The Therapeutic Landscape of Salivary Gland Malignancies—Where Are We Now?
Abstract
1. Introduction
| Subtype | Prevalence | Treatment | Prognosis |
|---|---|---|---|
| MEC | 29–35% [3,4,6] | - Surgery in case of resectable tumor - Adjuvant radiotherapy in case of perineural invasion, lymph node involvement, advanced high-grade tumors, positive margins after resection and extra-glandular extension [8] | Depends on grading 5-year OS = 79.3% 5-year DFS = 76.5% [7] |
| AdCC | 20–22% [9,10,11] | - Complete surgical resection - Postoperative radiotherapy is almost always used for AdCC [12] | 5-year OS = 55–98% 15-year OS = 23–40% [11] |
| SDC | 5–10% [13,14] | - Surgery and radiation therapy [13] | 5-year OS = 30–48% [15] |
| AcCC | 10–25% [16,17,18,19] | - Complete surgical removal via total or subtotal parotidectomy - Postoperative radiotherapy in all cases except T1N0 or T2N0 [16] | 5-year OS = 91% 10-year OS = 88% [16] |
2. Systemic Therapies
2.1. Chemotherapy
2.2. Target-Based Therapy
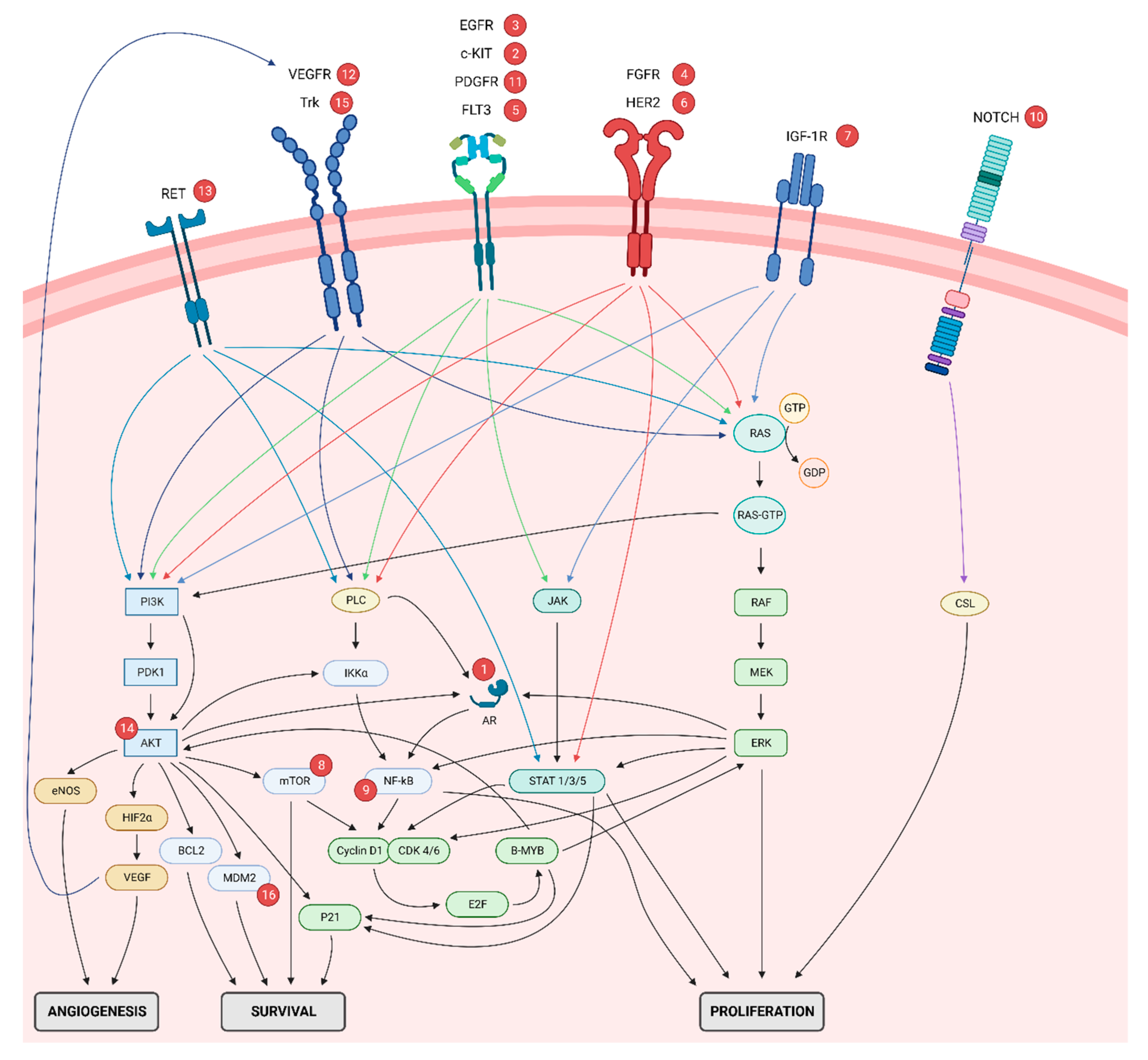
2.2.1. Receptors
2.2.2. Intracellular and Epigenetic Alterations
| Phase | Setting | Agent | Target | Pts, n | Subtype * | ORR, n (%) * | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|---|---|---|
| A. Tyrosine kinase inhibitors | ||||||||
| II [145] | R/M Any line | Apatinib | VEGFR, RET, c-KIT | 65 | AdCC | 30 (46) | 19.7 | Not reached |
| II [118] | R/M Any line | Axitinib | VEGFR, PDGFR, c-KIT | 33 | AdCC | 3 (9) | 5.7 | – |
| II [130] | R/M First- or second-line | Axitinib | VEGFR, PDGFR, c-KIT | 26 | AdCC: 6 Non-AdCC: 20 (1 SDC, 3 AcCC, 6 adeno, 2 clear cell, 1 ex pleomorphic adenoma, 1 myoepithelial, 1 epithelial-myoepithelial, 5 undifferentiated) | ITT: 2 (8) AdCC: 1 (17) Non-AdCC: 1 (5) (1 undifferentiated) | 5.5 | 26.2 |
| II [124] | R/M Any line | Axitinib | VEGFR, PDGFR, c-KIT | 27 | AdCC | 0 (0) | 10.8 | NR |
| II [124] | R/M Any line | Axitinib (after corss-over) | VEGFR, PDGFR, c-KIT | 26 | AdCC | 3 (12) | 14.5 | 27.2 |
| II [143] | R/M Any line | Cabozantinib | MET, RET, AXL, VEGFR2, FLT3, c-KIT. | 21 | AdCC: 15 Non-AdCC: 6 (1 MEC, 4 SDC, 1 AcCC, 1 ex pleomorphic adenoma) | ITT: 2 (10) AdCC: 1 (7) Non-AdCC: 1 (17) (1 SDC) | AdCC: 9.4 Non-AdCC: 7.2 | AdCC: 27.5 Non-AdCC: 14.2 |
| II [81] | R/M First-, second- or third-line | Cetuximab | EGFR | 30 | AdCC: 23 Non-AdCC: 7 (2 MEC, 1 AcCC, 1 adeno, 3 myoepithelial) | ITT: 0 (0) AdCC: 0 (0) Non-AdCC: 0 (0) | ITT: 6.0 AdCC: 6.0 Non-AdCC: 2.0 | – |
| I/II [99] | Non-R/M No prior line | Cetuximab (+ IMRT) | EGFR | 23 | AdCC | 8 (35) | NR (DFS) | 54.0 |
| II [117] | Locally advanced | Cetuximab + cisplatin | Cetuximab: EGFR Cisplatin: cytostatic drug | 9 | EGFR+ AdCC | 4 (44) | 64.0 | Not reached |
| II [117] | R/M | Cetuximab + cisplatin + 5-fluorouracil | Cetuximab: EGFR Cisplatin: cytostatic drug 5-fluorouracil: cytostatic drug | 12 | EGFR+ AdCC | 5 (42) | 13.0 | 24.0 |
| II [144] | R/M Any line | Dasatinib | c-kit, BCR-ABL, SRC family, PDGFβ, EPHA2 | 54 | c-kit+ AdCC: 40 Non-AdCC: 14 | ITT: 1 (2) AdCC: 1 (3) Non-AdCC: 0 (0) | AdCC: 4.8 | AdCC: 14.5 |
| II [125] | R/M Any line | Dovotinib | VEGFR, c-Kit, PDGFR, CSF-1R, RET, TrkA, FLT3 | 32 | AdCC | 1 (3) | 6.0 | Not reached |
| II [107] | R/M Any line | Dovotinib | VEGFR, c-Kit, PDGFR, CSF-1R, RET, TrkA, FLT3 | 34 | AdCC | 2 (6) | 8.2 | 20.6 |
| I [104] | R/M Any line | Figitumumab + dacomitinib | Figitumumab: IGF1R Dacomitinib: HER1/EGFR, HER2, HER4 | 5 | AdCC: 3 Non-AdCC: 2 | 1 (20) | – | – |
| II [123] | R/M Any line | Gefitinib | EGFR | 36 | AdCC: 19 Non-AdCC: 17 (2 MEC, 3 SDC, 2 AcCC, 9 adeno, 1 myoepithelial) | ITT: 0 (0) AdCC: 0 (0) Non-AdCC: 0 (0) | AdCC: 4.3 Non-AdCC: 2.1 | AdCC: 25.9 Non-AdCC: 16.0 |
| II [122] | R/M Any line | Imatinib | c-kit, BCR-ABL, PDGFR | 16 | c-kit+ AdCC | 0 (0) | 2.3 | 7.0 |
| II [136] | R/M Any line | Imatinib | c-kit, BCR-ABL, PDGFR | 10 | AdCC | 0 (0) | 6.0 | – |
| Retro [103] | R/M First-line | Imatinib | c-kit, BCR-ABL, PDGFR | 8 | c-kit+ AdCC | 0 (0) | 3.0 | – |
| II [114] | R/M Any line | Imatinib + cisplatin | Imatinib: c-kit, BCR-ABL, PDGFR Cisplatin: cytostatic drug | 28 | c-kit+ AdCC | 3 (11) | 15.0 | 35.0 |
| II [100] | R/M Any line | Lapatinib | HER2, EGFR | 40 | EGFR+ or HER2+ AdCC: 20 Non-AdCC: 20 (2 MEC, 4 SDC, 1 AcCC, 7 adeno, 3 SCC, 3 undifferentiated) | ITT: 0 (0) AdCC: 0 (0) Non-AdCC: 0 (0) | ITT: 3.4 AdCC: 3.5 Non-AdCC: 2.1 | ITT: Not reached AdCC: Not reached Non-AdCC: 13.8 |
| II [141] | R/M Any line | Lenvatinib | VEGFR, FGFR, PDGFR, RET, KIT | 32 | AdCC | 5 (16) | 17.5 | – |
| II [132] | R/M Any line | Lenvatinib | VEGFR, FGFR, PDGFR, RET, KIT | 26 | AdCC | 3 (12) | 9.1 | 27.0 |
| Retro [111] | R/M Any line | Lenvatinib | VEGFR, FGFR, PDGFR, RET, KIT | 21 | AdCC | 0 (0) | 4.5 | 12.0 |
| II [127] | R/M Any line | Nintedanib | VEGFR, FGFR, PDGFR | 20 | AdCC: 13 Non-AdCC: 7 (2 MEC, 1 SDC, 1 AcCC, 3 adeno) | ITT: 0 (0) AdCC: 0 (0) Non-AdCC: 0 (0) | ITT: 7.9 AdCC: 7.9 Non-AdCC: 6.3 | Not reached |
| II [120] | R/M Any line | Regorafenib | VEGFR, FGFR, PDGFR | 38 | AdCC | 0 (0) | – | – |
| II [142] | R/M Any line | Sorafenib | b-Raf, c-Raf, c- KIT, VEGFR, PDGFR, FLT3 | 23 | AdCC | 2 (11) | 11.3 | 19.6 |
| II [133] | R/M Any line | Sorafenib | b-Raf, c-Raf, c- KIT, VEGFR, PDGFR, FLT3 | 37 | AdCC: 19 Non-AdCC: 18 (5 MEC, 2 SDC, 7 adeno, 3 myoepithelial, 1 undifferentiated) | ITT: 6 (16) AdCC: 2 (11) Non-AdCC: 4 (22) (1 MEC, 1 SDC, 1 adeno, 1 undifferentiated) | ITT: 5.9 AdCC: 8.9 Non-AdCC: 4.2 | ITT: 23.4 AdCC: 26.4 Non-AdCC: 12.3 |
| I [134] | R/M Any line | Sorafenib + R1507 | Sorafenib: b-Raf, c-Raf, c- KIT, VEGFR, PDGFR, FLT3 R1507: IGF1R | ? | AdCC | 1 | – | – |
| II [105] | R/M Any line | Sunitinib | VEGFR, c-KIT, PDGFR, RET, FLT3 | 13 | AdCC | 0 (0) | 7.2 | 18.7 |
| B. Immune checkpoint inhibition | ||||||||
| Ib [106] | R/M Any line | Pembrolizumab | PD-1 | 26 | PD-L1+ (CPS ≥ 1) AdCC: 2 Non-AdCC: 24 (3 MEC, 1 SDC, 1 AcCC, 11 adeno, 1 myoepithelial, 1 carcinoid, 2 SCC, 1 serous, 3 undifferentiated) | ITT: 3 (12) AdCC: 0 (0) Non-AdCC: 3 (13) (2 adeno, 1 serous) | 4.0 | 13.0 |
| II [135] | R/M Any line | Pembrolizumab | PD-1 | 10 | AdCC | 0 (0) | 6.6 | 27.2 |
| II [135] | R/M Any line | Pembrolizumab (+ IMRT) | PD-1 | 10 | AdCC | 0 (0) | 4.5 | Not reached |
| II [109] | R/M Any line | Pembrolizumab | PD-1 | 109 | AdCC: 59 Non-AdCC: 50 (4 MEC, 2 SDC, 1 AcCC, 25 adeno, 5 mucinous, 2 SCC, 3 cystadeno, 2 ex pleomorphic adenoma, 1 cystic, 2 myoepithelial, 1 epithelial-myoepithelial, 2 undifferentiated) | 5 (5) PD-L1− (CPS 0): 2/77 (3) PD-L1+ (CPS ≥ 1): 3/28 (11) | 4.0 | 21.1 |
| II [138] | R/M Any line | Pembrolizumab + Vorinostat | Pembrolizumab: PD-1 Vorinostat: histone deacetylase | 25 | AdCC: 12 Non-AdCC: 13 (3 MEC, 1 SDC, 3 AcCC, 1 adeno, 1 myoepithelial, 2 ex pleomorphic adenoma, 1 lymphoepithelioma, 1 clear cell) | ITT: 4 (16) AdCC: 1 (8) Non-AdCC: 3 (23) (2 AcCC, 1 lymphoepithelioma) | 6.9 | 14.0 |
| C. HER2-targeted therapy | ||||||||
| II [116] | R/M First-, second- or third-line | Trastazumab | HER2 | 14 | HER2+ AdCC: 2 Non-AdCC: 12 (3 MEC, 7 adeno, 2 SCC) | AdCC: 0 (0) Non-AdCC: 1 (8) (1 MEC) | 4.2 | – |
| II [128] | R/M Any line | Trastuzumab + Pertuzumab | Trastuzumab: HER2 Pertuzumab: HER2 | 16 | HER2+ non-AdCC (1 MEC, 3 SDC, 8 adeno, 4 unspecified) | 9 (56) | 9.1 | 20.4 |
| II [140] | R/M Any line | Trastuzumab + Docetaxel | Trastuzumab: HER2 Docetaxel: cytostatic drug | 57 | HER2+ SDC | 40 (70) | 8.9 | 39.7 |
| II [129] | R/M Any line | Ado-trastuzumab emtansine | HER2-targeted ADC | 10 | HER2+ | 9 (90) | Not reached | Not reached |
| D. AR-targeted therapy | ||||||||
