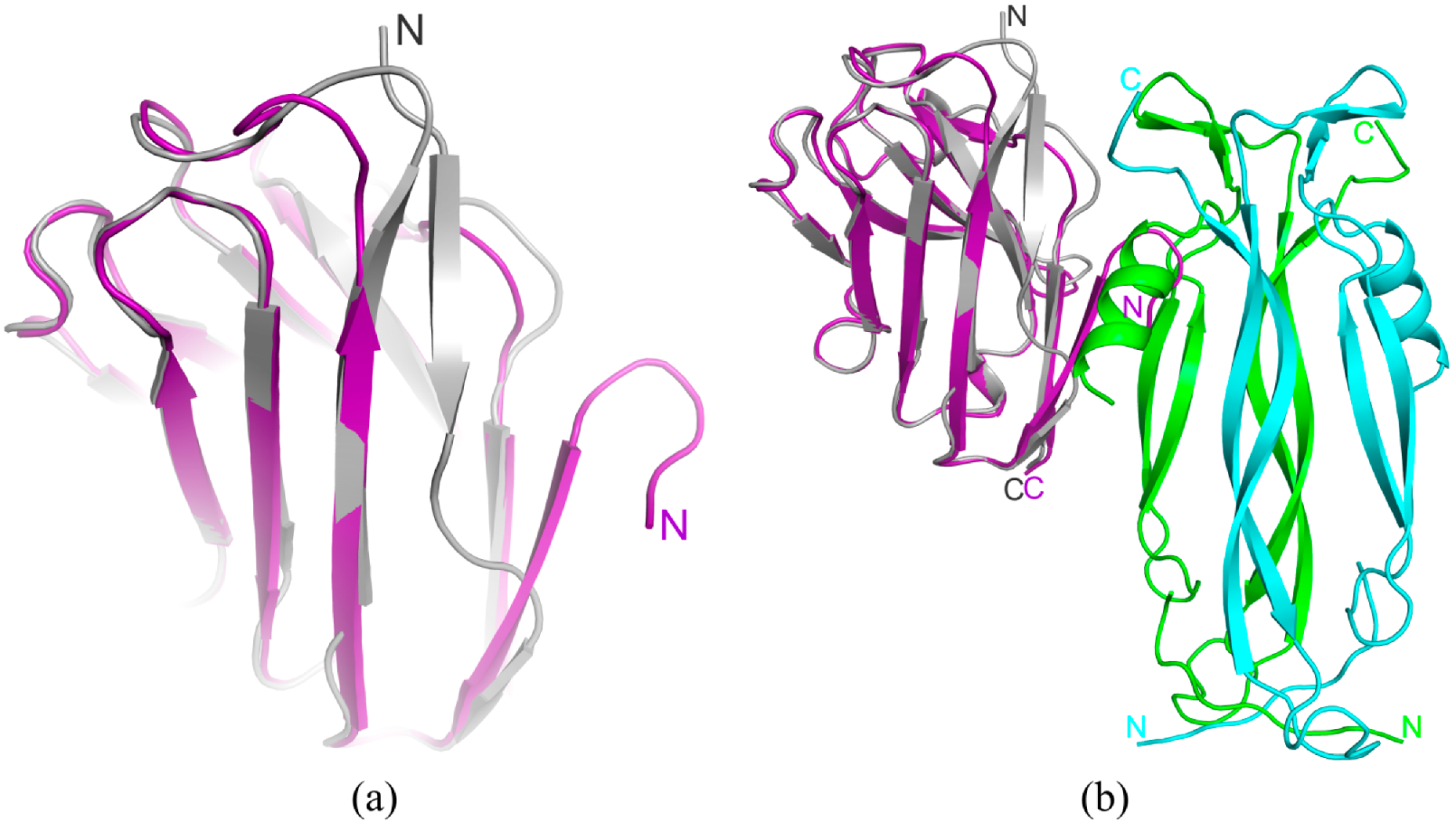
3.3. Comparative Structural Analysis of the IL-17A Epitope Regions
3.3.1. IL-17A/Secukinumab Fab Fragment Complex
3.3.2. IL-17A/CAT-2200 Fab Fragment Complex
3.3.3. Comparative Analysis of the Epitope-Paratope Regions in the IL-17A/HB0017 Fab Fragment and IL-17A/Anti-IL-17A-76 Complexes
4. Materials and Methods
4.1. Production and Purification of VHH Domain of Netakimab Antibody
4.2. Production and Purification of Mutant Forms of Anti-IL-17A
4.3. Production and Purification of the IL-17A
4.4. Analysis of the Anti-IL-17A and Its Mutant Forms with IL-17A Interactions by Surface Plasmon Resonance Approach (SPR)
4.5. Preparation of IL-17A/Anti-IL-17A-76 Complex and Crystallization
4.6. Diffraction Data Collection and Determining the IL-17A/Anti-IL-17A-76 Complex and Anti-IL-17A-N Mutant Form Structures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 family of cytokines in health and disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Fossiez, F.; Djossou, O.; Chornarat, P.; Flores-Romo, L.; Ait-Yahia, S.; Maat, C.; Pin, J.-J.; Garrone, P.; Garcia, E.; Saeland, S.; et al. T Cell Interleukin-17 Induces Stromal Cells to Produce Proinflammatory and Hematopoietic Cytokines. J. Exp. Med. 1996, 183, 2593–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajima, M.; Wakita, D.; Noguchi, D.; Chamoto, K.; Yue, Z.; Fugo, K.; Ishigame, H.; Iwakura, Y.; Kitamura, H.; Nishimura, T. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17—Producing CD8+ T cells. J. Exp. Med. 2008, 205, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, E.; Green, A.M.; Flynn, J.L. IL-17 Production Is Dominated by γδ T Cells rather than CD4 T Cells during Mycobacterium tuberculosis Infection. J. Immunol. 2006, 177, 4662–4669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, K.; Yamada, H.; Hara, H.; Kishihara, K.; Yoshikai, Y. Resident Vδ1+ γδ T Cells Control Early Infiltration of Neutrophils after Escherichia coli Infection via IL-17 Production. J. Immunol. 2007, 178, 4466–4472. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.-L.; Keller, A.C.; Paget, C.; Fujio, M.; Trottein, F.; Savage, P.B.; Wong, C.-H.; Schneider, E.; Dy, M.; Leite-de-Moraes, M.C. Identification of an IL-17–producing NK1.1neg iNKT cell population involved in airway neutrophilia. J. Exp. Med. 2007, 204, 995–1001. [Google Scholar] [CrossRef]
