Enlarging the Scope of 5-Aminolevulinic Acid-Mediated Photodiagnosis towards Breast Cancers
Abstract
1. Introduction
2. Results and Discussion
2.1. PpIX Induction
2.2. Dark Toxicity
2.3. Photodynamic Therapy
3. Materials and Methods
3.1. Cell Culture
3.2. PpIX Induction
3.3. Dark and Photo Toxicities
3.4. Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sardanelli, F.; Aase, H.S.; Alvarez, M.; Azavedo, E.; Baarslag, H.J.; Balleyguier, C.; Baltzer, P.A.; Beslagic, V.; Bick, U.; Bogdanovic-Stojanovic, D.; et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur. Radiol. 2017, 27, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Athanasiou, A.; Baltzer, P.A.T.; Camps-Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; Fuchsjager, M.H.; Helbich, T.H.; Killburn-Toppin, F.; et al. Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 2022, 32, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Grosenick, D.; Bremer, C. Fluorescence Imaging of Breast Tumors and Gastrointestinal Cancer. Recent Results Cancer Res. 2020, 216, 591–624. [Google Scholar] [CrossRef]
- Verbeek, F.P.; Troyan, S.L.; Mieog, J.S.; Liefers, G.J.; Moffitt, L.A.; Rosenberg, M.; Hirshfield-Bartek, J.; Gioux, S.; van de Velde, C.J.; Vahrmeijer, A.L.; et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: A multicenter experience. Breast Cancer Res. Treat. 2014, 143, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef]