| II [119] | R/M Any line | Enzalutamide | AR | 46 | AR+ AdCC:1 Non-AdCC: 45 (41 SDC, 1 adeno, 3 ex pleomorphic adenoma) | 7 (15) | 5.6 | 17.0 |
| II [131] | R/M Any line | Abiraterone acetate | CYP17A1 | 24 | AR+ non-AdCC (19 SDC, 5 adeno) | 5 (21) | ITT: 3.7 SDC: 4.0 Adeno: 2.5 | ITT: 22.5 SDC: Not reached Adeno: 8.8 |
| II [113] | R/M Any line | Leuprorelin acetate + Bicalutamide | Leuprorelin acetate: GnRH receptor agonist Bicalutamide: AR | 36 | AR+ non-AdCC (34 SDC, 2 adeno) | 15 (42) | 8.8 | 30.5 |
| E. Notch | ||||||||
| I [112] | R/M Any line | Brontictuzumab | NOTCH1 | 12 | AdCC | 2 (17) | – | – |
| I [102] | R/M Any line | BMS-986115 | pan-NOTCH | 5 | AdCC | 0 (0) | – | – |
| I [110] | R/M Any line | Crenigacestat | pan-NOTCH | 22 | AdCC | 1 (5) | ITT: 5.3 1st-line: Not reached 2nd-line: 7.7 3rd+-line: 2.4 | – |
| F. Other therpies | ||||||||
| II [121] | R/M Any line | Nelfinavir | Akt pathway | 15 | AdCC | 0 (0) | 5.5 | – |
| II [126] | R/M Any line | Everolimus | mTOR | 34 | AdCC | 0 (0) | 11.2 | 23.7 |
| II [101] | R/M Any line | Bortezomib | NF-κB | 21 | AdCC | 0 (0) | 6.4 | 21.0 |
| II [101] | R/M Any line | Bortezomib + doxorubcin | Bortezomib: NF-κB Doxorubcin: cytostatic drug | 10 | AdCC | 1 (10) | 6.4 | 21.0 |
| II [137] | R/M Any line | Eribulin mesylate | Microtubule inhibitor | 29 | AdCC: 11 Non-AdCC: 18 (2 MEC, 1 SDC, 2 AcCC, 4 adeno, 3 myoepithelial, 2 ex pleomorphic adenoma, 1 mammary analogue, 1 clear cell, 2 undifferentiated) | ITT: 3 (10) AdCC: 2 (18) Non-AdCC: 1 (6) (1 ex pleomorphic adenoma) | ITT: 3.5 AdCC: 3.5 Non-AdCC: 3.3 | ITT: 16.0 AdCC: 20.0 Non-AdCC: 15.5 |
| I [139] | R/M Any line | GSK3326595 | PRMT5 | 14 | AdCC | 3 (21) | – | – |
| I [146] | R/M Any line | Chidamide | Histone deacetylase | 3 | Undefined | 1 (33) | – | – |
| II [115] | R/M Any line | Vorinostat | Histone deacetylase | 30 | AdCC | 2 (7) | 10.0 | 11.5 |
| Phase | Trial Identification | Setting | Agent | Target | Pts, n | Subtype |
|---|---|---|---|---|---|---|
| I | NCT03886831 | R/M-Any line | PRT543 | PRMT5 | 227 | AdCC (+other indications) |
| I | NCT03291002 | R/M-Any line | CV8102 + anti-PD-1 therapy | VMD-928: TLR7/8 agonist anti-PD-1 therapy: PD-1 | 98 | AdCC (+other indications) |
| I | NCT03556228 | R/M-Any line | VMD-928 | VMD-928: TrkA | 74 | AdCC (+other indications) |
| I | NCT03287427 | R/M-Any line | TetMYB vaccine + tislelizumab | TetMYB vaccine: cancer therapy vaccine Tislelizumab: PD-1 | 32 | AdCC (+CRC) |
| II | NCT03999684 | R/M-Any line | Tretinoin | retinoic acid receptor | 27 | AdCC |
| II | NCT03691207 | R/M-Any line | AL101 | pan-NOTCH | 87 | AdCC |
| II | NCT03422679 | LA or R/M-Any line | CB-103 | pan-NOTCH | 200 | AdCC (+other indications) |
| I/II | NCT03781986 | R/M-Any line | APG-115 ± carboplatin | APG-115: MDM2 Carboplatin: cytostatic drug | 32 | AdCC Non-AdCC |
| II | NCT03639168 | R/M-Any line | chidamide + cisplatin | chidamide: histone deacetylase cisplatin: cytostatic drug | 22 | AdCC |
| II | NCT04209660 | R/M-Any line | Lenvatinib + pembrolizumab | Lenvatinib: VEGFR, FGFR, PDGFR, RET, KIT Pembrolizumab: PD-1 | 64 | AdCC Non-AdCC |
| II | NCT04119453 | R/M-Any line | Apatinib | VEGFR, RET, c-KIT | 80 | AdCC |
3. Novel Targets and Biomarkers
4. Case Description
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHugh, J.B.; Visscher, D.W.; Barnes, E.L. Update on selected salivary gland neoplasms. Arch. Pathol. Lab. Med. 2009, 133, 1763–1774. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; Ljung, B.M.; Morrow, M. Environmental factors and the risk of salivary gland cancer. Epidemiology 1997, 8, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Mesolella, M.; Iengo, M.; Testa, D.; Di Lullo, A.M.; Salzano, G.; Salzano, F.A. Mucoepidermoid carcinoma of the base of tongue. Acta Otorhinolaryngol. Ital. 2015, 35, 58–61. [Google Scholar]
- Peraza, A.; Gomez, R.; Beltran, J.; Amarista, F.J. Mucoepidermoid carcinoma. An update and review of the literature. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 713–720. [Google Scholar] [CrossRef]
- Spiro, R.H. Salivary neoplasms: Overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986, 8, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.H.; Huvos, A.G.; Berk, R.; Strong, E.W. Mucoepidermoid carcinoma of salivary gland origin: A clinicopathologic study of 367 cases. Am. J. Surg. 1978, 136, 461–468. [Google Scholar] [CrossRef] [PubMed]
- McHugh, C.H.; Roberts, D.B.; El-Naggar, A.K.; Hanna, E.Y.; Garden, A.S.; Kies, M.S.; Weber, R.S.; Kupferman, M.E. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 2012, 118, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Sama, S.; Komiya, T.; Guddati, A.K. Advances in the Treatment of Mucoepidermoid Carcinoma. World J. Oncol. 2022, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Morais, E.F.; da Silva, L.P.; Moreira, D.G.L.; Mafra, R.P.; Rolim, L.S.A.; de Moura Santos, E.; de Souza, L.B.; de Almeida Freitas, R. Prognostic Factors and Survival in Adenoid Cystic Carcinoma of the Head and Neck: A Retrospective Clinical and Histopathological Analysis of Patients Seen at a Cancer Center. Head Neck Pathol. 2021, 15, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.C.; Barpande, S.R.; Bhavthankar, J.D.; Mandale, M.S. Adenoid cystic carcinoma of palate: Report of a solid variant. J. Oral Maxillofac. Pathol. JOMFP 2018, 22 (Suppl. 1), S65–S68. [Google Scholar] [CrossRef]
- Xu, B.; Drill, E.; Ho, A.; Ho, A.; Dunn, L.; Prieto-Granada, C.N.; Chan, T.; Ganly, I.; Ghossein, R.; Katabi, N. Predictors of Outcome in Adenoid Cystic Carcinoma of Salivary Glands: A Clinicopathologic Study with Correlation Between MYB Fusion and Protein Expression. Am. J. Surg. Pathol. 2017, 41, 1422–1432. [Google Scholar] [CrossRef]
- Cantu, G. Adenoid cystic carcinoma. An indolent but aggressive tumour. Part B: Treatment and prognosis. Acta Otorhinolaryngol. Ital. 2021, 41, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Sharma, A.; Schmitt, N.C.; Johnson, J.T.; Ferris, R.L.; Duvvuri, U.; Kim, S. A 20-Year Review of 75 Cases of Salivary Duct Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Nakaguro, M.; Tada, Y.; Faquin, W.C.; Sadow, P.M.; Wirth, L.J.; Nagao, T. Salivary duct carcinoma: Updates in histology, cytology, molecular biology, and treatment. Cancer Cytopathol. 2020, 128, 693–703. [Google Scholar] [CrossRef] [PubMed]
- D’Heygere, E.; Meulemans, J.; Vander Poorten, V. Salivary duct carcinoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 142–151. [Google Scholar] [CrossRef]
- Al-Zaher, N.; Obeid, A.; Al-Salam, S.; Al-Kayyali, B.S. Acinic cell carcinoma of the salivary glands: A literature review. Hematol./Oncol. Stem Cell Ther. 2009, 2, 259–264. [Google Scholar] [CrossRef]
- Ammad Ud Din, M.; Shaikh, H. Adenoid Cystic Cancer; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, N.; Xu, L.; Zhao, H.; El-Naggar, A.K.; Sturgis, E.M. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer 2012, 118, 3945–3953. [Google Scholar] [CrossRef]
- Pang, Y.; Sun, L.; Liu, H.; Ma, J. Differential diagnosis and treatment of salivary secretory carcinoma and acinic cell carcinoma. Oral Oncol. 2021, 119, 105370. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Cannavale, R.; Flamminio, F.; Foschini, M.P. Fine-needle aspiration cytology of salivary gland lesions: A systematic review. J. Oral Maxillofac. Surg. 2010, 68, 2146–2153. [Google Scholar] [CrossRef]
- Eom, H.J.; Lee, J.H.; Ko, M.S.; Choi, Y.J.; Yoon, R.G.; Cho, K.J.; Nam, S.Y.; Baek, J.H. Comparison of fine-needle aspiration and core needle biopsy under ultrasonographic guidance for detecting malignancy and for the tissue-specific diagnosis of salivary gland tumors. Am. J. Neuroradiol. 2015, 36, 1188–1193. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Wong, K.T.; King, A.D.; Ahuja, A.T. Imaging of salivary gland tumours. Eur. J. Radiol. 2008, 66, 419–436. [Google Scholar] [CrossRef]
