Whole-Head Functional Near-Infrared Spectroscopy as an Ecological Monitoring Tool for Assessing Cortical Activity in Parkinson’s Disease Patients at Different Stages
Abstract
1. Introduction
2. Results
2.1. Patient Data Characterization
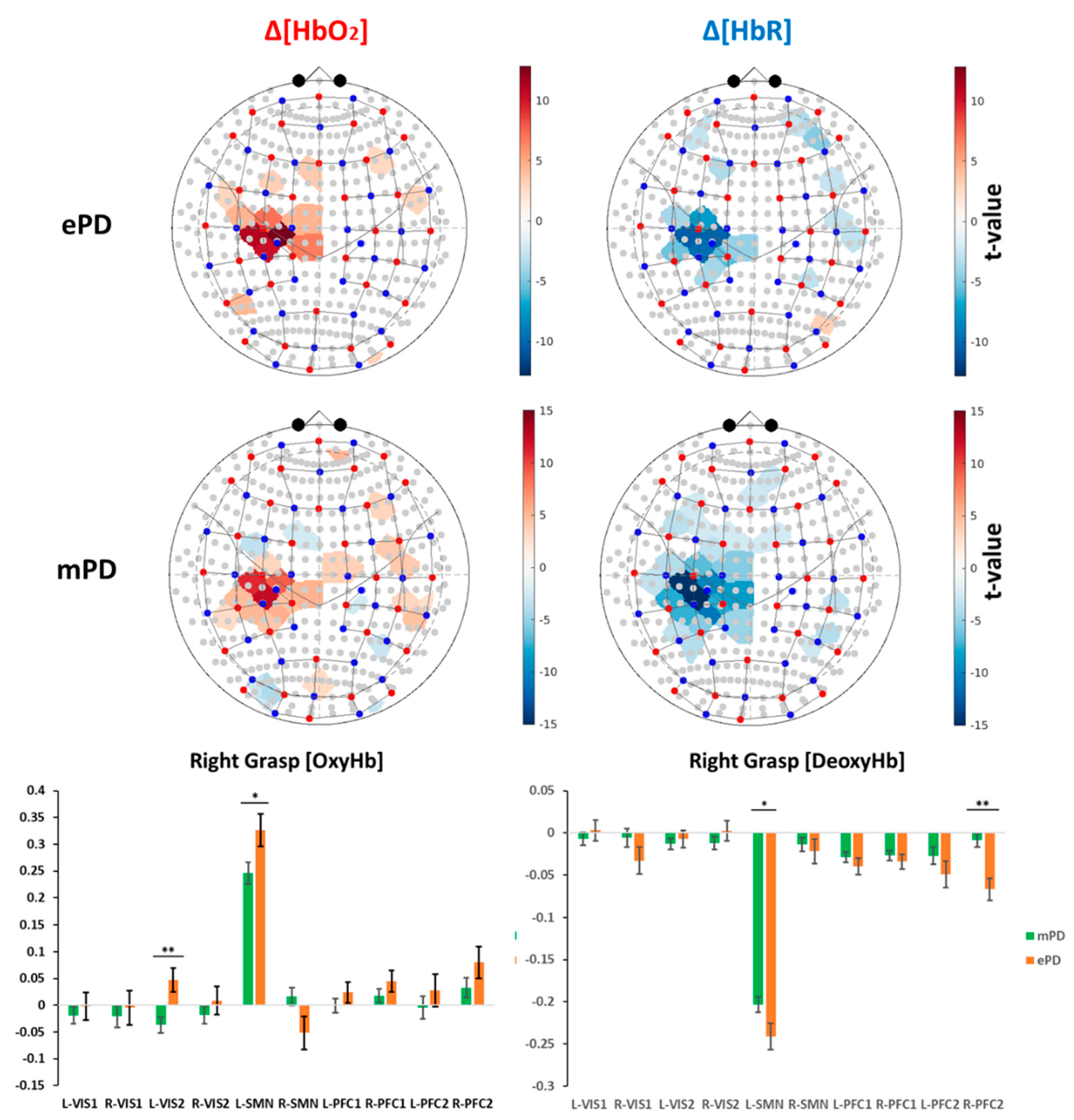
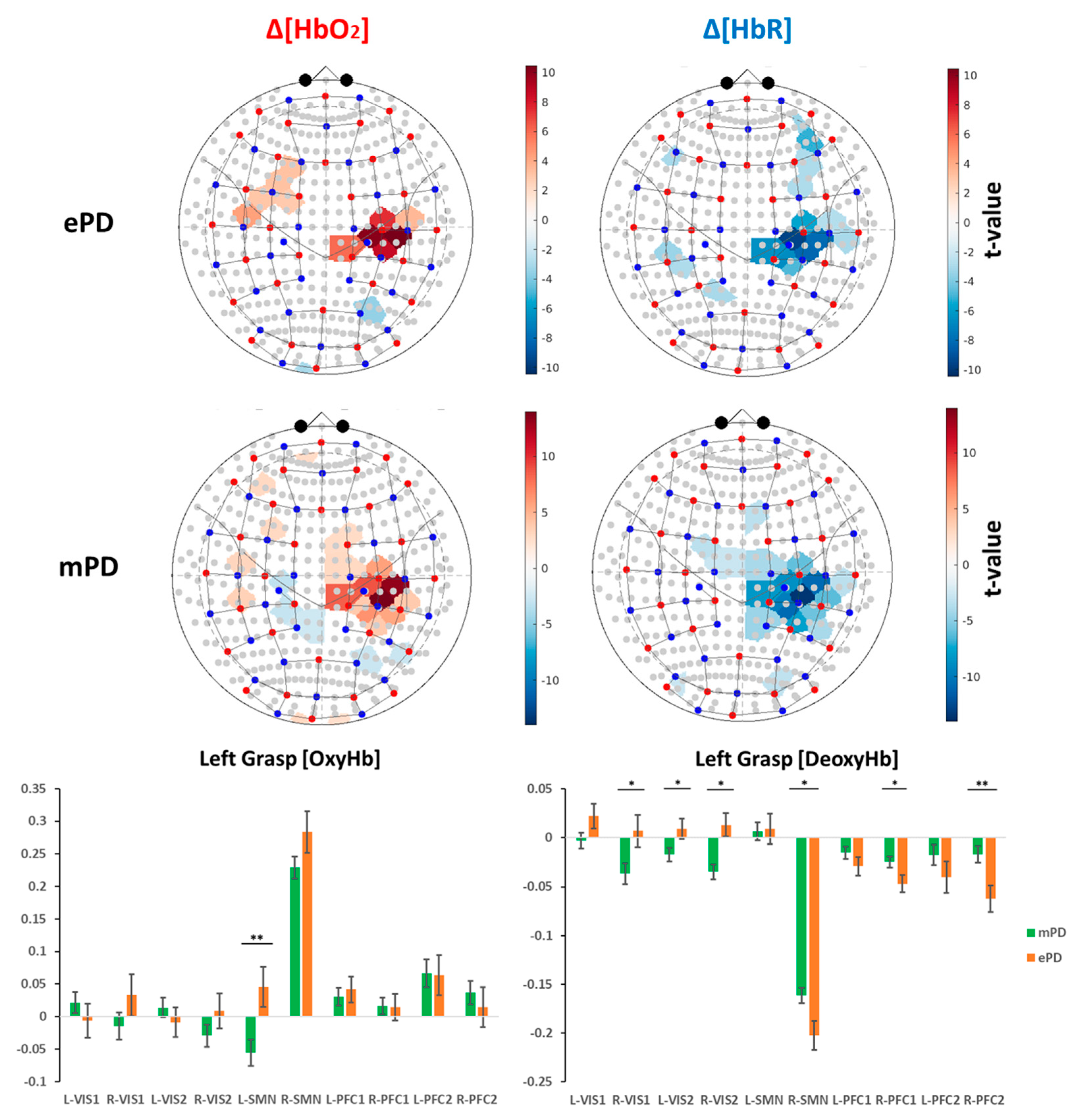
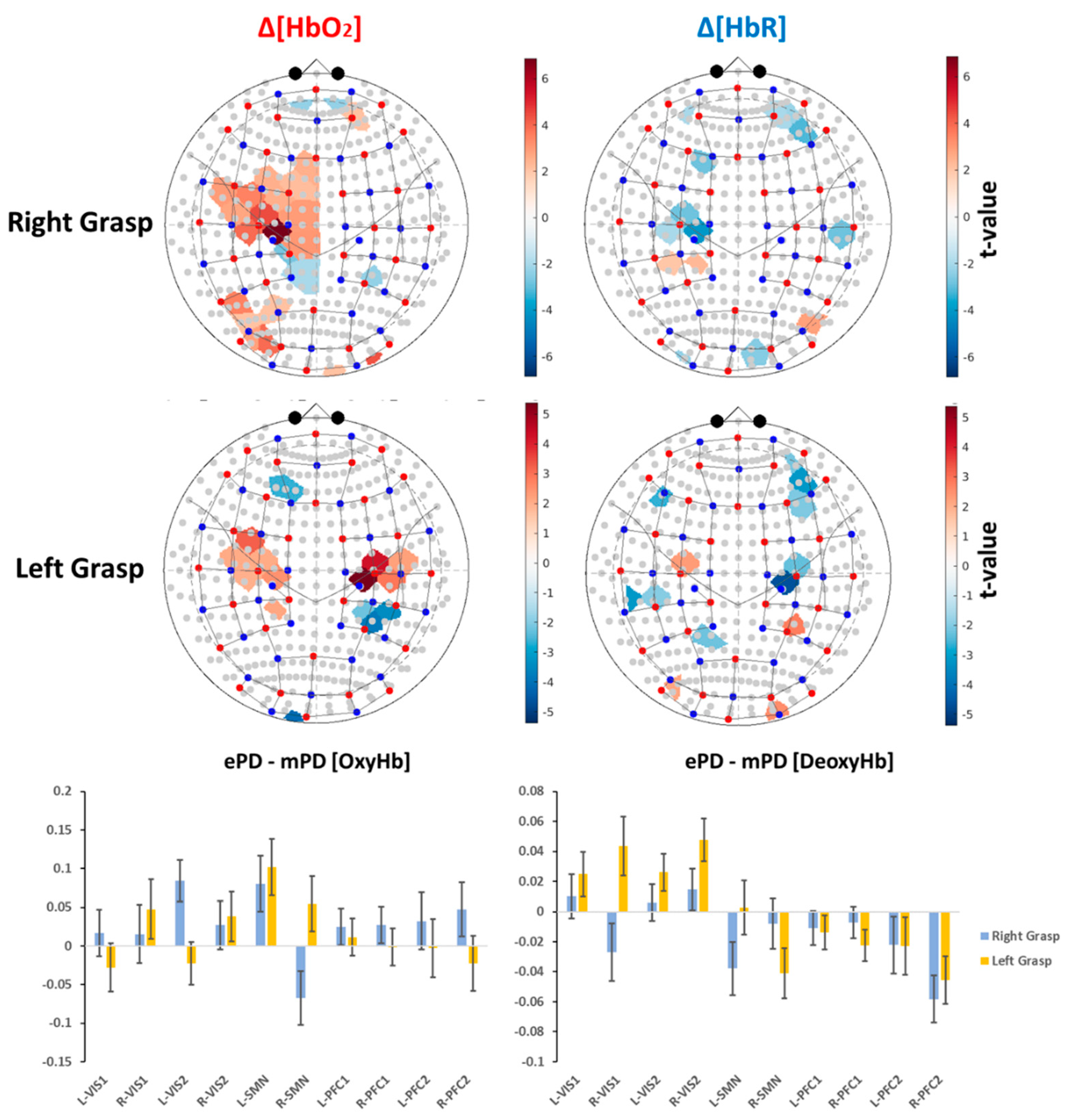
2.2. Group-Level Activation Maps
2.3. ROI-Based Correlation Analysis
2.4. fNIRS Results’ Interpretation
3. Discussion
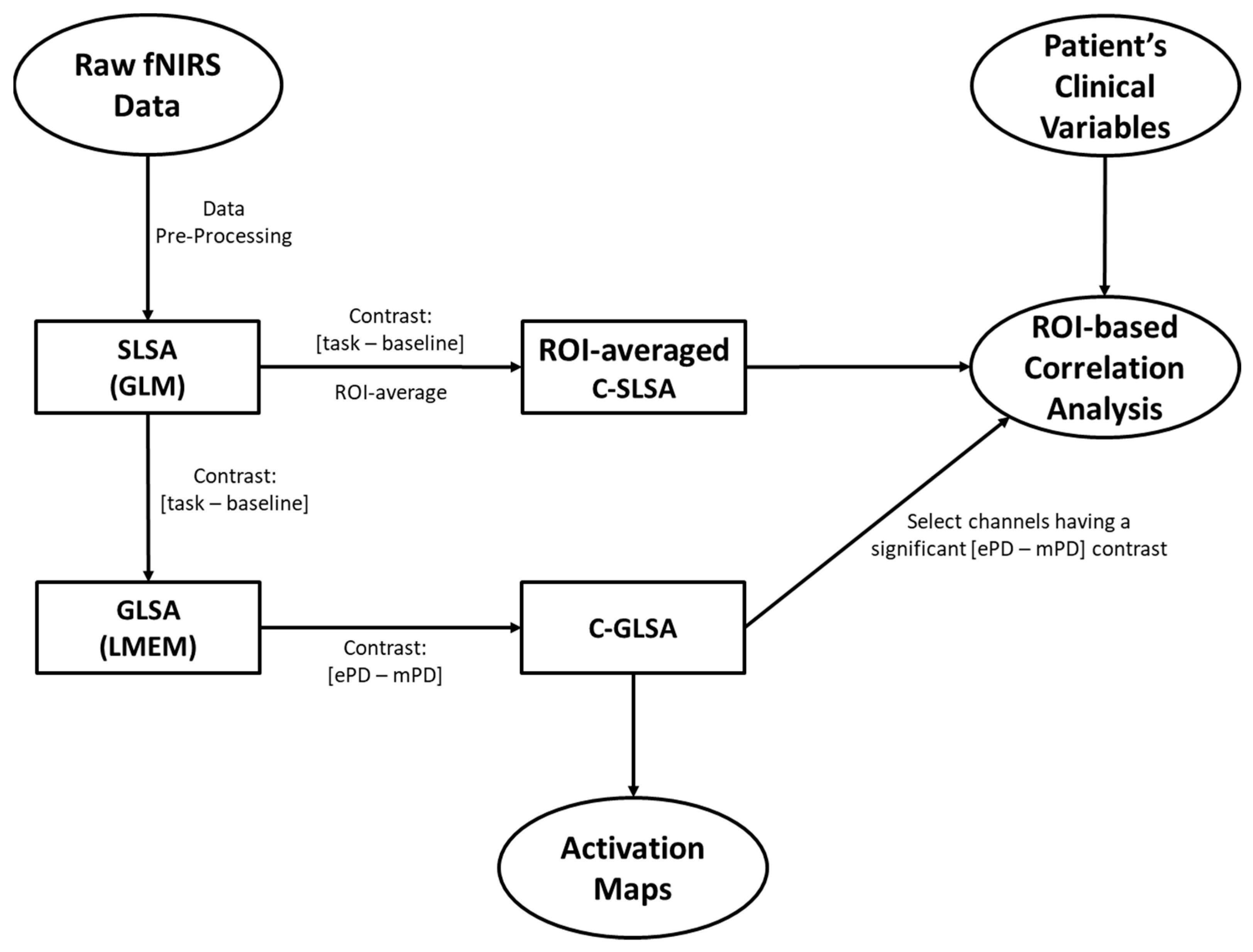
4. Materials and Methods
4.1. Participants
- age between 18 and 85 years (adult and older adult);
- agreement to participate, with signature on the informed consent form;
- presence of comorbidities that might determine clinical instability (i.e., severe orthopedic or severe cognitive deficits);
- overlapping between PD and other neurological pathologies or by PD with severe psychiatric complications, based on a pathological score in a screening test for cognitive impairment (Montreal Cognitive Assessment test—MoCA test < 17.54 [47])
4.2. Clinical Charaterization and Neuropsychological Assesment
4.3. fNIRS Assessment
4.4. Pre-Processing and Subject-Level Statistics of the fNIRS Data
4.5. Group-Level Statistics of fNIRS data
4.6. ROI-Based Correlation Analysis between fNIRS and Clinical Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boas, D.A.; Elwell, C.E.; Ferrari, M.; Taga, G. Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. NeuroImage 2013, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Pavia, J.M.; Wolf, U.; Wolf, M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 2014, 85, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-D.; Tsytsarev, V.; Delgado-Martínez, I.; Li, M.-L.; Erzurumlu, R.; Vipin, A.; Orellana, J.; Lin, Y.-R.; Lai, H.-Y.; Chen, Y.-Y.; et al. Neurovascular coupling: In vivo optical techniques for functional brain imaging. Biomed. Eng. Online 2013, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Delpy, D.T.; Cope, M.; Van Der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I. Review of functional near-infrared spectroscopy in neurorehabilitation. Neurophotonics 2016, 3, 031414. [Google Scholar] [CrossRef]
- Cutini, S.; Moro, S.B.; Bisconti, S. Functional near Infrared Optical Imaging in Cognitive Neuroscience: An Introductory Review. J. Near Infrared Spectrosc. 2012, 20, 75–92. [Google Scholar] [CrossRef]
- Gramigna, V.; Pellegrino, G.; Cerasa, A.; Cutini, S.; Vasta, R.; Olivadese, G.; Martino, I.; Quattrone, A. Near-Infrared Spectroscopy in Gait Disorders: Is It Time to Begin? Neurorehabilit. Neural Repair 2017, 31, 402–412. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Zappasodi, F.; Di Pompeo, F.; Merla, A. Simultaneous functional near-infrared spectroscopy and electroencephalography for monitoring of human brain activity and oxygenation: A review. Neurophotonics 2017, 4, 041411. [Google Scholar] [CrossRef] [PubMed]
- Aasted, C.M.; Yucel, M.; Cooper, R.; Dubb, J.; Tsuzuki, D.; Becerra, L.; Petkov, M.P.; Borsook, D.; Dan, I.; Boas, D.A. Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics 2015, 2, 020801. [Google Scholar] [CrossRef]