- Passos, S.T.; Silver, J.S.; O’Hara, A.C.; Sehy, D.; Stumhofer, J.S.; Hunter, C.A. IL-6 Promotes NK Cell Production of IL-17 during Toxoplasmosis. J. Immunol. 2010, 184, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Takatori, H.H.; Kanno, Y.; Watford, W.T.; Tato, C.M.; Weiss, G.; Ivanov, I.I.; Littman, D.R.; O’Shea, J.J. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 2009, 206, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Geremia, A.; Arancibia-Cárcamo, C.V.; Fleming, M.P.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.L.; Powrie, F. IL-23–responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, S.; Bonneau, O.; Dubois, G.R.; Jones, C.E.; Trifilieff, A. IL-17, Produced by Lymphocytes and Neutrophils, Is Necessary for Lipopolysaccharide-Induced Airway Neutrophilia: IL-15 as a Possible Trigger. J. Immunol. 2003, 170, 2106–2112. [Google Scholar] [CrossRef]
- Takahashi, N.; Vanlaere, I.; Rycke, R.; Cauwels, A.; Joosten, L.A.B.; Lubberts, E.; Berg, W.B.; Libert, C. IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 2008, 205, 1755–1761. [Google Scholar] [CrossRef] [Green Version]
- Hueber, A.J.; Asquith, D.L.; Miller, A.M.; Reilly, J.; Kerr, S.; Leipe, J.; Melendez, A.J.; McInnes, I.B. Mast Cells Express IL-17A in Rheumatoid Arthritis Synovium. J. Immunol. 2010, 184, 3336–3340. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Archer, N.; Adappa, N.; Palmer, J.; Cohen, N.; Harro, J.; Lee, S.; Miller, L.; Shirtliff, M. Interleukin-17A (IL-17A) and IL-17F Are Critical for Antimicrobial Peptide Production and Clearance of Staphylococcus aureus Nasal Colonization. Infect. Immun. 2016, 84, 3575–3583. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; William, C.; Fanslow, W.C.; Seldin, M.F.; Rousseau, A.-M.; Painter, S.L.; Comeau, M.R.; Cohen, J.I.; Spriggs, M.K. Herpesvirus Saimiri Encodes a New Cytokine, IL-17, Which Binds to a Novel Cytokine Receptor. Immunity 1995, 3, 811–821. [Google Scholar] [CrossRef] [Green Version]
- Toy, D.; Kugler, D.; Wolfson, M.; Bos, T.V.; Gurgel, J.; Derry, J.; Tocker, J.; Peschon, J. Interleukin 17 Signals through a Heteromeric Receptor Complex. J. Immunol. 2006, 177, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Zwicky, P.; Unger, S.; Becher, B. Targeting interleukin-17 in chronic inflammatory disease: A clinical perspective. J. Exp. Med. 2020, 217, e20191123. [Google Scholar] [CrossRef]