- Eida, S.; Sumi, M.; Nakamura, T. Multiparametric magnetic resonance imaging for the differentiation between benign and malignant salivary gland tumors. J. Magn. Reson. Imaging 2010, 31, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Ryu, C.H.; Choi, S.H.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Nam, S.Y.; Kim, S.Y. Clinical utility of 18F-FDG PET for patients with salivary gland malignancies. J. Nucl. Med. 2007, 48, 240–246. [Google Scholar] [PubMed]
- Keyes, J.W., Jr.; Harkness, B.A.; Greven, K.M.; Williams, D.W., III; Watson, N.E., Jr.; McGuirt, W.F. Salivary gland tumors: Pretherapy evaluation with PET. Radiology 1994, 192, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef]
- Lombardi, D.; McGurk, M.; Vander Poorten, V.; Guzzo, M.; Accorona, R.; Rampinelli, V.; Nicolai, P. Surgical treatment of salivary malignant tumors. Oral Oncol. 2017, 65, 102–113. [Google Scholar] [CrossRef]
- Mannelli, G.; Comini, L.V.; Sacchetto, A.; Santoro, R.; Spinelli, G.; Bonomo, P.; Desideri, I.; Bossi, P.; Orlandi, E.; Alderotti, G.; et al. Estimating survival after salvage surgery for recurrent salivary gland cancers: Systematic review. Head Neck 2022, 44, 1961–1975. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Peng, X. Conservative and functional surgery in the treatment of salivary gland tumours. Int. J. Oral Sci. 2019, 11, 22. [Google Scholar] [CrossRef]
- Lewis, A.G.; Tong, T.; Maghami, E. Diagnosis and Management of Malignant Salivary Gland Tumors of the Parotid Gland. Otolaryngol. Clin. North Am. 2016, 49, 343–380. [Google Scholar] [CrossRef]
- Voulgarelis, M.; Ziakas, P.D.; Papageorgiou, A.; Baimpa, E.; Tzioufas, A.G.; Moutsopoulos, H.M. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjogren syndrome. Medicine 2012, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, M.; Fornari, G.; Pedani, F.; Marchionatti, S.; Gabriele, P.; Succo, G.; Bumma, C. Paclitaxel and carboplatin for recurrent salivary gland malignancies. Anticancer Res. 2000, 20, 3781–3783. [Google Scholar] [PubMed]
- Airoldi, M.; Garzaro, M.; Pedani, F.; Ostellino, O.; Succo, G.; Riva, G.; Sensini, M.; Naqe, N.; Bellini, E.; Raimondo, L.; et al. Cisplatin+Vinorelbine Treatment of Recurrent or Metastatic Salivary Gland Malignancies (RMSGM): A Final Report on 60 Cases. Am. J. Clin. Oncol. 2017, 40, 86–90. [Google Scholar] [CrossRef]
- Airoldi, M.; Pedani, F.; Succo, G.; Gabriele, A.M.; Ragona, R.; Marchionatti, S.; Bumma, C. Phase II randomized trial comparing vinorelbine versus vinorelbine plus cisplatin in patients with recurrent salivary gland malignancies. Cancer 2001, 91, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Eisenberger, M.A.; Gray, W.C. Preliminary experience with chemotherapy in advanced salivary gland neoplasms. Med. Pediatr. Oncol. 1988, 16, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Creagan, E.T.; Woods, J.E.; Rubin, J.; Schaid, D.J. Cisplatin-based chemotherapy for neoplasms arising from salivary glands and contiguous structures in the head and neck. Cancer 1988, 62, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- De Haan, L.D.; De Mulder, P.H.; Vermorken, J.B.; Schornagel, J.H.; Vermey, A.; Verweij, J. Cisplatin-based chemotherapy in advanced adenoid cystic carcinoma of the head and neck. Head Neck 1992, 14, 273–277. [Google Scholar] [CrossRef]
- Dimery, I.W.; Legha, S.S.; Shirinian, M.; Hong, W.K. Fluorouracil, doxorubicin, cyclophosphamide, and cisplatin combination chemotherapy in advanced or recurrent salivary gland carcinoma. J. Clin. Oncol. 1990, 8, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, A.I.; Clark, J.R.; Fallon, B.G.; Posner, M.R.; Norris, C.M., Jr.; Miller, D. Cyclophosphamide, doxorubicin, and cisplatin combination chemotherapy for advanced carcinomas of salivary gland origin. Cancer 1987, 60, 2869–2872. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Li, Y.; Pinto, H.A.; Jennings, T.; Kies, M.S.; Silverman, P.; Forastiere, A.A. Phase II trial of taxol in salivary gland malignancies (E1394): A trial of the Eastern Cooperative Oncology Group. Head Neck 2006, 28, 197–204. [Google Scholar] [CrossRef]
- Hill, M.E.; Constenla, D.O.; A’Hern, R.P.; Henk, J.M.; Rhys-Evans, P.; Breach, N.; Archer, D.; Gore, M.E. Cisplatin and 5-fluorouracil for symptom control in advanced salivary adenoid cystic carcinoma. Oral Oncol. 1997, 33, 275–278. [Google Scholar] [CrossRef]
- Hong, M.H.; Kim, C.G.; Koh, Y.W.; Choi, E.C.; Kim, J.; Yoon, S.O.; Kim, H.R.; Cho, B.C. Efficacy and safety of vinorelbine plus cisplatin chemotherapy for patients with recurrent and/or metastatic salivary gland cancer of the head and neck. Head Neck 2018, 40, 55–62. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, S.J.; Park, S.; Jung, H.A.; Lee, S.H.; Jeong, H.S.; Chung, M.K.; Ahn, M.J. A Single-Arm, Prospective, Phase II Study of Cisplatin Plus Weekly Docetaxel as First-Line Therapy in Patients with Metastatic or Recurrent Salivary Gland Cancer. Cancer Res. Treat. 2022, 54, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Siu, L.L.; Winquist, E.; Maksymiuk, A.; Harnett, E.L.; Walsh, W.; Tu, D.; Parulekar, W.R. A phase 2 study of platinum and gemcitabine in patients with advanced salivary gland cancer: A trial of the NCIC Clinical Trials Group. Cancer 2010, 116, 362–368. [Google Scholar] [CrossRef]
- Licitra, L.; Cavina, R.; Grandi, C.; Palma, S.D.; Guzzo, M.; Demicheli, R.; Molinari, R. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. Ann. Oncol. 1996, 7, 640–642. [Google Scholar] [CrossRef]
- Licitra, L.; Marchini, S.; Spinazze, S.; Rossi, A.; Rocca, A.; Grandi, C.; Molinari, R. Cisplatin in advanced salivary gland carcinoma. A phase II study of 25 patients. Cancer 1991, 68, 1874–1877. [Google Scholar] [CrossRef]
- Mattox, D.E.; Von Hoff, D.D.; Balcerzak, S.P. Southwest Oncology Group study of mitoxantrone for treatment of patients with advanced adenoid cystic carcinoma of the head and neck. Investig. New Drugs 1990, 8, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Sato, Y.; Sasaki, T.; Shimbashi, W.; Fukushima, H.; Yonekawa, H.; Mitani, H.; Kawabata, K.; Takahashi, S. Combination chemotherapy of carboplatin and paclitaxel for advanced/metastatic salivary gland carcinoma patients: Differences in responses by different pathological diagnoses. Acta Oto-Laryngol. 2016, 136, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Schramm, V.L., Jr.; Srodes, C.; Myers, E.N. Cisplatin therapy for adenoid cystic carcinoma. Arch. Otolaryngol. 1981, 107, 739–741. [Google Scholar] [CrossRef]
- Tannock, I.F.; Sutherland, D.J. Chemotherapy for adenocystic carcinoma. Cancer 1980, 46, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, P.L.; Brantley, A.; Fisher, S.; Cole, T.B.; Crocker, I.; Huang, A.T. 5-Fluorouracil, cyclophosphamide, and vincristine for adenoid cystic carcinoma of the head and neck. Cancer 1987, 59, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Van Herpen, C.M.; Locati, L.D.; Buter, J.; Thomas, J.; Bogaerts, J.; Lacombe, D.; de Mulder, P.; Awada, A.; Licitra, L.; Bernier, J.; et al. Phase II study on gemcitabine in recurrent and/or metastatic adenoid cystic carcinoma of the head and neck (EORTC 24982). Eur. J. Cancer 2008, 44, 2542–2545. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Verweij, J.; de Mulder, P.H.; Cognetti, F.; Clavel, M.; Rodenhuis, S.; Kirkpatrick, A.; Snow, G.B. Epirubicin in patients with advanced or recurrent adenoid cystic carcinoma of the head and neck: A phase II study of the EORTC Head and Neck Cancer Cooperative Group. Ann. Oncol. 1993, 4, 785–788. [Google Scholar] [CrossRef]