- Forbes, S.H.; Wijeakumar, S.; Eggebrecht, A.T.; Magnotta, V.A.; Spencer, J.P. Processing pipeline for image reconstructed fNIRS analysis using both MRI templates and individual anatomy. Neurophotonics 2021, 8, 025010. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, D.; Dan, I. Spatial registration for functional near-infrared spectroscopy: From channel position on the scalp to cortical location in individual and group analyses. NeuroImage 2014, 85, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, Z.; Yuan, T.; Feng, W.; Wang, P. A Systemic Review of Functional Near-Infrared Spectroscopy for Stroke: Current Application and Future Directions. Front. Neurol. 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Obrig, H. NIRS in clinical neurology—A ‘promising’ tool? Neuroimage 2014, 85, 535–546. [Google Scholar] [CrossRef]
- Bonilauri, A.; Intra, F.S.; Pugnetti, L.; Baselli, G.; Baglio, F. A Systematic Review of Cerebral Functional Near-Infrared Spectroscopy in Chronic Neurological Diseases—Actual Applications and Future Perspectives. Diagnostics 2020, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Liu, T.; Tang, W.C.; Song, C.; Jin, M.; Ren, L.; Liang, Z. Greater prefrontal activation during sitting toe tapping predicts severer freezing of gait in Parkinson’s disease: An fNIRS study. Cereb. Cortex 2022, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Maidan, I.; Bernad-Elazari, H.; Gazit, E.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: An fNIRS study of transient motor-cognitive failures. J. Neurol. 2015, 262, 899–908. [Google Scholar] [CrossRef]
- Maidan, I.; Bernad-Elazari, H.; Giladi, N.; Hausdorff, J.M.; Mirelman, A. When is Higher Level Cognitive Control Needed for Locomotor Tasks Among Patients with Parkinson’s Disease? Brain Topogr. 2017, 30, 531–538. [Google Scholar] [CrossRef]
- Dagan, M.; Herman, T.; Bernad-Elazari, H.; Gazit, E.; Maidan, I.; Giladi, N.; Mirelman, A.; Manor, B.; Hausdorff, J.M. Dopaminergic therapy and prefrontal activation during walking in individuals with Parkinson’s disease: Does the levodopa overdose hypothesis extend to gait? J. Neurol. 2020, 268, 658–668. [Google Scholar] [CrossRef]
- Orcioli-Silva, D.; Vitório, R.; Nóbrega-Sousa, P.; Beretta, V.S.; da Conceição, N.R.; Oliveira, A.S.; Pereira, M.P.; Gobbi, L.T.B. Cortical Activity Underlying Gait Improvements Achieved With Dopaminergic Medication During Usual Walking and Obstacle Avoidance in Parkinson Disease. Neurorehabilit. Neural Repair 2021, 35, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.R.; Holtzer, R.; Izzetoglu, M.; Zemon, V.; Verghese, J.; Allali, G. The role of prefrontal cortex during postural control in Parkinsonian syndromes a functional near-infrared spectroscopy study. Brain Res. 2015, 1633, 126–138. [Google Scholar] [CrossRef]
- Nieuwhof, F.; Reelick, M.F.; Maidan, I.; Mirelman, A.; Hausdorff, J.M.; Rikkert, M.G.O.; Bloem, B.R.; Muthalib, M.; Claassen, J.A. Measuring prefrontal cortical activity during dual task walking in patients with Parkinson’s disease: Feasibility of using a new portable fNIRS device. Pilot Feasibility Stud. 2016, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Pelicioni, P.H.S.; Lord, S.R.; Okubo, Y.; Sturnieks, D.L.; Menant, J.C. People With Parkinson’s Disease Exhibit Reduced Cognitive and Motor Cortical Activity When Undertaking Complex Stepping Tasks Requiring Inhibitory Control. Neurorehabilit. Neural Repair 2020, 34, 1088–1098. [Google Scholar] [CrossRef]