- Su, Y.; Huang, J.; Zhao, X.; Lu, H.; Wang, W.; Yang, X.O.; Shi, Y.; Wang, X.; Lai, Y.; Dong, C. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci. Immunol. 2019, 4, eaau9657. [Google Scholar] [CrossRef] [PubMed]
- Ely, L.K.; Fischer, S.; Garcia, K.C. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 2009, 10, 1245–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facheris, P.; Valenti, M.; Pavia, G.; Guanziroli, E.; Narcisi, A.; Borroni, R.G.; Costanzo, A. Brodalumab: A new way to inhibit IL-17 in psoriasis. Dermatol. Ther. 2020, 33, e13403. [Google Scholar] [CrossRef] [PubMed]
- Rafael-Vidal, C.; Pérez, N.; Altabás, I.; Garcia, S.; Pego-Reigosa, J.M. Blocking IL-17: A Promising Strategy in the Treatment of Systemic Rheumatic Diseases. Int. J. Mol. Sci. 2020, 21, 7100. [Google Scholar] [CrossRef]
- Sanford, M.; McKeage, K. Secukinumab: First Global Approval. Drugs 2015, 75, 329–338. [Google Scholar] [CrossRef]
- Blauvelt, A.; Gooderham, M.; Iversen, L.; Ball, S.; Zhang, L.; Agada, N.O.; Reich, K. Efficacy and safety of ixekizumab for the treatment of moderate-to-severe plaque psoriasis: Results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER-3). J. Am. Acad. Dermatol. 2017, 77, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Ulitin, A.; Evdokimov, S.; Solovyev, V.; Chernykh, Y.; Goncharova, O.; Korzhavin, D.; Chernovskaya, T.; Nemankin, T.; Ivanov, R.; Morozov, D.; et al. High Affinity and Aggregatively Stable Antibodies on the Basis of Variable Domains vl and a Derivative vhh. WO Patent WO2016048188A1, 31 March 2016. [Google Scholar]
- Ulitin, A.; Evdokimov, S.; Solovyev, V.; Chernykh, Y.; Goncharova, O.; Korzhavin, D.; Chernovskaya, T.; Nemankin, T.; Ivanov, R.; Morozov, D.; et al. Method for Treatment of an IL-17A-Mediated Disease through Antibody Administration Specific to Human IL-17A. U.S. Patent 11447574B2, 20 September 2022. [Google Scholar]
- Kostareva, O.; Kolyadenko, I.; Ulitin, A.; Ekimova, V.; Evdokimov, S.; Garber, M.; Tishchenko, S.; Gabdulkhakov, A. Fab Fragment of VHH-Based Antibody Netakimab: Crystal Structure and Modeling Interaction with Cytokine IL-17A. Crystals 2019, 9, 177. [Google Scholar] [CrossRef] [Green Version]
