Fc Gamma Receptor IIIB NA1/NA2/SH Polymorphisms Are Associated with Malaria Susceptibility and Antibody Levels to P. falciparum Merozoite Antigens in Beninese Children
Abstract
1. Introduction
2. Results
2.1. Characteristics of Study Participants
2.2. Associations between FCGR2A SNP, FCGR3B SNPs, and Malaria Infections or Parasite Density
2.3. Associations between FCGR3B-Combined SNPs and Malaria Infection or Parasite Density
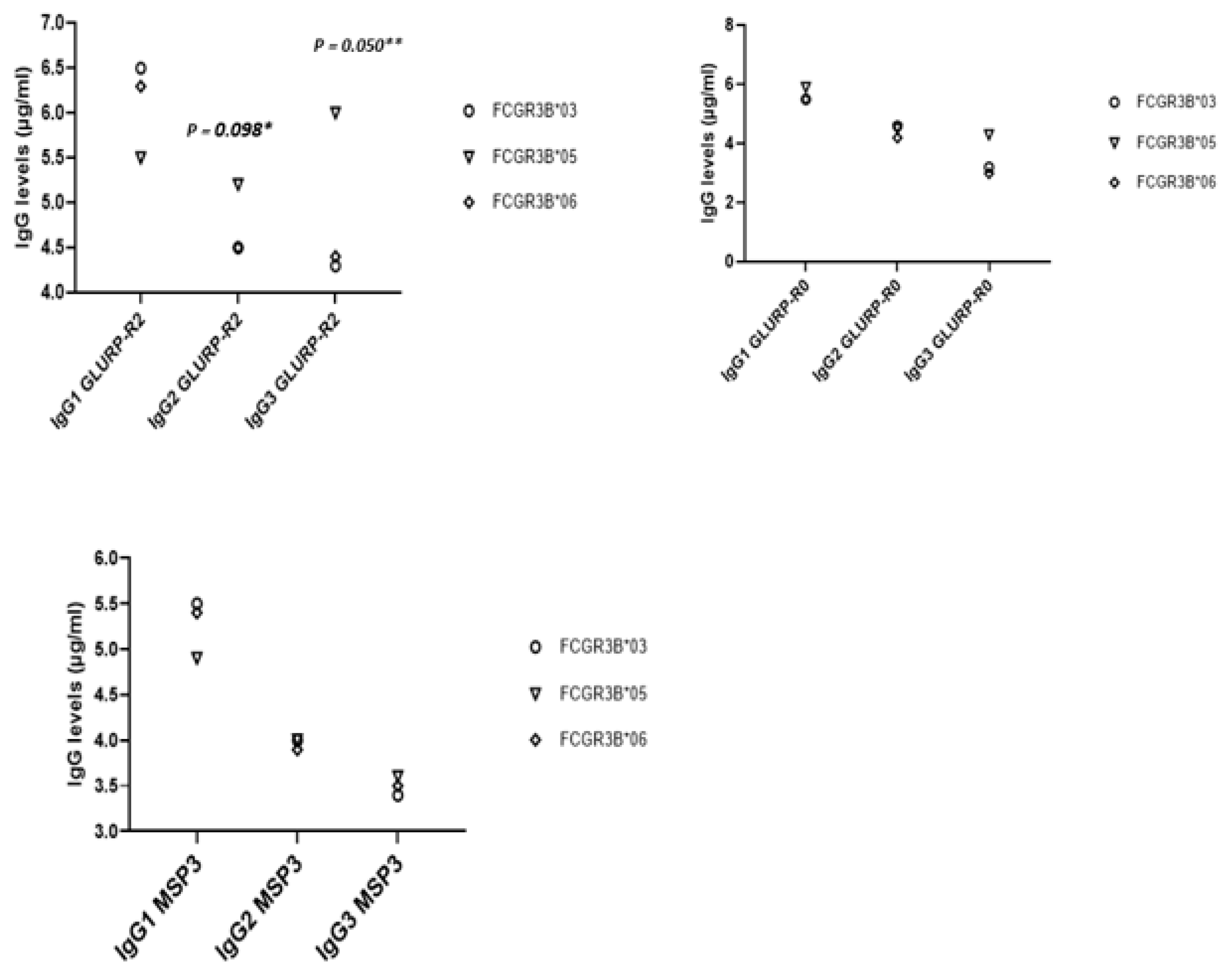
2.4. IgG Isotype Levels to P. Falciparum Merozoite Antigens, FCGR3B SNPs, and FCGR3B-Combined SNPs
3. Discussions
4. Materials and Methods
4.1. Study Area and Design
4.2. FcgRIIA Genotyping
4.3. Sequencing of FCGR3B Exon 3
4.4. ELISA Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aribot, G.; Druilhe, P.; Trape, J.-F.; Balde, A.T.; Sarthou, J.-L.; Rogier, C.; Roussilhon, C. Pattern of immunoglobulin isotype response to Plasmodium falciparum blood-stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, west Africa). Am. J. Trop. Med. Hyg. 1996, 54, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.P.; Felger, I.; Genton, B.; Alexander, N.; Al-Yaman, F.; Anders, R.F.; Alpers, M. Humoral and cell-mediated immunity to the Plasmodium falciparum ring-infected erythrocyte surface antigen in an adult population exposed to highly endemic malaria. Infect. Immun. Feb. 1995, 63, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.U.; Kimura, E.A.S.; Katzin, A.M.; Santos-Neto, L.L.; Ferrari, J.O.; Villalobos, J.M.; de Carvalho, M.E. The IgG-subclass distribution of naturally acquired antibodies to Plasmodium falciparum, in relation to malaria exposure and severity. Ann. Trop. Med. Parasitol. 1998, 92, 245–256. [Google Scholar] [CrossRef]
- Pleass, R.J.; Ogun, S.A.; McGuinness, D.H.; van de Winkel, J.G.J.; Holder, A.A.; Woof, J.M. Novel antimalarial antibodies highlight the importance of the antibody Fc region in mediating protection. Blood 2003, 102, 4424–4430. [Google Scholar] [CrossRef] [PubMed]
- Beeson, J.G.; Drew, D.R.; Boyle, M.; Feng, G.; Fowkes, F.; Richards, J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 2016, 40, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jönsson, F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front. Immunol. 2019, 10, 1958. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Wickramasinghe, S.N.; Phillips, R.E.; Looareesuwan, S.; Warrell, D.A.; Hughes, M. The bone marrow in human cerebral malaria: Parasite sequestration within sinusoids. Br. J. Haematol. 1987, 66, 295–306. [Google Scholar] [CrossRef]