- Pelicioni, P.; Lord, S.; Okubo, Y.; Menant, J. Cortical activation during gait adaptability in people with Parkinson’s disease. Gait Posture 2021, 91, 247–253. [Google Scholar] [CrossRef]
- Thumm, P.C.; Maidan, I.; Brozgol, M.; Shustak, S.; Gazit, E.; Shiratzki, S.S.; Bernad-Elazari, H.; Beck, Y.; Giladi, N.; Hausdorff, J.M.; et al. Treadmill walking reduces pre-frontal activation in patients with Parkinson’s disease. Gait Posture 2018, 62, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Bloem, B.R.; Giladi, N.; Hausdorff, J.M.; Claassen, J.A.H.R.; Mirelman, A. Evidence for Differential Effects of 2 Forms of Exercise on Prefrontal Plasticity During Walking in Parkinson’s Disease. Neurorehabilit. Neural Repair 2018, 32, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Reelick, M.F.; Bloem, B.R.; Giladi, N.; Deutsch, J.E.; Hausdorff, J.M.; Claassen, J.A.H.; Mirelman, A. The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson’s Disease and Healthy Older Adults. Neurorehabilit. Neural Repair 2016, 30, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.; Vitorio, R.; Morris, R.; Martini, D.N.; Fino, P.C.; Mancini, M. Cortical activity during walking and balance tasks in older adults and in people with Parkinson’s disease: A structured review. Maturitas 2018, 113, 53–72. [Google Scholar] [CrossRef]
- Hoang, I.; Ranchet, M.; Cheminon, M.; Derollepot, R.; Devos, H.; Perrey, S.; Luauté, J.; Danaila, T.; Paire-Ficout, L. An intensive exercise-based training program reduces prefrontal activity during usual walking in patients with Parkinson’s disease. Clin. Park. Relat. Disord. 2022, 6, 100128. [Google Scholar] [CrossRef]
- Stuart, S.; Belluscio, V.; Quinn, J.F.; Mancini, M. Pre-frontal Cortical Activity During Walking and Turning Is Reliable and Differentiates Across Young, Older Adults and People With Parkinson’s Disease. Front. Neurol. 2019, 10, 536. [Google Scholar] [CrossRef]
- Vitorio, R.; Stuart, S.; Rochester, L.; Alcock, L.; Pantall, A. fNIRS response during walking—Artefact or cortical activity? A systematic review. Neurosci. Biobehav. Rev. 2017, 83, 160–172. [Google Scholar] [CrossRef]
- Bishnoi, A.; Holtzer, R.; Hernandez, M. Brain Activation Changes While Walking in Adults with and without Neurological Disease: Systematic Review and Meta-Analysis of Functional Near-Infrared Spectroscopy Studies. Brain Sci. 2021, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, K.; Katayama, Y.; Yamamoto, T.; Suzuki, S. Changes in cerebral blood oxygenation of the frontal lobe induced by direct electrical stimulation of thalamus and globus pallidus: A near infrared spectroscopy study. J. Neurol. Neurosurg. Psychiatry 1999, 67, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.S.; Neimat, J.; Folley, B.S.; Bourne, S.K.; Konrad, P.E.; Charles, D.; Park, S. Deep brain stimulation of the subthalamic nucleus alters frontal activity during spatial working memory maintenance of patients with Parkinson’s disease. Neurocase 2016, 22, 369–378. [Google Scholar] [CrossRef]
- Morishita, T.; Higuchi, M.-A.; Saita, K.; Tsuboi, Y.; Abe, H.; Inoue, T. Changes in Motor-Related Cortical Activity Following Deep Brain Stimulation for Parkinson’s Disease Detected by Functional Near Infrared Spectroscopy: A Pilot Study. Front. Hum. Neurosci. 2016, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.K.; Schumacher, L.V.; Amtage, F.; Horn, A.; Egger, K.; Piroth, T.; Weiller, C.; Schelter, B.O.; Coenen, V.A.; Kaller, C.P. The rostro-caudal gradient in the prefrontal cortex and its modulation by subthalamic deep brain stimulation in Parkinson’s disease. Sci. Rep. 2021, 11, 2138. [Google Scholar] [CrossRef]