- Verweij, J.; de Mulder, P.H.; de Graeff, A.; Vermorken, J.B.; Wildiers, J.; Kerger, J.; Schornagel, J.; Cognetti, F.; Kirkpatrick, A.; Sahmoud, T.; et al. Phase II study on mitoxantrone in adenoid cystic carcinomas of the head and neck. Ann. Oncol. 1996, 7, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Sahara, S.; Herzog, A.E.; Nör, J.E. Systemic therapies for salivary gland adenoid cystic carcinoma. Am. J. Cancer Res. 2021, 11, 4092–4110. [Google Scholar] [PubMed]
- Holst, V.A.; Marshall, C.E.; Moskaluk, C.A.; Frierson, H.F., Jr. KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod. Pathol. 1999, 12, 956–960. [Google Scholar]
- Kondo, S.; Mukudai, Y.; Soga, D.; Nishida, T.; Takigawa, M.; Shirota, T. Differential expression of vascular endothelial growth factor in high- and low-metastasis cell lines of salivary gland adenoid cystic carcinoma. Anticancer Res. 2014, 34, 671–677. [Google Scholar] [PubMed]
- Park, S.; Nam, S.J.; Keam, B.; Kim, T.M.; Jeon, Y.K.; Lee, S.H.; Hah, J.H.; Kwon, T.K.; Kim, D.W.; Sung, M.W.; et al. VEGF and Ki-67 Overexpression in Predicting Poor Overall Survival in Adenoid Cystic Carcinoma. Cancer Res. Treat. 2016, 48, 518–526. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, B. In vitro angiogenesis and expression of nuclear factor kappaB and VEGF in high and low metastasis cell lines of salivary gland Adenoid Cystic Carcinoma. BMC Cancer 2007, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, B.; Chen, X. Expressions of nuclear factor kappaB, inducible nitric oxide synthase, and vascular endothelial growth factor in adenoid cystic carcinoma of salivary glands: Correlations with the angiogenesis and clinical outcome. Clin. Cancer Res. 2005, 11, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Myoken, Y.; Myoken, Y.; Okamoto, T.; Sato, J.D.; Kan, M.; McKeehan, W.L.; Nakahara, M.; Takada, K. Immunohistochemical study of overexpression of fibroblast growth factor-1 (FGF-1), FGF-2, and FGF receptor-1 in human malignant salivary gland tumours. J. Pathol. 1996, 178, 429–436. [Google Scholar] [CrossRef]
- Vekony, H.; Ylstra, B.; Wilting, S.M.; Meijer, G.A.; van de Wiel, M.A.; Leemans, C.R.; van der Waal, I.; Bloemena, E. DNA copy number gains at loci of growth factors and their receptors in salivary gland adenoid cystic carcinoma. Clin. Cancer Res. 2007, 13, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, M.D.; Manne, U.; Carroll, W.R.; Peters, G.E.; Weiss, H.L.; Grizzle, W.E. Molecular differences in mucoepidermoid carcinoma and adenoid cystic carcinoma of the major salivary glands. Laryngoscope 2001, 111, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Vered, M.; Braunstein, E.; Buchner, A. Immunohistochemical study of epidermal growth factor receptor in adenoid cystic carcinoma of salivary gland origin. Head Neck 2002, 24, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Iwai, K.; Okada, Y.; Mori, M. Immunohistochemical expression of epidermal growth factor receptor in salivary gland tumours. Virchows. Arch. A 1989, 415, 523–531. [Google Scholar] [CrossRef]
- Baserga, R. The insulin-like growth factor-I receptor as a target for cancer therapy. Expert Opin. Ther. Targets 2005, 9, 753–768. [Google Scholar] [CrossRef]
- Sachdev, D.; Yee, D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol. Cancer Ther. 2007, 6, 1–12. [Google Scholar] [CrossRef]
- Tao, Y.; Pinzi, V.; Bourhis, J.; Deutsch, E. Mechanisms of disease: Signaling of the insulin-like growth factor 1 receptor pathway—Therapeutic perspectives in cancer. Nat. Clin. Pract. Oncol. 2007, 4, 591–602. [Google Scholar] [CrossRef]
- Kato, T.; Hayama, S.; Yamabuki, T.; Ishikawa, N.; Miyamoto, M.; Ito, T.; Tsuchiya, E.; Kondo, S.; Nakamura, Y.; Daigo, Y. Increased expression of insulin-like growth factor-II messenger RNA–binding protein 1 is associated with tumor progression in patients with lung cancer. Clin. Cancer Res. 2007, 13, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.E.; Gee, J.M.; Hutcheson, I.R.; Knowlden, J.M.; Barrow, D.; Nicholson, R.I. Growth factor receptor interplay and resistance in cancer. Endocr. Relat. Cancer 2006, 13 (Suppl. 1), S45–S51. [Google Scholar] [CrossRef]
- Suda, K.; Mizuuchi, H.; Sato, K.; Takemoto, T.; Iwasaki, T.; Mitsudomi, T. The insulin-like growth factor 1 receptor causes acquired resistance to erlotinib in lung cancer cells with the wild-type epidermal growth factor receptor. Int. J. Cancer 2014, 135, 1002–1006. [Google Scholar] [CrossRef]
- Morgillo, F.; Woo, J.K.; Kim, E.S.; Hong, W.K.; Lee, H.Y. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006, 66, 10100–10111. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Ran, L.; Pan, F.; Zhao, X.; Ding, Z.; Chen, Y.; Peng, Q.; Liang, H. Phosphorylated insulin-like growth factor 1 receptor is implicated in resistance to the cytostatic effect of gefitinib in colorectal cancer cells. J. Gastrointest. Surg. 2011, 15, 942–957. [Google Scholar] [CrossRef] [PubMed]
- Boon, E.; Bel, M.; van Boxtel, W.; van der Graaf, W.T.A.; van Es, R.J.J.; Eerenstein, S.E.J.; Baatenburg de Jong, R.J.; van den Brekel, M.W.M.; van der Velden, L.A.; Witjes, M.J.H.; et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands. Int. J. Cancer 2018, 143, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Dalin, M.G.; Desrichard, A.; Katabi, N.; Makarov, V.; Walsh, L.A.; Lee, K.W.; Wang, Q.; Armenia, J.; West, L.; Dogan, S.; et al. Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer. Clin. Cancer Res. 2016, 22, 4623–4633. [Google Scholar] [CrossRef]
- Masubuchi, T.; Tada, Y.; Maruya, S.; Osamura, Y.; Kamata, S.E.; Miura, K.; Fushimi, C.; Takahashi, H.; Kawakita, D.; Kishimoto, S.; et al. Clinicopathological significance of androgen receptor, HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int. J. Clin. Oncol. 2015, 20, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Gay, L.M.; Wang, K.; Vergilio, J.A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.M.; Russell, J.; Ali, S.; et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann. Oncol. 2017, 28, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Tada, Y.; Hirai, H.; Kawakita, D.; Kano, S.; Tsukahara, K.; Shimizu, A.; Takase, S.; Imanishi, Y.; Ozawa, H.; et al. Prognostic and histogenetic roles of gene alteration and the expression of key potentially actionable targets in salivary duct carcinomas. Oncotarget 2018, 9, 1852–1867. [Google Scholar] [CrossRef] [PubMed]
- Takase, S.; Kano, S.; Tada, Y.; Kawakita, D.; Shimura, T.; Hirai, H.; Tsukahara, K.; Shimizu, A.; Imanishi, Y.; Ozawa, H.; et al. Biomarker immunoprofile in salivary duct carcinomas: Clinicopathological and prognostic implications with evaluation of the revised classification. Oncotarget 2017, 8, 59023–59035. [Google Scholar] [CrossRef] [PubMed]
- Santana, T.; Pavel, A.; Martinek, P.; Steiner, P.; Grossmann, P.; Baneckova, M.; Skalova, A. Biomarker immunoprofile and molecular characteristics in salivary duct carcinoma: Clinicopathological and prognostic implications. Hum. Pathol. 2019, 93, 37–47. [Google Scholar] [CrossRef]
- Locati, L.D.; Perrone, F.; Losa, M.; Mela, M.; Casieri, P.; Orsenigo, M.; Cortelazzi, B.; Negri, T.; Tamborini, E.; Quattrone, P.; et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs). Oral Oncol. 2009, 45, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.M.; Faquin, W.C.; Dayal, Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors: Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am. J. Clin. Pathol. 2003, 119, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Radtke, F. Notch signaling in solid tumors. Curr. Top. Dev. Biol. 2010, 92, 411–455. [Google Scholar] [PubMed]