- Morita, M.; Tanaka, H.; Kumamoto, Y.; Nakamura, A.; Harada, Y.; Ogata, T.; Sakaguchi, K.; Taguchi, T.; Takamatsu, T. Fluorescence-based discrimination of breast cancer cells by direct exposure to 5-aminolevulinic acid. Cancer Med. 2019, 8, 5524–5533. [Google Scholar] [CrossRef]
- Ottolino-Perry, K.; Shahid, A.; DeLuca, S.; Son, V.; Sukhram, M.; Meng, F.; Liu, Z.A.; Rapic, S.; Anantha, N.T.; Wang, S.C.; et al. Intraoperative fluorescence imaging with aminolevulinic acid detects grossly occult breast cancer: A phase II randomized controlled trial. Breast Cancer Res. 2021, 23, 72. [Google Scholar] [CrossRef]
- Frei, K.A.; Bonel, H.M.; Frick, H.; Walt, H.; Steiner, R.A. Photodynamic detection of diseased axillary sentinel lymph node after oral application of aminolevulinic acid in patients with breast cancer. Br. J. Cancer 2004, 90, 805–809. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.; Musial, J.; Kordek, R. Oncologic photodynamic diagnosis and therapy: Confocal Raman/fluorescence imaging of metal phthalocyanines in human breast cancer tissue in vitro. Analyst 2014, 139, 5547–5559. [Google Scholar] [CrossRef]
- Jendželovský, R.; Jendželovská, Z.; Kuchárová, B.; Fedoročko, P. Breast cancer resistance protein is the enemy of hypericin accumulation and toxicity of hypericin-mediated photodynamic therapy. Biomed. Pharmacother. 2019, 109, 2173–2181. [Google Scholar] [CrossRef]