- Yu, N.; Liang, S.; Lu, J.; Shu, Z.; Li, H.; Yu, Y.; Wu, J.; Han, J. Quantified assessment of deep brain stimulation on Parkinson’s patients with task fNIRS measurements and functional connectivity analysis: A pilot study. Chin. Neurosurg. J. 2021, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, A.T.; Ferradal, S.L.; Robichaux-Viehoever, A.; Hassanpour, M.S.; Dehghani, H.; Snyder, A.Z.; Hershey, T.; Culver, J.P. Mapping distributed brain function and networks with diffuse optical tomography. Nat. Photon 2014, 8, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Baglieri, A.; Marino, M.A.; Morabito, R.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Differences between conventional and non-conventional MRI techniques in Parkinson’s disease. Funct. Neurol. 2013, 28, 73–82. [Google Scholar]
- Pagano, G.; Niccolini, F.; Politis, M. Imaging in Parkinson’s disease. Clin. Med. 2016, 16, 371–375. [Google Scholar] [CrossRef]
- Pelizzari, L.; Laganà, M.M.; Rossetto, F.; Bergsland, N.; Galli, M.; Baselli, G.; Clerici, M.; Nemni, R.; Baglio, F. Cerebral blood flow and cerebrovascular reactivity correlate with severity of motor symptoms in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419838354. [Google Scholar] [CrossRef]
- Laganà, M.M.; Pirastru, A.; Pelizzari, L.; Rossetto, F.; Di Tella, S.; Bergsland, N.; Nemni, R.; Meloni, M.; Baglio, F. Multimodal Evaluation of Neurovascular Functionality in Early Parkinson’s Disease. Front. Neurol. 2020, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Baglio, F.; Pirastru, A.; Bergsland, N.; Cazzoli, M.; Tavazzi, E. Neuroplasticity mediated by motor rehabilitation in Parkinson’s disease: A systematic review on structural and functional MRI markers. Rev. Neurosci. 2021, 33, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. The influence of normal human ageing on automatic movements. J. Physiol. 2005, 562, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, F.; Borgnis, F.; Blasi, V.; Banfi, P.I.; Tavanelli, M.; Realdon, O.; Mantovani, F.; Foglia, E.; Garagiola, E.; Croce, D.; et al. System Integrated Digital Empowerment and Rehabilitation to promote patient Activation and well-Being (SIDERA^B): Protocol for a Randomized Crossover Trial on Effectiveness and Implementation. medRxiv 2022. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. MovementDisorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Conti, S.; Bonazzi, S.; Laiacona, M.; Masina, M.; Coralli, M.V. Montreal Cognitive Assessment (MoCA)-Italian version: Regression based norms and equivalent scores. Neurol. Sci. 2015, 36, 209–214. [Google Scholar] [CrossRef]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar]
- Janssen, M.F.; Pickard, A.S.; Golicki, D.; Gudex, C.; Niewada, M.; Scalone, L.; Swinburn, P.; Busschbach, J. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: A multi-country study. Qual. Life Res. 2012, 22, 1717–1727. [Google Scholar] [CrossRef]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. A short version of the Stroop test: Normative data in an Italian population sample | Una versione abbreviata del test di Stroop: Dati normativi nella popolazione Italiana. Nuova Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Jurcak, V.; Tsuzuki, D.; Dan, I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. Neuroimage 2007, 34, 1600–1611. [Google Scholar] [CrossRef]