- Miele, L.; Golde, T.; Osborne, B. Notch signaling in cancer. Curr. Mol. Med. 2006, 6, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Valero Mayor, C.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef]
- Sajed, D.P.; Faquin, W.C.; Carey, C.; Severson, E.A.; Afrogheh, A.; Johnson, C.; Blacklow, S.C.; Chau, N.G.; Lin, D.T.; Krane, J.F.; et al. Diffuse Staining for Activated NOTCH1 Correlates with NOTCH1 Mutation Status and Is Associated with Worse Outcome in Adenoid Cystic Carcinoma. Am. J. Surg. Pathol. 2017, 41, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Mukaigawa, T.; Hayashi, R.; Hashimoto, K.; Ugumori, T.; Hato, N.; Fujii, S. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J. Surg. Oncol. 2016, 114, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef]
- Sridharan, V.; Gjini, E.; Liao, X.; Chau, N.G.; Haddad, R.I.; Severgnini, M.; Hammerman, P.; El-Naggar, A.; Freeman, G.J.; Hodi, F.S.; et al. Immune Profiling of Adenoid Cystic Carcinoma: PD-L2 Expression and Associations with Tumor-Infiltrating Lymphocytes. Cancer Immunol. Res. 2016, 4, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Drier, Y.; Cotton, M.J.; Williamson, K.E.; Gillespie, S.M.; Ryan, R.J.; Kluk, M.J.; Carey, C.D.; Rodig, S.J.; Sholl, L.M.; Afrogheh, A.H.; et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat. Genet. 2016, 48, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mitani, Y.; Li, J.; Rao, P.H.; Zhao, Y.J.; Bell, D.; Lippman, S.M.; Weber, R.S.; Caulin, C.; El-Naggar, A.K. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin. Cancer Res. 2010, 16, 4722–4731. [Google Scholar] [CrossRef]
- Persson, M.; Andren, Y.; Mark, J.; Horlings, H.M.; Persson, F.; Stenman, G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc. Natl. Acad. Sci. USA 2009, 106, 18740–18744. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.H.; Zhao, F.; Yang, W.W.; Chen, C.W.; Du, Z.H.; Fu, M.; Ge, X.Y.; Li, S.L. MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int. J. Oncol. 2019, 54, 1579–1590. [Google Scholar] [CrossRef]
- Younes, M.N.; Park, Y.W.; Yazici, Y.D.; Gu, M.; Santillan, A.A.; Nong, X.; Kim, S.; Jasser, S.A.; El-Naggar, A.K.; Myers, J.N. Concomitant inhibition of epidermal growth factor and vascular endothelial growth factor receptor tyrosine kinases reduces growth and metastasis of human salivary adenoid cystic carcinoma in an orthotopic nude mouse model. Mol. Cancer Ther. 2006, 5, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, B. NF-kappaB promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell Prolif. 2009, 42, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Magner, W.J.; Kazim, A.L.; Stewart, C.; Romano, M.A.; Catalano, G.; Grande, C.; Keiser, N.; Santaniello, F.; Tomasi, T.B. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immunol. 2000, 165, 7017–7024. [Google Scholar] [CrossRef] [PubMed]
- Adeberg, S.; Akbaba, S.; Lang, K.; Held, T.; Verma, V.; Nikoghosyan, A.; Bernhardt, D.; Münter, M.; Freier, K.; Plinkert, P.; et al. The Phase 1/2 ACCEPT Trial: Concurrent Cetuximab and Intensity Modulated Radiation Therapy with Carbon Ion Boost for Adenoid Cystic Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 167–173. [Google Scholar] [CrossRef]
- Agulnik, M.; Cohen, E.W.; Cohen, R.B.; Chen, E.X.; Vokes, E.E.; Hotte, S.J.; Winquist, E.; Laurie, S.; Hayes, D.N.; Dancey, J.E.; et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non–adenoid cystic carcinoma malignant tumors of the salivary glands. J. Clin. Oncol. 2007, 25, 3978–3984. [Google Scholar] [CrossRef]
- Argiris, A.; Ghebremichael, M.; Burtness, B.; Axelrod, R.S.; Deconti, R.C.; Forastiere, A.A. A phase 2 trial of bortezomib followed by the addition of doxorubicin at progression in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: A trial of the Eastern Cooperative Oncology Group (E1303). Cancer 2011, 117, 3374–3382. [Google Scholar] [CrossRef]
- Aung, K.L.; El-Khoueiry, A.B.; Gelmon, K.; Tran, B.; Bajaj, G.; He, B.; Chen, T.; Zhu, L.; Poojary, S.; Basak, S.; et al. A multi-arm phase I dose escalating study of an oral NOTCH inhibitor BMS-986115 in patients with advanced solid tumours. Investig. New Drugs 2018, 36, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Panda, N.K.; Elangovan, A.; Bakshi, J.; Verma, R.; Mohindra, S.; Gupta, R.; Oinam, A.S.; Kaur, S.; Vashishta, R.K.; et al. Evaluation of Multimodality Management of Adenoid Cystic Carcinoma of the Head and Neck. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. 1), 628–632. [Google Scholar] [CrossRef]
- Calvo, E.; Soria, J.C.; Ma, W.W.; Wang, T.; Bahleda, R.; Tolcher, A.W.; Gernhardt, D.; O’Connell, J.; Millham, R.; Giri, N.; et al. A Phase I Clinical Trial and Independent Patient-Derived Xenograft Study of Combined Targeted Treatment with Dacomitinib and Figitumumab in Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.G.; Hotte, S.J.; Chen, E.X.; Chin, S.F.; Turner, S.; Wang, L.; Siu, L.L. A phase II study of sunitinib in recurrent and/or metastatic adenoid cystic carcinoma (ACC) of the salivary glands: Current progress and challenges in evaluating molecularly targeted agents in ACC. Ann. Oncol. 2012, 23, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.L.; Le Tourneau, C.; et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am. J. Clin. Oncol. 2018, 41, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P.M.; Petroni, G.R.; Horton, B.J.; Moskaluk, C.A.; Fracasso, P.M.; Douvas, M.G.; Varhegyi, N.; Zaja-Milatovic, S.; Thomas, C.Y. A Phase II Study of Dovitinib in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma. Clin. Cancer Res. 2017, 23, 4138–4145. [Google Scholar] [CrossRef]
- Dogan, S.; Ng, C.K.Y.; Xu, B.; Kumar, R.; Wang, L.; Edelweiss, M.; Scott, S.N.; Zehir, A.; Drilon, A.; Morris, L.G.T.; et al. The repertoire of genetic alterations in salivary duct carcinoma including a novel HNRNPH3-ALK rearrangement. Hum. Pathol. 2019, 88, 66–77. [Google Scholar] [CrossRef]
- Even, C.; Delord, J.P.; Price, K.A.; Nakagawa, K.; Oh, D.Y.; Burge, M.; Chung, H.C.; Doi, T.; Fakih, M.; Takahashi, S.; et al. Evaluation of pembrolizumab monotherapy in patients with previously treated advanced salivary gland carcinoma in the phase 2 KEYNOTE-158 study. Eur. J. Cancer 2022, 171, 259–268. [Google Scholar] [CrossRef]
- Even, C.; Lassen, U.; Merchan, J.; Le Tourneau, C.; Soria, J.C.; Ferte, C.; Ricci, F.; Diener, J.T.; Yuen, E.; Smith, C.; et al. Safety and clinical activity of the Notch inhibitor, crenigacestat (LY3039478), in an open-label phase I trial expansion cohort of advanced or metastatic adenoid cystic carcinoma. Investig. New Drugs 2020, 38, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Feeney, L.; Jain, Y.; Beasley, M.; Donnelly, O.; Kong, A.; Moleron, R.; Nallathambi, C.; Rolles, M.; Sanghera, P.; Tin, A.; et al. Centralised RECIST Assessment and Clinical Outcomes with Lenvatinib Monotherapy in Recurrent and Metastatic Adenoid Cystic Carcinoma. Cancers 2021, 13, 4336. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Eckhardt, G.; Patnaik, A.; LoRusso, P.; Faoro, L.; Heymach, J.V.; Kapoun, A.M.; Xu, L.; Munster, P. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 2018, 29, 1561–1568. [Google Scholar] [CrossRef]