- Gustalik, J.; Aebisher, D.; Bartusik-Aebisher, D. Photodynamic therapy in breast cancer treatment. J. Appl. Biomed. 2022, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Herceg, V.; Lange, N.; Allemann, E.; Babic, A. Activity of phosphatase-sensitive 5-aminolevulinic acid prodrugs in cancer cell lines. J. Photochem. Photobiol. B 2017, 171, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Self-immolative chemistry in nanomedicine. Chem. Eng. J. 2018, 340, 24–31. [Google Scholar] [CrossRef]
- Babic, A.; Herceg, V.; Ateb, I.; Allemann, E.; Lange, N. Tunable phosphatase-sensitive stable prodrugs of 5-aminolevulinic acid for tumor fluorescence photodetection. J. Control Release 2016, 235, 155–164. [Google Scholar] [CrossRef]
- Ortel, B.; Chen, N.; Brissette, J.; Dotto, G.P.; Maytin, E.; Hasan, T. Differentiation-specific increase in ALA-induced protoporphyrin IX accumulation in primary mouse keratinocytes. Br. J. Cancer 1998, 77, 1744–1751. [Google Scholar] [CrossRef]
- Bourre, L.; Giuntini, F.; Eggleston, I.M.; Wilson, M.; MacRobert, A.J. 5-Aminolaevulinic acid peptide prodrugs enhance photosensitization for photodynamic therapy. Mol. Cancer Ther. 2008, 7, 1720–1729. [Google Scholar] [CrossRef]
- Yang, D.F.; Chen, J.H.; Chiang, C.P.; Huang, Z.; Lee, J.W.; Liu, C.J.; Chang, J.L.; Hsu, Y.C. Improve efficacy of topical ALA-PDT by calcipotriol through up-regulation of coproporphyrinogen oxidase. Photodiagnosis Photodyn. Ther. 2014, 11, 331–341. [Google Scholar] [CrossRef]