- Kashou, N.H.; Giacherio, B.M.; Nahhas, R.W.; Jadcherla, S.R. Hand-grasping and finger tapping induced similar functional near-infrared spectroscopy cortical responses. Neurophotonics 2016, 3, 25006. [Google Scholar] [CrossRef]
- Yücel, M.A.; Lühmann, A.V.; Scholkmann, F.; Gervain, J.; Dan, I.; Ayaz, H.; Boas, D.; Cooper, R.J.; Culver, J.; Elwell, C.E.; et al. Best practices for fNIRS publications. Neurophotonics 2021, 8, 012101. [Google Scholar] [CrossRef]
- Pinti, P.; Scholkmann, F.; Hamilton, A.; Burgess, P.; Tachtsidis, I. Current Status and Issues Regarding Pre-processing of fNIRS Neuroimaging Data: An Investigation of Diverse Signal Filtering Methods Within a General Linear Model Framework. Front. Hum. Neurosci. 2019, 12, 505. [Google Scholar] [CrossRef]
- Pfeifer, M.D.; Scholkmann, F.; Labruyère, R. Signal Processing in Functional Near-Infrared Spectroscopy (fNIRS): Methodological Differences Lead to Different Statistical Results. Front. Hum. Neurosci. 2018, 11, 641. [Google Scholar] [CrossRef]
- Hocke, L.M.; Oni, I.K.; Duszynski, C.C.; Corrigan, A.V.; Frederick, B.D.; Dunn, J.F. Automated Processing of fNIRS Data—A Visual Guide to the Pitfalls and Consequences. Algorithms 2018, 11, 67. [Google Scholar] [CrossRef]
- Bonilauri, A.; Intra, F.S.; Baselli, G.; Baglio, F. Assessment of fNIRS Signal Processing Pipelines: Towards Clinical Applications. Appl. Sci. 2021, 12, 316. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The NIRS Brain AnalyzIR Toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef]
- Molavi, B.; Dumont, G.A. Wavelet-based motion artifact removal for functional near-infrared spectroscopy. Physiol. Meas. 2012, 33, 259–270. [Google Scholar] [CrossRef]
- Yücel, M.A.; Selb, J.; Aasted, C.M.; Lin, P.-Y.; Borsook, D.; Becerra, L.; Boas, D.A. Mayer waves reduce the accuracy of estimated hemodynamic response functions in functional near-infrared spectroscopy. Biomed. Opt. Express 2016, 7, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics 2016, 3, 031405. [Google Scholar] [CrossRef] [PubMed]
- Tak, S.; Ye, J.C. Statistical analysis of fNIRS data: A comprehensive review. NeuroImage 2014, 85, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J. Commentary on the statistical properties of noise and its implication on general linear models in functional near-infrared spectroscopy. Neurophotonics 2016, 3, 010401. [Google Scholar] [CrossRef]
- Barker, J.W.; Aarabi, A.; Huppert, T.J. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed. Opt. Express 2013, 4, 1366–1379. [Google Scholar] [CrossRef]
- Penny, W.; Friston, K.; Ashburner, J.; Kiebel, S.; Nichols, T. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Collins, D.; Zijdenbos, A.; Kollokian, V.; Sled, J.; Kabani, N.; Holmes, C.; Evans, A. Design and construction of a realistic digital brain phantom. IEEE Trans. Med. Imaging 1998, 17, 463–468. [Google Scholar] [CrossRef]