- Aitken, E.H.; Alemu, A.; Rogerson, S.J. Neutrophils and Malaria. Front. Immunol. 2018, 9, 3005. [Google Scholar] [CrossRef]
- Kapelski, S.; Klockenbring, T.; Fischer, R.; Barth, S.; Fendel, R. Assessment of the neutrophilic antibody-dependent respiratory burst (ADRB) response to Plasmodium falciparum. J. Leukoc. Biol. 2014, 96, 1131–1142. [Google Scholar] [CrossRef]
- Cohen, S.; McGregor, I.A.; Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature 1961, 192, 733–737. [Google Scholar] [CrossRef]
- Ouma, C.; Keller, C.C.; Opondo, D.A.; Were, T.; Otieno, R.O.; Otieno, M.F.; Orago, A.S.S.; Ong’Echa, J.M.; Vulule, J.M.; Ferrell, R.E.; et al. Association of FCgamma receptor IIA (CD32) polymorphism with malarial anemia and high-density parasitemia in infants and young children. Am. J. Trop. Med. Hyg. 2006, 74, 573–577ss. [Google Scholar] [CrossRef] [PubMed]
- Courtin, D.; Oesterholt, M.; Huismans, H.; Kusi, K.; Milet, J.; Badaut, C.; Gaye, O.; Roeffen, W.; Remarque, E.J.; Sauerwein, R.; et al. The Quantity and Quality of African Children’s IgG Responses to Merozoite Surface Antigens Reflect Protection against Plasmodium falciparum Malaria. PLoS ONE 2009, 4, e7590. [Google Scholar] [CrossRef] [PubMed]
- Fall, A.K.D.J.; Dechavanne, C.; Sabbagh, A.; Guitard, E.; Milet, J.; Garcia, A.; Dugoujon, J.-M.; Courtin, D.; Migot-Nabias, F. Susceptibility to Plasmodium falciparum Malaria: Influence of Combined Polymorphisms of IgG3 Gm Allotypes and Fc Gamma Receptors IIA, IIIA, and IIIB. Front. Immunol. 2020, 11, 608016. [Google Scholar] [CrossRef] [PubMed]
- Parren, P.W.; Warmerdam, P.A.; Boeije, L.C.; Arts, J.; Westerdaal, N.A.; Vlug, A.; Capel, P.J.; Aarden, L.A.; van de Winkel, J.G. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J. Clin. Investig. 1992, 90, 1537–1546. [Google Scholar] [CrossRef]
- Maiga, B.; Dolo, A.; Touré, O.; Dara, V.; Tapily, A.; Campino, S.; Sepulveda, N.; Corran, P.; Rockett, K.; Clark, T.; et al. Fc gamma Receptor II a-H 131 R Polymorphism and Malaria Susceptibility in Sympatric Ethnic Groups, Fulani and Dogon of M ali. Scand. J. Immunol. 2014, 79, 43–50. [Google Scholar] [CrossRef]
- Warmerdam, P.A.; van de Winkel, J.G.; Gosselin, E.J.; Capel, P.J. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32). J. Exp. Med. 1990, 172, 19–25. [Google Scholar] [CrossRef]