- Berrahmoune, S.; Fotinos, N.; Bezdetnaya, L.; Lange, N.; Guedenet, J.C.; Guillemin, F.; D’Hallewin, M.A. Analysis of differential PDT effect in rat bladder tumor models according to concentrations of intravesical hexyl-aminolevulinate. Photochem. Photobiol. Sci. 2008, 7, 1018–1024. [Google Scholar] [CrossRef]
- Millon, S.R.; Ostrander, J.H.; Yazdanfar, S.; Brown, J.Q.; Bender, J.E.; Rajeha, A.; Ramanujam, N. Preferential accumulation of 5-aminolevulinic acid-induced protoporphyrin IX in breast cancer: A comprehensive study on six breast cell lines with varying phenotypes. J. Biomed. Opt. 2010, 15, 018002. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Deveci Ozkan, A.; Sozen Kucukkara, E.; Kamanli, A.F.; Gunoglu, B.; Yildiz, M.Z. Optimization of 5-aminolevulinic acid-based photodynamic therapy protocol for breast cancer cells. Photodiagnosis Photodyn. Ther. 2020, 31, 101854. [Google Scholar] [CrossRef] [PubMed]

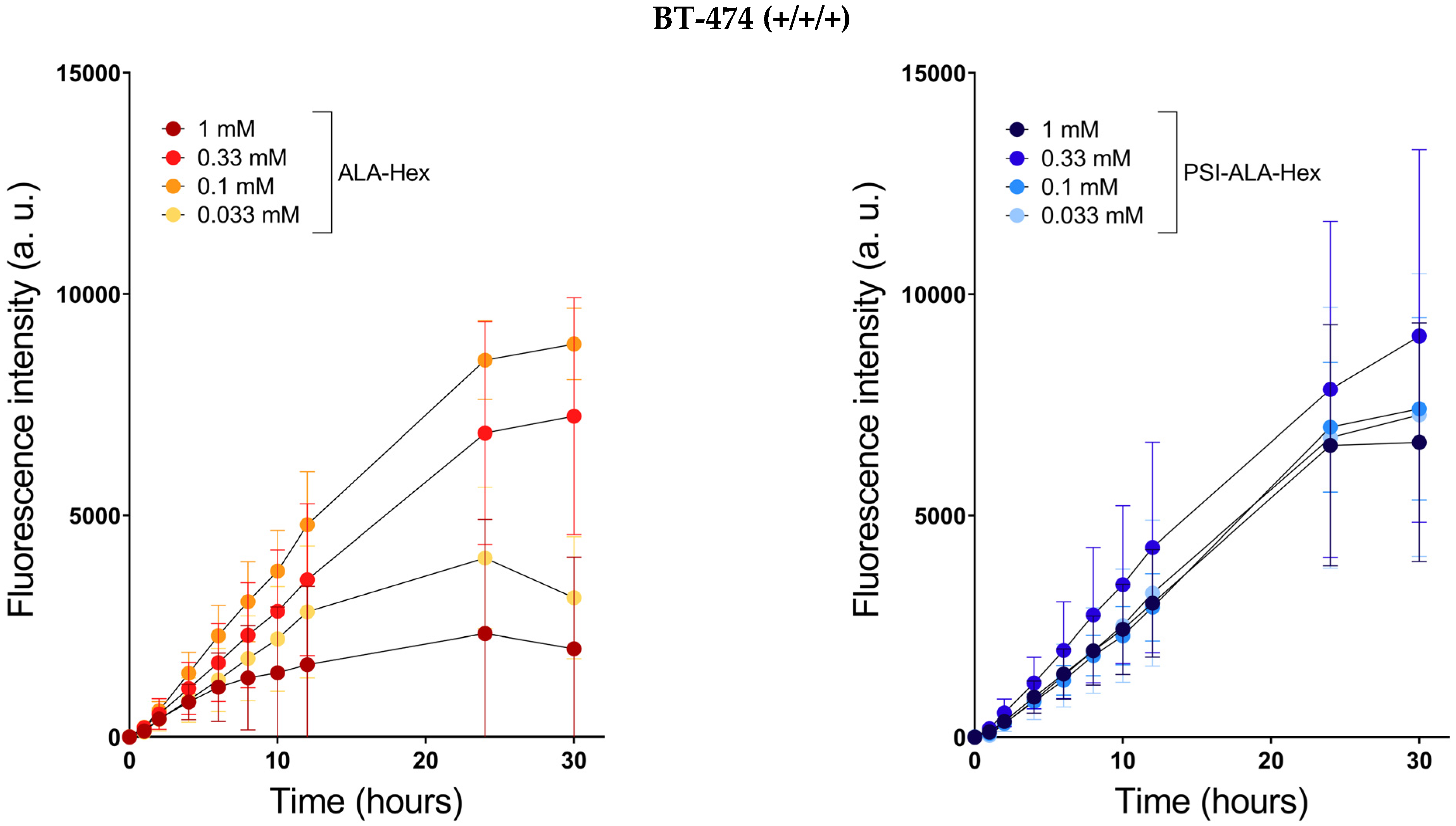
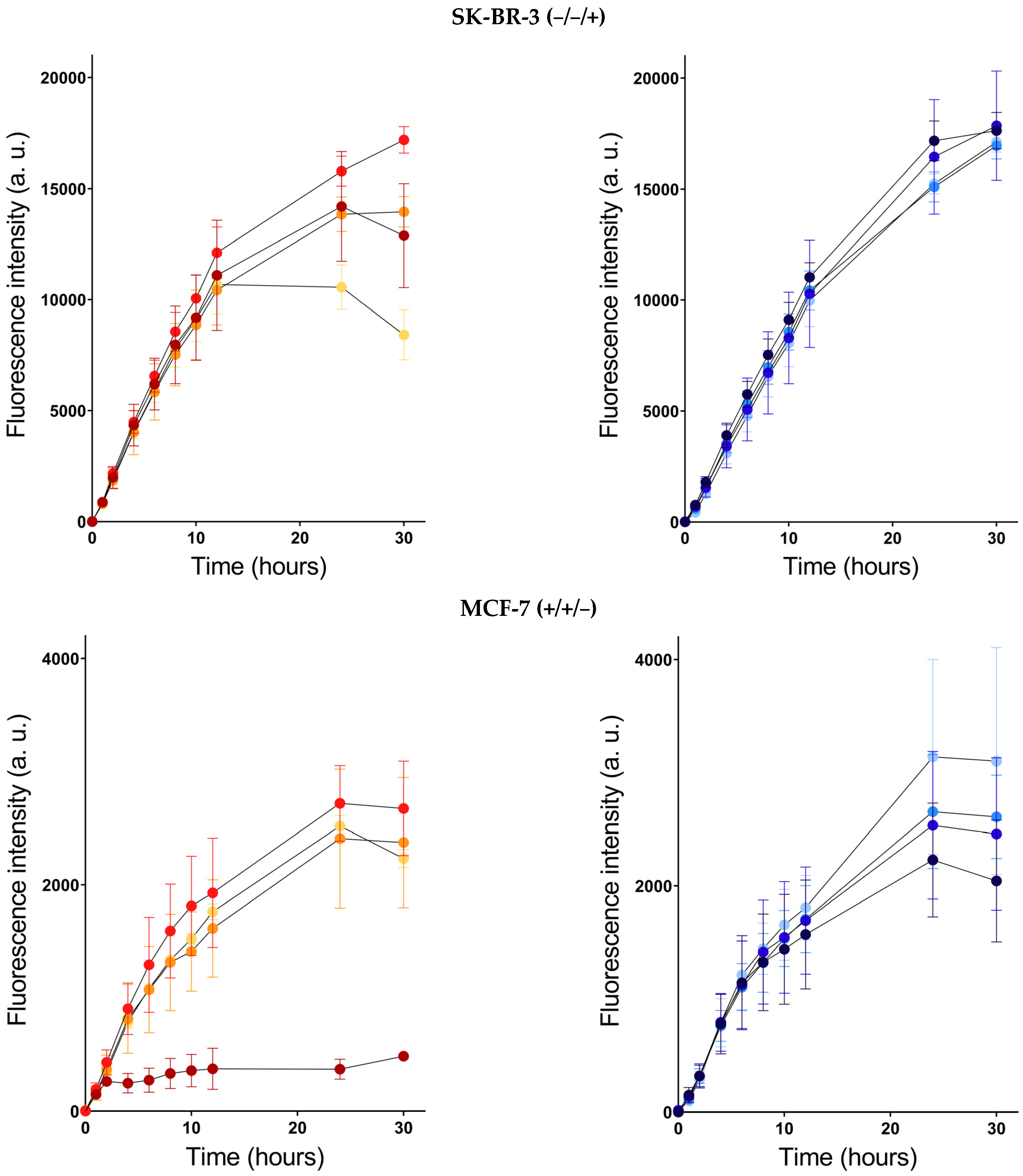

 ) and PSI-ALA-Hex (
) and PSI-ALA-Hex ( ) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM after 30 h of incubation by WST-1 viability measurement in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro.
) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM after 30 h of incubation by WST-1 viability measurement in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro.