- Strangman, G.E.; Li, Z.; Zhang, Q. Depth Sensitivity and Source-Detector Separations for Near Infrared Spectroscopy Based on the Colin27 Brain Template. PLoS ONE 2013, 8, e66319. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Cooper, R.J.; Caffini, M.; Dubb, J.; Fang, Q.; Custo, A.; Tsuzuki, D.; Fischl, B.; Wells, W.; Dan, I.; Boas, D.A. Validating atlas-guided DOT: A comparison of diffuse optical tomography informed by atlas and subject-specific anatomies. NeuroImage 2012, 62, 1999–2006. [Google Scholar] [CrossRef]
| ePD (N = 13) Mean (SE) | mPD (N = 26) Mean (SE) | ePD vs. mPD (dof, p) | |
|---|---|---|---|
| Age | 63.519 (1.6549) | 71.676 (1.3652) | 55.5 (37, <0.001) (**) |
| Sex | M/F 6/7 (46.15%) | M/F 14/12 (53.85%) | - |
| Handedness | R/L 12/1 (92.31%) | R/L 26/0 (100%) | - |
| Education | 13.308 (1.0824) | 11.423 (0.7425) | 1.4508 (37, 0.155) (*) |
| CRIQ | 131.385 (6.1899) | 122.577 (3.8021) | 1.272 (37, 0.211) (*) |
| Disease duration | 2.903 (0.8005) | 5.049 (0.4695) | 87 (37, 0.014) (**) |
| HY | 1.423 (0.0521) | 2.327 (0.0731) | - |
| UPDRS | 16.077 (1.5626) | 35.654 (2.3993) | −5.4598 (37, <0.001) (*) |
| LEDD | 405.385 (56.430) | 538.961 (44.282) | 1.797 (37, 0.08) (*) |
| MOCA | 25.162 (0.798) | 23.764 (0.536) | −1.479 (37, 0.148) |
| SCWE | 9.083 (3.2543) | 8.88 (1.6677) | 132.5 (35, 0.58) (**) |
| SCWT | 12.693 (2.4129) | 18.716 (3.1002) | 105 (34, 0.196) (**) |
| EQ5D5L | 0.841 (0.0321) | 0.754 (0.0248) | 96.5 (37, 0.031) **) |
| ROI | Type | Contrast | Beta | T | p | |||
|---|---|---|---|---|---|---|---|---|
| ePD | mPD | ePD | mPD | ePD | mPD | |||
| L-SMN | Left Grasp | 0.046 | −0.056 | 1.499 | −2.748 | 0.136 | 0.007 (*) | |
| Right Grasp | 0.326 | 0.246 | 10.837 | 12.321 | 0.000 (*) | 0.000 (*) | ||
| Right Grasp | −0.241 | −0.203 | −15.762 | −22.111 | 0.000 (*) | 0.000 (*) | ||
| R-SMN | Left Grasp | 0.283 | 0.229 | 8.972 | 13.543 | 0.000 (*) | 0.000 (*) | |
| Left Grasp | −0.203 | −0.161 | −13.815 | −19.861 | 0.000 (*) | 0.000 (*) | ||
| R-VIS1 | Left Grasp | 0.007 | −0.037 | 0.437 | −3.348 | 0.663 | 0.001 (*) | |
| Right Grasp | −0.033 | −0.006 | −2.046 | −0.549 | 0.043 | 0.584 | ||
| L-VIS2 | Left Grasp | 0.009 | −0.017 | 0.877 | −2.518 | 0.382 | 0.013 (*) | |
| Right Grasp | 0.047 | −0.037 | 2.112 | −2.480 | 0.036 | 0.014 | ||
| R-VIS2 | Left Grasp | 0.013 | −0.035 | 1.099 | −4.558 | 0.273 | 0.000 (*) | |
| L-PFC2 | Left Grasp | 0.064 | 0.067 | 2.067 | 3.150 | 0.040 | 0.002 (*) | |
| Left Grasp | −0.040 | −0.017 | −2.510 | −1.647 | 0.013 | 0.102 | ||
| Right Grasp | −0.049 | −0.027 | −3.106 | −2.588 | 0.002 (*) | 0.011 (*) | ||
| R-PFC2 | Left Grasp | 0.014 | 0.037 | 0.464 | 2.024 | 0.643 | 0.045 | |
| Left Grasp | −0.062 | −0.017 | −4.678 | −1.916 | 0.000 (*) | 0.057 | ||
| Right Grasp | 0.080 | 0.033 | 2.653 | 1.823 | 0.009 (*) | 0.070 | ||
| Right Grasp | −0.067 | −0.009 | −5.069 | −0.994 | 0.000 (*) | 0.322 | ||
| L-PFC1 | Left Grasp | 0.042 | 0.030 | 2.107 | 2.245 | 0.037 | 0.026 | |
| Left Grasp | −0.029 | −0.015 | −3.008 | −2.470 | 0.003 (*) | 0.015 | ||
| Right Grasp | −0.040 | −0.029 | −4.168 | −4.732 | 0.000 (*) | 0.000 (*) | ||
| R-PFC1 | Left Grasp | −0.047 | −0.024 | −5.297 | −4.238 | 0.000 (*) | 0.000 (*) | |
| Right Grasp | 0.045 | 0.018 | 2.253 | 1.402 | 0.026 | 0.163 | ||
| Right Grasp | −0.034 | −0.027 | −3.905 | −4.701 | 0.000 (*) | 0.000 (*) | ||
| ROI | Type | Contrast | Beta | SE | T | p | |
|---|---|---|---|---|---|---|---|
| L-SMN | Left Grasp | 0.102 | 0.037 | 2.768 | 0.006 | 0.029 | |