- Fanger, M.W.; Shen, L.; Graziano, R.F.; Guyre, P.M. Cytotoxicity mediated by human Fc receptors for IgG. Immunol. Today 1989, 10, 92–99. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Edberg, J.C.; Salmon, J.E.; Kimberly, R.P. Functional capacity of Fc gamma receptor III (CD16) on human neutrophils. Immunol. Res. 1992, 11, 239–251. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Masson, P.L.; Pillai, S.; Scott, J. Les Bases de L’immunologie Fondamentale et Clinique; Elsevier Masson SAS, Elsevier Health Sciences: Issy-les-Moulineaux, France, 2020. [Google Scholar]
- Powell, M.S.; Hogarth, P.M. Fc Receptors. Multichain Immune Recognition Receptor Signaling: From Spatiotemporal Organization to Human Disease; Springer: Berlin/Heidelberg, Germany, 2008; pp. 24–34. [Google Scholar]
- Sondermann, P.; Kaiser, J.; Jacob, U. Molecular Basis for Immune Complex Recognition: A Comparison of Fc-Receptor Structures. J. Mol. Biol. 2001, 309, 737–749. [Google Scholar] [CrossRef]
- Zhang, Y.; Boesen, C.C.; Radaev, S.; Brooks, A.G.; Fridman, W.H.; Sautes-Fridman, C.; Sun, P.D. Crystal structure of the extracellular domain of a human Fc gamma RIII. Immunity 2000, 13, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.J.; Brown, E.J. CR3 (Mac-1, alpha M beta 2, CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: Requirement for Fc gamma RIII and tyrosine phosphorylation. J. Cell Biol. 1994, 125, 1407–1416. [Google Scholar] [CrossRef]
- Bux, J.; Kissel, K.; Hofmann, C.; Santoso, S. The use of allele-specific recombinant Fc gamma receptor IIIb antigens for the detection of granulocyte antibodies. Blood 1999, 93, 357–362. [Google Scholar] [CrossRef]
- Huizinga, T.W.; Kleijer, M.; Tetteroo, P.A.; Roos, D.; Von dem Borne, A.E. Biallelic neutrophil Na-antigen system is associated with a polymorphism on the phospho-inositol-linked Fc gamma receptor III (CD16). Blood 1990, 75, 213–217. [Google Scholar] [CrossRef]
- Ory, P.A.; Clark, M.R.; Kwoh, E.E.; Clarkson, S.B.; Goldstein, I.M. Sequences of complementary DNAs that encode the NA1 and NA2 forms of Fc receptor III on human neutrophils. J. Clin. Investig. 1989, 84, 1688–1691. [Google Scholar] [CrossRef]
- Bux, J.; Stein, E.L.; Bierling, P.; Fromont, P.; Clay, M.; Stroncek, D.; Santoso, S. Characterization of a new alloantigen (SH) on the human neutrophil Fc gamma receptor IIIb. Blood 1997, 89, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Adu, B.; Dodoo, D.; Adukpo, S.; Hedley, P.L.; Arthur, F.K.; Gerds, T.A.; Larsen, S.O.; Christiansen, M.; and Theisen, M. Fc gamma receptor IIIB (FcgammaRIIIB) polymorphisms are associated with clinical malaria in Ghanaian children. PLoS ONE 2012, 7, e46197. [Google Scholar] [CrossRef]