- Sundstrom, M.; Lundqvist, T.; Rodin, J.; Giebel, L.B.; Milligan, D.; Norstedt, G. Crystal structure of an antagonist mutant of human growth hormone, G120R, in complex with its receptor at 2.9 Å resolution. J. Biol. Chem. 1996, 271, 32197–32203. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Song, X.; Chrunyk, B.; Shanker, S.; Hoth, L.; Marr, E.; Griffor, M. Crystal Structures of Interleukin 17A and Its Complex with IL-17 Receptor A. Nat. Commun. 2013, 4, 1888. [Google Scholar] [CrossRef] [Green Version]
- Ekimova, V.; Ulitin, A.; Evdokimov, S.; Sofronova, E.; Nemankin, T.; Solovyev, V.; Ustugov, I.; Nedorubov, A.; Chernykh, Y.; Goncharova, O.; et al. High Affinity Anti-Il-17a Monoclonal Antibody. In Proceedings of the Conference: PEGS, Boston, MA, USA, 4–8 May 2015. [Google Scholar]
- Padova, D.; Gram, H.; Hofstetter, H.; Jeschke, M.; Rondeau, J.; Van Den Berg, W. IL-17 Antagonistic Antibodies. U.S. Patent 7,807,155 B2, 5 October 2010. [Google Scholar]
- Gerhardt, S.; Abbott, W.; Hargreaves, D.; Pauptit, R.; Davies, R.; Needham, M.; Langham, C.; Barker, W.; Aziz, A.; Snow, M.; et al. Structure of IL-17A in Complex with a Potent, Fully Human Neutralizing Antibody. J. Mol. Biol. 2009, 394, 905–921. [Google Scholar] [CrossRef]
- Xu, J.; Jia, H.; Chen, S.; Xu, J.; Zhan, Y.; Yu, H.; Wang, W.; Kang, X.; Cui, X.; Feng, Y.; et al. Structural and functional insights into a novel pre-clinical-stage antibody targeting IL-17A for treatment of autoimmune diseases. Int. J. Biol. Macromol. 2022, 202, 529–538. [Google Scholar] [CrossRef]
- Nimrod, G.; Fischman, S.; Austin, M.; Herman, A.; Keyes, F.; Leiderman, O.; Hargreaves, D.; Strajbl, M.; Breed, J.; Klompus, S.; et al. Computational design of epitope-specific functional antibodies. Cell. Rep. 2018, 25, 2121–2131. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Zhang, L.; Wei, H.; Li, F.; Hu, L.; Ma, H.; Liu, Q.; Li, X.; Liu, Z. Optimized methods for IL-17A refolding and anti-IL-17A Fab production for co-crystallization with small molecules. Biotechniques 2020, 69, 427–433. [Google Scholar] [CrossRef]
- Murshudov, G.; Skulak, P.; Lebedev, A.; Pannu, N.; Steiner, R.; Nicholls, R.; Winn, M.; Long, F.; Vagin, A. A REFMAC5 for the Refinement of Macromolecular Crystal Structures. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.; Cowtan, K. Features and Development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System. Available online: https://pymol.org/2/ (accessed on 1 October 2022).