| Right Grasp | 0.080 | 0.036 | 2.228 | 0.027 | 0.088 | ||
| Right Grasp | −0.038 | 0.018 | −2.120 | 0.036 | 0.103 | ||
| R-SMN | Left Grasp | −0.041 | 0.017 | −2.452 | 0.015 | 0.054 | |
| R-VIS1 | Left Grasp | 0.044 | 0.020 | 2.223 | 0.028 | 0.088 | |
| L-VIS2 | Left Grasp | 0.026 | 0.012 | 2.112 | 0.036 | 0.103 | |
| Right Grasp | 0.084 | 0.027 | 3.129 | 0.002 | 0.012 | ||
| R-VIS2 | Left Grasp | 0.048 | 0.014 | 3.377 | 0.001 | 0.006 | |
| R-PFC2 | Left Grasp | −0.046 | 0.016 | −2.880 | 0.005 | 0.022 | |
| Right Grasp | −0.058 | 0.016 | −3.706 | 0.000 | 0.002 | ||
| R-PFC1 | Left Grasp | −0.022 | 0.011 | −2.119 | 0.036 | 0.103 |
| Contrast | ROI | Age | CRIQ | Disease Duration | UPDRS | SCWE | SCWT |
|---|---|---|---|---|---|---|---|
| Right Grasp Δ[HbO2] | R-VIS1 | 0.077 (0.639) | −0.083 (0.617) | −0.397 (0.012) | −0.035 (0.832) | 0.162 (0.338) | 0.005 (0.976) |
| R-VIS2 | −0.156 (0.343) | −0.009 (0.956) | −0.366 (0.022) | −0.388 (0.015) | −0.063 (0.711) | −0.174 (0.311) | |
| Left Grasp Δ[HbO2] | - | - | - | - | - | - | - |
| Right Grasp Δ[HbR] | R-VIS1 | 0.159 (0.334) | −0.159 (0.333) | −0.29 (0.073) | 0.04 (0.81) | 0.371 (0.024) | −0.047 (0.787) |
| L-SMN | 0.441 (0.005) | −0.059 (0.72) | 0.241 (0.139) | 0.22 (0.179) | −0.049 (0.773) | 0.248 (0.145) | |
| L-PFC2 | 0.275 (0.091) | −0.027 (0.873) | 0.05 (0.762) | 0.142 (0.388) | 0.245 (0.144) | 0.476 (0.003) | |
| R-PFC2 | 0.394 (0.013) | −0.236 (0.149) | −0.056 (0.734) | 0.192 (0.241) | 0.539 (<0.001) | 0.144 (0.403) | |
| L-PFC1 | 0.193 (0.239) | −0.1 (0.546) | 0.006 (0.969) | 0.208 (0.205) | 0.057 (0.736) | 0.354 (0.034) | |
| R-PFC1 | 0.329 (0.041) | −0.18 (0.274) | 0.001 (0.998) | 0.184 (0.263) | 0.464 (0.004) | 0.167 (0.33) | |
| Left Grasp Δ[HbR] | L-VIS2 | −0.366 (0.022) | 0.259 (0.112) | −0.217 (0.185) | −0.19 (0.248) | 0.13 (0.442) | 0.138 (0.421) |
| L-SMN | 0.152 (0.356) | −0.531 (<0.001) | 0.091 (0.583) | 0.071 (0.666) | 0.284 (0.089) | −0.057 (0.743) | |
| R-SMN | 0.345 (0.031) | −0.137 (0.405) | 0.242 (0.138) | 0.185 (0.26) | 0.132 (0.437) | 0.184 (0.282) | |
| L-PFC2 | 0.456 (0.004) | −0.145 (0.377) | 0.259 (0.111) | 0.335 (0.037) | 0.067 (0.693) | −0.016 (0.925) | |
| R-PFC2 | 0.115 (0.486) | 0.01 (0.953) | 0.138 (0.402) | 0.208 (0.204) | 0.252 (0.132) | 0.394 (0.017) | |
| R-PFC1 | 0.047 (0.777) | −0.024 (0.886) | 0.168 (0.307) | 0.2 (0.222) | 0.214 (0.203) | 0.398 (0.016) |
| Contrast | ROI | CRIQtotal | Disease Duration | UPDRS | SCWE | SCWT |
|---|---|---|---|---|---|---|
| Right Grasp Δ[HbO2] | R-VIS1 | −0.064 (0.704) | −0.41 (0.011) | −0.088 (0.6) | 0.158 (0.359) | −0.011 (0.95) |
| R-VIS2 | −0.055 (0.742) | −0.355 (0.029) | −0.363 (0.025) | 0.006 (0.971) | −0.147 (0.4) | |
| Left Grasp Δ[HbO2] | - | - | - | - | - | - |
| Right Grasp Δ[HbR] | R-VIS1 | −0.121 (0.468) | −0.314 (0.055) | −0.05 (0.765) | 0.347 (0.038) | −0.084 (0.63) |
| L-PFC2 | 0.054 (0.748) | 0.02 (0.903) | 0 (0.999) | 0.147 (0.391) | 0.448 (0.007) | |
| R-PFC2 | −0.143 (0.392) | −0.11 (0.512) | −0.014 (0.932) | 0.457 (0.005) | 0.073 (0.675) | |
| L-PFC1 | −0.049 (0.771) | −0.016 (0.926) | 0.128 (0.442) | −0.018 (0.919) | 0.318 (0.063) (*) | |
| R-PFC1 | −0.097 (0.561) | −0.039 (0.818) | 0.017 (0.919) | 0.382 (0.022) | 0.106 (0.543) | |
| Left Grasp Δ[HbR] | L-SMN | −0.515 (<0.001) | 0.075 (0.654) | −0.008 (0.961) | 0.259 (0.127) | −0.075 (0.67) |