- Huizinga, T.W.; Kerst, M.; Nuyens, J.H.; Vlug, A.; von dem Borne, A.E.; Roos, D.; Tetteroo, P.A. Binding characteristics of dimeric IgG subclass complexes to human neutrophils. J. Immunol. 1989, 142, 2359–2364. [Google Scholar] [PubMed]
- Adu, B.; Jepsen, M.P.G.; Gerds, T.A.; Kyei-Baafour, E.; Christiansen, M.; Dodoo, D.; Theisen, M. Fc Gamma Receptor 3B (FCGR3B-c.233C>A-rs5030738) Polymorphism Modifies the Protective Effect of Malaria Specific Antibodies in Ghanaian Children. J. Infect. Dis. 2014, 209, 285–289. [Google Scholar] [CrossRef]
- Van Sorge, N.; Van Der Pol, W.-L.; Van De Winkel, J. FcγR polymorphisms: Implications for function, disease susceptibility and immunotherapy. Tissue Antigens 2003, 61, 189–202. [Google Scholar] [CrossRef]
- Iriemenam, N.C.; Khirelsied, A.H.; Nasr, A.; El Ghazali, G.; Giha, H.A.; A-Elgadir, T.M.E.; Agab-Aldour, A.A.; Montgomery, S.M.; Anders, R.F.; Theisen, M.; et al. Antibody responses to a panel of Plasmodium falciparum malaria blood-stage antigens in relation to clinical disease outcome in Sudan. Vaccine 2009, 27, 62–71. [Google Scholar] [CrossRef]
- Stanisic, D.I.; Richards, J.S.; McCallum, F.J.; Michon, P.; King, C.L.; Schoepflin, S.; Gilson, P.R.; Murphy, V.J.; Anders, R.F.; Mueller, I.; et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 2009, 77, 1165–1174. [Google Scholar] [CrossRef]
- Roussilhon, C.; Oeuvray, C.; Müller-Graf, C.; Tall, A.; Rogier, C.; Trape, J.F.; Theisen, M.; Balde, A.; Pérignon, J.-L.; Druilhe, P. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007, 4, e320. [Google Scholar] [CrossRef] [PubMed]
- Koene, H.R.; Kleijer, M.; Roos, D.; de Haas, M.; dem Borne, A.E. Fc gamma RIIIB gene duplication: Evidence for presence and expression of three distinct Fc gamma RIIIB genes in NA(1+,2+) SH(+) individuals. Blood 1998, 91, 673–679. [Google Scholar] [CrossRef]
- Munde, E.O.; Okeyo, W.A.; Raballah, E.; Anyona, S.B.; Were, T.; Ong’Echa, J.M.; Perkins, D.J.; Ouma, C. Association between Fcg receptor IIA, IIIA and IIIB genetic polymorphisms and susceptibility to severe malaria anemia in children in western Kenya. BMC Infect Dis. 2017, 17, 289. [Google Scholar] [CrossRef]