 ) and PSI-ALA-Hex (
) and PSI-ALA-Hex ( ) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM after 30 h of incubation by WST-1 viability measurement in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro.
) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM after 30 h of incubation by WST-1 viability measurement in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro.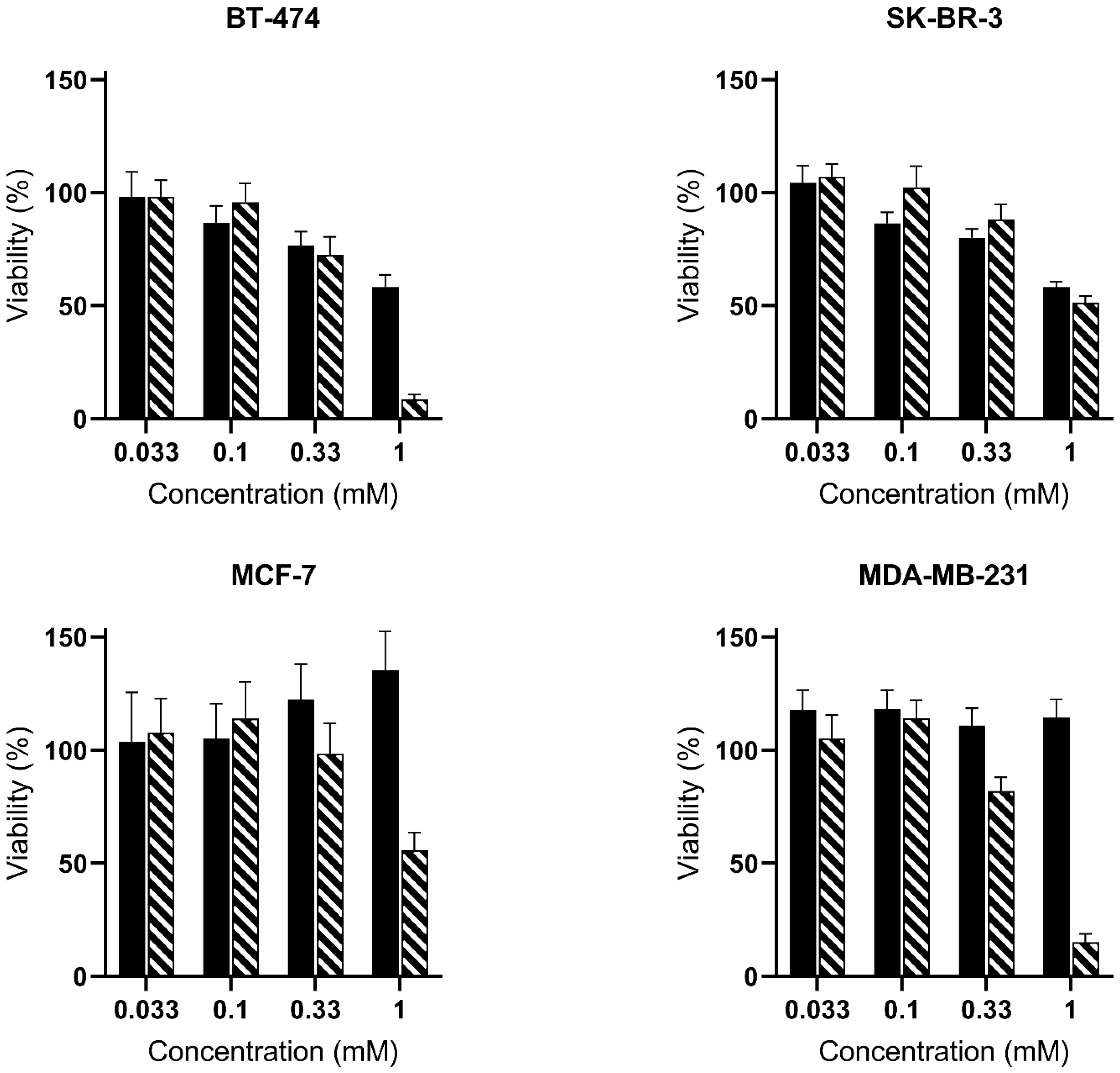
 ) and PSI-ALA-Hex (
) and PSI-ALA-Hex ( ) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro. Cells were incubated for 6 h then exposed to blue light (5 J/cm2), and the PDT efficacy was assessed 24 h post-irradiation by WST-1 viability measurement. Values are normalized against the untreated control exposed to the same blue light fluence.
) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro. Cells were incubated for 6 h then exposed to blue light (5 J/cm2), and the PDT efficacy was assessed 24 h post-irradiation by WST-1 viability measurement. Values are normalized against the untreated control exposed to the same blue light fluence.
 ) and PSI-ALA-Hex (
) and PSI-ALA-Hex ( ) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro. Cells were incubated for 6 h then exposed to blue light (5 J/cm2), and the PDT efficacy was assessed 24 h post-irradiation by WST-1 viability measurement. Values are normalized against the untreated control exposed to the same blue light fluence.