| Kon (M−1 s−1) | Koff (s−1) | KD (nM) | |
|---|---|---|---|
| anti-IL-17A | 5.0 × 105 | 4.3 × 10−5 | 0.09 |
| anti-IL-17A-76 | 4.7 × 105 | 2.2 × 10−5 | 0.06 |
| Kon (M−1 s−1) | Koff (s−1) | KD (nM) | |
|---|---|---|---|
| Netakimab | 2.0 × 105 | 1.0 × 10−7 | <0.001 |
| Fab fragment | 6.3 × 104 | 2.0 × 10−5 | 0.3 |
| VHH domain | 5.0 × 105 | 4.3 × 10−5 | 0.09 |
| Number of Amino Acid | 1 11 |
|---|---|
| Anti-IL-17A | QVQLVQSGGGLVQA |
| Anti-IL-17A-N | RGSEFEVQ-------- LVQA |
| Kon (M−1 s−1) | Koff (s−1) | KD (nM) | |
|---|---|---|---|
| anti-IL-17A-76 | 4.7 × 105 | 2.2 × 10−5 | 0.06 |
| anti-IL-17A-N | 4.8 × 105 | 2.5 × 10−5 | 0.05 |
| KD (nM) | IC50 (pM) | Reference | |
|---|---|---|---|
| netakimab Fab fragment | 0.30 | 66 | [29] |
| netakimab VHH domain | 0.09 | 30 | [24] |
| secukinumab | 0.12 | 201 | [30] |
| CAT-2200 Fab fragment | 2.10 | 1560 | [31] |
| HB0017 | 0.01 | 56 | [32] |
| Data Collection | ||
|---|---|---|
| IL-17A/Anti-IL-17A-76 Complex | Mutant Form Anti-IL-17A-N | |
| Space group | P3121 | P22121 |
| a, b, c, (Å) | 71.05, 106.04 | 54.87, 72.49, 129.38 |
| α, β, γ, (°) | 90.0, 120.0, 90 | 90, 90, 90 |
| Resolution limits, Å | 50.0–2.85 (2.92–2.85) | 50.0–2.38 (2.52–2.38) |
| Rsigma, % | 25.3 (182.9) | 13.8 (116.9) |
| Mean I/σ(I) | 12.00 (1.58) | 9.10 (1.40) |
| Completeness, % | 98.1 (99.4) | 98.2 (90.7) |
| Redundancy | 6.73 (7.11) | 5.18 (5.06) |
| CC1/2 (%) | 95.9 (46.2) | 99.6 (80.8) |
| Unique reflections | 7466 (538) | 21,024 (3 076) |
| Refinement statistics | ||
| Resolution, Å | 40.17–2.85 (3.26–2.85) | 41.85–2.45 (2.51–2.45) |
| Total number of reflections | 7460 (2336) | 19,563 (2702) |
| Rwork/Rfree, % | 22.2/26.8 (23.9/31.8) | 21.5/28.01 (33.75/38.07) |
| Average B-factor, Å2 | 71.0 | 41/0 |
| Ramachandran plot | ||
| Most favorable regions, % | 94.2 | 96.84 |
| Allowed regions, % | 5.8 | 3.16 |
| R.m.s. deviations | ||
| Bond length, Å | 0.009 | 0.009 |
| Bond angle, ° | 1.109 | 1.053 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostareva, O.; Svoeglazova, A.; Kolyadenko, I.; Nikulin, A.; Evdokimov, S.; Dzhus, U.; Gabdulkhakov, A.; Tishchenko, S. Two Epitope Regions Revealed in the Complex of IL-17A and Anti-IL-17A VHH Domain. Int. J. Mol. Sci. 2022, 23, 14904. https://doi.org/10.3390/ijms232314904
Kostareva O, Svoeglazova A, Kolyadenko I, Nikulin A, Evdokimov S, Dzhus U, Gabdulkhakov A, Tishchenko S. Two Epitope Regions Revealed in the Complex of IL-17A and Anti-IL-17A VHH Domain. International Journal of Molecular Sciences. 2022; 23(23):14904. https://doi.org/10.3390/ijms232314904
Chicago/Turabian StyleKostareva, Olga, Arina Svoeglazova, Ilya Kolyadenko, Alexey Nikulin, Stanislav Evdokimov, Uliana Dzhus, Azat Gabdulkhakov, and Svetlana Tishchenko. 2022. "Two Epitope Regions Revealed in the Complex of IL-17A and Anti-IL-17A VHH Domain" International Journal of Molecular Sciences 23, no. 23: 14904. https://doi.org/10.3390/ijms232314904
APA StyleKostareva, O., Svoeglazova, A., Kolyadenko, I., Nikulin, A., Evdokimov, S., Dzhus, U., Gabdulkhakov, A., & Tishchenko, S. (2022). Two Epitope Regions Revealed in the Complex of IL-17A and Anti-IL-17A VHH Domain. International Journal of Molecular Sciences, 23(23), 14904. https://doi.org/10.3390/ijms232314904