- Ouma, C.; Davenport, G.C.; Garcia, S.; Kempaiah, P.; Chaudhary, A.; Were, T.; Anyona, S.B.; Raballah, E.; Konah, S.N.; Hittner, J.B.; et al. Functional haplotypes of Fc gamma (Fcgamma) receptor (FcgammaRIIA and FcgammaRIIIB) predict risk to repeated episodes of severe malarial anemia and mortality in Kenyan children. Hum Genet. 2012, 131, 289–299. [Google Scholar] [CrossRef]
- Cottrell, G.; Kouwaye, B.; Pierrat, C.; Le Port, A.; Bouraima, A.; Fonton, N.; Hounkonnou, M.N.; Massougbodji, A.; Corbel, V.; Garcia, A. Modeling the influence of local environmental factors on malaria transmission in Benin and its implications for cohort study. PLoS ONE 2012, 7, e28812. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-H.; Gibson, J.P. Genetic polymorphisms in the leptin gene and their association with fatness in four pig breeds. Mamm. Genome 1999, 10, 191–193. [Google Scholar] [CrossRef] [PubMed]



| Characteristics of the Study Group | At Least One P. Falciparum Infection in Infants * | ||
|---|---|---|---|
| No (n = 65) | Yes (n = 280) | p-Value | |
| Mothers | |||
| Married (n, %): | Yes: 64 (98.46) No: 1 (1.53) | Yes: 275 (98.21) No: 5 (1.78) | 0.893 a |
| Job (n, %): | Yes: 32 (49.23) No: 33 (50.77) | Yes: 118 (42.14) No: 162 (57.85) | 0.298 a |
| Number of infants, (median, Q1-Q3) | 2 (1–3) | 2 (1–4) | 0.071 a |
| Mother’s age (median, Q1-Q3) | 25 (22–28) | 25 (21.5–30) | 0.116 a |
| Infection during pregnancy (n, %): | Yes: 15 (23.07) No: 50 (76.92) | Yes: 87 (31.07) No: 193 (68.92) | 0.203 a |
| Infants | |||
| Birth weight (g, median, Q1-Q3) | 2950 (2710–3190) | 3030 (2810–3300) | 0.216 a |
| Sex (n, %): Male Female | 36 (55.38) 29 (44.61) | 147 (52.5) 133 (47.5) | 0.673 a |
| Ethnic group (n, %): Aïzo Fon Others | 42 (73.84) 12 (18.46) 11 (16.92) | 195 (69.64) 65 (23.21) 20 (7.14) | 0.041a |
| P. falciparum infections in infants: Mean number of infections Min-Max Parasite density (mean nb parasites/µL) | 0 | 4.44 1–16 9105.77 | 0.0001 a |
| Socio-economic Score c (mean ± SD) | 1.692 (1.08) | 1.646 (1.06) | 0.098 a |
| Bednet use score b (mean of use ± SD) | 3.62 (0.84) | 3.51 (0.86) | 0.315 a |
| Mosquito exposure (med of exposure, Q1–Q3) | 0.9 (0.6–1.2) | 1 (0.7–1.5) | 0.0001 a |
| Genetic Modification | Variation | Protein Variant | Genotype (Haplotype) n (%) | Minor Allele Frequency (%) | HWE Test a (p Value) | ||
|---|---|---|---|---|---|---|---|
| FCGR2A 494 A/G | rs1801274 | R131H | HH 58 (18.98) | RH 163 (53.09) | RR 86 (27.73) | H (45) | 1.52 (0.21) |
| FCGR3B | |||||||