- Fushimi, C.; Tada, Y.; Takahashi, H.; Nagao, T.; Ojiri, H.; Masubuchi, T.; Matsuki, T.; Miura, K.; Kawakita, D.; Hirai, H.; et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann. Oncol. 2018, 29, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, N.; Mais, K.; Shenjere, P.; Julyan, P.; Hastings, D.; Ward, T.; Ryder, W.D.; Bruce, I.; Homer, J.; Slevin, N.J. Phase II study of cisplatin and imatinib in advanced salivary adenoid cystic carcinoma. Br. J. Oral Maxillofac. Surg. 2011, 49, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, P.H.; Heilbrun, L.K.; Barrett, M.T.; Kummar, S.; Hansen, A.R.; Siu, L.L.; Piekarz, R.L.; Sukari, A.W.; Chao, J.; Pilat, M.J.; et al. A phase 2 study of vorinostat in locally advanced, recurrent, or metastatic adenoid cystic carcinoma. Oncotarget 2017, 8, 32918–32929. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Colevas, A.D.; Krane, J.F.; Cooper, D.; Glisson, B.; Amrein, P.C.; Weeks, L.; Costello, R.; Posner, M. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003, 39, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Hitre, E.; Budai, B.; Takácsi-Nagy, Z.; Rubovszky, G.; Tóth, E.; Remenár, É.; Polgár, C.; Láng, I. Cetuximab and platinum-based chemoradio- or chemotherapy of patients with epidermal growth factor receptor expressing adenoid cystic carcinoma: A phase II trial. Br. J. Cancer 2013, 109, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Dunn, L.; Sherman, E.J.; Fury, M.G.; Baxi, S.S.; Chandramohan, R.; Dogan, S.; Morris, L.G.; Cullen, G.D.; Haque, S.; et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann. Oncol. 2016, 27, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Foster, N.R.; Zoroufy, A.J.; Campbell, J.D.; Worden, F.; Price, K.; Adkins, D.; Bowles, D.W.; Kang, H.; Burtness, B.; et al. Phase II Study of Enzalutamide for Patients with Androgen Receptor–Positive Salivary Gland Cancers (Alliance A091404). J. Clin. Oncol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Ho, A.L.; Sherman, E.J.; Baxi, S.S.; Haque, S.; Ni, A.; Antonescu, C.R.; Katabi, N.; Morris, L.G.; Chan, T.A.; Pfister, D.G. Phase II study of regorafenib in progressive, recurrent/metastatic adenoid cystic carcinoma. J. Clin. Oncol. 2016, 34, 6096. [Google Scholar] [CrossRef]
- Hoover, A.C.; Milhem, M.M.; Anderson, C.M.; Sun, W.; Smith, B.J.; Hoffman, H.T.; Buatti, J.M. Efficacy of nelfinavir as monotherapy in refractory adenoid cystic carcinoma: Results of a phase II clinical trial. Head Neck 2015, 37, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Hotte, S.J.; Winquist, E.W.; Lamont, E.; MacKenzie, M.; Vokes, E.; Chen, E.X.; Brown, S.; Pond, G.R.; Murgo, A.; Siu, L.L. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: A Princess Margaret Hospital phase II consortium study. J. Clin. Oncol. 2005, 23, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Jakob, J.A.; Kies, M.S.; Glisson, B.S.; Kupferman, M.E.; Liu, D.D.; Lee, J.J.; El-Naggar, A.K.; Gonzalez-Angulo, A.M.; Blumenschein, G.R., Jr. Phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck 2015, 37, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.J.; Ahn, M.J.; Ock, C.Y.; Lee, K.W.; Kwon, J.H.; Yang, Y.; Choi, Y.H.; Kim, M.K.; Ji, J.H.; Yun, T.; et al. Randomized Phase II Study of Axitinib versus Observation in Patients with Recurred or Metastatic Adenoid Cystic Carcinoma. Clin. Cancer Res. 2021, 27, 5272–5279. [Google Scholar] [CrossRef] [PubMed]
- Keam, B.; Kim, S.B.; Shin, S.H.; Cho, B.C.; Lee, K.W.; Kim, M.K.; Yun, H.J.; Lee, S.H.; Yoon, D.H.; Bang, Y.J. Phase 2 study of dovitinib in patients with metastatic or unresectable adenoid cystic carcinoma. Cancer 2015, 121, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Oh, D.Y.; Shin, S.H.; Kang, J.H.; Cho, B.C.; Chung, J.S.; Kim, H.; Park, K.U.; Kwon, J.H.; Han, J.Y.; et al. A multicenter phase II study of everolimus in patients with progressive unresectable adenoid cystic carcinoma. BMC Cancer 2014, 14, 795. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, S.J.; Lee, J.Y.; Lee, S.H.; Sun, J.M.; Park, K.; An, H.J.; Cho, J.Y.; Kang, E.J.; Lee, H.Y.; et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: A multicenter phase 2 study (Korean Cancer Study Group HN14-01). Cancer 2017, 123, 1958–1964. [Google Scholar] [CrossRef]
- Kurzrock, R.; Bowles, D.W.; Kang, H.; Meric-Bernstam, F.; Hainsworth, J.; Spigel, D.R.; Bose, R.; Burris, H.; Sweeney, C.J.; Beattie, M.S.; et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: Results from MyPathway, a phase IIa multiple basket study. Ann. Oncol. 2020, 31, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Shen, R.; Offin, M.; Buonocore, D.J.; Myers, M.L.; Venkatesh, A.; Razavi, P.; Ginsberg, M.S.; Ulaner, G.A.; Solit, D.B.; et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): Results from a phase II basket trial. J. Clin. Oncol. 2019, 37, 6001. [Google Scholar] [CrossRef]
- Locati, L.D.; Cavalieri, S.; Bergamini, C.; Resteghini, C.; Alfieri, S.; Calareso, G.; Bossi, P.; Perrone, F.; Tamborini, E.; Quattrone, P.; et al. Phase II trial with axitinib in recurrent and/or metastatic salivary gland cancers of the upper aerodigestive tract. Head Neck 2019, 41, 3670–3676. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Cavalieri, S.; Bergamini, C.; Resteghini, C.; Colombo, E.; Calareso, G.; Mariani, L.; Quattrone, P.; Alfieri, S.; Bossi, P.; et al. Abiraterone Acetate in Patients with Castration-Resistant, Androgen Receptor-Expressing Salivary Gland Cancer: A Phase II Trial. J. Clin. Oncol. 2021, 39, 4061–4068. [Google Scholar] [CrossRef] [PubMed]
- Locati, L.D.; Galbiati, D.; Calareso, G.; Alfieri, S.; Singer, S.; Cavalieri, S.; Bergamini, C.; Bossi, P.; Orlandi, E.; Resteghini, C.; et al. Patients with adenoid cystic carcinomas of the salivary glands treated with lenvatinib: Activity and quality of life. Cancer 2020, 126, 1888–1894. [Google Scholar] [CrossRef]
- Locati, L.D.; Perrone, F.; Cortelazzi, B.; Bergamini, C.; Bossi, P.; Civelli, E.; Morosi, C.; Lo Vullo, S.; Imbimbo, M.; Quattrone, P.; et al. A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: Translational analyses and clinical impact. Eur. J. Cancer 2016, 69, 158–165. [Google Scholar] [CrossRef]
- Mahadevan, D.; Sutton, G.R.; Arteta-Bulos, R.; Bowden, C.J.; Miller, P.J.; Swart, R.E.; Walker, M.S.; Haluska, P.; Munster, P.N.; Marshall, J.; et al. Phase 1b study of safety, tolerability and efficacy of R1507, a monoclonal antibody to IGF-1R in combination with multiple standard oncology regimens in patients with advanced solid malignancies. Cancer Chemother. Pharmacol. 2014, 73, 467–473. [Google Scholar] [CrossRef]
- Mahmood, U.; Bang, A.; Chen, Y.H.; Mak, R.H.; Lorch, J.H.; Hanna, G.J.; Nishino, M.; Manuszak, C.; Thrash, E.M.; Severgnini, M.; et al. A Randomized Phase 2 Study of Pembrolizumab with or without Radiation in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.R.; Talmi, Y.; Catane, R.; Symon, Z.; Yosepovitch, A.; Levitt, M. A phase II study of Imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. 2007, 43, 33–36. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Martins, R.G.; Baik, C.; Chow, L.Q.; Santana-Davila, R.; Goulart, B.H.; Lee, S.; Eaton, K.D. Phase II trial of eribulin mesylate in recurrent or metastatic salivary gland malignancies. Head Neck 2018, 40, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.P.; Wu, Q.V.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Siu, L.L.; Rasco, D.W.; Postel Vinay, S.; Martin Romano, P.; Menis, J.; Opdam, F.L.; Heinhuis, K.M.; Egger, J.L.; Gorman, S.A.; Parasrampuria, R.; et al. METEOR-1: A phase I study of GSK3326595, a first-in-class protein arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumours. Ann. Oncol. 2019, 30, v159. [Google Scholar] [CrossRef]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II Trial of Trastuzumab and Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2–Positive Salivary Duct Carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef]