| L-PFC2 | −0.022 (0.897) | 0.236 (0.154) | 0.131 (0.433) (*) | −0.105 (0.544) | −0.102 (0.559) | |
| R-PFC2 | 0.044 (0.795) | 0.127 (0.447) | 0.175 (0.294) | 0.218 (0.202) | 0.381 (0.024) | |
| R-PFC1 | −0.01 1 (0.947) | 0.164 (0.326) | 0.206 (0.215) | 0.205 (0.231) | 0.399 (0.018) |
| N | Mean (SE) | Skewness (SE) | Kurtosis (SE) | Shapiro–Wilk W (p) | |
|---|---|---|---|---|---|
| age | 39 | 68.957 (1.2226) | −0.538 (0.378) | −0.706 (0.741) | 0.944 (0.051) |
| sex | M/F 20/19 (51.28%) | - | - | - | - |
| handedness | R/L 38/1 (97.44%) | - | - | - | - |
| education | 39 | 12.051 (0.6212) | 0.179 (0.378) | −0.389 (0.741) | 0.94 (0.038) |
| CRIQ | 39 | 125.513 (3.2905) | −0.439 (0.378) | −0.231 (0.741) | 0.958 (0.154) |
| disease duration | 39 | 4.333 (0.4371) | 0.467 (0.378) | −0.223 (0.741) | 0.964 (0.244) |
| HY | 39 | 2.026 (0.0861) | 0.162 (0.378) | −0.623 (0.741) | 0.913 (0.005) |
| UPDRS | 39 | 29.128 (2.2412) | 0.574 (0.378) | −0.145 (0.741) | 0.954 (0.114) |
| MOCA | 39 | 24.23 (0.45) | 0.088 (0.378) | −0.344 (0.741) | 0.982 (0.768) |
| LEDD | 39 | 494.436 (36.05) | 0.839 (0.378) | 1.565 (0.741) | 0.947 (0.065) |
| SCWE | 37 | 8.946 (1.5174) | 0.922 (0.388) | −0.397 (0.759) | 0.855 (<0.001) |
| SCWT | 36 | 16.708 (2.2474) | 1.995 (0.393) | 6.544 (0.768) | 0.844 (<0.001) |
| EQ5D5L | 39 | 0.783 (0.0205) | −0.726 (0.378) | 0.194 (0.741) | 0.944 (0.051) |
| Functional Label | Functional Areas | Anatomical Areas |
|---|---|---|
| L-SMN/R-SMN | BA1–2–3–4 | Sensorimotor Network |
| L-VIS1/R-VIS1 | BA17 | Visual Network 1 (Occipital Cortex) |
| L-VIS2/R-VIS2 | BA18–19 | Visual Network 2 (Occipital Cortex) |
| L-PFC1/R-PFC1 | BA46–9 | Prefronatal Cortex 1 (Dorsolateral) |
| L-PFC2/R-PFC2 | BA45–47 | Prefronatal Cortex 2 (Ventrolateral) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonilauri, A.; Sangiuliano Intra, F.; Rossetto, F.; Borgnis, F.; Baselli, G.; Baglio, F. Whole-Head Functional Near-Infrared Spectroscopy as an Ecological Monitoring Tool for Assessing Cortical Activity in Parkinson’s Disease Patients at Different Stages. Int. J. Mol. Sci. 2022, 23, 14897. https://doi.org/10.3390/ijms232314897
Bonilauri A, Sangiuliano Intra F, Rossetto F, Borgnis F, Baselli G, Baglio F. Whole-Head Functional Near-Infrared Spectroscopy as an Ecological Monitoring Tool for Assessing Cortical Activity in Parkinson’s Disease Patients at Different Stages. International Journal of Molecular Sciences. 2022; 23(23):14897. https://doi.org/10.3390/ijms232314897
Chicago/Turabian StyleBonilauri, Augusto, Francesca Sangiuliano Intra, Federica Rossetto, Francesca Borgnis, Giuseppe Baselli, and Francesca Baglio. 2022. "Whole-Head Functional Near-Infrared Spectroscopy as an Ecological Monitoring Tool for Assessing Cortical Activity in Parkinson’s Disease Patients at Different Stages" International Journal of Molecular Sciences 23, no. 23: 14897. https://doi.org/10.3390/ijms232314897
APA StyleBonilauri, A., Sangiuliano Intra, F., Rossetto, F., Borgnis, F., Baselli, G., & Baglio, F. (2022). Whole-Head Functional Near-Infrared Spectroscopy as an Ecological Monitoring Tool for Assessing Cortical Activity in Parkinson’s Disease Patients at Different Stages. International Journal of Molecular Sciences, 23(23), 14897. https://doi.org/10.3390/ijms232314897