) at concentrations of 0.033 mM, 0.1 mM, 0.33 mM, and 1 mM in BT-474, SK-BR-3, MCF-7, and MDA-MB-231 breast cancer cells in vitro. Cells were incubated for 6 h then exposed to blue light (5 J/cm2), and the PDT efficacy was assessed 24 h post-irradiation by WST-1 viability measurement. Values are normalized against the untreated control exposed to the same blue light fluence.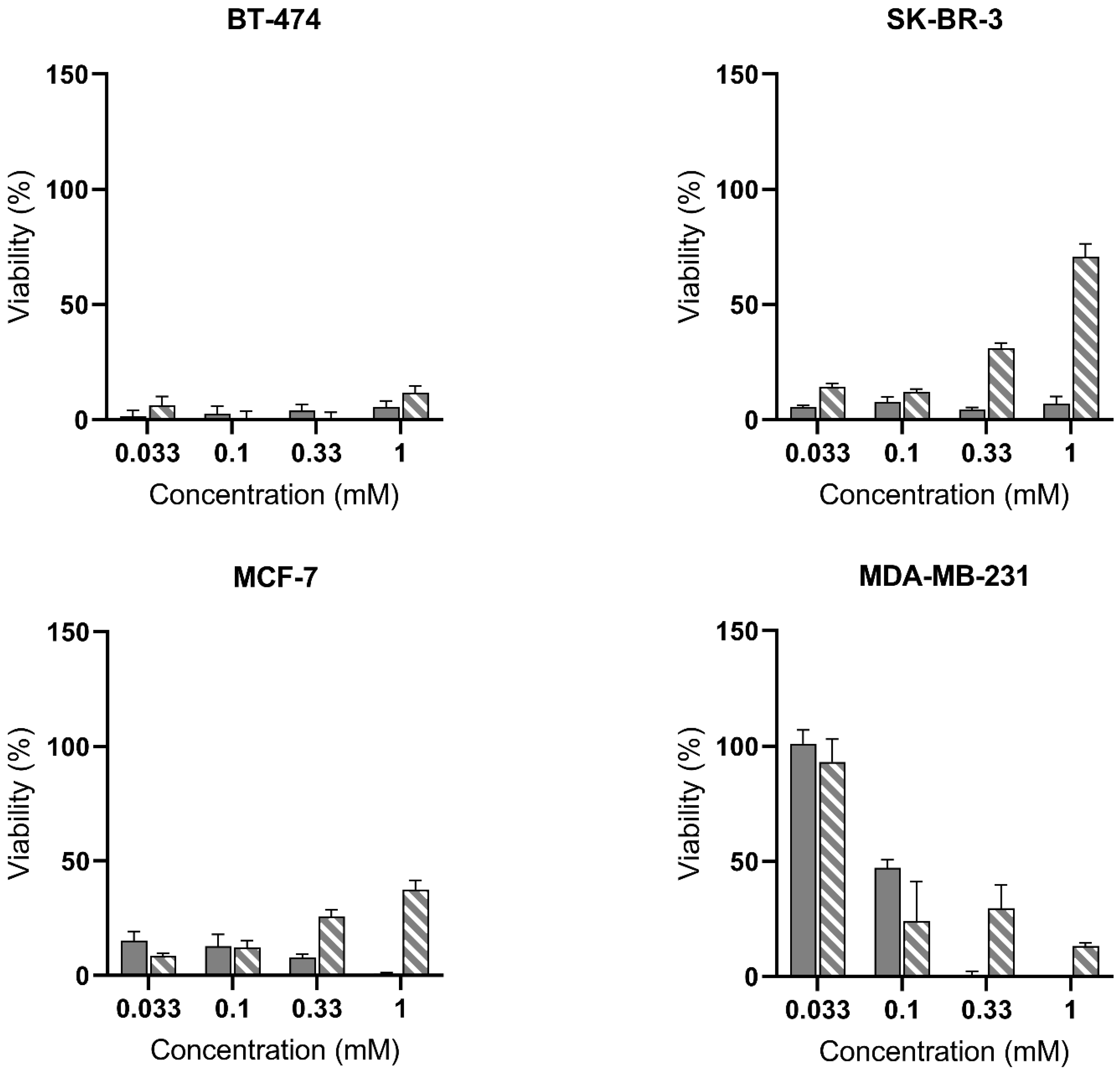
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiening, M.; Lange, N. Enlarging the Scope of 5-Aminolevulinic Acid-Mediated Photodiagnosis towards Breast Cancers. Int. J. Mol. Sci. 2022, 23, 14900. https://doi.org/10.3390/ijms232314900
Kiening M, Lange N. Enlarging the Scope of 5-Aminolevulinic Acid-Mediated Photodiagnosis towards Breast Cancers. International Journal of Molecular Sciences. 2022; 23(23):14900. https://doi.org/10.3390/ijms232314900
Chicago/Turabian StyleKiening, Martin, and Norbert Lange. 2022. "Enlarging the Scope of 5-Aminolevulinic Acid-Mediated Photodiagnosis towards Breast Cancers" International Journal of Molecular Sciences 23, no. 23: 14900. https://doi.org/10.3390/ijms232314900
APA StyleKiening, M., & Lange, N. (2022). Enlarging the Scope of 5-Aminolevulinic Acid-Mediated Photodiagnosis towards Breast Cancers. International Journal of Molecular Sciences, 23(23), 14900. https://doi.org/10.3390/ijms232314900