- Tchekmedyian, V.; Sherman, E.J.; Dunn, L.; Tran, C.; Baxi, S.; Katabi, N.; Antonescu, C.R.; Ostrovnaya, I.; Haque, S.S.; Pfister, D.G.; et al. Phase II Study of Lenvatinib in Patients with Progressive, Recurrent or Metastatic Adenoid Cystic Carcinoma. J. Clin. Oncol. 2019, 37, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.J.; Silva, P.; Denton, K.; Bonington, S.; Mak, S.K.; Swindell, R.; Homer, J.; Sykes, A.J.; Lee, L.W.; Yap, B.K.; et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck 2015, 37, 182–187. [Google Scholar] [CrossRef]
- Van Boxtel, W.; Uijen, M.J.M.; Krens, S.D.; Dijkema, T.; Willems, S.M.; Jonker, M.A.; Pegge, S.A.H.; van Engen-van Grunsven, A.C.H.; van Herpen, C.M.L. Excessive toxicity of cabozantinib in a phase II study in patients with recurrent and/or metastatic salivary gland cancer. Eur. J. Cancer 2022, 161, 128–137. [Google Scholar] [CrossRef]
- Wong, S.J.; Karrison, T.; Hayes, D.N.; Kies, M.S.; Cullen, K.J.; Tanvetyanon, T.; Argiris, A.; Takebe, N.; Lim, D.; Saba, N.F.; et al. Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors. Ann. Oncol. 2016, 27, 318–323. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, L.; Dou, S.; Li, R.; Li, J.; Ye, L.; Jiang, W.; Dong, M.; Ruan, M.; Yang, W.; et al. Apatinib in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: A single-arm, phase II prospective study. Ther. Adv. Med. Oncol. 2021, 13, 17588359211013626. [Google Scholar] [CrossRef]
- Dong, M.; Ning, Z.Q.; Xing, P.Y.; Xu, J.L.; Cao, H.X.; Dou, G.F.; Meng, Z.Y.; Shi, Y.K.; Lu, X.P.; Feng, F.Y. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother. Pharmacol. 2012, 69, 1413–1422. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Krijger, G.C.; de Bree, R.; Willems, S.M.; de Keizer, B. Prostate-specific membrane antigen PET imaging and immunohistochemistry in adenoid cystic carcinoma-a preliminary analysis. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; van Es, R.J.J.; Willems, S.M.; Braat, A.; Devriese, L.A.; de Bree, R.; de Keizer, B. First experiences with (177)Lu-PSMA-617 therapy for recurrent or metastatic salivary gland cancer. EJNMMI Res. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Van Boxtel, W.; Lutje, S.; van Engen-van Grunsven, I.C.H.; Verhaegh, G.W.; Schalken, J.A.; Jonker, M.A.; Nagarajah, J.; Gotthardt, M.; van Herpen, C.M.L. (68)Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: A phase 2 imaging study. Theranostics 2020, 10, 2273–2283. [Google Scholar] [CrossRef] [PubMed]
- Kerche, L.E.; de Sousa, E.A.; Squarize, C.H.; Oliveira, K.K.; Marchi, F.A.; Bettim, B.B.; Kowalski, L.P.; Soares, F.A.; Lourenco, S.V.; Coutinho-Camillo, C.M. EMT in salivary gland tumors: The expression of microRNAs miR-155 and miR-200c is associated with clinical-pathological parameters. Mol. Biol. Rep. 2022, 49, 2157–2167. [Google Scholar] [CrossRef]
- Margolis, M.; Janovitz, T.; Laird, J.; Mata, D.A.; Montesion, M.; Lee, J.K.; Madison, R.W.; Schrock, A.B.; Tukachinsky, H.; Allen, J.M.; et al. Activating IGF1R hotspot non-frameshift insertions define a novel, potentially targetable molecular subtype of adenoid cystic carcinoma. Mod. Pathol. 2022, 35, 1618–1623. [Google Scholar] [CrossRef]
- De Sousa, L.G.; Jovanovic, K.; Ferrarotto, R. Metastatic Adenoid Cystic Carcinoma: Genomic Landscape and Emerging Treatments. Curr. Treat. Options Oncol. 2022, 23, 1135–1150. [Google Scholar] [CrossRef]
- Feeney, L.; Hapuarachi, B.; Adderley, H.; Rack, S.; Morgan, D.; Walker, R.; Rauch, R.; Herz, E.; Kaye, J.; Harrington, K.; et al. Clinical disease course and survival outcomes following disease recurrence in adenoid cystic carcinoma with and without NOTCH signaling pathway activation. Oral Oncol. 2022, 133, 106028. [Google Scholar] [CrossRef]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Mitani, Y.; McGrail, D.J.; Li, K.; Karpinets, T.V.; Bell, D.; Frank, S.J.; Song, X.; Kupferman, M.E.; Liu, B.; et al. Proteogenomic Analysis of Salivary Adenoid Cystic Carcinomas Defines Molecular Subtypes and Identifies Therapeutic Targets. Clin. Cancer Res. 2021, 27, 852–864. [Google Scholar] [CrossRef]
- Karpinets, T.V.; Mitani, Y.; Liu, B.; Zhang, J.; Pytynia, K.B.; Sellen, L.D.; Karagiannis, D.T.; Ferrarotto, R.; Futreal, A.P.; El-Naggar, A.K. Whole-Genome Sequencing of Common Salivary Gland Carcinomas: Subtype-Restricted and Shared Genetic Alterations. Clin. Cancer Res. 2021, 27, 3960–3969. [Google Scholar] [CrossRef]
- Miller, L.E.; Au, V.; Mokhtari, T.E.; Goss, D.; Faden, D.L.; Varvares, M.A. A Contemporary Review of Molecular Therapeutic Targets for Adenoid Cystic Carcinoma. Cancers 2022, 14, 992. [Google Scholar] [CrossRef]
- Mueller, S.K.; Haderlein, M.; Lettmaier, S.; Agaimy, A.; Haller, F.; Hecht, M.; Fietkau, R.; Iro, H.; Mantsopoulos, K. Targeted Therapy, Chemotherapy, Immunotherapy and Novel Treatment Options for Different Subtypes of Salivary Gland Cancer. J. Clin. Med. 2022, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, M.; Blandino, G.; Di Agostino, S. MicroRNAs in head and neck squamous cell carcinoma: A possible challenge as biomarkers, determinants for the choice of therapy and targets for personalized molecular therapies. Transl. Cancer Res. 2021, 10, 3090–3110. [Google Scholar] [CrossRef] [PubMed]
- Witte, H.M.; Gebauer, N.; Steinestel, K. Mutational and immunologic Landscape in malignant Salivary Gland Tumors harbor the potential for novel therapeutic strategies. Crit. Rev. Oncol. Hematol. 2022, 170, 103592. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Morita, K.I.; Kayamori, K.; Tange, S.; Shibata, H.; Harazono, Y.; Michi, Y.; Ikeda, T.; Harada, H.; Imoto, I.; et al. Detection of novel fusion genes by next-generation sequencing-based targeted RNA sequencing analysis in adenoid cystic carcinoma of head and neck. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Saade, R.E.; Bell, D.; Garcia, J.; Roberts, D.; Weber, R. Role of CRTC1/MAML2 Translocation in the Prognosis and Clinical Outcomes of Mucoepidermoid Carcinoma. JAMA Otolaryngol.–Head Neck Surg. 2016, 142, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, J.; Gu, Y.; Hu, C.; Li, J.L.; Lin, S.; Shen, H.; Cao, C.; Gao, R.; Li, J.; et al. Aberrantly activated AREG–EGFR signaling is required for the growth and survival of CRTC1–MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene 2014, 33, 3869–3877. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, W.; Li, J.L.; Lin, S.; Zhou, X.; Sun, Y.; Li, J.W.; Leon, M.E.; Hurtado, M.D.; Zolotukhin, S.; et al. The CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight 2021, 6, e139497. [Google Scholar] [CrossRef]
- Ni, W.; Chen, Z.; Zhou, X.; Yang, R.; Yu, M.; Lu, J.; Kaye, F.J.; Wu, L. Targeting Notch and EGFR signaling in human mucoepidermoid carcinoma. Signal Transduct. Target. Ther. 2021, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Li, X.; Wang, Y.; Li, H.; Zhang, C.; Zhao, X.; Zhang, Z.; Ruan, M.; Zhang, C. HIF-1alpha induced NID1 expression promotes pulmonary metastases via the PI3K-AKT pathway in salivary gland adenoid cystic carcinoma. Oral Oncol. 2022, 131, 105940. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.X.; Wang, L.; Cai, M.; Meng, Y.; Tang, Y.T.; Zhu, Q.H.; Han, W.; Sun, N.N.; Ma, B.; Hu, Y.; et al. Sphk1 promotes salivary adenoid cystic carcinoma progression via PI3K/Akt signaling. Pathol. Res. Pract. 2021, 227, 153620. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Li, D.; Lu, Z.; Dong, X.; Wang, X. Type III TGF-beta receptor inhibits cell proliferation and migration in salivary glands adenoid cystic carcinoma by suppressing NF-kappaB signaling. Oncol. Rep. 2016, 35, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef]