| 108 G/C | rs403016 | R36S | GG [NA1] 83 (26.01) | GC 137 (42.94) | CC [NA2/SH] 99 (31.03) | G (47) | 6.15 (0.01) * |
| 114 C/T | rs447536 | synonymous coding (L38L) | CC [NA1] 82 (25.78) | CT 139 (43.71) | TT [NA2/SH] 97 (30.05) | C (47) | 4.88 (0.02) * |
| 194 A/G | rs448740 | N65S | AA [NA1] 66 (20.68) | AG 134 (42.00) | GG [NA2/SH] 119 (37.30) | A (42) | 5.90 (0.01) * |
| 197 G/T | rs374112391 | R66L | GG 5 (1.56) | GT 49 (15.36) | TT 265 (83.07) | G (9) | 2.29 (0.12) |
| 233 C/A | rs5030738 | A78D | CC [NA1/NA2] 217 (62.35) | CA 83 (23.85) | AA [SH] 18 (5.17) | A (19) | 6.41 (0.01) * |
| 244 A/G | rs147574249 | N82D | GG [NA1] 101 (31.66) | AG 127 (39.81) | AA [NA2/SH] 91 (28.52) | A (48) | 13.14 (<0.01) * |
| 297 G/T | rs368410676 | synonymous coding (P99P) | GG 292 (91.53) | GT 22 (6.89) | TT 5 (1.56) | T (5) | 24.33 (<0.01) * |
| 316 A/G | rs2290834 | I106V | GG [NA1] 37 (11.59) | GA 113 (35.42) | AA [NA2/SH] 169 (48.42) | G (29) | 6.72 (<0.01) * |
| 371 A/G | rs1373400409 | H124R | AA 53 (16.61) | GA 130 (40.75) | GG 136 (42.63) | A (37) | 5.04 (0.02) * |
| (A) | ||||
| n | IRR | CI 95% | p-Value | |
| Number of P. falciparum infections | ||||
| Genotypes | ||||
| RR | 86 | |||
| RH | 163 | 0.89 | 0.73; 1.09 | 0.272 |
| HH | 58 | 0.94 | 0.73; 1.21 | 0.649 |
| Alleles | ||||
| R | 0.95 | 0.76; 1.19 | 0.712 | |
| H | 0.92 | 0.76; 1.12 | 0.436 | |
| P. falciparum parasite density | ||||
| Genotypes | ||||
| RR | 86 | |||
| RH | 163 | 798.43 | −2662.13; 4259.00 | 0.651 |
| HH | 58 | −141.44 | −4602.44; 4319.54 | 0.950 |
| Alleles | ||||
| R | 289.88 | −3357.67; 3937.45 | 0.876 | |
| H | 669.48 | −2449.99; 3788.96 | 0.674 | |
| (B) | ||||
| n | IRR | CI 95% | p-Value | |
| Number of P. falciparum infections | ||||
| Genotypes | ||||
| FCGR3B 194 A/G | ||||
| GG | 119 | |||
| AG | 134 | 1.05 | 0.87; 1.28 | 0.553 |
| AA | 66 | 1.28 | 1.02; 1.61 | 0.027 |
| FCGR3B 297 G/T | ||||
| GG | 292 | |||
| GT | 22 | 0.73 | 0.50; 1.06 | 0.100 |
| TT | 5 | 0.46 | 0.21; 1.00 | 0.050 |
| Alleles | ||||
| FCGR3B 194 A/G | ||||
| A | 1.13 | 0.95; 1.35 | 0.157 | |
| G | 0.80 | 0.65; 0.97 | 0.029 | |
| FCGR3B 297 G/T | ||||
| G | 2.06 | 0.95; 4.47 | 0.067 | |
| T | 0.66 | 0.47; 0.93 | 0.018 | |
| Parasite density | ||||
| Genotypes | ||||
| FCGR3B 297 G/T | ||||
| GG | 292 | |||
| GT | 22 | −3984.73 | −9927.02; 1957.55 | 0.189 |
| TT | 5 | −8874.19 | −19,390.84; 1642.45 | 0.098 |
| Alleles | ||||
| FCGR3B 297 G/T | ||||
| G | 8656.59 | −1879.81; 19,193.01 | 0.100 | |