- Allard, D.; Allard, B.; Stagg, J. On the mechanism of anti-CD39 immune checkpoint therapy. J. Immunother. Cancer 2020, 8, e000186. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Bauer, A.; Gebauer, N.; Knief, J.; Tharun, L.; Arnold, N.; Riecke, A.; Steinestel, K.; Witte, H.M. The expression of the adenosine pathway markers CD39 and CD73 in salivary gland carcinomas harbors the potential for novel immune checkpoint inhibition. J. Cancer Res. Clin. Oncol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Alame, M.; Cornillot, E.; Cacheux, V.; Tosato, G.; Four, M.; De Oliveira, L.; Gofflot, S.; Delvenne, P.; Turtoi, E.; Cabello-Aguilar, S.; et al. The molecular landscape and microenvironment of salivary duct carcinoma reveal new therapeutic opportunities. Theranostics 2020, 10, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- De Lima-Souza, R.A.; Scarini, J.F.; Lavareze, L.; Emerick, C.; Dos Santos, E.S.; Paes Leme, A.F.; Egal, E.S.A.; Altemani, A.; Mariano, F.V. Protein markers of primary salivary gland tumors: A systematic review of proteomic profiling studies. Arch. Oral Biol. 2022, 136, 105373. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.F.; Gong, L.; Cao, Z.; Ma, H.; Ji, W.; Deng, M.; Liu, M.; Hu, X.H.; Chen, P.; Yan, Q.; et al. alphaA- and alphaB-crystallins interact with caspase-3 and Bax to guard mouse lens development. Curr. Mol. Med. 2012, 12, 177–187. [Google Scholar] [CrossRef] [PubMed]
| Study | Setting | Agent | Target | Pts, n | Subtype * | ORR, n (%) * | Median PFS (Months) | Median OS (Months) |
|---|---|---|---|---|---|---|---|---|
| A. Monotherapy | ||||||||
| Retro [50] | R/M Any line | 5-fluorouracil | cytostatic drug | 12 | AdCC | 4 (33) | – | ITT: 21.0 |
| II [49] | R/M Any line | Cisplatin | cytostatic drug | 10 | AdCC | 7 (70) | – | – |
| II [46] | R/M First- or second-line | Cisplatin | cytostatic drug | 25 | AdCC: 13 Non-AdCC: 12 (5 MEC, 5 adeno, 1 AcCC, 1 SCC) | ITT: 4 (16) AdCC: 2 (15) Non-AdCC: 2 (17) (1 MEC, 1 SCC) | 7.0 | 14.0 |
| II [37] | R/M First- or second-line | Cisplatin | cytostatic drug | 10 | AdCC | 0 (0) | 3.0 | 21.0 |
| Retro [36] | R/M First-line | Cisplatin | cytostatic drugs | 34 | AdCC: 11 Non-AdCC: 23 (7 MEC, 14 adeno, 2 mixed) | ITT: 13 (38) AdCC: 2 (18) Non-AdCC: 11 (48) (3 MEC, 7 adeno, 1 mixed) | 7.0 | 15.0 |
| II [53] | R/M First- or second-line | Epirubicin | cytostatic drug | 20 | AdCC | 2 (10) | 3.7 | 15.6 |
| II [52] | R/M First-line | Gemcitabine | cytostatic drug | 21 | AdCC | 0 (0) | – | – |
| Retro [50] | R/M Any line | Methotrexate | cytostatic drug | 7 | AdCC | 0 (0) | – | ITT: 21.0 |
| II [54] | R/M First-line | Mitoxantrone | cytostatic drug | 32 | AdCC | 4 (13) | – | 18.0 |
| II [47] | R/M Any line | Mitoxantrone | cytostatic drug | 18 | AdCC | 1 (5) | – | 19.0 |
| II [40] | R/M First-line | Paclitaxel | cytostatic drug | 45 | AdCC: 14 Non-AdCC: 31 (14 MEC, 17 adeno) | ITT: 8 (18) AdCC: 0 (0) Non-AdCC: 8 (26) (3 MEC, 5 adeno) | 4.0 | ITT: 12.5 |
| II [34] | R/M First- or second line | Vinorelbine | cytostatic drug | 20 | AdCC: 13 Non-AdCC: 7 (5 adeno, 1 mixed, 1 undifferentiated carcinoma) | ITT: 4 (20) AdCC: 2 (15) Non-AdCC: 2 (29) (2 adeno) | 5.0 | 8.5 |
| B. Combination therapy | ||||||||
| II [32] | R/M Any line | Carboplatin + paclitaxel | cytostatic drugs | 14 | AdCC: 10 Non-AdCC: 9 (1 MEC, 1 adeno, 2 undifferentiated) | ITT: 2 (14) AdCC: 2 (20) Non-AdCC: 0 (0) | 6.0–13.5 | 12.5 |
| Retro [48] | R/M Any line | Carboplatin + paclitaxel | cytostatic drugs | 38 | AdCC: 9 Non-AdCC: 29 (1 MEC, 18 SDC, 4 adeno, 1 myoepithelial, 1 epithelial-myoepithelial, 4 ex pleomorphic adenoma) | ITT: 15 (39) AdCC: 1 (9) Non-AdCC: 14 (48) | ITT: 6.5 AdCC: 9.7 Non-AdCC: 6.2–6.5 | ITT: 26.5 AdCC: 21.9 Non-AdCC: 34.7–44.0 |
| II [41] | R/M Any line | Cisplatin + 5-fluorouracil | cytostatic drugs | 11 | AdCC | 0 (0) | 9.0 | 12.0 |
| II [37] | R/M First- or second line | Cisplatin + doxorubicin + bleomycin | cytostatic drugs | 9 | AdCC | 3 (33) | 10.0 | 12.0 |
| Retro [39] | R/M Any line | Cisplatin + doxorubicin + cyclophosphamide | cytostatic drugs | 13 | AdCC: 9 Non-AdCC: 4 (4 adeno) | ITT: 6 (46) AdCC: 3 (33) Non-AdCC: 3 (75) (3 adeno) | – | – |
| Retro [35] | R/M Any line | Cisplatin + doxorubicin + cyclophosphamide | cytostatic drugs | 8 | AdCC: 4 Non-AdCC: 4 (3 MEC, 1 adeno) | ITT: 5 (63) AdCC: 1 (25) Non-AdCC: 4 (100) (3 MEC, 1 adeno) | 5.0 | 11.0 |
| II [45] | R/M Any line | Cisplatin + doxorubicin + cyclophosphamide | cytostatic drugs | 22 | AdCC: 12 Non-AdCC: 10 (1 MEC, 2 SDC, 2 adeno, 1 NET, 3 myoepithelioma, 1 undifferentiated) | ITT: 6 (27) AdCC: 3 (25) Non-AdCC: 3 (30) (1 MEC, 1 SDC, 1 NET) | – | 21.0 |
| II [38] | R/M First-line | Cisplatin + doxorubicin + cyclophosphamide + 5-fluorouracil | cytostatic drugs | 16 | AdCC: 7 Non-AdCC: 9 (1 MEC, 8 adeno) | ITT: 8 (50) AdCC: 3 (42) Non-AdCC: 5 (56) (1 MEC, 4 adeno) | – | 16.8 |
| II [34] | R/M First- or second line | Cisplatin + vinorelbine | cytostatic drugs | 16 | AdCC: 9 Non-AdCC: 7 (1 MEC, 4 adeno, 2 undifferentiated) | ITT: 7 (44) AdCC: 4 (44) Non-AdCC: 3 (43) (2 adeno, 1 undifferentiated) | 7.0 | 11.0 |
| II [33] | R/M First-line | Cisplatin + vinorelvine | cytostatic drugs | 42 | AdCC: 24 Non-AdCC: 18 (2 MEC, 11 adeno, 2 mixed, 3 undifferentiated) | ITT: 13 (31) AdCC: 7 (17) Non-AdCC: 7 (39) (7 adeno) | 6.0 | 10.0 |
| II [33] | R/M Second-line | Cisplatin + vinorelvine | cytostatic drugs | 18 | AdCC: 10 Non-AdCC: 8 (9 MEC, 4 adeno, 2 undifferentiated) | ITT: 1 (6) AdCC: 0 (0) Non-AdCC: 1 (13) (1 adeno) | 3.5 | 4.0 |
| II [42] | R/M Any line | Cisplatin + vinorelvine | cytostatic drugs | 40 | AdCC: 19 Non-AdCC: 21 (6 MEC, 10 adeno, 2 AcCC, 1 myoepithelial, 1 ex pleomorphic adenoma, 1 undifferentiated) | ITT: 14 (35) AdCC: 6 (32) Non-AdCC: 8 (38) | 6.3 | 16.9 |
| II [51] | R/M First-line | Cyclophosphamide + vincristine + 5-fluorouracil | cytostatic drugs | 8 | AdCC | 2 (25) | 28.0 | 62.0 |
| II [43] | R/M First-line | Platin + docetaxel | cytostatic drugs | 41 | AdCC: 26 Non-AdCC: 15 (1 MEC, 10 SDC, 3 adeno, 1 SCC) | ITT: 19 (46) AdCC: 6 (23) Non-AdCC: 13 (87) (1 MEC, 9 SDC, 2 adeno, 1 SCC | ITT: 9.4 AdCC: 8.9 Non-AdCC: 10.5 | ITT: 28.2 AdCC: 27.6 Non-AdCC: 29.3 |
| II [44] | R/M First- or second line | Platin + gemcitabine | cytostatic drugs | 33 | AdCC: 10 Non-AdCC: 23 (4 MEC, 1 SDC, 5 AcCC, 9 adeno, 4 undifferentiated) | ITT: 8 (27) AdCC: 2 (20) Non-AdCC: 6 (26) (1 MEC, 1 SDC, 1 AcCC, 3 adeno) | – | 13.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cleymaet, R.; Vermassen, T.; Coopman, R.; Vermeersch, H.; De Keukeleire, S.; Rottey, S. The Therapeutic Landscape of Salivary Gland Malignancies—Where Are We Now? Int. J. Mol. Sci. 2022, 23, 14891. https://doi.org/10.3390/ijms232314891
Cleymaet R, Vermassen T, Coopman R, Vermeersch H, De Keukeleire S, Rottey S. The Therapeutic Landscape of Salivary Gland Malignancies—Where Are We Now? International Journal of Molecular Sciences. 2022; 23(23):14891. https://doi.org/10.3390/ijms232314891
Chicago/Turabian StyleCleymaet, Robbert, Tijl Vermassen, Renaat Coopman, Hubert Vermeersch, Stijn De Keukeleire, and Sylvie Rottey. 2022. "The Therapeutic Landscape of Salivary Gland Malignancies—Where Are We Now?" International Journal of Molecular Sciences 23, no. 23: 14891. https://doi.org/10.3390/ijms232314891
APA StyleCleymaet, R., Vermassen, T., Coopman, R., Vermeersch, H., De Keukeleire, S., & Rottey, S. (2022). The Therapeutic Landscape of Salivary Gland Malignancies—Where Are We Now? International Journal of Molecular Sciences, 23(23), 14891. https://doi.org/10.3390/ijms232314891