| T | −5134.44 | −10,382.69; 113.79 | 0.050 | |
| (A) | ||||
| FCGR3B combined polymorphisms | ||||
| Number of infections | n | IRR | CI 95% | p-Value |
| FCGR3B*05 108C–114T–194A–233C–244A–316A | 11 | 2.44 | 1.23; 4.86 | 0.011 |
| FCGR3B*06 108C–114T–194G–233C–244G–316A | 140 | 0.58 | 0.37; 0.91 | 0.018 |
| Parasite density | Coef. | CI 95% | p-Value | |
| FCGR3B*05 108C–114T–194A–233C–244A–316A | 11 | 29,699.73 | 16,242.34; 43,157.13 | 0.0001 |
| FCGR3B*06 108C–114T–194G–233C–244G–316A | 140 | −10,373.26 | −20,409.75; −336.7668 | 0.043 |
| (B) | ||||
| Number of infections | n | IRR | CI 95% | p-Value |
| FCGR3B*03 108C–114T–194G–233A–244A–316A | 65 | 0.53 | 0.29; 0.95 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fall, A.K.D.J.; Courtin, D.; Adamou, R.; Edslev, S.; Hansen, A.; Domingo, N.; Christiansen, M.; Adu, B.; Milet, J.; Garcia, A.; et al. Fc Gamma Receptor IIIB NA1/NA2/SH Polymorphisms Are Associated with Malaria Susceptibility and Antibody Levels to P. falciparum Merozoite Antigens in Beninese Children. Int. J. Mol. Sci. 2022, 23, 14882. https://doi.org/10.3390/ijms232314882
Fall AKDJ, Courtin D, Adamou R, Edslev S, Hansen A, Domingo N, Christiansen M, Adu B, Milet J, Garcia A, et al. Fc Gamma Receptor IIIB NA1/NA2/SH Polymorphisms Are Associated with Malaria Susceptibility and Antibody Levels to P. falciparum Merozoite Antigens in Beninese Children. International Journal of Molecular Sciences. 2022; 23(23):14882. https://doi.org/10.3390/ijms232314882
Chicago/Turabian StyleFall, Abdou Khadre Dit Jadir, David Courtin, Rafiou Adamou, Sofie Edslev, Anita Hansen, Nadia Domingo, Michael Christiansen, Bright Adu, Jacqueline Milet, André Garcia, and et al. 2022. "Fc Gamma Receptor IIIB NA1/NA2/SH Polymorphisms Are Associated with Malaria Susceptibility and Antibody Levels to P. falciparum Merozoite Antigens in Beninese Children" International Journal of Molecular Sciences 23, no. 23: 14882. https://doi.org/10.3390/ijms232314882
APA StyleFall, A. K. D. J., Courtin, D., Adamou, R., Edslev, S., Hansen, A., Domingo, N., Christiansen, M., Adu, B., Milet, J., Garcia, A., Theisen, M., Migot-Nabias, F., & Dechavanne, C. (2022). Fc Gamma Receptor IIIB NA1/NA2/SH Polymorphisms Are Associated with Malaria Susceptibility and Antibody Levels to P. falciparum Merozoite Antigens in Beninese Children. International Journal of Molecular Sciences, 23(23), 14882. https://doi.org/10.3390/ijms232314882





